Abstract
Application of umbilical cord blood (UCB) transplantation in adults as a treatment post-chemotherapy is hampered due to delayed platelet recovery. A potential solution suggested is the transfusion of ex vivo expanded megakaryocytes (Mks) from hematopoietic stem cells (HSCs). Alternatively, large-scale production of platelets in vitro has also been attempted with the goal of transfusing them into patients with thrombocytopenia. Glycosaminoglycans (GAGs) have been shown to influence the proliferation and differentiation of HSCs. This study sought to examine the effects of immobilized GAGs on the expansion, apoptosis, and platelet release activity of CD41a+ Mk progenitors in vitro. Freshly isolated HSCs from UCB were cultured in serum-free media supplemented with thrombopoietin on GAG-derivatized chitosan membranes for 17 days. Controls consisted of uncoated and chitosan-coated wells. Wells were demidepopulated at periodic intervals and analyzed by flow cytometry. Heparin and dermatan sulfate surfaces significantly enhanced total cell and Mk cell expansion (p < 0.05) compared to both the controls. The apoptotic Mk fraction was significantly lower on GAG surfaces (p < 0.05) compared to the polystyrene control during the early stages of the culture (days 7 and 11). However, by day 17, the apoptotic Mk fraction was comparable on all surfaces. The cumulative number of platelets generated on dermatan sulfate and heparan sulfate surfaces was significantly higher (p < 0.05) than on both the controls. These results suggest that immobilized GAGs delay Mk apoptosis and thereby enhance Mk expansion and platelet production.
Keywords: umbilical cord blood, glycosaminoglycans, megakaryocytes, apoptosis, platelets
INTRODUCTION
Umbilical cord blood (UCB) is a preferred source of hematopoietic stem cells (HSC) due to its limitless supply, risk-free collection, and reduced incidence of graft-versus-host disease (GVHD).1 However, implementation of UCB transplantation as a treatment in adult patients after radiation therapy and chemotherapy is severely limited due to delayed platelet recovery post-transplantation. Studies have shown that it takes a median time of approximately 71 days for the platelet count to return to normal after UCB transplantation.2 During this time, patients need to be subjected to platelet transfusions. Reports suggest that in the United States alone, approximately 1.5 million patients undergo platelet transfusions every year.3 Since platelets are derived from volunteer donors, there is always a scarcity. Moreover, platelet transfusions are often accompanied by significant risks of bacterial and viral infections from the donors.4,5in vitro expansion of megakaryocytes (Mks) from CD34+ stem cells, followed by their transfusion to expedite the regeneration of platelets has been suggested as a potential solution.6 Efforts have also been made for ex vivo production of human platelets from UCB CD34+ cells that are morphologically and functionally similar to platelets in the peripheral blood.7
Thrombopoietin (TPO) is the primary cytokine that supports the proliferation and differentiation of Mks.8–10 It is also known to play an important role in platelet production and function.11,12 Platelets have been shown to form on long filopodial extensions called proplatelets, which originate from the mature Mk membrane.13 It is believed that Mk apoptosis plays a critical role in this phenomenon by initiating the final stages of platelet formation.14,15 Recently, Junt et al.16 reported that proplatelets are extended into the blood vessel by the Mks in the mouse bone marrow and the shear stress imparted by blood flow results in the fragmentation of these proplatelets to release platelets.
The hematopoietic microenvironment in the bone marrow mainly consists of hematopoietic cells, stromal cells, and the extra cellular matrix (ECM). Linear heteropolysaccharides termed glycosaminoglycans (GAGs) form a major component of the ECM. Previous work has shown that HSC proliferation and differentiation can be influenced by the use of GAGs. Specifically, GAGs have been shown to modulate the activity of various cytokines and growth factors.17–24 It has also been reported that GAGs can bind to cytokines and protect them from chemical and physiological degradation.25 In the recent past, a few studies have reported that GAGs promote the expansion of human Mks in the presence of TPO. hyaluronan and heparan sulfate have been shown to promote the production of Mk colony forming units (CFU-Meg) from UCB CD34+ cells, whereas dermatan sulfate has been shown to significantly enhance the clonal growth of CFU-Meg from human peripheral blood CD34+ cells.26,27 Heparin has been shown to enhance the activity of TPO and thereby positively regulate megakaryopoiesis.28 Although the results of the aforementioned studies are promising, little is known about the effect of GAGs on the apoptosis of Mks and the subsequent platelet release. In addition, soluble GAGs used in these culture systems are susceptible to rapid internalization and enzymatic degradation. These phenomena can produce transient changes in GAG concentration with the duration of the culture. Furthermore, application of soluble GAGs may not embody the signaling dynamics of matrix-bound GAGs in vivo. Therefore, there is a need for a more stable, scalable, ex vivo expansion culture system that would better reproduce the in vivo signaling dynamics.
