Abstract
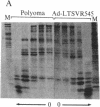
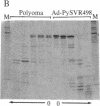
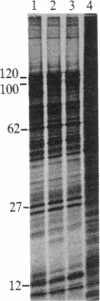
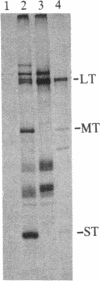
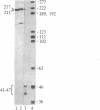
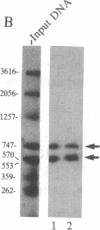
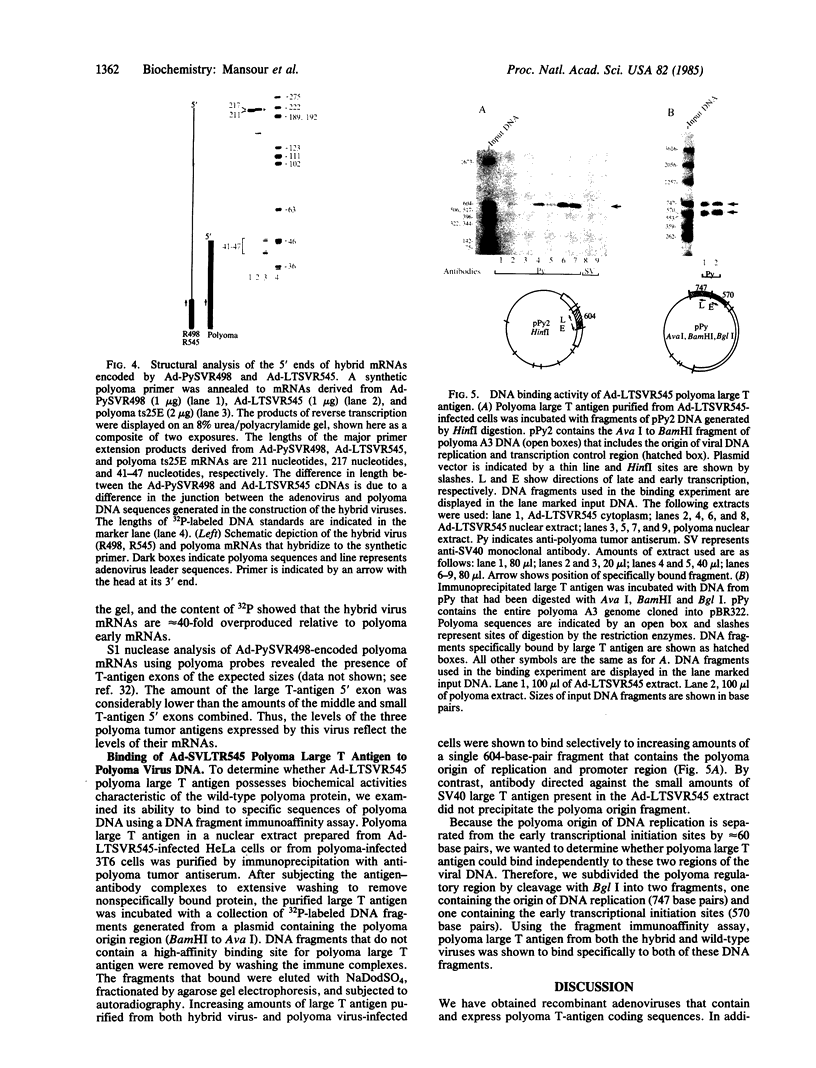
We have used a generalized adenovirus vector system to express the three polyoma tumor (T) antigen proteins under the control of the adenovirus major late promoter. One hybrid virus, Ad-PySVR498, expresses high levels of polyoma middle and small T antigens. A second hybrid virus, Ad-LTSVR545, which contains a cDNA copy of the polyoma A gene, overproduces large T antigen. The T antigens produced are indistinguishable from their authentic polyoma counterparts as determined by immunoprecipitation and partial cleavage by V8 protease. Analysis of polyoma mRNAs encoded by the recombinant viruses showed that they initiate from the adenovirus major late promoter and contain the tripartite leader at their 5' ends. Large T antigen isolated from Ad-LTSVR545-infected cells by immunoaffinity was shown to bind selectively to polyoma DNA sequences that contain the origin of viral DNA replication as well as the sites for transcription initiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Clark R., Peden K., Pipas J. M., Nathans D., Tjian R. Biochemical activities of T-antigen proteins encoded by simian virus 40 A gene deletion mutants. Mol Cell Biol. 1983 Feb;3(2):220–228. doi: 10.1128/mcb.3.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cowie A., Jat P., Kamen R. Determination of sequences at the capped 5' ends of polyoma virus early region transcripts synthesized in vivo and in vitro demonstrates an unusual microheterogeneity. J Mol Biol. 1982 Aug 5;159(2):225–255. doi: 10.1016/0022-2836(82)90494-6. [DOI] [PubMed] [Google Scholar]
- Deninger P. L., Esty A., LaPorte P., Hsu H., Friedmann T. The nucleotide sequence and restriction enzyme sites of the polyoma genome. Nucleic Acids Res. 1980 Feb 25;8(4):855–860. [PMC free article] [PubMed] [Google Scholar]
- Dilworth S. M., Cowie A., Kamen R. I., Griffin B. E. DNA binding activity of polyoma virus large tumor antigen. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1941–1945. doi: 10.1073/pnas.81.7.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart W. Complementation and transformation by temperature-sensitive mutants of polyoma virus. Virology. 1969 May;38(1):120–125. doi: 10.1016/0042-6822(69)90133-0. [DOI] [PubMed] [Google Scholar]
- Eckhart W., Hutchinson M. A., Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979 Dec;18(4):925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Gingeras T. R., Sciaky D., Gelinas R. E., Bing-Dong J., Yen C. E., Kelly M. M., Bullock P. A., Parsons B. L., O'Neill K. E., Roberts R. J. Nucleotide sequences from the adenovirus-2 genome. J Biol Chem. 1982 Nov 25;257(22):13475–13491. [PubMed] [Google Scholar]
- Griffin B. E., Fried M., Cowie A. Polyoma DNA: a physical map. Proc Natl Acad Sci U S A. 1974 May;71(5):2077–2081. doi: 10.1073/pnas.71.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzicker T., Lewis J. B., Anderson C. W. Conditional lethal mutants of adenovirus type 2-simian virus 40 hybrids. II. Ad2+ND1 host-range mutants that synthesize fragments of the Ad2+ND1 30K protein. J Virol. 1976 Aug;19(2):559–571. doi: 10.1128/jvi.19.2.559-571.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson M. A., Hunter T., Eckhart W. Characterization of T antigens in polyoma-infected and transformed cells. Cell. 1978 Sep;15(1):65–77. doi: 10.1016/0092-8674(78)90083-1. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Tjian R. Essential contact residues within SV40 large T antigen binding sites I and II identified by alkylation-interference. Cell. 1984 Jan;36(1):155–162. doi: 10.1016/0092-8674(84)90084-9. [DOI] [PubMed] [Google Scholar]
