Abstract
Background
The nematode C. elegans is widely used as a model for understanding the neuronal and genetic bases of behavior. Recent studies have required longitudinal assessment of individual animal’s behavior over extended periods.
New Method
Here we present a technique for automated monitoring of multiple worms for several days. Our method uses an array of plano-concave glasswells containing standard agar media. The concave well geometry allows worms to be imaged even at the edge of the agar surface and prevents them from burrowing under the agar. We transfer one worm or embryo into each well, and perform imaging of the array of wells using a single camera. Machine vision software is used to quantify size, activity, and/or fluorescence of each worm over time.
Results
We demonstrate the utility of our method in two applications: (1) quantifying behavioral quiescence and developmental rate in wild-type and mutant animals, and (2) characterizing differences in mating behavior between two C. elegans strains.
Comparison with Existing Method(s)
Current techniques for tracking behavior in identified worms are generally restricted to imaging either single animals or have not been shown to work with arbitrary developmental stages; many are also technically complex. Our system works with up to 24 animals of any stages and is technically simple.
Conclusions
Our multi-well imaging method is a powerful tool for quantification of long-term behavioral phenotypes in C. elegans.
Keywords: C. elegans, behavior, development, imaging, lethargus, mating
1. Introduction
The nematode Caenorhabditis elegans is widely used in studies of neurobiology. Its advantages include its well-mapped, anatomically compact nervous system, genetic manipulability, and optical transparency. Traditional methods for assaying behavioral phenotypes largely rely on visual observation of worms through a stereo microscope. However, measurement of more subtle behavioral or growth characteristics, particularly those requiring long-term observation, is often only possible or practical using automated analysis with machine vision systems.
A number of ‘worm trackers’ for quantifying worm behavior have been described (Husson et al., 2012), including systems which image single or multiple worms. Where longitudinal imaging of defined worms is required, worms must be either imaged singly or confined to multiple compartments to avoid confusion between individuals. Strategies for compartmentalization include microfluidic devices (Lockery et al., 2008), microfabricated agar chambers (Bringmann, 2011), and nanodroplets (Belfer et al, 2013). However, these methods have been demonstrated with only a limited range of developmental stages or are associated with impaired survival.
Here we describe a simple and inexpensive multi-well technique for imaging up to 24 worms of any developmental stage for periods of several days. In our method, individual worms are segregated into small glass wells, allowing each animal to be tracked independently and at high resolution (~10–20 μm per pixel).
To demonstrate the utility of our multi-well method, we performed two sets of experiments. First we used it to continuously monitor the activity of multiple worms throughout their development from embryo to adulthood. We measured behavioral quiescence occurring during the larval transition stage known as lethargus (Singh and Sulston, 1978) as well as growth rates of wild-type and mutant animals. In previous work, such longitudinal imaging was limited to single animals (Raizen et al., 2008) or to single developmental stages (Singh et al., 2011; Nagy et al., 2013). As a proof of principle, we assay a mutant with a slow development phenotype, a mutant with a lethargus quiescence phenotype, and a mutant for a neurotransmitter synthesis pathway.
Second, we use our system to characterize differences in mating behavior between two C. elegans isolates. A strain of C. elegans (CB4856, isolated in Hawaii) is more efficient at mating than the standard laboratory strain (N2, isolated in Bristol, England): the probability that a single CB4856 male/hermaphrodite pair will mate successfully in a 48 h period is more than twice that of N2 (Bahrami and Zhang, 2013). However, it is unclear if this increased efficiency is due to an increased frequency of mating, increased duration of mating, and/or other factors. We sought to uncover behavioral reasons for increased mating in CB4856 compared to N2.
2. Materials and Methods
2.1 Well Preparation
Each well consists of a single plano-concave glass lens with the concave surface oriented upward. Each well is partially filled with standard Nematode Growth Medium agar (Sulston and Hodgkin, 1988) seeded with OP50 E. coli food bacteria (Fig. 1a). We arrange arrays of 6–24 of these wells, each containing a single worm (or a single pair of worms for mating experiments), and image them under dark field illumination (Fig. 1b–d). The concave well geometry allows for a clear image of the worms throughout the agar surface, unlike vertical-walled chambers which can obscure imaging at the edge of the agar. In addition, the acute angle formed at the edge of the agar prevents worms from burrowing under the agar surface.
Fig. 1.
