Abstract
Objective
Diabetes and hypertension, common conditions in antiretroviral (ART) treated HIV-infected individuals, are associated with glomerular hyperfiltration, which precedes the onset of proteinuria and accelerated kidney function decline. In the Multicenter AIDS Cohort Study, we examined the extent to which hyperfiltration is present and associated with metabolic, cardiovascular, HIV and treatment risk factors among HIV-infected men.
Design
Cross-sectional cohort using direct measurement of glomerular filtration rate (GFR) by iohexol plasma clearance for 367 HIV-infected men and 241 HIV-uninfected men who were free of CKD.
Methods
Hyperfiltration was defined as GFR >140 ml/min/1.73m2 - 1 ml/min/1.73m2 per each year over age 40. Multivariate logistic regression was used to estimate the odds ratios (OR) of prevalent hyperfiltration for metabolic, cardiovascular, HIV and cumulative ART exposure factors.
Results
Among subjects without CKD, the prevalence of hyperfiltration was higher for HIV-infected participants (25%) compared to uninfected participants (17%; p=0.01). HIV infection was associated with hyperfiltration (OR: 1.70, 95%CI: 1.11, 2.61) and modified the association between diabetes and hyperfiltration, such that the association among HIV-uninfected men (OR: 2.56 95%CI: 1.33, 5.54) was not observed among HIV-infected men (OR: 1.19, 95%CI: 0.69, 2.05). These associations were independent of known risk factors for hyperfiltration. Indicators of hyperglycemia and hypertension were also associated with hyperfiltration as was cumulative zidovudine exposure.
Conclusions
Hyperfiltration, a potential modifiable predictor of kidney disease progression, is common among ART-treated HIV-infected men. HIV infection is associated with significant odds of hyperfiltration in addition to known risk factors for kidney damage.
Keywords: Glomerular hyperfiltration, glomerular filtration rate, HIV, antiretroviral therapy, iohexol
Introduction
Highly active antiretroviral therapy (HAART) use has resulted in marked reductions in AIDS-related mortality and opportunistic disease among HIV infected persons, [1,2] yet these patients are now at increased risk of death as a result of chronic non-infectious age-related co-morbid conditions, including chronic kidney disease (CKD) [3,4]. In the context of HAART-treated HIV infection and aging, CKD often results from metabolic abnormalities, such as diabetes mellitus, and hypertension [5,6]. In the general population, proteinuria and impaired kidney function related to these conditions can be preceded by glomerular hyperfiltration, in which the glomerular filtration rate (GFR) increases to above normal levels [7–11], due to compensatory hemodynamic alterations within the kidney. Metabolic dysfunction characterized by impaired fasting glucose [12], diabetes mellitus [9,11,13], hypertension, and obesity [9] -conditions for which HIV-infected persons receiving HAART are at higher risk [14,15] - have also been linked to hyperfiltration.
Since hyperfiltration is an early indicator of kidney dysfunction and is potentially reversible by aggressive antihypertensive therapy or dietary changes [7,13], establishing its relevance for HIV clinical care is important. However, whether and to what extent glomerular hyperfiltration is present among HIV-infected persons receiving HAART, and whether metabolic and cardiovascular risk factors and HIV-related factors are associated with hyperfiltration in this population are unclear. To address these questions, we used directly measured GFR data from a representative subsample of the Multicenter AIDS Cohort Study (MACS), a contemporary cohort of men with or at risk for HIV infection. We aimed to: 1) determine the prevalence of hyperfiltration; 2) investigate the association of HIV infection and metabolic, cardiovascular and behavioral factors with hyperfiltration; and 3) investigate HIV disease severity and HAART as potential risk factors for hyperfiltration.
Methods
Study population
The MACS is a prospective observational cohort study of the natural and treated histories of HIV infection among 6972 homosexual and bisexual men enrolled in four United States metropolitan areas from 1984 to 2003. Details of the study design have been previously described [16]. In brief, demographics, medical history and clinical characteristics were collected semi-annually. Standard protocols included physical examinations, blood pressure measurements, blood and urine collection and self-administered interviews.
The current study included men who underwent direct iohexol-based GFR measurement (iGFR) [17–19] between August 2008 and January 2011. These participants were a subsample of the full MACS cohort [19], chosen by random selection using a ratio of approximately 2 HIV-infected participants for each HIV-uninfected participant. Additionally, all participants co-infected with hepatitis C virus (HCV) infection, as determined by presence of serum anti-HCV antibody or plasma HCV RNA [20], were eligible for iGFR measurement. Participants who received renal replacement therapy, were diagnosed with cancer in the preceding 3 years, or were unable to complete data collection due to contrast allergy were excluded. A central laboratory measured blood glucose, insulin, lipid panels (Heinz, Pittsburgh) and HCV antibodies (Tricore, New Mexico). HIV-1 infection was defined by positive serum ELISA with confirmatory Western blot. Plasma HIV RNA levels were ascertained by the Roche Amplicor assay (Hoffman-LaRoche, Nutley, NJ) sensitive to 50 copies/mL, and CD4 lymphocyte counts/ml were measured using standardized flow cytometry [21]. This study was approved by Institutional Review Boards at all participating sites.
Dependent variables
The primary outcome was hyperfiltration, defined from GFR measured by the plasma disappearance of iohexol using a 2-compartment 4-point model standardized to a body surface area of 1.73m2 as previously described [19]. To address the expected decline in GFR associated with age, we defined hyperfiltration as iGFR ≥140 ml/min/1.73m2 for men 40 years and younger and subtracted 1 ml/min/1.73m2 for each year over age 40 [22]. Among men with GFR below this hyperfiltration threshold, we excluded subjects with GFR <60 ml/min/1.73m2 or with proteinuria (urine protein-to-creatinine ratio (UPCR) >200 mg/g) [7,23,24]. The remaining men without markedly impaired GFR (i.e., “normofiltration”) served as the comparison group. UPCR was measured at each site, and was based on the mean of three measurements for 1 year prior to iGFR measurement. Subjects with missing UPCR data were excluded (n= 8).