Chitosan is widely studied as a potential biomaterial for tissue engineering applications.29,30 Apart from being biocompatible and biodegradable, the primary amine groups on the chitosan molecule allow the attachment of various other biologically active substances. In this study, GAGs were covalently immobilized onto chitosan membranes to mimic the in vivo extracellular environment. This method also results in less temporal variation in GAG exposure, reduced GAG requirements, and possibly a reduction in the likelihood of GAG-mediated cytokine sequestration. Furthermore, binding of cytokines to covalently immobilized GAGs could reduce cytokine requirements, by limiting cytokine degradation and internalization. Finally, the immobilization method may facilitate scale up and development of more efficient, three-dimensional bioreactor culture systems. UCB CD34+ cells were seeded onto immobilized GAG surfaces in TPO supplemented, serum-free culture medium and the effect of immobilized GAGs on Mk expansion, apoptosis and the subsequent platelet release was examined.
MATERIALS AND METHODS
Sources of materials and reagents
Medium molecular weight chitosan, glacial acetic acid, hyaluronic acid sodium salt from streptococcus equi sp., Histopaque-1077, bovine serum albumin (BSA), and 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide (EDC) were obtained from Sigma-Aldrich (St. Louis, MO). StemSpan Serum-Free Expansion Medium (SFEM) and StemSep CD34+ Positive Selection Kits were purchased from Stem Cell Technologies (Vancouver, Canada). MiniMACS Columns were obtained from Miltenyi Biotec (Auburn, CA). Heparin, dermatan sul-fate, and heparan sulfate from bovine intestinal mucosa were purchased from Celsus Laboratories (Cincinnati, OH). Mouse-anti-human-CD34-PE, mouse-anti-human-CD41a-FITC, and Annexin V-PE were purchased from Southern Biotech (Birmingham, AL). 7-Aminoactinomycin D (7AAD) was obtained from BD Biosciences (San Diego, CA). Recombinant Human TPO was purchased from Peprotech (Rocky Hill, NJ). Collagen Type I was isolated in house from Sprague Dawley rat tail ten-dons by the method of Elsdale and Bard.31
Preparation of GAG-modified chitosan surfaces
In an effort to generate culture surfaces with constant characteristics, GAGs were covalently immobilized onto chitosan membranes at saturating densities. Briefly, 250 μL of sterile, 1.5 wt % chitosan solution in 1% acetic acid was added to each well of a 24-well plate, and the excess chitosan solution was aspirated. The plate was then air dried in a sterile hood under laminar flow for 3 h to form chitosan membranes. These membranes were neutralized with 0.2M NaOH and washed three times with sterile PBS. Sterile GAG solutions, either heparin, dermatan sulfate, hyaluronan, or heparan sulfate (1 mg/mL in PBS) was preactivated by adding equal volume of 10 mM EDC (in PBS) for 15–20 min. Five hundred microliter of the GAG-EDC complex was added to each well and allowed to react for 24 h on an orbital shaker. The GAG-derivatized chitosan surfaces formed were then washed three times with 1 mL sterile PBS for 1 h each. The GAGs evaluated in this study were heparin, dermatan sulfate, hyaluronan, and heparan sulfate.
Evaluation of the stability of immobilized GAG culture surfaces
Chitosan membranes were covalently immobilized with GAGs as described earlier. Once the reaction was complete, the unreacted GAG solution was collected and 0.5 mL of PBS (substitute instead of culture medium) was added to each culture well and the culture plate was incubated at 37°C for 3 weeks. Half volume changes of PBS were done twice a week. The amount of GAG present in the initial unreacted GAG solution and the amount of GAG released (desorbed) at periodic intervals was determined by a colorimetric assay using Safranin-O dye. Briefly, 30 μL of each sample was added to a 96-well plate. To this, 240 μL of 0.05 mg/mL Safranin O dye (in 50 mM sodium acetate buffer) was added, and the absorbance was recorded at 510 nm. A standard curve was plotted by recording the absorbance of serially diluted known concentrations of GAG. The linear equations generated from the standard curves were used for GAG quantification. The amount of GAG initially bound to the chitosan surface was evaluated by calculating the difference between the initial amount of GAG added and the amount of unbound GAG remaining after the reaction. The amount of GAG desorbed was evaluated by determining the GAG concentration in the PBS solution retrieved at intervals over a period of 3 weeks.
Isolation of CD34+ cord blood cells
UCB samples were collected from Hutzel Hospital in 50 mL tubes containing 5 mL of acid citrate dextrose, in accordance with an approved protocol from Wayne State University Institutional Review Board. Mononuclear cells were separated by density gradient centrifugation (300g for 30 min at 20°C) using Histopaque-1077. The cell suspension was washed twice with 2 mM EDTA in PBS, and the remaining red blood cells were lysed using ammonium chloride lysis buffer (150 mM ammonium chloride, 10 mM sodium bicarbonate, and 1 mM EDTA). CD34+ cells were isolated using the StemSep CD34+ Positive Selection Kit and the Mini-MACS magnetic separation column according to manufacturer's instructions. The purity of CD34+ cells was verified by flow cytometry (FACSCalibur, Becton Dickinson) and was consistently >80%.
Cell culture
The enriched CD34+ cell suspension was seeded onto GAG-modified chitosan surfaces in 24-well plates at a density of 25,000 cells/well. Uncoated and chitosan coated surfaces were used as controls. Cells were cultured in StemSpan serum-free medium supplemented with 50 ng/mL TPO, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a 5% CO2 atmosphere. Half volume medium changes were done twice per week. The total culture duration was 17 days. The culture wells were demidepopulated at days 7, 11, 14, and 17. Cells were counted and analyzed using a FACSCalibur flow cytometer (Becton Dickinson).