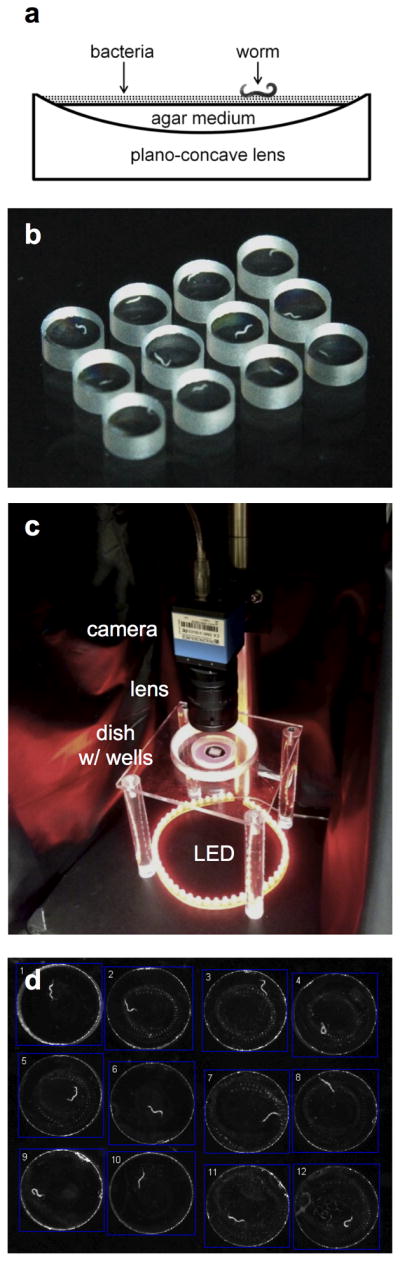
Experimental setup of multi-well imaging. (a) Schematic of one well. Optional mineral oil layer not shown. (b) Twelve wells each containing culture media and a single N2 L4 hermaphrodite. (c) Dark field imaging setup. Wells are surrounded by an annulus of NGM agar and wet tissue for humidification. (d) Camera image of 12 wells loaded with adult worms. Squares show regions of interest.
For single worm assays, we used uncoated plano-concave lenses with 2.5mm diameter and focal length −5.8 mm (concave surface radius of curvature R = 2.9mm, Anchor Optics AX27314). The agar volume was approximately 1 μl per well.
We aligned wells into a rectangular array at the center of a 60 mm or 100 mm diameter polystyrene Petri dish with vented lids (T3315 or T3301, Tritech Research). To reduce dehydration, an annulus (60 mm outer diameter, approximately 20 mm inner diameter) of solid NGM agar was positioned around the wells. We found that wet tissues or hydrated sodium polyacrylate crystals were also effective at humidifying the chamber. The Petri dish was covered by the vented lid to reduce dehydration while permitting gas exchange.
For development assays we covered the agar surfaces in each well with a thin layer (~100 μm) of mineral oil, which is sufficiently gas-permeable to support normal growth and behavior. While not strictly required, this step helped reduce evaporation from the agar during the 3-day experiments.
For mating assays, we used larger wells. Uncoated plano-concave lenses with 12.5 mm diameter and −15 mm focal length (approximate radius of curvature R = 7.5 mm, Anchor Optics AX75584) were filled with 100 μL agar per well. After the agar solidified, we added 10 μL of OP50 E. coli suspended in LB Broth onto each agar surface and allowed the excess liquid to evaporate. A single virgin adult hermaphrodite and single virgin adult male, prepared by transferring L4 worms from a standard mixed hermaphrodite/male population onto separate plates one day earlier, were then transferred to each well. The 100 mm diameter Petri dish carrying the array of wells was humidified during imaging with a ring of wet laboratory tissues. Due to the shorter imaging duration, mineral oil was not used in mating assays.
We performed activity analyses using 4 strains: N2, which was used as the reference strain, CB4876 (clk-1 (e2519), which contains a mutation in a hydroxylase responsible for ubiquinone synthesis), CB1112 (cat-2(e1112), which contains a mutation in an enzyme responsible for the rate-limiting step of dopamine synthesis) and MT1074 (egl-4(n479), which contains a loss-of-function mutation in a cGMP-dependent protein kinase) (Horvitz, 1982).
Mating experiments were performed using N2 and CB4856 animals.