Independent variables
Metabolic variables included body mass index (BMI, kg/m2; obesity defined as ≥30 kg/m2), serum HDL and non-HDL cholesterol, dyslipidemia (fasting total cholesterol ≥200 mg/dL, LDL ≥130 mg/dL, HDL <40 mg/dL, triglycerides ≥150 mg/dL or use of lipid lowering medications with self-reported/clinical diagnosis of dyslipidemia), fasting glucose level, hemoglobin A1c (HbA1c), insulin resistance (HOMA-IR), diabetes (HbA1c ≥6.5%, fasting glucose >126 mg/dL or diagnosis of diabetes with use of medications) and metabolic syndrome [25]. High fasting glucose (>100 mg/dL) and HbA1c (≥6.5%) were also used as indicators of hyperglycemia [12]. Cardiovascular variables were systolic and diastolic blood pressure (SBP, DBP), uncontrolled hypertension (SBP ≥140 or DBP ≥90 mmHg), and history of hypertension (uncontrolled hypertension or diagnosis of hypertension with use of antihypertensive medications). Behavioral variables included current smoking status at the time of iGFR measurement, stimulant use (cocaine, amphetamines or methamphetamines), and other drug use (marijuana and inhalant nitrates). For all continuous variables, the mean of available data from the visits in 1 year prior to and including the iGFR visit was used as a summary level. For binary variables, such as diabetes, the presence of these conditions was determined by at least 2 occurrences in the visits prior to and including the iGFR measurement in a 1 year period (i.e., 2 out of 3 measurements, about 6 months apart). Since variables within metabolic and cardiovascular domains were expected to be highly collinear, we did not simultaneously include them in multivariate analyses.
To investigate the association of HIV and HAART on hyperfiltration, we restricted the sample to men receiving HAART at the time of iGFR measurement since few HIV-infected subjects were HAART-naïve (n= 4). Exposure to antiretroviral therapy (ART) was characterized by: 1) years from any ART initiation to the time of iGFR measurement (i.e., time since ART initiation, either monotherapy, combination therapy or potent therapy with 3 or more agents); 2) years from HAART initiation (i.e., a combination regimen of 3 or more ART agents) to the time of iGFR measurement; and 3) cumulative exposure, i.e., person-years of use, for each subclass of ARTs: non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs) and nucleoside reverse transcriptase inhibitors (NRTIs). Cumulative exposure was also calculated for specific antiretroviral drugs that have been associated with either metabolic or cardiovascular derangements (such as hyperglycemia or dyslipidemia, associated with thymidine-analog reverse transcriptase inhibitor medications used in earlier HAART regimens [15,26]) or for drugs that are considered nephrotoxic [27]. The specific medications evaluated were indinavir (IDV), ritonavir (RTV), atazanavir (ATV) for PIs; and zidovudine (AZT), abacavir (ABC), tenofovir (TDF) and stavudine (d4T) for NRTIs. If the subject discontinued ART use between study visits, exposure time was calculated as half the time between study visits. For IDV, RTV, ATV, ABC and d4T, in which more than 50% of subjects were unexposed, these variables were classified by “any exposure”. As a sensitivity analysis, we also categorized ART exposed years as no exposure and by 4 year intervals.
Statistical analyses
The one-way Wilcoxon rank-sum test and Fisher's exact test were used to compare differences by filtration status within each HIV infection group with the null hypothesis that comorbidities were more common among participants with hyperfiltration. Multivariate logistic regression was used to estimate odds ratios (OR) of prevalent hyperfiltration. Confounders were identified a priori based on biological plausibility and published research, and included age, race, BMI, ACE-inhibitor (ACEi)/ angiotensin receptor blocker (ARB) use, stimulant drug use, current smoking status [28,29] and HIV disease severity (as measured by CD4+ cell counts) [30]. Although age after 40 years was part of the hyperfiltration definition, age was also included in multivariate models to control for potential residual confounding since it is strongly related to renal function. In investigating the effect of ART exposure on the odds of prevalent hyperfiltration, we additionally adjusted for time since any ART initiation and ART use in the pre-HAART era (i.e., prior to July 1, 1996 [31]). As weight gain may be in the causal pathway between ART exposure and hyperfiltration, we also performed a sensitivity analysis excluding BMI as a confounder, instead using height to indicate body size.
Interactions between HIV status and categorical putative hyperfiltration risk factors (diabetes, hypertension, and metabolic syndrome) were assessed and included if significant. Multiple imputation (10 repetitions) was used to complete missing data for unbiased estimates of effects, and the corresponding 95% confidence intervals (95%CI) are presented. A dataset comprising complete cases was analyzed as a sensitivity analysis and yielded similar inferences as the multiple imputation approach. Statistical significance was assessed at the 0.05 level. All statistical analyses were completed using SAS 9.2 (SAS Institute, Cary, NC).
Results
Cohort characteristics
Of 741 men who underwent an iGFR study, 720 (97%) had a valid iGFR and were included in our analysis (260 HIV-uninfected and 460 HIV-infected subjects). Of the HIV-infected men, 456 (99%) had initiated ART prior to the iGFR study. The HIV-uninfected group was older, with a median age of 53 years versus 50 years in the infected group. The median iGFR was 105 and 109 ml/min/1.73m2 for HIV-uninfected and infected men, respectively. Among the HIV-uninfected subjects, 16% had iGFR above the hyperfiltration threshold compared to 20% of the HIV-infected subjects (p= 0.194). About 4% (n=10) of HIV-uninfected and 3% (n=16) of HIV-infected subjects had iGFR <60 ml/min/1.73m2; these subjects were excluded from further analysis. We also excluded 6 HIV-uninfected and 69 HIV-infected subjects with other evidence of CKD: near-normal iGFR (i.e., 60 ml/min/1.73m2 < iGFR < hyperfiltration threshold) and proteinuria [24], resulting in an analytic sample of 608 men free of CKD. Among HIV-uninfected subjects with hyperfiltration, 2.6% had proteinuria. In contrast, 10% of HIV-infected subjects with hyperfiltration had proteinuria.
Metabolic, cardiovascular and behavioral characteristics by HIV status and filtration status
Table 1 compares participant demographic and clinical characteristics by HIV serostatus and filtration status, comprising 241 HIV-uninfected participants (17% with hyperfiltration) and 367 HIV-infected participants (25% with hyperfiltration), without CKD (17% vs. 25%; p= 0.01). Among HIV-uninfected subjects, men with hyperfiltration had a higher proportion of diabetes (39% vs. 21%) compared to men with normofiltration; however, both groups had similar proportions of individuals with obesity, hypertension and metabolic syndrome.
Table 1.