Measurement of Mk fraction and Mk apoptosis by flow cytometry
A three color flow cytometry analysis was used for the simultaneous measurement of Mk CD41a expression, Mk apoptosis (via Annexin V binding), and cell viability (via 7AAD binding). Briefly, the demidepopulated cell suspension was washed and centrifuged at 200g for 10 minutes to pellet the cells. The pellet was resuspended, stained with anti-CD41a-FITC and incubated in the dark for 30 min at 4°C. Following this, the cell suspension was washed, stained with Annexin V-PE in Annexin V binding buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2, 0.1% BSA) and incubated in the dark for 15 min at 4°C. To test for viability, the cells were stained with 7-Aminoactinomycin D (7AAD) and immediately analyzed by flow cytometry. Ten thousand events were counted per sample, and the data was analyzed using WinMDI 2.8 software. The Mk apoptotic fraction was determined by taking the ratio of the number of apoptotic Mks (CD41a+, Annexin V+) to the total number of Mks (CD41a+). Viability was assessed by considering the 7AAD+ (bright) cells as dead cells.
Analysis of Mk ploidy by flow cytometry
A double staining technique was used for the simultaneous measurement of DNA content and surface immunopheno-typing to determine the DNA ploidy of Mks. Briefly, cells demidepopulated at periodic intervals were washed, stained with anti-CD41-FITC, and incubated for 30 min in the dark at 4°C. Following this, the cells were fixed in 1% paraformaldehyde for 10 min, permeabilized with ice cold 70% methanol (–20°C) for 15 min, washed and incubated with 100 μg/mL RNase for 15 min at 37°C. Cells were stained with propidium iodide (50 μg/mL) and immediately analyzed by flow cytometry. Ten thousand events were counted per sample, and the data was analyzed using WinMDI 2.8 software. CD41a+ cells were gated and analyzed for ploidy.
Platelet analysis by flow cytometry
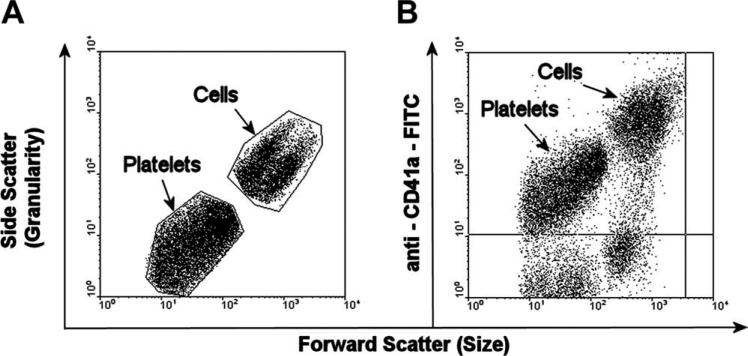
Platelets generated in the culture were enumerated using flow cytometry as CD41a+ events with low forward scatter properties in the size range of blood platelets. Briefly, the demidepopulated cell suspension was first centrifuged at 200g for 10 min to form the first pellet. The supernatant was then collected and centrifuged at 1000g for 10 min to form the second pellet. Both the pellets were washed, stained with anti-CD41a-FITC, incubated for 30 min in the dark at 4°C and analyzed by flow cytometry. Ten thousand events were counted per sample, and the platelets were enumerated via back gating of CD41a+ events that are smaller in size as determined by low forward scatter (Fig. 6). The number of platelets reported is the combined number from both the pellets.
FIGURE 6.
Flow cytometry analysis for platelets. (A) Forward scatter (FSC) versus side scatter (SSC) plot to select events based on size. (B) FSC versus anti-CD41a-FITC plot to determine smaller size events that express positive for CD41a (platelets).
Morphological and functional analysis of platelets by scanning electron microscopy
Platelet morphology and functionality was examined using scanning electron microscopy. A clean glass slide was first coated with collagen solution (1.3 wt %) and air dried. Thirty microliter of a day 14 culture sample (medium + cells) was deposited onto the coated slide and incubated at 37°C for 45 min. The culture medium was then carefully aspirated, and the cells were fixed with 2% glutaraldehyde in PBS for 15 min at room temperature. After fixation, the sample was washed with PBS, dehydrated with an ethanol series, and vacuum dried for 24 h. Slides were then sputter coated with gold and observed under a scanning electron microscope.
Statistical analysis
Results are expressed as mean ± standard deviation of data obtained from at least two independent experiments, with three replicate culture wells per group from each experiment. The data sets from multiple experiments were combined by normalizing the data from each experimental run to its polystyrene control. The individual normalized data points from all experiments were then multiplied by the mean value of the polystyrene control across experiments to obtain a combined data set of n ≥ 6 per group. Statistical significance was determined by using Student's t-test. Probability values of less than 0.05 were considered statistically significant.