- Kamen R., Jat P., Treisman R., Favaloro J., Folk W. R. 5' termini of polyoma virus early region transcripts synthesized in vivo by wild-type virus and viable deletion mutants. J Mol Biol. 1982 Aug 5;159(2):189–224. doi: 10.1016/0022-2836(82)90493-4. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Levine A. S., Levin M. J., Oxman M. N., Lewis A. M., Jr Studies of nondefective adenovirus 2-simian virus 40 hybrid viruses. VII. Characterization of the simian virus 40 RNA species induced by five nondefective hybrid viruses. J Virol. 1973 May;11(5):672–681. doi: 10.1128/jvi.11.5.672-681.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J., Shenk T. Adenovirus tripartite leader sequence enhances translation of mRNAs late after infection. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3655–3659. doi: 10.1073/pnas.81.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Rio D. C., Robbins A. K., Tjian R. SV40 gene expression is modulated by the cooperative binding of T antigen to DNA. Cell. 1981 Aug;25(2):373–384. doi: 10.1016/0092-8674(81)90056-8. [DOI] [PubMed] [Google Scholar]
- Pomerantz B. J., Mueller C. R., Hassell J. A. Polyomavirus large T antigen binds independently to multiple, unique regions on the viral genome. J Virol. 1983 Sep;47(3):600–610. doi: 10.1128/jvi.47.3.600-610.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABSON A. S., O'CONOR G. T., BEREZESKY I. K., PAUL F. J. ENHANCEMENT OF ADENOVIRUS GROWTH IN AFRICAN GREEN MONKEY KIDNEY CELL CULTURES BY SV40. Proc Soc Exp Biol Med. 1964 May;116:187–190. doi: 10.3181/00379727-116-29197. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Cowie A., Carr A., Glaichenhaus N., Kamen R., Cuzin F. The roles of individual polyoma virus early proteins in oncogenic transformation. Nature. 1982 Dec 23;300(5894):713–718. doi: 10.1038/300713a0. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Skehel J. J. The polypeptides of adenovirus-infected cells. J Gen Virol. 1972 Apr;15(1):45–57. doi: 10.1099/0022-1317-15-1-45. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Benjamin T. L. Phosphorylation of polyoma T antigens. Cell. 1979 Dec;18(4):935–946. doi: 10.1016/0092-8674(79)90206-x. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. Transforming genes and gene products of polyoma and SV40. CRC Crit Rev Biochem. 1982;13(3):215–286. doi: 10.3109/10409238209114230. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Thummel C. S., Burgess T. L., Tjian R. Properties of simian virus 40 small t antigen overproduced in bacteria. J Virol. 1981 Feb;37(2):683–697. doi: 10.1128/jvi.37.2.683-697.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel C., Tjian R., Grodzicker T. Construction of adenovirus expression vectors by site-directed in vivo recombination. J Mol Appl Genet. 1982;1(5):435–446. [PubMed] [Google Scholar]
- Thummel C., Tjian R., Grodzicker T. Expression of SV40 T antigen under control of adenovirus promoters. Cell. 1981 Mar;23(3):825–836. doi: 10.1016/0092-8674(81)90447-5. [DOI] [PubMed] [Google Scholar]
- Thummel C., Tjian R., Hu S. L., Grodzicker T. Translational control of SV40 T antigen expressed from the adenovirus late promoter. Cell. 1983 Jun;33(2):455–464. doi: 10.1016/0092-8674(83)90427-0. [DOI] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- Treisman R., Cowie A., Favaloro J., Jat P., Kamen R. The structures of the spliced mRNAs encoding polyoma virus early region proteins. J Mol Appl Genet. 1981;1(2):83–92. [PubMed] [Google Scholar]
- Treisman R., Novak U., Favaloro J., Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle-T protein. Nature. 1981 Aug 13;292(5824):595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- Zhu Z. Y., Veldman G. M., Cowie A., Carr A., Schaffhausen B., Kamen R. Construction and functional characterization of polyomavirus genomes that separately encode the three early proteins. J Virol. 1984 Jul;51(1):170–180. doi: 10.1128/jvi.51.1.170-180.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]