Description of study participants by HIV and filtration status. Median [IQR], n (%).
| HIV-uninfected | HIV-infected | |||||
|---|---|---|---|---|---|---|
| Characteristic | Normofiltrationa n= 200 | Hyperfiltrationb n= 41 | P | Normofiltrationa n= 275 | Hyperfiltrationb n= 92 | P |
| Age, years | 54 [48, 61] | 53 [47, 61] | 0.33 | 49 [44, 55] | 51 [47, 57] | 0.04 |
| Black race | 63 (32) | 15 (37) | 0.32 | 99 (36) | 22 (24) | 0.99 |
| HCV infection | 23 (12) | 4 (10) | 0.75 | 34 (13) | 4 (5) | 0.99 |
| Metabolic and cardiovascular variables | ||||||
| BMI, kg/m2 | 26.6 [24.2, 29.6] | 25.7 [23.7, 29.3] | 0.31 | 25.8 [23.6, 28] | 26.3 [23.9, 29.7] | 0.09 |
| Obese (BMI > 26 kg/m2) | 46 (23) | 10 (24) | 0.49 | 41 (15) | 24 (26) | 0.01 |
| HDL cholesterol, mg/dL | 49 [41, 58] | 54 [45, 59] | 0.06 | 44 [38, 53] | 44 [37, 52] | 0.20 |
| Non-HDL cholesterol, mg/dL | 138 [114, 160] | 131 [114, 151] | 0.14 | 143 [119, 165] | 143 [119, 166] | 0.40 |
| Dyslipidemia | 137 (73) | 24 (65) | 0.88 | 221 (84) | 73 (84) | 0.56 |
| Hemoglobin A1c, % | 5.6 [5.4, 5.8] | 5.7 [5.4, 5.9] | 0.22 | 5.5 [5.2, 5.7] | 5.5 [5.2, 5.8] | 0.35 |
| Insulin, μIU/mL | 11.6 [8.8, 17.3] | 11.8 [8.4, 15.7] | 0.46 | 13.1 [9.8, 18.7] | 14 [9.8, 22.7] | 0.11 |
| Fasting glucose,mg/dL | 98 [91, 105] | 100 [94, 109] | 0.08 | 100 [94, 106] | 105 [97, 113] | <0.01 |
| FG > 100 mg/dL and ≤ 126 mg/dL | 71 (39) | 14 (41) | 0.47 | 115 (47) | 41 (54) | 0.16 |
| FG > 126 mg/dL | 14 (8) | 5 (15) | 0.16 | 15 (6) | 8 (11) | 0.14 |
| HOMA-IR | 3.0 [2.0, 4.6] | 3.1 [2.1, 4.4] | 0.39 | 3.2 [2.2, 4.7] | 3.9 [2.6, 6.3] | 0.04 |
| Diabetes | 41 (21) | 15 (38) | 0.02 | 72 (26) | 26 (28) | 0.40 |
| Metabolic syndromec | 112 (57) | 24 (62) | 0.36 | 180 (65) | 66 (72) | 0.16 |
| Systolic BP, mmHg | 126 [118, 135] | 128 [115, 136] | 0.39 | 123 [115, 132] | 127 [118, 136] | 0.02 |
| Diastolic BP, mmHg | 76 [72, 83] | 77 [71, 84] | 0.49 | 77 [71, 82] | 78 [72, 83] | 0.32 |
| SBP > 140 or DBP > 90 mmHg | 45 (23) | 10 (28) | 0.36 | 41 (15) | 22 (26) | 0.02 |
| History of hypertension | 87 (44) | 22 (56) | 0.11 | 101 (37) | 46 (50) | 0.02 |
| ACEi/ARB use | 38 (19) | 5 (12) | 0.90 | 38 (14) | 13 (14) | 0.53 |
| Behavioral variables | ||||||
| Current smoker | 47 (24) | 11 (27) | 0.39 | 91 (33) | 29 (32) | 0.66 |
| Stimulant use | 30 (15) | 6 (16) | 0.55 | 38 (14) | 20 (22) | 0.05 |
| Other drug use (marijuana/inhalant nitrates) | 72 (37) | 16 (42) | 0.32 | 126 (46) | 47 (51) | 0.22 |
One-sided p-values based on Wilcoxon rank sum test for continuous variables and Fisher's exact test for categorical variables, comparing normofiltration and hyperfiltration within HIV infection status group.
Normofiltration defined as iGFR < 140 ml/min/1.73m2 – 1 ml/min per year over age 40 and iGFR > 60 ml/min/1.73m2 and urine protein : creatinine < 200 mg/g.
Hyperfiltration defined as iGFR > 140 ml/min/1.73m2 – 1 ml/min per year over age 40.
Metabolic syndrome defined as having three or more of: waist ≥ 102cm, fasting triglycerides ≥ 150 mg/dL, HDL-cholesterol < 40 mg/dL, fasting glucose ≥ 100 mg/dL, or diabetes diagnosis with medication use.
Abbreviations: FG= fasting glucose; HOMA-IR= homeostatic model assessment – insulin resistance; ACEi/ARB= angiotensin-converting-enzyme-inhibitor, angiotensin receptor blocker; HCV = hepatitis C virus infection
Among HIV-infected men, those with hyperfiltration were older (medians= 51 vs. 49 years) than men with normofiltration. HIV-infected participants with hyperfiltration also had higher fasting blood glucose (105 vs. 100 mg/dL) and HOMA-IR levels (3.9 vs. 3.2) and were more likely to be obese (26% vs. 15%). Men with hyperfiltration were more likely to have a history of hypertension (50% vs. 37%) and uncontrolled hypertension (26% vs. 15%), but the same proportion reported ACEi/ARB use as those with normofiltration (14%). Stimulant use was more common among men with hyperfiltration (22% vs. 14%), but current smoking and other drug use did not differ between groups.
Table 2 presents odds ratios of prevalent hyperfiltration associated with HIV-infection adjusted for age, race, stimulant use, current smoking status, ACEi/ ARB use and BMI. HIV-infected subjects were 1.70-times more likely to exhibit hyperfiltration than HIV-uninfected subjects (95%CI: 1.11, 2.61). The effect of stimulant use was similar and borderline significant (OR: 1.71, 95%CI: 0.99, 2.95). In contrast, race, age and current ACEi/ARB use were not associated with odds of hyperfiltration.
Table 2.
