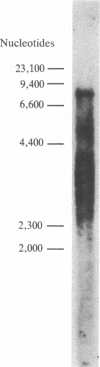
Abstract
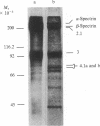
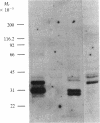
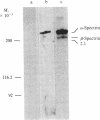
A cloned segment of mouse alpha-spectrin mRNA has been identified by immunological techniques. Double-stranded cDNA derived from spleens of anemic mice was introduced into a bacterial expression vector, pUC, and transformed Escherichia coli colonies were screened by using an antiserum to erythrocyte membrane ghost proteins. Of 17 positive colonies, 2 bound antibody to mouse spectrin, and these 2 colonies contained 750-base-pair inserts that cross-hybridized. Transfer of the 750-base-pair insert to an expression vector containing the PL promoter of phage lambda produced larger amounts of peptides that were bound by antibody to mouse spectrin. The spectrin-like peptides made in E. coli elicited antibody that reacted only with the alpha-spectrin subunit of erythrocyte membranes. This clone will be useful for the study of the structure and expression of the spectrin gene, particularly in understanding the role of spectrin in human inherited hemolytic anemias.
Full text
PDF
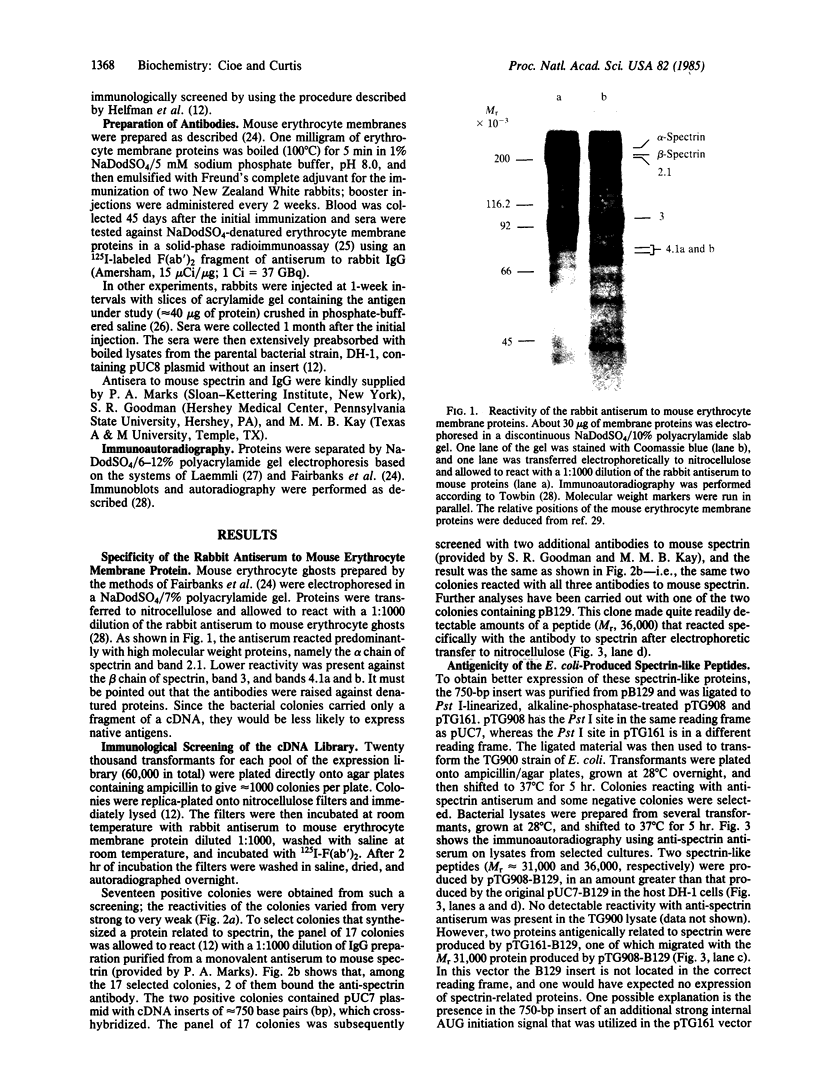



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baines A. J. The spread of spectrin. Nature. 1983 Feb 3;301(5899):377–378. doi: 10.1038/301377b0. [DOI] [PubMed] [Google Scholar]
- Branton D., Cohen C. M., Tyler J. Interaction of cytoskeletal proteins on the human erythrocyte membrane. Cell. 1981 Apr;24(1):24–32. doi: 10.1016/0092-8674(81)90497-9. [DOI] [PubMed] [Google Scholar]
- Courtney M., Buchwalder A., Tessier L. H., Jaye M., Benavente A., Balland A., Kohli V., Lathe R., Tolstoshev P., Lecocq J. P. High-level production of biologically active human alpha 1-antitrypsin in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Feb;81(3):669–673. doi: 10.1073/pnas.81.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis P. J. Cloning of mouse carbonic anhydrase mRNA and its induction in mouse erythroleukemic cells. J Biol Chem. 1983 Apr 10;258(7):4459–4463. [PubMed] [Google Scholar]
