Abstract
Background and Purpose:
Participant recruitment is central to all clinical trials. Any delay in recruitment affects the completion and ultimate success of the trial. We report our experience with patient screening and randomization in CombiRx, which may inform the design of other trials.
CombiRx was a multi-center, phase III, double-blind, randomized clinical trial comparing the combined use of interferon beta-1a and glatiramer acetate to either agent alone in patients with relapsing-remitting multiple sclerosis (RRMS). This trial was launched in January 2005 in 69 centers in the U.S. and Canada under a co-operative agreement with the National Institute of Neurological Disorders and Stroke (NINDS). The goal was to recruit 1000 patients over 1.5 years after a 6 month startup period. Instead, the investigators required 4.25 years to enroll 1008 patients.
Methods:
During this trial, we assessed the effectiveness of various recruitment strategies, utility of rescreening prior screen failures, and potential factors and strategies used in study conduct, research and infrastructure, all of which affected recruitment of participants and ultimately time to completion of CombiRx. We particularly were interested in the variability in time to site initiation between academic centers and private practice sites.
Results:
Physicians who were directly involved in the medical care of patients with RRMS were the primary source of patients recruited to CombiRx. A flexible study design that allowed for re-screening of the initial screen failures after a period of time was useful due to the relapsing/remitting course of the disease. Academic centers took longer to implement the trial than the private practice centers, but once sites were approved for enrollment, there was no important difference in the number of participants enrolled.
Limitations:
The CombiRx trial was conducted during a period when multiple new medications were being tested, thus affecting the pace of recruitment and limiting ability to generalize our experiences. However, the lessons we learned about process are relevant.
Conclusion:
Participants can be enrolled successfully in a clinical trial for RRMS, but factors affecting the time to achieve the requirements needed to start screening can be unpredictable and problematic. Prospective planning by the sponsors and investigators, use of central IRBs, master trial agreements and secure remote desktop access to the trial database may expedite trial implementation and participant recruitment. A good scientific research question with flexible study design and active involvement of the clinicians are important factors driving recruitment. Clinical trials can be implemented successfully both in private practices and at academic centers, a consideration when selecting sites.
Keywords: CombiRx, relapsing remitting, multiple sclerosis, recruitment strategies, rescreening, clinical trial, clinical research sites
Introduction and background
Achieving successful recruitment of participants to a clinical trial can be an expensive and a time intensive process1, 2. Participant recruitment to a trial remains a key factor affecting completion and ultimate success of the trial.1 Delays in recruitment result in postponement of trial completion that may be protracted when a large number of participants are sought.3 Innovative designs that allow reassessment and modification of inclusion and exclusion criteria without compromising generalizability and the potential to rescreen prior screening failures may contribute to efficient completion of trial enrollment.. One example of a trial in which these strategies were employed is the combination therapy in patients with relapsing-remitting multiple sclerosis (CombiRx) trial. 4,5
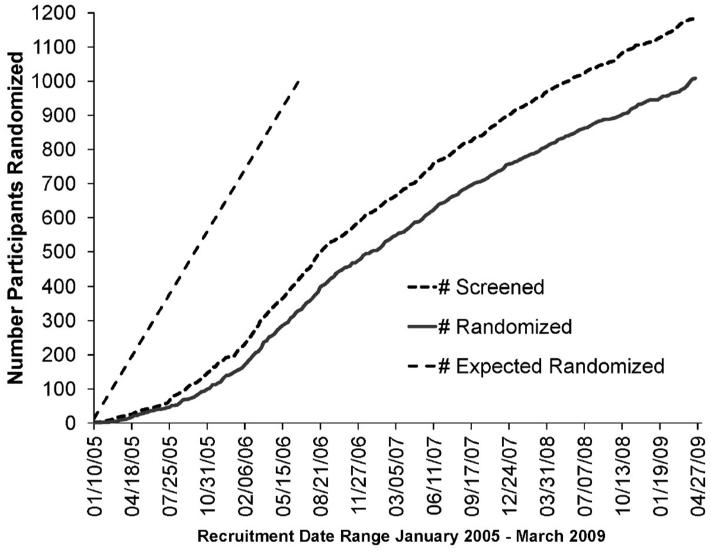