RESULTS
GAG desorption kinetics
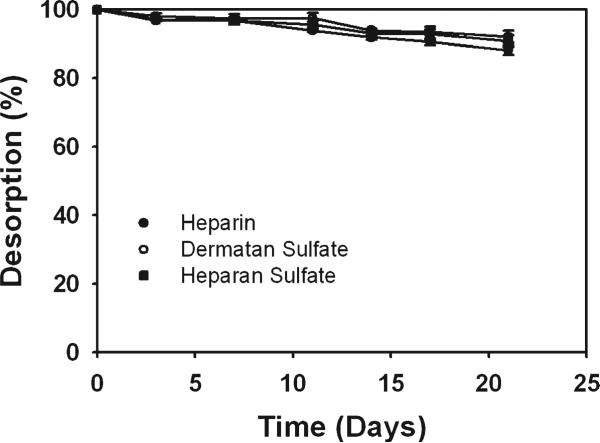
Covalent immobilization of GAGs was performed to generate culture surfaces with constant characteristics. GAG desorption studies were performed to evaluate the stability of the covalently immobilized GAG surfaces. Heparin, dermatan sulfate, and heparan sulfate surfaces showed similar release kinetics. They were observed to release slowly into the medium and by the end of 3 weeks >88% of the initial GAG was still bound to the surface (Fig. 1). The Safranin O dye used in the assay binds to the GAG sulfate groups. Since hyaluronan is not sulfated, the solutions collected from hyaluronan surfaces could not be analyzed using Safranin O. Nevertheless, the loss rate of hyaluronan is expected to be similar to the other GAGs. These results indicate that covalent immobilization of GAGs onto chitosan resulted in a stable culture system with near constant characteristics.
FIGURE 1.
Percentage desorption of covalently immobilized GAGs from chitosan membranes over 3 weeks. More than 88% of GAG remained bound to the chitosan surface indicating that covalent GAG immobilization resulted in a stable culture system.
Cell morphology
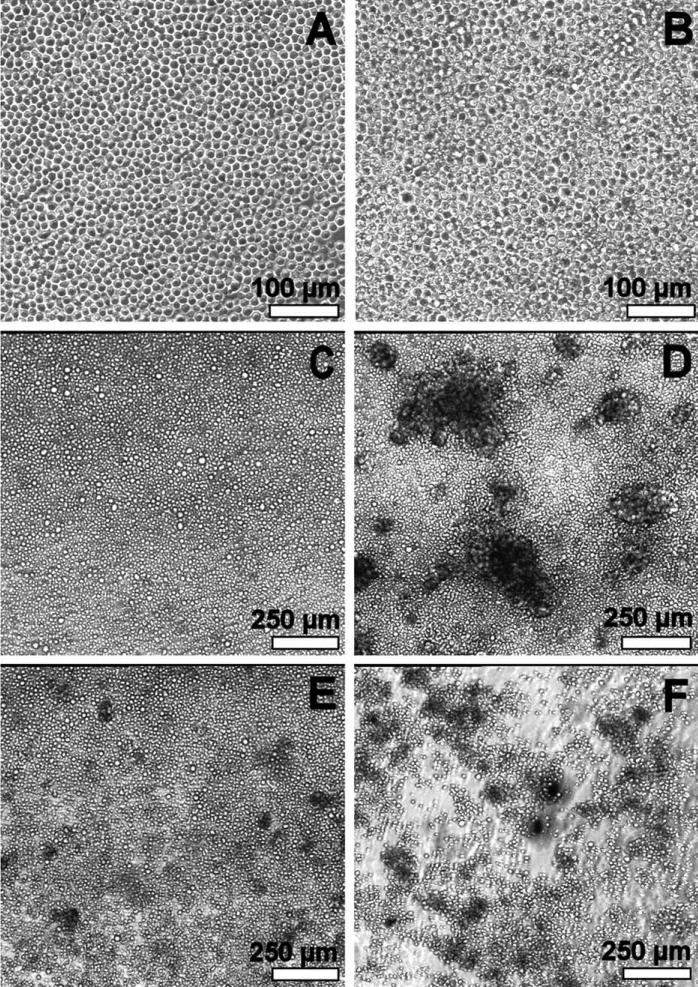
Most cells were weakly adherent and maintained a spherical morphology on all surfaces until day 14 (Fig. 2). During week 2, some cells with larger diameters (compared to week 1) were observed. After day 14, cell aggregation was observed on some surfaces. At day 17, chitosan control, heparin, and heparan sulfate surfaces showed large adherent cell aggregates. Aggregates were also observed on dermatan sulfate surfaces but were smaller in size. On hyaluronan surfaces a large number of non-adherent, smaller-sized cell aggregates were observed. On polystyrene, no cell aggregation was observed and cells maintained their spherical morphology.
FIGURE 2.
Phase contrast microscopy. (A) Week 1: similar morphology on all surfaces. (B) Week 2: similar morphology on all surfaces. Some cells with larger diameter compared to week 1. (C–F) Day 17: polystyrene control, heparin, dermatan sulfate, and hyaluronan, respectively. Chitosan control and heparan sulfate surfaces exhibited cell morphology similar to the heparin surface.