2.2 Imaging System
The imaging system consisted of a camera, a lens, an LED illuminator, and mechanical components (Fig. 1). We used a monochrome camera at 1024×768 pixel resolution (DMK 31BU03, The Imaging Source) connected to a Pentax C22525KP C-Mount lens (focal length 25 mm, f/1.4–22) with a 10-mm C-mount extension ring to permit close focusing. The illuminator was a red Flexible LED Strip strip (48 cm long, 210 lumens, peak wavelength 619 nm, Oznium Inc.) curved into a 15 cm diameter ring and driven by a 12V DC power supply. The camera was secured to a 1.5” diameter verticalpost (Thorlabs P10) via a rail carrier (Thorlabs C1501) and pointed down toward the sample placed on an acrylic or glass stage plate. We attached the bottom of the post to a 12” × 12” × ¼” aluminum plate covered with black cloth and the top of the post to a 12” × 12” × ¼” black acrylic sheet. To minimize stray light, we covered the system with black cloth during experiments. We acquired time lapse images at 1 frame per 3 seconds and an exposure time of 1/10 – 1/15 second. Images were saved to hard drive in BMP format using the camera’s Image Capture software on a Windows 7 or XP computer. Experiments were performed at a temperature T ≈ 25±1°C.
2.3 Image analysis for development experiments
Worms in the glass wells were clearly visible during all developmental stages. Movie 1 shows a single N2 worm from the embryo stage to adulthood.
After completion of image acquisition we used custom MATLAB software to calculate behavioral quantities from the image data. First we manually selected regions of interest surrounding each well (Fig. 1d). We defined worm activity as the sum of the absolute difference in gray scale values between consecutive images (Fig. 2c). We chose this definition, rather than one based on the centroid or mid-body position of the animal, because it is sensitive to any type of movement; for example, worms frequently move only their anterior parts of the body while feeding. Next, we calculated a moving average across a 15-minute window. Fraction of quiescence is defined as the proportion of frames in a 30-minute window during which the activity level is below a custom-defined threshold. Because the activity level varies with the size of the animals, which increases greatly during development, the threshold for quiescence was set to be 0.1% of the total of pixel values within the region of interest. Total of pixel values was used because it gradually increases over time due to bacterial and animal growth.
Fig. 2.
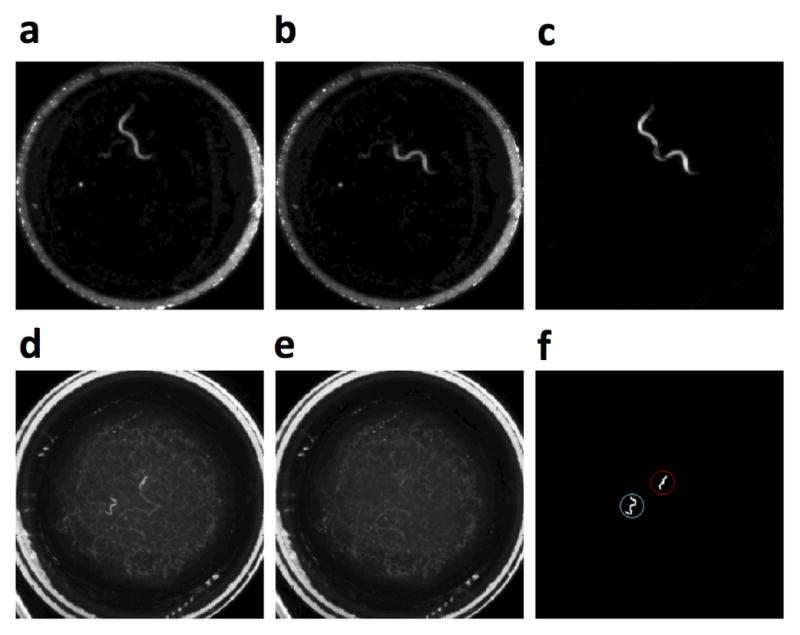
Image processing. (a–c) Development experiments. (a) A region of interest containing a single N2 L4 hermaphrodite. (b) Same region 3 seconds later. (c) Absolute difference between (a) and (b). Worm activity is represented by the sum of all pixel intensities in this image. (d–f) Mating experiments. (d) A region of interest containing an N2 adult male and an N2 adult hermaphrodite. (e) Calculated background image of the same region of interest. (f) Image containing only the animals, generated by subtracting (e) from (d). Red (upper right) and blue (lower left) circles denote the hermaphrodite and male, respectively.