CombiRx was a multi-center, phase III, double-blind, randomized clinical trial that compared the combined use of interferon beta-1a and glatiramer acetate to either agent alone in patients with relapsing-remitting multiple sclerosis (RRMS). Half of the participants in the study received the combination of interferon beta-1a and glatiramer acetate versus 25% in each arm of a single agent plus matching placebo for the other agent. The primary endpoint of the study was comparison of the Annualized Relapse Rate (ARR) between the three treatment arms. This trial was launched in January 2005 in 69 centers (65 sites within 32 states of the US and 4 sites within 3 provinces of Canada) under a co-operative agreement with the National Institute of Neurological Disorders and Stroke (NINDS). The goal was to recruit 1000 participants over 1.5 years after a 6 month startup period. This goal was based on statistical estimation of the sample size required to provide adequate power to detect a clinically and statistically significant difference in outcomes and on estimates of numbers of potential participants and patients with RRMS provided by the sites on initial survey. However, contractual and Institutional Review Board (IRB) review processes delayed individual site initiation. Furthermore, the approval of a new drug natalizumab, a humanized monoclonal antibody against the cell adhesion molecule alpha-4 integrin, further slowed recruitment because of the promise of efficacy of this drug. Nevertheless, the CombiRx trial investigators recruited 1008 patients but required 4.25 years to do so. (Figure 1)
Figure 1.
Projected number of participants at the start of the study versus cumulative number of candidates screened and eligible candidates randomized in the CombiRx trial, by time.
We report our experience with patient screening and randomization in CombiRx and highlight issues and lessons learned in relapsing remitting multiple sclerosis (RRMS) participant recruitment that may generalize to future trials. We also report the strategies incorporated in the study design and research infrastructure that enabled investigators to achieve the target enrollment for CombiRx.
Methods
Sites were initiated in 3 waves, starting in July 2004 (wave 1), April 2005 (wave 2), January 2006 and later (wave 3). Beginning in August 2007, all new participants were asked to complete a questionnaire that requested information regarding trial referral to assess the effectiveness of various recruitment approaches, i.e. referral by physician, staff or other patients, in-clinic and media advertising, mass mailings, internet or listing on clinicaltrials.gov. We advertised the trial to neurologists at scientific conferences, local medical meetings, and corresponded directly with community neurologists and patient advocacy groups such as the National Multiple Sclerosis Society. We compared the characteristics of participants who were randomized with screenees who were terminal screen failures (TSF) i.e. those who could not rescreened.
Participating Sites
Of the 68 centers that randomized at least one participant, 39 were academic centers and 29 were considered private practices. Eighteen private practice sites and 3 academic centers used a central IRB (Western IRB); the rest of the sites used local IRBs. Following regulatory approvals, the sites had to complete MRI certification, training in the use of laboratory and outcome rating scales, and Data Entry System certification. Magnetic resonance imaging (MRI) certification required that the imaging site associated with each of the clinical sites perform a test MRI according to trial protocol on a volunteer with known cerebral lesions consistent with the disease. The images were transmitted to the central MRI Analysis Center in Houston. Following central review of the images to confirm that they met specifications and were of adequate quality for semi-automated image analysis, the center and the MRI scanner used to produce the test images were certified to participate in the trial. Data entry system certification required local assistance with installation of the data entry application (requiring firewall access and test data entry) before participant randomization could be initiated.