Odds ratios of prevalent hyperfiltration from multivariate logistic regression.
| Characteristic | OR (95%CI) |
|---|---|
| HIV-infected | 1.70 (1.11, 2.61) |
| Per 1 year increase in age | 1.01 (0.98, 1.03) |
| Black race | 0.66 (0.41, 1.05) |
| Stimulant use | 1.71 (0.99, 2.95) |
| ACEi/ARB use | 0.77 (0.43, 1.38) |
| Current smoker | 1.06 (0.66, 1.69) |
| Per 1 log increase in BMI | 2.44 (0.71, 8.34) |
Abbreviations: OR= Odds ratio; ACEi= Angiotensin-converting enzyme inhibitor; ARB= Angiotensin-renin blocker; BMI= Body mass index (kg/m2)
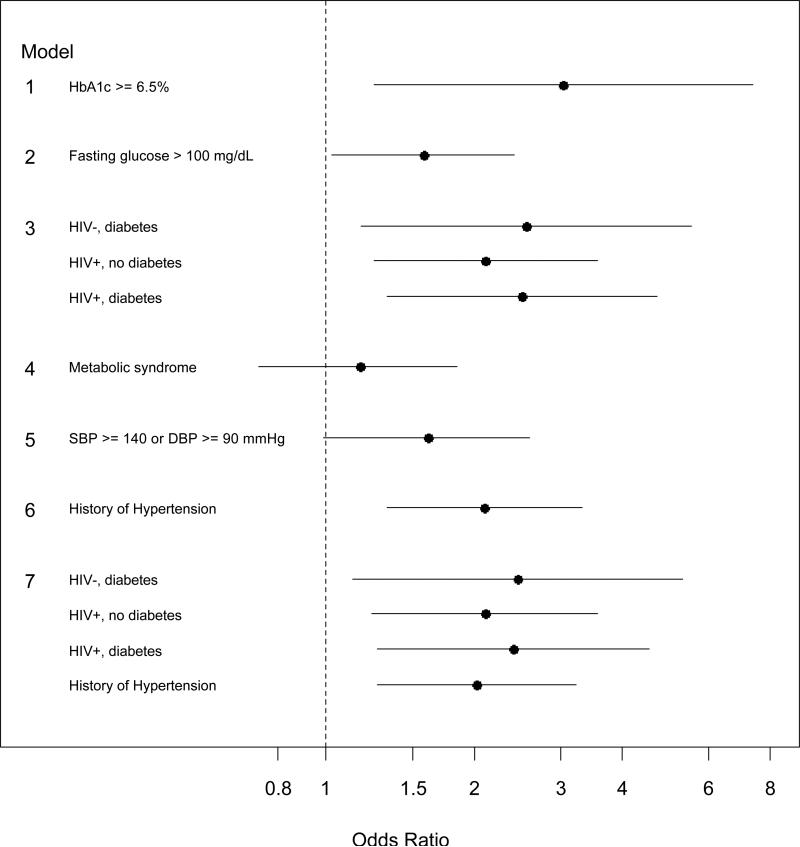
Figure 1 presents results when extending the above model to include the main effects for metabolic and cardiovascular factors. The effect of HIV remained consistently strong across all models, with ORs ranging from 1.63 to 1.80. Indicators of hyperglycemia were associated with higher odds of prevalent hyperfiltration (HbA1c ≥ 6.5% OR: 3.03, 95%CI: 1.25, 7.38; fasting glucose >100 mg/dL OR: 1.58, 95%CI: 1.03, 2.42). The prevalence of hyperfiltration was similar by metabolic syndrome status. The association of diabetes with hyperfiltration differed by HIV status (p=0.05 for interaction). Specifically, diabetes was associated with hyperfiltration among HIV-uninfected individuals (OR: 2.56, 95%CI: 1.33, 5.54), but not among HIV-infected subjects (OR: 1.19, 95%CI: 0.69, 2.05; not shown). However, compared to HIV-uninfected individuals without diabetes (reference in this model represented by the vertical line at 1), HIV-infected individuals (regardless of diabetes status) were more than twice as likely to have hyperfiltration; this comparison was statistically significant. Uncontrolled hypertension was associated with higher odds of hyperfiltration (OR= 1.61, 95%CI: 0.99, 2.60) as was history of hypertension (OR= 2.10, 95%CI: 1.33, 3.33). The latter association was similar by HIV status (p=0.91 for interaction). The inclusion of hypertension in the model assessing the interaction of diabetes and HIV on hyperfiltration yielded similar results (Model 7, Figure 1).
Figure 1.
The odds ratios (OR) with 95% confidence intervals of prevalent hyperfiltration associated with metabolic and cardiovascular factors among men without CKD (n= 608). The reference group (indicated by the vertical line at 1.0) are those without the variable of interest in each model. Each model was adjusted for HIV infection, age, race, stimulant drug use, ACEi/ARB use, current smoking status and body mass index, whose effects are shown in Table 2. Footnote: The reference groups for Model 3 and Model 7 comprise HIV-uninfected subjects with no diabetes; and HIV-uninfected subjects with no diabetes and no history of hypertension, respectively.
HIV and ART characteristics associated with hyperfiltration
Table 3 displays HIV- and treatment-related factors among HIV-infected subjects stratified by filtration status. Indices of HIV disease severity were similar by hyperfiltration status while some ART factors differed between the two groups. The hyperfiltration and normofiltration groups had similar median times since any ART initiation, but the median cumulative HAART use was longer in the hyperfiltration group than the normofiltration group (median time was 7.3 vs. 6.3 years). Cumulative PI and NNRTI years were similar between the two groups, while men with hyperfiltration had higher median cumulative NRTI years (19.7 vs. 17.4). This difference in cumulative ART use was primarily accounted for by AZT use (medians: 4.1 vs. 2.0 years).
Table 3.
HIV and HIV-treatment related characteristics by filtration status and adjusted odds ratios of prevalent hyperfiltration, n (%), median [IQR] and OR (95%CI).