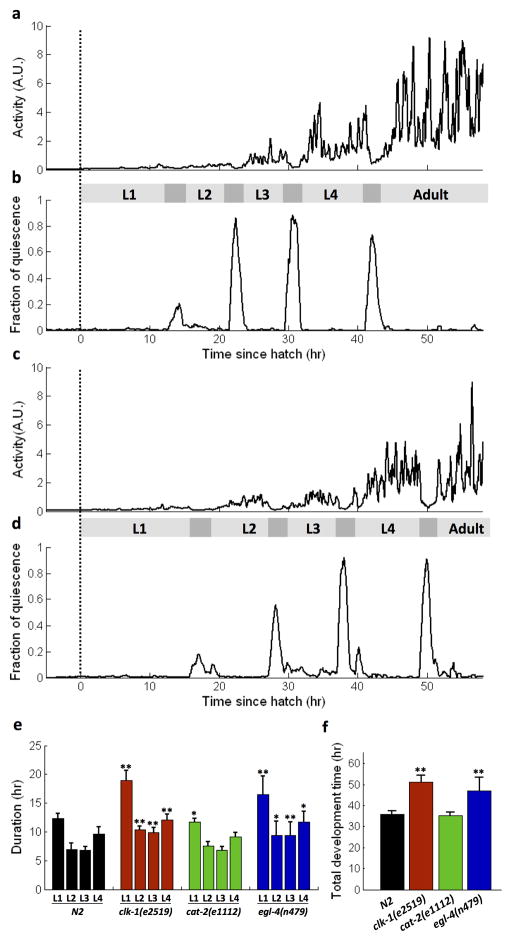
Plotting the activity level over time for one worm shows reduced activity during lethargus (Fig. 3a,c). The fraction of quiescence, which measures the level of inactivity of the animal, is derived from the activity value (Fig. 3b,d). Since lethargus occurs at the end of each larval stage, the duration of each larval stage can be measured by the time difference between the ends of two consecutive lethargus periods (or between hatching and the end of the first lethargus, for L1 animals). Total developmental time is defined as the interval from hatching to the end of L4 lethargus.
Fig. 3.
Monitoring animal development from embryo to adulthood. (a–b) A representative N2 wild type animal. (c–d) A representative clk-1(e2519) mutant. Dotted line represents hatch time of the animal. The gray bar between the activity and fraction of quiescence plots indicates non-lethargic (light) and lethargic (dark) periods. Activity is averaged over a 15-minute window, whereas the fraction of quiescence is average over a 30-minute window. (e) Duration of developmental stages strains N2 (n=39), clk-1(c2519) (n=10), cat-2(e1112) (n=11), egl-4(n479) (n=11). Duration of each stage includes both non-lethargic and lethargic periods. Significance tests are performed in comparison to equivalent stages in N2. *P<0.05, **P<0.005 (2-tailed Student’s t-test). Absence of label represents P>0.05. (f) total development time for each strain.
2.4 Image analysis for mating experiments
For mating experiments, images were divided into regions of interest as in development experiments. Next, for each image the background image was generated by calculating the pixel-by-pixel minimum of 5 equally-spaced frames within 10 minutes of the image being analyzed. The pixel-wise minimum method filters out bright moving objects, namely the animals (Fig. 2e). We choose a 10-minute window because it is long enough to guarantee locomotion between the frames in a mating assay, and short enough that changes in the background image are minimal. (A larger window reduces the amount of details, such as the traces on bacterial lawns, in the background image. Consequently, failure to capture non-animal objects in the background image increases segmentation error rate.) The background image is subtracted from the image, leaving an image containing only the animals (Fig. 2d–f). After background subtraction, the image is converted into a binary image using a threshold value, which is manually adjusted for each ROI (but constant over time) to provide clear segmentation of the two worms while rejecting background noise. Next, contiguous pixels in the binary image are grouped into regions. The regions whose areas are under a certain area threshold are removed. The area threshold is selected to be a value below the smallest area of the animal, but great enough to remove clusters of noise or other non-animal objects. This dual-threshold filtering process removes the background and the singleton high-intensity pixels (noise) from the image; only the regions with significant brightness and size are preserved in the end image. At this point, the centroids and areas of the two largest objects in the image are recorded. Next we calculate the inter-worm distance, i.e. the distance between the two centroids. If the processed image includes only one object (as is the case when the animals are mating) the inter-worm distance is assigned a value of zero. We define an encounter as a sequence of zero inter-worm distance that last for more than 5-frames (15 seconds). This threshold effectively removes occasional segmentation errors, in which the machine vision algorithm fails to capture one of the worms and erroneously assign a zero inter-worm distance to a frame in which the worms do not touch. To validate this algorithm we manually scored images acquired from 6 pairs of animals over of a total of 18h. We found that the machine vision identification of significant encounters agreed with that of visual inspection with 99.5% sensitivity and 93% specificity. Movie 2 shows the camera images and machine vision annotation of 3 pairs of N2 animals and 3 pairs of CB4856 animals over 10 minutes.