Participant screening and randomization
All potential participants were screened for eligibility based on disease history, disease activity, extended disability status scale (EDSS) score, multiple sclerosis functional composite (MSFC) score, and an MRI scan. The MRI was scheduled as the last eligibility hurdle to minimize the cost of screening. The randomization window for a CombiRx candidate initially was a minimum of 14 days to a maximum of 30 days after screening was initiated, in order to ensure that a baseline MRI had been obtained.
Participant re-screening
Participants who initially failed screening on a reversible criterion were reassessed and ultimately could become a randomized participant as the initial reasons for exclusion resolved. The reasons for initial screen failures were protocol defined failures associated with disease status and medical conditions and included ongoing/recent relapse activity in the prior 30 days, failure to meet prior relapse criteria, other medical conditions, abnormal laboratory values, and failure to meet RRMS diagnostic criteria (Table 1).
Table 1.
Reasons for initial and terminal screen failure.
| Failure Reason | Initial screen failure, n (%) |
Rescreened after initial failure | ||
|---|---|---|---|---|
|
| ||||
| Second failure, n (%) |
Exclusion resolved for inclusion, n (%) |
Total rescreened, n (%) |
||
| Total number of participants |
176 (100) | 4 (2.2) | 55 (31) | 59 (33.5) |
| Exacerbation within 30 days |
32 (18.2) | 1 (25) | 16 (29.1) | |
| < 2 exacerbations in prior 3 years |
22 (12.5) | 6 (10.9) | ||
| Other medical condition | 19 (10.8) | 1 (25) | 5 (9.1) | |
| Abnormal lab value | 17 (9.7) | 1 (25) | 7 (12.7) | |
| Inconclusive for / Not RRMS |
13 (7.4) | 1 (1.8) | ||
| Unable to have MRI/Gad sensitivity |
7 (4.0) | 2 (3.6) | ||
| EDSS > 5.5 | 2 (1.1) | 1 (25) | ||
| Other reason* | 64 (36.3) | 18 (32.7) | ||
Other non-medical reasons, including: site error, exceeding screening window, and participant decision
Protocol amendments
During the trial, the following amendments were made to address the slow accrual rate during the first 2 years:
Addition of sites: Up to 100 active sites were invited to participate in the study, instead of the prior limit of 90 sites.
Longer screening-to-randomization window: In the first year of the trial it became apparent that the logistics of the time frame for obtaining the MRI within 30 days after screening of a candidate patient was problematic due to scheduling problems at a number of sites. Hence the screening-to-randomization window was increased to 45 days as long as there were no intervening relapses. Any patient scheduled for an MRI more than 45 days after screening was allowed to participate provided the MRI was obtained within a time frame that was deemed reasonable by the CombiRx steering committee, from a scheduling perspective and the delay was admitted as a protocol exception. This change significantly reduced the site burden by eliminating protocol exceptions and the consequent reporting process. This change also led to a more realistic time frame for screening and flexibility to accommodate MRI scheduling.
Incentives: In order to increase recruitment, we initiated a series of supplemented financial incentives of $5,000 each to sites after 5, 10 and 20 participants had been enrolled.
Data Analysis
The CombiRx starting time for administrative benchmarks was the IRB approval date at the Clinic Coordinating Center at Mt. Sinai School of Medicine. Subsequent benchmarks were measured in days and reported in 30-day months or 12-month years.
We compared the characteristics of participants who were randomized with screenees who were Terminal Screen Failures (TSF) i.e. those who could not be rescreened. Group comparisons were made with the t-test, Wilcoxon rank-sum test, or Chi-square test, as appropriate; p-values are presented for descriptive purposes only, with a p < 0.05 considered meaningful. Analyses were performed using JMP v8 and SAS v9.1.