- Curtis P. J., Withers E., Demuth D., Watt R., Venta P. J., Tashian R. E. The nucleotide sequence and derived amino acid sequence of cDNA coding for mouse carbonic anhydrase II. Gene. 1983 Nov;25(2-3):325–332. doi: 10.1016/0378-1119(83)90237-8. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Helfman D. M., Feramisco J. R., Fiddes J. C., Thomas G. P., Hughes S. H. Identification of clones that encode chicken tropomyosin by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1983 Jan;80(1):31–35. doi: 10.1073/pnas.80.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles W., Marchesi S. L., Marchesi V. T. Spectrin: structure, function, and abnormalities. Semin Hematol. 1983 Jul;20(3):159–174. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Nelson W. J. Expression of spectrin in nonerythroid cells. Cell. 1982 Dec;31(3 Pt 2):505–508. doi: 10.1016/0092-8674(82)90306-3. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T. The red cell membrane skeleton: recent progress. Blood. 1983 Jan;61(1):1–11. [PubMed] [Google Scholar]
- Nelson W. J., Lazarides E. Switching of subunit composition of muscle spectrin during myogenesis in vitro. 1983 Jul 28-Aug 3Nature. 304(5924):364–368. doi: 10.1038/304364a0. [DOI] [PubMed] [Google Scholar]
- Privalsky M. L., Sealy L., Bishop J. M., McGrath J. P., Levinson A. D. The product of the avian erythroblastosis virus erbB locus is a glycoprotein. Cell. 1983 Apr;32(4):1257–1267. doi: 10.1016/0092-8674(83)90307-0. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Shohet S. B. Reconstitution of spectrin-deficient, spherocytic mouse erythrocyte membranes. J Clin Invest. 1979 Aug;64(2):483–494. doi: 10.1172/JCI109486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speicher D. W., Davis G., Marchesi V. T. Structure of human erythrocyte spectrin. II. The sequence of the alpha-I domain. J Biol Chem. 1983 Dec 25;258(24):14938–14947. [PubMed] [Google Scholar]
- Speicher D. W., Davis G., Yurchenco P. D., Marchesi V. T. Structure of human erythrocyte spectrin. I. Isolation of the alpha-I domain and its cyanogen bromide peptides. J Biol Chem. 1983 Dec 25;258(24):14931–14937. [PubMed] [Google Scholar]
- Summers J. Physical map of polyoma viral DNA fragments produced by cleavage with a restriction enzyme from Haemophilus aegyptius, endonuclease R-HaeIII. J Virol. 1975 Apr;15(4):946–953. doi: 10.1128/jvi.15.4.946-953.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Whitfield C. F., Mylin L. M., Goodman S. R. Species-dependent variations in erythrocyte membrane skeletal proteins. Blood. 1983 Mar;61(3):500–506. [PubMed] [Google Scholar]
- Wunner W. H., Curtis P. J., Wiktor T. J. Rabies mRNA translation in Xenopus laevis oocytes. J Virol. 1980 Oct;36(1):133–142. doi: 10.1128/jvi.36.1.133-142.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]