| Variable | Normofiltration n= 275 | Hyperfiltration n= 92 | Adjusted OR (95%CI)a |
|---|---|---|---|
| Nadir CD4 cell count < 350 per μL | 225 (82) | 73 (79) | 0.69 (0.36, 1.30) |
| Current CD4 cell count < 350 per μL | 49 (18) | 21 (23) | 1.48 (0.79, 2.78) |
| Current detectable HIV RNA | 57 (21) | 16 (18) | 0.91 (0.45, 1.86) |
| History of AIDS diagnosis | 39 (14) | 10 (11) | 0.71 (0.33, 1.54) |
| Time since ART initiationb, years | 12.3 [7.6, 19.8] | 12.8 [8.4, 19.1] | 1.48 (0.86, 2.55)d |
| Cumulative HAARTc years | 6.3 [3.8, 9] | 7.3 [4.4, 9.3] | 1.31 (0.87, 1.99)d |
| Cumulative NNRTI years | 2.7 [0.3, 6.2] | 3.8 [0.3, 6.9] | 1.15 (0.81, 1.64)d |
| Cumulative PI years | 4.1 [0.1, 8] | 4.3 [0.2, 8] | 1.00 (0.75, 1.33)d |
| Cumulative IDV years | 0.0 [0.0, 0.3] | 0.0 [0.0, 0.7] | |
| Any IDV exposure | 76 (29) | 31 (35) | 1.41 (0.81, 2.46) |
| Any RTV exposure | 25 (9) | 9 (10) | 1.21 (0.58, 2.55) |
| Cumulative ATV years | 0.0 [0.0, 1.2] | 0.0 [0.0, 0.2] | |
| Any ATV exposure | 87 (33) | 22 (25) | 0.71 (0.41, 1.24) |
| Cumulative NRTI years | 17.4 [10.5, 23.3] | 19.7 [11.9, 25.6] | 1.12 (0.96, 1.31)d |
| Cumulative AZT years | 2.0 [0.0, 5.9] | 4.1 [0.2, 8.0] | 1.53 (1.12, 2.10) d |
| Cumulative TDF years | 2.4 [0.0, 4.6] | 2.0 [0.0, 3.9] | 0.67 (0.40, 1.14) |
| Cumulative ABC years | 0.0 [0.0, 3.1] | 0.0 [0.0, 3.1] | |
| Any ABC exposure | 114 (43) | 36 (41) | 0.91 (0.55, 1.50) |
| Cumulative d4T years | 0.0 [0.0, 3.3] | 0.0 [0.0, 3.5] | |
| Any d4T exposure | 130 (47) | 46 (50) | 1.03 (0.60, 1.76) |
Odds ratios are calculated from multiple logistic regression models adjusting for age, race, BMI, ACEi/ARB use, current smoking status, stimulant drug use, current and nadir CD4+ cell count < 350, time since ART initiation and any ART use prior to July 1, 1996 (pre-HAART era).
Time since ART initiation refers to first known date of any antiretroviral therapy (i.e., monotherapy, combination therapy, or potent therapy).
HAART refers to potent combination therapy according to December 2009 guidelines (a regimen of three or more drugs).
Odds ratios for a 5 year increase in cumulative medication exposure.
Abbreviations: OR= Odds ratio; 95%CI= 95% confidence interval; HAART= highly active antiretroviral therapy; NNRTI= non-nucleoside reverse transcriptase inhibitors; PI= protease inhibitors; NRTI= nucleoside reverse transcriptase inhibitors; IDV= Indinavir; RTV= Ritonavir; ATV= Atazanavir; AZT= Zidovudine; TDF= Tenofovir; ABC= Abacavir; d4T= Stavudine.
The association of HIV disease severity indicators and therapy with hyperfiltration, adjusted for age, race, current and nadir CD4 cell count, time since first ART exposure and ART use in the pre-HAART era, BMI, ACEi/ARB use, current smoking status and stimulant drug use, are presented by the adjusted odds of prevalent hyperfiltration ratios in the right-most column of Table 3. None of the indicators of HIV disease stage were associated with hyperfiltration, including nadir/ current CD4 cell count <350/μl, and detectable plasma HIV RNA level. Indicators of ART exposure were not associated with hyperfiltration, with the exception of AZT. For a 5-year increase in AZT exposure, the odds of hyperfiltration significantly increased by 53% (95%CI: 1.12, 2.09). A sensitivity analysis of cumulative ART exposure by 4-year categories yielded similar results. For AZT, with no exposure to AZT as the reference group, there was no association for less than 4 years of exposure (OR= 0.85, 95%CI: 0.42, 1.70). However, the odds ratio of prevalent hyperfiltration was 1.88 for >4 to 8 years of exposure (95%CI: 0.93, 3.81) and was 2.11 for more than 8 years of AZT exposure (95%CI: 0.99, 4.49).
Discussion
In this MACS GFR substudy, the overall prevalence of measured glomerular hyperfiltration for men without CKD was significantly higher among those infected with HIV compared to uninfected men, 25% and 17%, respectively. From this group, subjects with HIV had 1.7 times higher odds of hyperfiltration than uninfected subjects, independent of demographic, metabolic and cardiovascular factors. We further confirmed the findings of previous studies which identified hyperglycemia [12], diabetes mellitus [32] and hypertension [9], as independently associated with hyperfiltration in this unique population, although the effect of diabetes was mainly observed among HIV-uninfected men. Our findings suggest that hyperfiltration is common in this cohort and may be an important clinical consideration as an early indicator of kidney dysfunction among HIV-infected persons.
HIV infects renal epithelial cells [33] and disturbs podocyte structure and function [34]. The latter appears to involve upregulation of the renin-angiotensin system [35], which is central to the pathophysiology of hyperfiltration. Alternatively, HIV itself, ART and associated metabolic derangements may contribute to hyperfiltration via perturbations in insulin-like growth factor [36] leading to visceral adiposity and abnormal glucose metabolism [14,37]. In our cohort, since nearly all HIV-infected men were also being treated with ART, we were unable to discern whether the elevated prevalence of hyperfiltration was due to HIV infection alone, ART use, or both.
In the general population, previous studies have found that elevated blood glucose and HbA1c are associated with higher odds of hyperfiltration, with ORs ranging from 1.3 [9] and 1.6 to 2.2 [12,38], as well as hypertension (OR: 1.8) [9,39]. Studies also suggest hyperfiltration precedes the onset of albuminuria and kidney function decline and is therefore an important marker of future CKD [23]. Indeed, among diabetic individuals, hyperfiltration has been associated with increased risk of diabetic nephropathy and CKD progression [32]. Among individuals with hypertension, the proportion of those with persistent hyperfiltration and those with hyperfiltration progressing to normofiltration who developed microalbuminuria was 16% and 36%, respectively, compared to only 5% of those who never experienced hyperfiltration, over a median follow-up of 8.5 years [40].
Our results show that HIV is also associated with higher odds of hyperfiltration, and that HIV-infection modifies the diabetes-hyperfiltration relationship: among HIV-uninfected subjects, diabetes was strongly associated with hyperfiltration; however, among HIV-infected subjects, there was no further association between diabetes and hyperfiltration, but these subjects were at higher risk for hyperfiltration than their HIV-uninfected peers. The effect of diabetes on hyperfiltration may be secondary to the dominant effect of HIV and its treatment.