Results
Patient referral and participant identification for the trial
By April 2012, 842 (83.5%) of 1008 randomized participants and 51 of the 176 screen failures (28.9 %) defined by not meeting at least one of the inclusion criteria (no one category of which exceeded 15%), had provided the referral information. The majority of referrals were physician related, with 63% already treated by an investigator or physician involved in CombiRx. Another 29% were referred by other treating neurologists to a CombiRx participating physician. The remaining participants were referred to a CombiRx physician by clinic staff (4%), another physician (1%), or two specific websites, www.nmss.org (1%) and www.mayo.edu (1%). Less than 1% of participants were referred to the trial for screening by www.clinicaltrials.gov, a friend or another patient, in-clinic advertising or some other method. CombiRx personnel did not track the source of “other physician” referrals and could not determine how non-CombiRx physicians heard about the study.
Altogether, 1129 potentially eligible participants were screened for the trial of which 953 patients were randomized after their first screening and 176 screenees were deemed initial screening failures; 55 initial screening failures were randomized after additional screening; 121 screenees failed to meet inclusion criteria.
Participant re-screening
Of 176 initial screen failures, the majority of reasons for failure (64%) were protocol defined failures associated with disease status and medical conditions including: ongoing/recent relapse activity in the prior 30 days in18.2%, failure to meet prior relapse criteria in 12.5%, other medical conditions in 10.8%, abnormal laboratory values (most common was elevated liver function test) in 9.7%, and failure to meet RRMS diagnostic criteria in 7.4% (Table 1). Of the 176 initial screening failures, 59 were re-screened; for 55 of re-screenees (93%) the initial reasons for exclusion had resolved and they were randomized. Of those successfully rescreened, 29% waited at least 30 days for disease activity to stabilize, 23% were due to a site error or delay that resulted in exceeding the 45 day window for randomization, thus requiring a rescreen, and 11% enrolled after a second relapse to satisfy the requirement of having experienced 2 relapses in the prior 3 years. Altogether, 121 participants (10.7%) of the total number screened were classified as terminal screening failures (TSF).
Characteristics of participants recruited
By self-report of the 1008 randomized participants, 87.6 % were Caucasian, 7.2 % African American and 89.6% of non-Hispanics/ Latino ethnicity. Similarly, among the 121 TSF, 85.3% were Caucasian, 8.6% were African American and 5.3% were Hispanics.5 Since MS is more common among Caucasians, these differences reflect well-recognized features of this disease.
In regard to the disease severity, the proportion of screenees with EDSS scores > 2 was somewhat higher at 49.5% among TSFs compared to 39.2% among randomized participants (p=0.06). The proportion of TSFs with no gadolinium (Gd) enhancing lesions on the baseline brain MRI also was higher, 71.7% in TSF compared to 60.4% in randomized participants. However, only 60 of 121 TSFs had MRI scans (p=0.08) as participants deemed ineligible for other reasons often were excluded prior to obtaining an MRI. There were no differences in the volume of Gd enhancing lesions, when present, or the number and volume of T2 lesions. Only small differences were observed between randomized participants and TSFs in CombiRx; none of the observed differences was related to disease course. 5,6
Potential factors influencing recruitment for CombiRx
Description of the participating sites
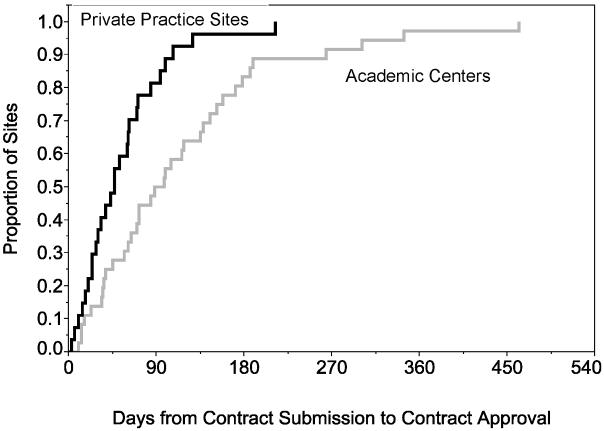
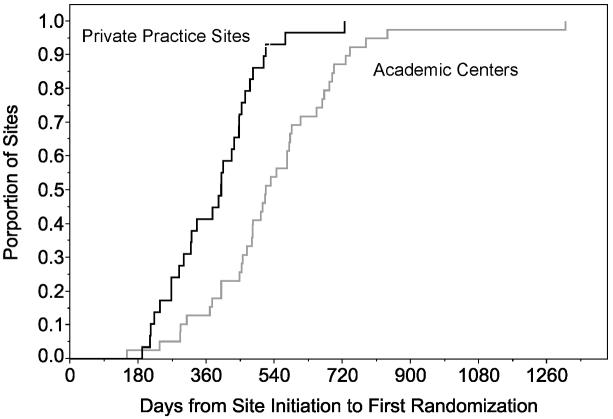