Cell proliferation
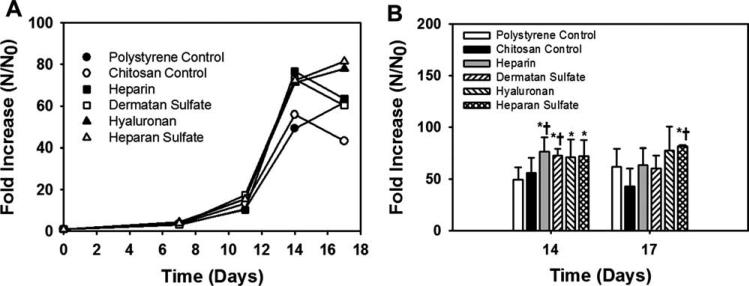
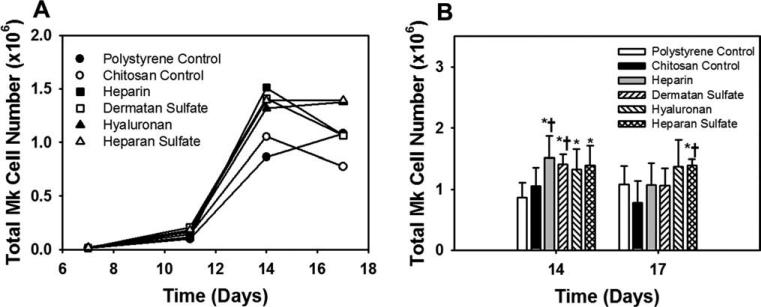
The total cell number increased on all surfaces until day 14 (Fig. 3). Higher total cell expansion was observed on GAG surfaces compared to the controls. Notably, heparin and dermatan sulfate surfaces showed significantly (p < 0.05) higher total cell expansion compared to both the controls. The decline in the total cell number observed on some GAG surfaces after day 14 might have been due to the formation of cell aggregates which register as single events in flow cytometry analyses, thus reducing the number of counted cells. More rigorous mechanical and enzymatic methods for aggregate dissociation were not used due to the likelihood of generating artifacts in cell viability or antigen expression in the subsequent flow cytometry analysis.
FIGURE 3.
Effect of immobilized GAGs on total cell expansion. (A) All time points: the total cell expansion increased on all surfaces till day 14. Error bars have been omitted for clarity. (B) Days 14 and 17: significantly higher total cell expansion was observed on the heparin and dermatan sulfate surfaces compared to both the controls at day 14. *Comparison with polystyrene control, p < 0.05. †Comparison with chitosan control, p < 0.05.
The cell viability decreased on all surfaces with the duration of the culture (Table I). Sulfated-GAG surfaces showed a higher fraction of viable cells (p < 0.05) during the early stages of the culture (days 7 and 11) compared to the controls. Heparin and heparan sulfate surfaces showed higher cell viability compared to the controls even at day 17. Viability on the hyaluronan surface was comparable to the controls at all time points.
TABLE I.
Effect of Immobilized GAGs on Cell Viability
| Cell Viability (%) |
||||
|---|---|---|---|---|
| Culture Surface | Day 7 | Day 11 | Day 14 | Day 17 |
| Polystyrene control | 83.9 ± 2.6 | 72.0 ± 1.8 | 70.0 ± 1.8 | 41.2 ± 3.6 |
| Chitosan control | 84.6 ± 0.8 | 73.2 ± 2.2 | 70.8 ± 7.1 | 39.9 ± 6.0 |
| Heparin | 94.6 ± 0.3a,b | 81.7 ± 1.4a,b | 75.2 ± 7.3 | 58.0 ± 4.0a,b |
| Dermatan sulfate | 90.1 ± 1.9a,b | 80.8 ± 1.0a,b | 70.0 ± 3.5 | 48.8 ± 4.3 |
| Hyaluronan | 84.0 ± 0.6 | 75.3 ± 2.9 | 70.4 ± 4.8 | 38.6 ± 7.9 |
| Heparan sulfate | 93.9 ± 1.0a,b | 79.9 ± 2.8a,b | 73.4 ± 5.1 | 57.4 ± 2.6a,b |
Comparison with polystyrene surface, p < 0.05.
Comparison with chitosan surface, p < 0.05.
The Mk fraction increased with culture time and by day 14, nearly 70% of cells on all surfaces expressed CD41a, indicating a high purity Mk culture. The GAG surfaces showed a significantly higher CD41a+ expression (p < 0.05) compared to the polystyrene control at day 14 (Table II). The Mk cell numbers followed a trend similar to the total cell numbers. Heparin and dermatan sulfate surfaces showed significantly higher (p < 0.05) Mk cell numbers compared to the controls at day 14 (Fig. 4). The results indicate that immobilized GAGs promote total cell expansion, Mk cell expansion, and cell viability.
TABLE II.