Next we calculated statistics concerning the encounters between the animals. For every image sequence from a pair we calculated three parameters: (1) proportion of mating frames, (2) mean duration of significant encounters and (3) frequency of significant encounters.
3. Results and discussion
3.1 Behavior Quiescence/Developmental Time Assay
We found a longer total development time in clk-1(e2519) relative to the wild-type (51.0 ± 3.2 h vs. 35.7 ± 1.7 h) at a temperature 25±1°C (Fig. 3e, p < 0.001). This 43 ± 5% longer developmental time in clk-1(e2519) animals is consistent with previous reports of 51.5 ± 20.0% longer developmental time (experiments conducted at 20°C) (Wong et al, 1995).
The egl-4 (n479) mutants, for which no developmental phenotypes have been described to our knowledge, exhibited a significantly longer developmental time than the wild-type (p < 0.001). Other published results indicated that loss-of-function egl-4 mutants have increased longevity (Hirose et al., 2003) and reduced quiescence during lethargus (Raizen et al., 2008). Our results further suggest that EGL-4 is required for normal developmental time.
We did not find a difference between cat-2 (e1112) mutants and wild type animals, suggesting that the neurotransmitter dopamine is not required for normal developmental timing.
3.2 Mating Assay
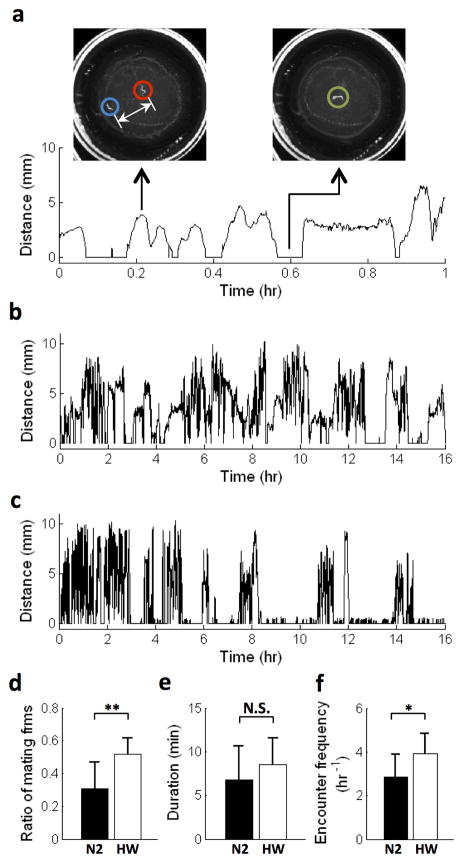
We found that pairs of CB4856 (Hawaiian) animals spend significantly higher proportions of time with each other compared to N2 (0.52 ± 0.10 h vs. 0.31 ± 0.16 h, all values reported as mean ± SEM). This could arise from a higher frequency of encounters, longer duration of encounters, or both. We found no difference between the duration of encounters in the two strains (Fig. 4e). However we found that CB4856 animals exhibited a significantly higher encounter frequency compared to N2 animals (2.86 ± 1.03 times per hour in CB4856 vs. 3.91 ± 0.95 times per hour for N2). These results suggest that the higher mating efficiency in CB4856 animals compared to N2 is due at least in part to a higher frequency of encounters.
Fig. 4.
Characterizing C. elegans mating behavior. (a–b) Distance between a pair of adult N2 worms. The red and blue circles denote the hermaphrodite and the male animals, respectively. The green circle denotes the two animals during an encounter, in which the distance is assigned a value of zero. (c) Distance between a pair of adult CB4856 worms. (d–f) Characteristics of mating behavior in N2 (n=9) and CB4856 (n=9). N.S., P>0.05. *P<0.05, **P<0.005. (2-tailed Student’s t-test)
3.3 Conclusions
In summary, we have developed a method for longitudinal imaging of multiple C. elegans. This method is technically simple, inexpensive, and works for all developmental stages. Our proof-of-principle experiments demonstrate that this method is well-suited for quantitatively comparing long-term behavioral phenotypes. While the experiments described here employ custom-built imaging setups and software, the multi-well technique can also be readily used with other imaging systems and with other worm tracking software. In addition, the multi-well method is compatible with other imaging modalities such as fluorescence microscopy.