From overall study IRB approval at the central coordinating institution, a median 3 months (range 1-22 months) elapsed before all local investigators submitted the contract and protocol for local approval (Figures 2a, b). Overall, contract approval took approximately 6 months and IRB approval about 6.5 months. Following regulatory approvals, completion of MRI certification, laboratory and outcome rating scales training, and data entry system certification were completed at 10 months (2.4 months – 2.5 years). An additional 4 months, on average, elapsed before the first participant was randomized at a site.
Figure 2a.
Kaplan-Meier curves for number of days from contract submission to contract approval at private practice sites (black) in comparison to the academic centers (grey).
Figure 2b.
Kaplan-Meier curves for number of days from site initiation to the first randomization at private practice sites (black) in comparison to the academic centers (grey).
Site comparisons
The first participant was randomized into CombiRx about 7 months after the Clinical Coordinating Center (CCC) received IRB approval at a private practice site in January 2005. The mean time from site initiation to first randomization for all sites was more than twice as long, at just over 15 months (5 months to 3.6 years). The mean time was 13 months (6.3 months to 2 years) in private practices and 17 months (5 months to 3.6 years) in academic centers. The largest delays occurred with IRB and contract approvals at academic centers. While the minimum time from study initiation to first randomization was similar in academic centers versus private practice (8.5 vs. 7 months), the median time to first enrollment was 4 months longer for academic centers compared to private practice sites, with the largest differences occurring during the contract approval process (4.7 months), MRI certification (3.5 months) and first randomization (4 months). After adjusting for the difference in the delays of contract submission, academic centers had more than a two-month delay in contract approval compared to private practice sites. (Figures 2a, b)
Other Considerations
Administrative supplements and incentives
Most of the sites (63 of total 67 active sites) enrolled at least 5 participants and received the first monetary supplement of $5000, 44 sites received a second supplement after enrolling 10 or more participants, and 18 sites (11 academic and 7 private practice sites) that enrolled more than 20 participants received a third administrative supplement.
Seasonal fluctuations
The average accrual rate for the trial remained steady at about 20 participants per month after the first 18 months. Although it has been suggested that MS activity or symptoms may fluctuate seasonally, 7 we did not notice any significant seasonal effect in the CombiRx recruitment pattern. There was a slight, but not significant, decline in the accrual rate during the July to September quarter annually.
Discussion
We learned from the CombiRx recruitment experience that a) rescreening patients with RRMS, and possibly other diseases that have a relapsing/remitting course, and flexible entry criteria are useful strategies for recruitment; b) an area for improvement and targeted effort for future trials include reducing delays in start-up times at individual sites; and c) buy-in from the investigators and clinicians involved in the medical care of the target patients was important.. While there were differences in academic versus private practice in getting started, after initiating trial activities, there were no differences in the median number of participants screened (15 vs. 12) or randomized (13 vs. 11), the months open for recruitment months vs. 35.5 months), the number of staff trained (a surrogate for study personnel turnover), or centers switching to remote data entry system access.