Effect of Immobilized GAGs on Total Mk Fraction
| Total Mk Fraction (%) |
||||
|---|---|---|---|---|
| Culture Surface | Day 7 | Day 11 | Day 14 | Day 17 |
| Polystyrene control | 16.8 ± 3.8 | 37.8 ± 9.8 | 69.7 ± 2.9 | 70.3 ± 1.2 |
| Chitosan control | 18.3 ± 4.1 | 43.4 ± 14.2 | 75.0 ± 2.5a | 72.9 ± 5.1 |
| Heparin | 19.7 ± 4.9 | 45.5 ± 18.1 | 78.3 ± 5.5a | 69.7 ± 8.0 |
| Dermatan sulfate | 18.3 ± 3.4 | 47.4 ± 19.5 | 76.9 ± 3.4a | 72.8 ± 4.5 |
| Hyaluronan | 19.9 ± 6.1 | 42.7 ± 16.1 | 73.9 ± 2.2a | 69.2 ± 2.1 |
| Heparan sulfate | 20.2 ± 5.1 | 47.5 ± 19.8 | 76.5 ± 2.0a | 69.2 ± 4.7 |
Comparison with polystyrene surface, p < 0.05.
FIGURE 4.
Effect of immobilized GAGs on Mk cell number. (A) All time points: The Mk cell number increased on all surfaces till day 14. Error bars have been omitted for clarity. (B) Days 14 and 17: significantly higher Mk cell number was observed on the heparin and dermatan sulfate surfaces compared to both the controls at day 14. *Comparison with polystyrene control, p < 0.05. †Comparison with chitosan control, p < 0.05.
Megakaryocyte apoptosis and ploidy
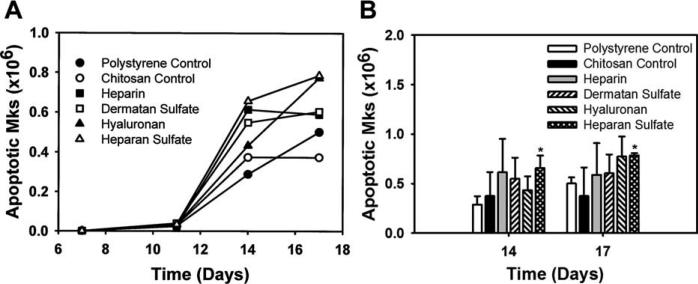
Mk apoptosis is believed to play an important role in platelet release. The effect of various immobilized GAGs on Mk apoptosis was analyzed via Annexin V binding. The analysis revealed that significantly lower Mk apoptotic fractions were observed (p < 0.05) on GAG surfaces compared to the polystyrene control during the early time points of the culture (Table III). However, by day 14 the Mk apoptotic fraction on all surfaces was comparable. The higher Mk numbers combined with comparable apoptotic fractions by the end of the culture resulted in a higher number of apoptotic Mks on GAG surfaces compared to the controls (Fig. 5). Mks generated in the culture had a ploidy level of <8N on all surfaces and immobilized GAGs had no effect on Mk ploidy (data not shown). The results indicate that immobilized GAGs reduced Mk apoptosis during the early time points of the culture, resulting in larger numbers of Mks on GAG surfaces later in the culture.
TABLE III.
Effect of Immobilized GAGs on Apoptotic Mk Fraction within the Megakaryocyte Population
| Apoptotic Mk Fraction (%) |
||||
|---|---|---|---|---|
| Culture Surface | Day 7 | Day 11 | Day 14 | Day 17 |
| Polystyrene control | 5.2 ± 0.2 | 26.3 ± 3.6 | 33.5 ± 4.1 | 46.3 ± 8.0 |
| Chitosan control | 5.2 ± 0.6 | 21.6 ± 3.3a | 33.2 ± 11.1 | 48.3 ± 8.0 |
| Heparin | 4.3 ± 0.5a | 21.5 ± 2.4a | 36.6 ± 13.3 | 55.2 ± 10.9 |
| Dermatan sulfate | 3.3 ± 0.9a,b | 19.9 ± 3.5a | 38.4 ± 12.2 | 56.8 ± 5.1 |
| Hyaluronan | 4.0 ± 0.3a,b | 20.1 ± 3.9a | 32.9 ± 7.8 | 56.2 ± 5.0 |
| Heparan sulfate | 4.1 ± 0.3a,b | 17.8 ± 2.6a | 45.6 ± 8.7a | 56.4 ± 4.7 |
Comparison with polystyrene surface, p < 0.05.
Comparison with chitosan surface, p < 0.05.
FIGURE 5.
Effect of immobilized GAGs on the number of apoptotic Mks. (A) All time points: the apoptotic Mk cell number increased on all surfaces till day 14. Error bars have been omitted for clarity. (B) Days 14 and 17: Higher apoptotic Mk cell numbers on the immobilized GAG surfaces compared to the controls. *Comparison with polystyrene control, p < 0.05. †Comparison with chitosan control, p < 0.05.