Supplementary Material
Highlights.
We describe a method for quantifying long-term behavioral phenotypes in C. elegans.
Individual worms are placed in an array of glass wells filled with agar media
Machine vision analysis is used to quantify worm behavior for periods up to 3 days
We use the system to assay development time and mating in several strains
Our method is simple, inexpensive, and broadly useful in C. elegans neuroscience
Acknowledgments
Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). We thank Adam Bahrami for useful discussions. C. Y. was supported by the Center of Undergraduate Research and Fellowships (CURF) at the University of Pennsylvania, and by the Littlejohn Research Fellowship Program. D. R. was supported by the National Institutes of Health (R01NS064030) and a NARSAD Young Investigator Award. C. F. Y. was supported by an Alfred P. Sloan Research Fellowship, the Ellison Medical Foundation, and the National Institutes of Health (R01NS084835).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Chih-Chieh (Jay) Yu, Email: jayyu0528@gmail.com.
David M. Raizen, Email: raizen@mail.med.upenn.edu.
Christopher Fang-Yen, Email: fangyen@seas.upenn.edu.
References
- Bahrami AK, Zhang Y. When females produce sperm: genetics of C.elegans hermaphrodite reproductive choice. G3 (Bethesda) 2013;3(10):1851–59. doi: 10.1534/g3.113.007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfer SJ, Chuang HS, Freedman BL, Yuan J, Norton M, Bau HH, et al. Caenorhabditis-in-drop array for monitoring C.elegans quiescent behavior. Sleep. 2013;36(5):689–98. doi: 10.5665/sleep.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann H. Agarose hydrogel microcompartments for imaging sleep- and wake-like behavior and nervous system development in Caenorhabditis elegans larvae. J Neurosci Methods. 2011;201(1):78–88. doi: 10.1016/j.jneumeth.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Daniels SA, Ailion M, Thomas JH, Sengupta P. egl-4 acts through a transforming growth factor-beta/SMAD pathway in Caenorhabditis elegans to regulate multiple neuronal circuits in response to sensory cues. Genetics. 2000;156(1):123–41. doi: 10.1093/genetics/156.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science. 1982;216(4549):1012–4. 1012–4. doi: 10.1126/science.6805073. [DOI] [PubMed] [Google Scholar]
- Husson SJ, Costa WS, Schmitt C, Gottschalk A. Keeping track of worm trackers. WormBook; 2012. http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T, Nakano Y, Nagamatsu Y, Misumi T, Ohta H, Ohshima Y. Cyclic GMP-dependent protein kinase EGL-4 controls body size and lifespan in C. elegans. Development. 2003;130(6):1089–99. doi: 10.1242/dev.00330. [DOI] [PubMed] [Google Scholar]
- Lockery SR, Lawto KJ, Doll JC, Faumont S, Coulthard SM, Thiele TR, et al. Artificial dirt: microfluidic substrates for nematode neurobiology and behavior. J Neurophysiol. 2008;99(6):3136–43. doi: 10.1152/jn.91327.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsalve GC, Van Buskirk C, Frand AR. LIN-42/PERIOD controls cyclical and developmental progression of C. elegans molts. Curr Biol. 2011;21(24):2033–45. doi: 10.1016/j.cub.2011.10.054. [DOI] [PubMed] [Google Scholar]
- Nagy S, Wright C, Tramm N, Labello N, Burov S, Biron D. A longitudinal study of Caenorhabditis elegans larvae reveals a novel locomotion switch, regulated by Gαs signaling. Elife. 2013;2:e00782. doi: 10.7554/eLife.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizen DM, Zimmerman JE, Maycock MH, Ta UD, You YJ, Sundaram MV, et al. Lethargus is a Caenorhabditis elegans sleep-like state. Nature. 2008;451(7178):569–72. doi: 10.1038/nature06535. [DOI] [PubMed] [Google Scholar]
- Singh K, Chao MY, Somers GA, Komatsu H, Corkins ME, Larkins-Ford J, et al. C. elegans Notch signaling regulates adult chemosensory response and larval molting quiescence. Curr Biol. 2011;21(10):825–34. doi: 10.1016/j.cub.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Sulston J. Nematologica. Vol. 24. Brill; Boston: 1978. Some observations on moulting in Caenorhabditis elegans; pp. 63–71. [Google Scholar]
- Sulston J, Hodgkin J. The Nematode Caenorhabditis Elegans. Cold Spring Harbor Laboratory Press; New York: 1988. Method; p. 587. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.