We do not know whether all eligible patients were invited to enroll, but about 90% of the participants screened for CombiRx enrolled in the trial. One of the factors that may have contributed to this high screening to randomization ratio is the fact that most of the referring neurologists were CombiRx investigators and hence familiar with the trial eligibility criteria and study design and were interested in the research question. We do not know how much the availability of medications at no cost was an inducement for the clinicians who otherwise might have to defend eligibility to payers. Free medication likely influenced patients’ willingness to participate.
Allowing for re-screening of the candidates after a period of time contributed an additional 55 (5%) of the randomized participants in the trial. Provisions for rescreening of initially ineligible patients and modification of eligibility criteria should be anticipated in the trial protocol. Changes made after initiation of the trial must be considered with full awareness that such changes have the potential to shift the cohort characteristics that may lead to differences in magnitude or direction of therapeutic outcomes in sub-cohorts defined by the implementation of changes. We addressed this issue by comparing the characteristics of participants in two groups, participants who were randomized after rescreening and patients who were TSFs.
When we compared the types of participating sites, it was clear that academic centers took longer to start a trial than the private practice centers typically did. Academic centers generally had more local administrative steps involved in contract negotiations and approvals. In addition, MRI site certification was a lengthy and iterative process for some sites. The time to complete data entry training, the final step in the process prior to enrollment, was significantly longer in academic centers, largely due to local security and firewall issues that had to be resolved before installation of the data acquisition system 8. Thus clinical trials can be conducted successfully in private practice settings as well as academic institutions. Our finding is similar to that reported in from the Sub macular Surgery Trials in the field of ophthalmology. 9 It may be prudent for trial organizers and sponsors to consider a balanced number of private practice and academic centers as participating sites for large phase III clinical trials in order to maintain a steady accrual rate, especially in the initial start-up period. Academic institutions are advised to assess local roadblocks that lead to delays in implementing multicenter research studies and to increase internal efficiency.
We provided monetary supplements or incentives for high enrollers. Potentially, we could have provided higher incentives to reward the high enrolling sites, Designers of future trials may wish to consider incentives to reward more rapidly enrolling centers; however, monetary incentives are likely to raise concerns by ethics boards in some countries.
Recommendations for future trials
Moving forward, it is important to incorporate a realistic recruitment plan in the study design. The, use of master trial agreements or a central IRB may reduce the start-up time. Academic centers should map their institutional procedures to identify redundant or unnecessary administrative steps and to develop and monitor internal metrics of performance. Potential clinical trial investigators at academic centers should become familiar with internal procedures, anticipate roadblocks, and address problems expeditiously. Use of a secure remote desktop option or web/cloud based technology that eliminates the need to install software programs and does not compromise the confidentiality of patient and research data may help to mitigate delays in future trials.
Limitations
The CombiRx trial was conducted while testing of other oral agents and monoclonal antibodies administered as monthly infusions was underway, thereby affecting on the pace of patient recruitment. This situation may limit ability to generalize our experiences, although the lessons learned about process are relevant.
Another limitation is that we made administrative changes across centers to facilitate recruitment, but we could not quantify the benefits of each of these specific changes to identify the most helpful strategy. Also, the availability of medications at no cost may have influenced physician referrals to CombiRx and patients’ willingness to participate but these effects cannot be quantified.
Conclusions
Our findings suggest that participants can be successfully recruited to a clinical trial for RRMS, but factors affecting the time to achieve the requirements necessary to start screening can be unpredictable and problematic. Sponsors must plan to provide support to clinical trials with large number of participants, multiple centers and long duration of follow-up beyond the typical 5-year funding period. More flexible funding strategies currently are under consideration by some institutes of the U.S. National Institutes of Health. Active involvement of the clinical investigators is essential to sustained recruitment of patients. Thus, a good scientific research question or hypothesis for which the answer from the trial will affect clinical care is essential to clinician engagement. Use of central IRBs, master trial agreements and secure access to the trial database for entering data may expedite implementation and recruitment. Clinical trials can be implemented successfully at private practice centers and academic centers, a consideration when selecting sites.