Two recent publications documented the association of diabetes and HIV on decreased renal function [41] and ESRD [42]. In these studies, the presence of either HIV or diabetes was associated with a higher risk of diminished GFR [41], and ESRD among African Americans [42]; a relationship which was similar to the increased odds of hyperfiltration in our study. In contrast to our findings, however, those who had both diabetes and HIV were at even greater risk of incidence of low eGFR <45 ml/min compared to those with HIV or diabetes only [41]. Similar to the results for ESRD [42], we did not find an additive effect of HIV and diabetes on hyperfiltration. These results suggest that hyperfiltration may mediate some portion of the effect of diabetes and/or HIV on ESRD, but future research is needed to investigate this relationship.
Although not all associations were statistically significant, we consistently found increased odds of hyperfiltration in association with most ART factors, suggesting that prolonged ART exposure may increase the risk of hyperfiltration. While this relationship was strongest for AZT use (OR: 1.53 per 5-year increase in AZT exposure), increased AZT exposure may be a marker for longer HIV infection duration, and longer HIV infection is the risk factor for hyperfiltration. Alternatively, AZT use may be a proxy for historically poorer HIV suppression, since it was commonly used in the pre-HAART era; this was a possibility we could not explore. Despite the diminished use of AZT, AZT exposure may be clinically relevant for identifying patients with hyperfiltration since there are HIV-infected patients in care with prior and/or current exposure to AZT. Indeed, there may also be a threshold effect of AZT on hyperfiltration that is more common with chronic use, or the effect may manifest later among persons with a longer duration of HIV infection. The protective, but non-significant trend, associated with TDF exposure was also notable since TDF is nephrotoxic. Subjects at-risk for kidney disease may have been less likely to be prescribed TDF than others (i.e., channeling bias). Alternatively, increased TDF exposure may have caused pathologic nephron loss, potentially counteracting any hyperfiltration effects due to other disease or therapy processes. Third, TDF renal toxicity is thought to be directed towards the proximal tubule rather than the glomerulus; therefore, TDF-treated individuals may not necessarily be at higher risk of glomerular hyperfiltration. Lastly, better virologic control and HIV management with TDF may reduce the risk of hyperfiltration.
Of note, in multivariate analysis, stimulant drug use was associated with increased odds of hyperfiltration (OR= 1.71). The relationship between stimulant drug use and hyperfiltration has not been well-characterized in the literature, but may be related to the effects of stimulant drug use on the sympathetic nervous system and on the regulation of beta cell function and dopamine (potentially affecting glomerular perfusion). Studies have suggested that cocaine and other stimulants may perturb insulin secretion and lipid/glucose homeostasis [43,44]. The observed association between stimulants and hyperfiltration may be related to a shared relationship with hormonal dysregulation, hyperglycemia and renal function. Alternatively, stimulant use may be an indicator for socioeconomic or lifestyle factors that adversely affect disease management, adherence to medications or overall general health, and thereby increase the risk of hyperfiltration.
While ACEi/ARB use was not significantly associated with decreased odds of hyperfiltration, the directionality of effect was consistent with reduced GFR due to preferential dilation of the efferent arteriole (OR= 0.77). In contrast, we were unable to explain the lower prevalence of hyperfiltration among black participants (OR= 0.66) although this effect was also non-significant. This was surprising given the higher risk of diabetes, diabetic nephropathy and ESRD in the black population; and the documented higher risk of hyperfiltration among black people with hypertension [45].
One limitation of this study is that we are unable to causally link identified metabolic, cardiovascular and HIV-infection factors with hyperfiltration. However, the physiologic framework of the HIV renal reservoir, the influence of ART on metabolic and cardiovascular health, as well as previous literature, suggest that metabolic and cardiovascular changes likely initiate hyperfiltration [10,32]. Furthermore, since previous direct measurements of GFR had not been obtained, we were unable to determine the duration of hyperfiltration, although a second iGFR measurement will be obtained in these subjects for future analyses. While hyperfiltration is a known precursor to kidney damage, the duration of hyperfiltration necessary to precipitate kidney function decline is unclear. An early report among Pima Indians demonstrated GFR declines over a 4 year period after hyperfiltration was identified [46]. Future longitudinal data of iGFR in our cohort are needed to further understand risk factors for and consequences of hyperfiltration. An additional limitation is that the study sample was exclusively men, and whether these inferences extend to women is unknown.
In summary, we found that among men without CKD, HIV infection was associated with a higher prevalence of hyperfiltration compared to being uninfected. A major strength of this study was the use of directly measured GFR by iohexol to detect hyperfiltration, a more reliable method than estimating equations for high GFR levels [10,47]. Non-infectious metabolic conditions found commonly in aging HIV-infected individuals were associated with hyperfiltration. Additionally, among HIV-infected men, increased exposure to ART, particularly AZT, was associated with higher odds of hyperfiltration. Since poor metabolic and cardiovascular health are known risk factors for glomerular hyperfiltration, which is an early clinical marker of kidney damage, evaluation and aggressive treatment of these risk factors [9,10] should be an important priority for health management in HIV-infected as well as non-HIV-infected populations.
Acknowledgements
The Multicenter AIDS Cohort Study (MACS) includes the following: Baltimore: Baltimore: The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (PI), Barbara Crain, Adrian Dobs, Homayoon Farzadegan, Joel Gallant, Lisette Johnson-Hill, Michael W. Plankey, Ned Sacktor, Ola Selnes, James Shepard, Chloe Thio. Chicago: Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: Steven M. Wolinsky (PI), John P. Phair, Sheila Badri, Maurice O'Gorman, David Ostrow, Frank Palella, Ann Ragin. Los Angeles: University of California, UCLA Schools of Public Health and Medicine: Roger Detels (PI), Otoniel Martínez-Maza (Co-P I), Aaron Aronow, Robert Bolan, Elizabeth Breen, Anthony Butch, Beth Jamieson, Eric N. Miller, John Oishi, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang. Pittsburgh: University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (PI), Lawrence A. Kingsley (Co-PI), James T. Becker, Ross D. Cranston, Jeremy J. Martinson, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall. Data Coordinating Center: The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (PI), Alvaro Muñoz (Co-PI), Alison Abraham, Keri Althoff, Christopher Cox, Gypsyamber D'Souza, Priya Duggal, Elizabeth Golub, Janet Schollenberger, Eric C. Seaberg, Sol Su, Pamela Surkan. NIH: National Institute of Allergy and Infectious Diseases: Robin E. Huebner; National Cancer Institute: Geraldina Dominguez. Website located at http://www.statepi.jhsph.edu/macs/macs.html. We are grateful to GE Healthcare, Amersham Division, for providing the MACS study with ioxehol (Omnipaque) for GFR measurements.