Platelet production
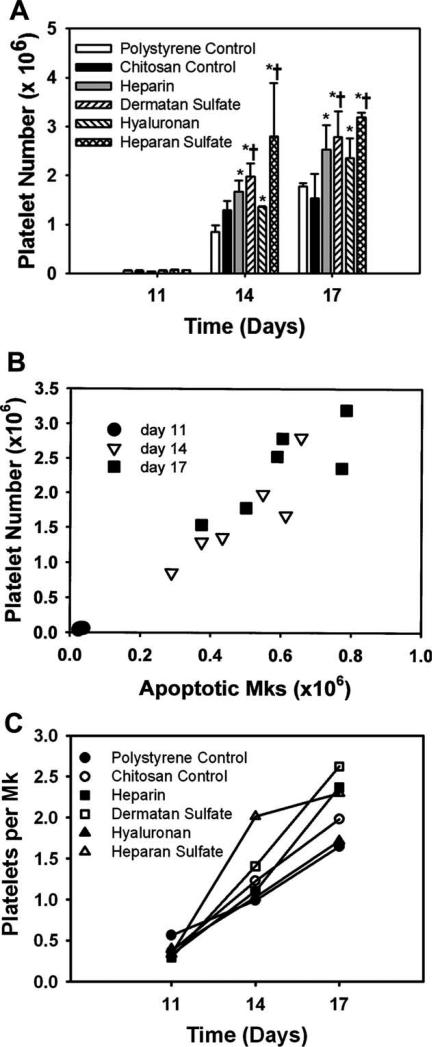
Mk-generated platelets were enumerated using flow cytometry analysis based on size and CD41a expression (Fig. 6). Platelet release was analyzed on days 11, 14, and 17 (Fig. 7). Platelet numbers are reported as a cumulative number up to the point of analysis. Up to day 11, platelets were scarce on all surfaces. However, by day 14, a distinct CD41a+ population with low forward scatter properties in the size range of blood platelets was observed. Higher platelet production was observed on the GAG surfaces compared to the controls. More importantly, dermatan sulfate and heparan sulfate surfaces showed significantly higher platelet numbers (p < 0.05) compared to both the controls [Fig. 7(A)] by the end of the culture. The number of platelets released in our culture correlated very closely with the number of apoptotic Mks [Fig. 7(B)]. Furthermore, a higher platelet number per Mk was observed on sulfated GAG surfaces compared to the polystyrene control on days 14 and 17 [Fig. 7(C)].
FIGURE 7.
Effect of immobilized GAGs on platelet release. (A) Number of platelets: significantly higher number of platelets was observed on dermatan sulfate and heparan sulfate surfaces compared to both the controls. (B) Correlation between apoptotic Mk number and platelet number—the number of platelets generated correlate closely with the number of apoptotic Mks. (C) Number of platelet per Mk—higher platelet number per Mk was observed on sulfated GAG surfaces at days 14 and 17. *Comparison with polystyrene control, p < 0.05. †Comparison with chitosan control, p < 0.05.
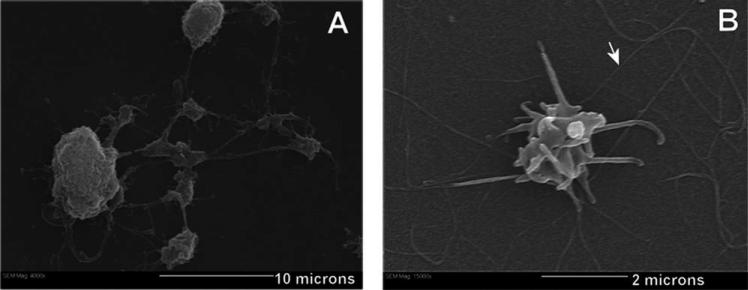
Platelets are known to be activated by contact with collagen.32 An activated platelet morphology (characterized by filopodial processes) suggests that the platelet is functional. While chitosan can induce platelet aggregation and adhesion,33 the mechanism is unclear. Therefore, to visually confirm the functionality of platelets generated in culture, the better-understood collagen activation method was used. At day 14, 30 μL of cell suspension was removed from culture and deposited onto a collagen-coated glass slide to activate the platelets. The slides were then fixed and examined using scanning electron microscopy. Figure 8(A) shows a Mk with pseudopodial extensions exhibiting a tubular and beaded appearance, characteristic of proplatelets. Figure 8(B) shows a culture-derived platelet (2 μm diameter) with thin filopodial extensions. These extensions are indicative of platelet activation34 in response to the collagen-coated substrate. The results indicate that immobilized GAGs enhance platelet production and that the platelets generated in this culture system are functional.
FIGURE 8.
Scanning electron microscopy of Mk, proplatelet and platelet morphology. (A) Long psuedopodial extensions originate from the Mk cytoplasm called proplatelets (scale bar: 10 microns). (B) 2 μm diameter platelet activated in response to the collagen surface (scale bar: 2 microns). Arrow head indicates collagen fibers.