Acknowledgements
We thank all the CombiRx site investigators and staff who enrolled participants: Drs. M Agius,UC Davis,CA, USA; K Bashir, UAB, AL, USA; R Bumhefner, VA Medical Center-West LA, CA, USA; G Birnbaum, Minneapolis Clinic, MN, USA; G Blevins, Capital Health and the University of Alberta, Canada; T Brown and J Dunn, MS Center at Evergreen, WA, USA; A Camac, Lahey Clinic, MA, USA; R Bomprezzi, T Vollmer and D Campagnolo, Barrow Neurological Institute, AZ, USA; J Carter, Mayo Clinic, AZ, USA; B Cohen, Northwestern University, IL, USA; J Cooper, Sutter East Bay Medical Foundation, CA, USA; J Corboy, U of Colorado, CO, USA; A Cross, Washington University, MO, USA; L D Dewitt, U of Utah, UT, USA; K Edwards, MS Center of Northeastern New York Empire Neurology PC, NY, USA; E Eggenberger, Michigan State University, MI, USA; J English and S William, MS Center of Atlanta, GA, USA; W Felton, Virginia Commmonwealth University, VA, USA; P Fodor, Patricia Fodor PC, CO, USA; C Ford, U of New Mexico, NM, USA; M Freedman, Ottawa hospital, Ottawa, Canada; D Jacobs and S Galetta, U of Pennsylvania, PA, USA; G Garmany, Alpine Clinical Research Center, CO, USA; A Goodman, U of Rochester, NY, USA; M Gottesman, Winthrop Neurology Faculty Practice, NY, USA; D Pelletier, J Preiningerova, C Gottschalk and C Riley, Yale University, CT, USA; K Pandy, N Lava and M Gruenthal, Albany Medical College, NY, USA; M Gudesblatt, South Shore Neurologic Assoc, Inc, NY, USA; J Herbert, Hospital for Joint Disease, NY; R Holub, Neuro Assoc of Albany PC, NY, USA; WD Honeycutt, Neurology Associates PA, FL, USA; B Hughes, Ruan Neurology Clinic and Research Center, IA, USA; G Hutton, Baylor college of Medicine, TX, USA; V Thadani, L Kasper, E Lallana, Dartmouth Medical School, NH, USA; J Kattah OSF St Francis Medical Center, IL, USA; A Katz, Centrstate MS Center, NJ, USA; M Kaufman, CMC-Neuroscience and Spine Institute, NC, USA; M Keegan, Mayo Clinic-Rochester, MN, USA; O Khan, Wayne State University, MI, USA; B Khatri, Regional MS Center at St. Luke’s Medical Center, WI, USA; M Kita, Virginia Mason MS Center, WA, USA; B Koffman, Medical College of Ohio, OH, USA; J W Linsey, UT-Houston, TX, USA; M Hagan, R Williams, P Loge, St. Vincent Healthcare, Neuroscience Center, MT, USA; S Lynch, U Kansas Med Center, KS, USA; L Mejico, SUNY Upstate Medical University, NY, USA; L Metz, M Yeung, University of Calgary, Canada; P O’Connor, St. Michael’s hospital, ON, Canada; R Hamill and H Panitch, U of Vermont, VT, USA; A Boster and K Rammohan, The Ohio State University MS Center, OH, USA, P Riskind, U Mass Memorial Medical Center, MA, USA; L Rolak, Marshfield Clinic, WI, USA; W Royal and K Johnson, Maryland Center for MS, MD, USA; S Scarberry, Sanford Clinic Neuroscience, ND, USA; A Schulman and F McGee, Neurological Associates, VA, USA; T Scott, Allegheny MS Treatment Center, PA, USA; C Sheppard, Oak Clinic for MS, OH, USA; W Sheremata, U of Miami, FL, USA; L Stone, Cleveland Clinic, OH; S Subramaniam, Vanderbilt Univ MS Center, TN, USA; F Thomas, St. Louis Univ-VA Medical Center, MO, USA; B Thrower, Shephard Center, GA, USA; M Tullman, Columbia University, NY; USA; S Waldman, Neurology Center North Orange County, CA, USA; B Weinstock-Guttman, The Jacobs Neurological Institute, NY, USA; J Wendt, Northwest NeuroSpecialists, PLLC, AZ, USA; D Wynn, Consultants in Neurology MS Center, IL, USA.