This work was supported by the National Institute of Allergy and Infectious Diseases and National Cancer Institute [UO1-AI-35042, UM1-AI-35043, UO1-AI-35039, UO1-AI-35040, UO1-AI-35041]; the National Center for Advancing Translational Sciences [UL1TR000424]; and the National Institutes of Health [1K23DK081317 to M.M.E.].
Footnotes
The authors have no conflict of interests to declare.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Wada N, Jacobson LP, Cohen M, French A, Phair J, Muñoz A. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome-infected and human immunodeficiency syndrome-negative individuals followed simultaneously in long- term cohort studies, 1984-2008. Am J Epidemiol. 2013;177:116–125. doi: 10.1093/aje/kws321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adih WK, Selik RM, Hu X. Trends in Diseases Reported on US Death Certificates That Mentioned HIV Infection, 1996-2006. J Int Assoc Physicians AIDS Care (Chic) 2011;10:5–11. doi: 10.1177/1545109710384505. [DOI] [PubMed] [Google Scholar]
- 4.Wyatt CM, Morgello S, Katz-Malamed R, Wei C, Klotman ME, Klotman PE, et al. The spectrum of kidney disease in patients with AIDS in the era of antiretroviral therapy. Kidney Int. 2009;75:428–434. doi: 10.1038/ki.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–1184. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 6.George E, Lucas GM, Nadkarni GN, Fine DM, Moore R, Atta MG. Kidney function and the risk of cardiovascular events in HIV-1-infected patients. AIDS. 2010;24:387–394. doi: 10.1097/QAD.0b013e3283359253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 1996;49:1774–1777. doi: 10.1038/ki.1996.265. [DOI] [PubMed] [Google Scholar]
- 8.Chaiken RL, Eckert-Norton M, Bard M, Banerji MA, Palmisano J, Sachimechi I, et al. Hyperfiltration in African-American patients with type 2 diabetes. Cross-sectional and longitudinal data. Diabetes Care. 1998;21:2129–2134. doi: 10.2337/diacare.21.12.2129. [DOI] [PubMed] [Google Scholar]
- 9.Tomaszewski M, Charchar FJ, Maric C, McClure J, Crawford L, Grzeszczak W, et al. Glomerular hyperfiltration: a new marker of metabolic risk. Kidney Int. 2007;71:816–821. doi: 10.1038/sj.ki.5002160. [DOI] [PubMed] [Google Scholar]
- 10.Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol. 2012;8:293–300. doi: 10.1038/nrneph.2012.19. [DOI] [PubMed] [Google Scholar]
- 11.Ruggenenti P, Porrini EL, Gaspari F, Motterlini N, Cannata A, Carrara F, et al. Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care. 2012;35:2061–2068. doi: 10.2337/dc11-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melsom T, Mathisen UD, Ingebretsen OC, Jenssen TG, Njølstad I, Solbu MD, et al. Impaired fasting glucose is associated with renal hyperfiltration in the general population. Diabetes Care. 2011;34:1546–1551. doi: 10.2337/dc11-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okada R, Yasuda Y, Tsushita K, Wakai K, Hamajima N, Matsuo S. Glomerular hyperfiltration in prediabetes and prehypertension. Nephrol Dial Transplant. 2012;27:1821–1825. doi: 10.1093/ndt/gfr651. [DOI] [PubMed] [Google Scholar]
- 14.Tebas P. Insulin Resistance and Diabetes Mellitus Associated With Antiretroviral Use in HIV-Infected Patients: Pathogenesis, Prevention, and Treatment Options. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2008;49:S86–S92. doi: 10.1097/QAI.0b013e31818651e6. [DOI] [PubMed] [Google Scholar]
- 15.Vu CN, Ruiz-Esponda R, Yang E, Chang E, Gillard B, Pownall HJ, et al. Altered relationship of plasma triglycerides to HDL cholesterol in patients with HIV/HAART-associated dyslipidemia: Further evidence for a unique form of Metabolic Syndrome in HIV patients. Metab Clin Exp Published Online First. 2013 Mar 19; doi: 10.1016/j.metabol.2013.01.020. doi:10.1016/j.metabol.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz GJ, Furth S, Cole SR, Warady B, Muñoz A. Glomerular filtration rate via plasma iohexol disappearance: pilot study for chronic kidney disease in children. Kidney Int. 2006;69:2070–2077. doi: 10.1038/sj.ki.5000385. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Abraham AG, Furth SL, Warady BA, Muñoz A. Optimizing iohexol plasma disappearance curves to measure the glomerular filtration rate in children with chronic kidney disease. Kidney Int. 2010;77:65–71. doi: 10.1038/ki.2009.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng DKS, Schwartz GJ, Jacobson LP, Palella FJ, Margolick JB, Warady BA, et al. Universal GFR determination based on two time points during plasma iohexol disappearance. Kidney Int. 2011;80:423–430. doi: 10.1038/ki.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caliendo AM, Valsamakis A, Zhou Y, Yen-Lieberman B, Andersen J, Young S, et al. Multilaboratory comparison of hepatitis C virus viral load assays. J Clin Microbiol. 2006;44:1726–1732. doi: 10.1128/JCM.44.5.1726-1732.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hultin LE, Menendez FA, Hultin PM, Jamieson BD, O'Gorman MRG, Borowski L, et al. Assessing immunophenotyping performance: proficiency-validation for adopting improved flow cytometry methods. Cytometry B Clin Cytom. 2007;72:249–255. doi: 10.1002/cyto.b.20176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Premaratne E, Macisaac RJ, Tsalamandris C, Panagiotopoulos S, Smith T, Jerums G. Renal hyperfiltration in type 2 diabetes: effect of age-related decline in glomerular filtration rate. Diabetologia. 2005;48:2486–2493. doi: 10.1007/s00125-005-0002-9. [DOI] [PubMed] [Google Scholar]
- 23.Palatini P. Glomerular hyperfiltration: a marker of early renal damage in pre-diabetes and pre-hypertension. Nephrol Dial Transplant. 2012;27:1708–1714. doi: 10.1093/ndt/gfs037. [DOI] [PubMed] [Google Scholar]
- 24.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 25.Mondy K, Overton ET, Grubb J, Tong S, Seyfried W, Powderly W, et al. Metabolic Syndrome in HIV-Infected Patients from an Urban, Midwestern US Outpatient Population. Clin Infect Dis. 2007;44:726–734. doi: 10.1086/511679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curran A, Ribera E. From old to new nucleoside reverse transcriptase inhibitors: changes in body fat composition, metabolic parameters and mitochondrial toxicity after the switch from thymidine analogs to tenofovir or abacavir. Expert Opin Drug Saf. 2011;10:389–406. doi: 10.1517/14740338.2011.542145. [DOI] [PubMed] [Google Scholar]
- 27.Kalyesubula R, Perazella MA. Nephrotoxicity of HAART. AIDS Res Treat. 2011;2011:562790. doi: 10.1155/2011/562790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crowe AV, Howse M, Bell GM, Henry JA. Substance abuse and the kidney. QJM. 2000;93:147–152. doi: 10.1093/qjmed/93.3.147. [DOI] [PubMed] [Google Scholar]
- 29.Maeda I, Hayashi T, Sato KK, Koh H, Harita N, Nakamura Y, et al. Cigarette smoking and the association with glomerular hyperfiltration and proteinuria in healthy middle-aged men. Clin J Am Soc Nephrol. 2011;6:2462–2469. doi: 10.2215/CJN.00700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shoptaw S, Stall R, Bordon J, Kao U, Cox C, Li X, et al. Cumulative exposure to stimulants and immune function outcomes among HIV-positive and HIV-negative men in the Multicenter AIDS Cohort Study. Int J STD AIDS. 2012;23:576–580. doi: 10.1258/ijsa.2012.011322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mateen FJ, Shinohara RT, Carone M, Miller EN, McArthur JC, Jacobson LP, et al. Neurologic disorders incidence in HIV+ vs HIV- men: Multicenter AIDS Cohort Study, 1996-2011. Neurology. 2012;79:1873–1880. doi: 10.1212/WNL.0b013e318271f7b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jerums G, Premaratne E, Panagiotopoulos S, MacIsaac RJ. The clinical significance of hyperfiltration in diabetes. Diabetologia. 2010;53:2093–2104. doi: 10.1007/s00125-010-1794-9. [DOI] [PubMed] [Google Scholar]
- 33.Bruggeman LA, Ross MD, Tanji N, Cara A, Dikman S, Gordon RE, et al. Renal Epithelium Is a Previously Unrecognized Site of HIV-1 Infection. JASN. 2000;11:2079–2087. doi: 10.1681/ASN.V11112079. [DOI] [PubMed] [Google Scholar]
- 34.Lu T-C, He JC, Klotman PE. Podocytes in HIV-associated nephropathy. Nephron Clin Pract. 2007;106:c67–71. doi: 10.1159/000101800. [DOI] [PubMed] [Google Scholar]
- 35.Chandel N, Sharma B, Husain M, Salhan D, Singh T, Rai P, et al. HIV Compromises Integrity of Podocyte Actin Cytoskeleton through down regulation of Vitamin D receptor. Am J Physiol Renal Physiol Published Online First. 2013 Mar 6; doi:10.1152/ajprenal.00717.2012. [Google Scholar]
- 36.Congote LF. Monitoring insulin-like growth factors in HIV infection and AIDS. Clin Chim Acta. 2005;361:30–53. doi: 10.1016/j.cccn.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Rao MN, Mulligan K, Tai V, Wen MJ, Dyachenko A, Weinberg M, et al. Effects of insulin- like growth factor (IGF)-I/IGF-binding protein-3 treatment on glucose metabolism and fat distribution in human immunodeficiency virus-infected patients with abdominal obesity and insulin resistance. J Clin Endocrinol Metab. 2010;95:4361–4366. doi: 10.1210/jc.2009-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okada R, Wakai K, Naito M, Morita E, Kawai S, Yin G, et al. Renal hyperfiltration in prediabetes confirmed by fasting plasma glucose and hemoglobin A1c. Ren Fail. 2012;34:1084–1090. doi: 10.3109/0886022X.2012.717516. [DOI] [PubMed] [Google Scholar]
- 39.Schmieder RE, Veelken R, Gatzka CD, Rüddel H, Schächinger H. Predictors for hypertensive nephropathy: results of a 6-year follow-up study in essential hypertension. J Hypertens. 1995;13:357–365. [PubMed] [Google Scholar]
- 40.Palatini P, Mos L, Ballerini P, Mazzer A, Saladini F, Bortolazzi A, et al. Relationship between GFR and albuminuria in stage 1 hypertension. Clin J Am Soc Nephrol. 2013;8:59–66. doi: 10.2215/CJN.03470412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medapalli RK, Parikh CR, Gordon K, Brown ST, Butt AA, Gibert CL, et al. Comorbid diabetes and the risk of progressive chronic kidney disease in HIV-infected adults: data from the Veterans Aging Cohort Study. J Acquir Immune Defic Syndr. 2012;60:393–399. doi: 10.1097/QAI.0b013e31825b70d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Volberding PA, O'Hare AM. Racial differences in end-stage renal disease rates in HIV infection versus diabetes. J Am Soc Nephrol. 2007;18:2968–2974. doi: 10.1681/ASN.2007040402. [DOI] [PubMed] [Google Scholar]
- 43.Wierup N, Björkqvist M, Kuhar MJ, Mulder H, Sundler F. CART regulates islet hormone secretion and is expressed in the beta-cells of type 2 diabetic rats. Diabetes. 2006;55:305–311. doi: 10.2337/diabetes.55.02.06.db04-1383. [DOI] [PubMed] [Google Scholar]
- 44.Banke E, Riva M, Shcherbina L, Wierup N, Degerman E. Cocaine- and amphetamine-regulated transcript is expressed in adipocytes and regulate lipid- and glucose homeostasis. Regul Pept. 2013;182:35–40. doi: 10.1016/j.regpep.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 45.Kotchen TA, Piering AW, Cowley AW, Grim CE, Gaudet D, Hamet P, et al. Glomerular hyperfiltration in hypertensive African Americans. Hypertension. 2000;35:822–826. doi: 10.1161/01.hyp.35.3.822. [DOI] [PubMed] [Google Scholar]
- 46.Nelson RG, Bennett PH, Beck GJ, Tan M, Knowler WC, Mitch WE, et al. Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. Diabetic Renal Disease Study Group. N Engl J Med. 1996;335:1636–1642. doi: 10.1056/NEJM199611283352203. [DOI] [PubMed] [Google Scholar]
- 47.Stevens LA, Coresh J, Greene T, Levey AS. Assessing Kidney Function — Measured and Estimated Glomerular Filtration Rate. New England Journal of Medicine. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]