Funding acknowledgement: The study was funded by the National Institutes of Health (NIH) and the National Institute of Neurological Disorders and Stroke (NINDS), U01 NS045719; with medications and matched placebos provided by Biogen Idec and Teva Pharmaceuticals. Design, analysis, and decision to publish results are the responsibility of the CCC, SDMC, MRI-AC. Additional funding was provided by the NINDS NIH Intramural Program for the Biomarker MS ancillary study and the National Multiple Sclerosis Society for the Contrast Sensitivity ancillary project. The trial is registered at www.clincaltrials.gov, NCT00211887.
Footnotes
Disclaimer: This report does not represent the official view of the NINDS, the NIH, or any part of the US Federal Government. No official support or endorsement of this article by the NINDS or NIH is intended or should be inferred.
Statement of Conflicts of Interest: Drs. M Bhanushali and R Conwit are employees of the NIH/ NINDS and declare that they have no other conflicts of interest. All other authors, Drs. Gustafson, Powell, Wolinsky, Cutter, Lublin and Cofield are members of the CombiRx steering committee and receive funding from the NIH/ NINDS.
References
- 1.Cofield S, Conwit R, Barsan W, et al. Recruitment and retention of patients into emergency medicine clinical trials. Acad Emerg Med. 2010;10:1104–12. doi: 10.1111/j.1553-2712.2010.00866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gul R, Ali P. Clinical trials: the challenges of recruitment and retention of participants. Clin Nurs. 2010;19:227–233. doi: 10.1111/j.1365-2702.2009.03041.x. [DOI] [PubMed] [Google Scholar]
- 3.Lee J, Foster H, Jr, McVary K, et al. Recruitment of participants to a clinical trial of botanical therapy for benign prostatic hyperplasia. J Altern Complement Med. 2011;17(5):469–72. doi: 10.1089/acm.2010.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lublin FD, Cofield SS, Cutter GR, et al. Randomized study combining interferon and glatiramer acetate in multiple sclerosis. Ann Neurol. 2013;73(3):327–340. doi: 10.1002/ana.23863. doi: 10.1002/ana.23863. Epub 2013 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindsey J, Scott T, Lynch S, et al. The CombiRx trial of combined therapy with interferon and glatiramer acetate in relapsing remitting MS: Design and baseline characteristics. Mult Scler Relat Disord. 2012 Apr 1;1(2):81–86. doi: 10.1016/j.msard.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Cofield SS, Cutter GR, Wolinsky JS, et al. Are randomized participants really different from non-Eligible ones in CombiRx? Neurology. 2011;76(S4):P04.191. [Google Scholar]
- 7.Meir DS, Balashov KE, Healy B, et al. Seasonal Prevalence of MS disease activity. Neurology. 2010;75(9):799–806. doi: 10.1212/WNL.0b013e3181f0734c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Probstfield JL, Frye RL. Strategies for recruitment and retention of participants in clinical trials. JAMA. 2011;306(16):1798–99. doi: 10.1001/jama.2011.1544. [DOI] [PubMed] [Google Scholar]
- 9.Submacular Surgery Trials Research Group Clinical trial performance of community-vs university-based practices in the Sub macular Surgery Trials (SST): SST reports no. 2. Arch Ophthalmol. 2004;122(6):857–63. doi: 10.1001/archopht.122.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]