DISCUSSION
A delay in Mk engraftment after UCB transplantation has limited its application as a posttreatment after chemotherapy and radiation therapy. Over the past few years, researchers have focused on expanding Mks and generating platelets from HSCs in vitro, with the goal of reducing thrombocytopenia. GAGs have been shown to interact with and modulate the activity of various growth factors and cytokines, thereby influencing cell signaling, proliferation, and differentiation.17–24 In this study, GAGs were covalently immobilized onto chitosan, and their effect on the process of megakaryopoiesis was investigated. The key findings of the current study are as follows: cell viability on sulfated GAG surfaces was significantly higher compared to both the polystyrene control and chitosan control during the early stages of the culture (days 7 and 11). At day 14, significantly higher total cell and Mk cell expansion was observed on heparin and dermatan sulfate surfaces compared to both the polystyrene control and chitosan control. At day 14, the GAG surfaces showed a significantly higher Mk fraction compared to the polystyrene control (Table II). The Mk apoptotic fraction was significantly lower on GAG surfaces compared to both the polystyrene control and chitosan control at day 7 and was significantly lower than the polystyrene control at day 11, suggesting that GAGs delay Mk apoptosis. The Mk ploidy levels were comparable on all surfaces. At days 14 and 17, dermatan sulfate and heparan sulfate surfaces showed significantly higher platelet numbers compared to both the polystyrene and chitosan controls. When comparing the two controls, chitosan showed a higher total Mk fraction at day 14 and a lower apoptotic Mk fraction at day 11 compared to the polystyrene control. However, the total cell numbers, Mk numbers and platelet numbers were comparable between both the controls at all time points.
GAGs are found localized within the ECM in vivo and are believed to mediate cell–cell and cell–ECM interactions. The soluble GAGs traditionally used in vitro may not embody the signaling dynamics of matrix bound GAGs in vivo. In an attempt to mimic the in vivo microenvironment, heparin was previously immobilized onto chitosan membranes via charge interactions (i.e., ionically).22 However, ionic immobilization led to 90% of the GAG being released into the culture medium over a 24-day incubation period. In contrast, covalent immobilization used in this study resulted in a more stable culture system and most of the GAG remained bound to the chitosan surface even after 3 weeks duration (Fig. 1). The slow but constant desorption of covalently immobilized GAGs is likely due to passive hydrolysis.
Platelet factor (PF4) and β-thromboglobulin (βTG) are negative modulators of megakaryopoiesis that have been shown to be released into the medium during Mk differentiation in vitro.35,36 Han et al.37 have reported that GAGs have the ability to neutralize the effect of these inhibitors and thereby promote megakaryopoiesis. This effect could be partly responsible for the increased proliferation (Fig. 3) and increased cell viability (Table I) observed on GAG surfaces in the current study. In addition, Han et al. reported that GAGs potentiate the activity of TPO and thereby enhance murine Mk growth in a serum-free agar system. The results from this study suggest a similar stimulating effect of GAGs on TPO, evidenced by the higher Mk proliferation (Fig. 4) compared to the controls.
The cell proliferation results indicate that the GAG surfaces promote total cell and Mk cell expansion. Specifically, heparin and dermatan sulfate surfaces were more promising and significantly enhanced cell expansion compared to both the controls. These results are in agreement with a study by Chen et al.28 which reported that heparin stimulates the activity of TPO and thereby enhances megakaryopoiesis. Teramachi et al.27 investigated the effect of various GAGs on the clonal growth of Mk progenitor cells. Unlike what was observed in this study, dermatan sulfate was shown to have no effect on the growth of Mks. This might possibly be due to the differences in the magnitude and duration of signaling produced by cytokines bound to soluble versus surface immobilized GAGs.
Nuclear polyploidization is essential for the maturation of Mks. It has been previously reported that the Mk ploidy levels correlate with the number of platelets released.35 Mks from bone marrow and peripheral blood have been shown to be capable of several cycles of DNA replication up to 128 N.38,39 However, Mks from cord blood fail to mature and do not attain high levels of ploidy.10,35 The results from this study showed that the ploidy level of cord blood Mks was below 8 N on all surfaces and immobilized GAGs had no effect on Mk ploidy. These results concur with a study by Teramachi et al.27 that show that GAGs do not influence the ploidy distribution of cord blood Mks.
It has become increasingly evident that apoptosis plays an intricate role in platelet production.14,15 TPO has been proven to be a key cytokine not only for Mk proliferation and differentiation but also for platelet production.8–12 Moreover, it has also been reported that TPO significantly reduces the percentage of apoptosis in primary Mks derived from bone marrow aspirates during the early time points in culture.40 Given the GAG enhancement of TPO activity and TPO effects on Mk apoptosis, we studied the effect of GAGs on Mk apoptosis in the presence of TPO. The significantly lower Mk apoptotic fractions observed on GAG surfaces compared to the polystyrene control during the early stages of the culture suggest a GAG-mediated delay in the onset of Mk apoptosis (Table III). This delay may have resulted in a higher number of viable cells capable of proliferation on GAG surfaces around day 11, thus possibly explaining the higher Mk numbers on GAG surfaces at day 14. Therefore, delaying Mk apoptosis is a desirable outcome because it prevents premature senescence and allows for greater expansion of Mk numbers prior to platelet release. However, it is important to note that at day 14 the apoptotic Mk fractions on GAG surfaces were comparable to that on the controls indicating that the GAGs did not inhibit Mk apoptosis. Higher Mk numbers and comparable apoptotic fractions by the end of the culture resulted in a higher number of apoptotic Mks on GAG surfaces compared to the controls (Fig. 5).
Higher platelet production was observed on GAG surfaces compared to the controls. The enhanced platelet production on GAG surfaces was due to both a higher total Mk number [Fig. 4(B)] and a higher number of platelets produced by each Mk [Fig. 7(C)]. Although all four GAG surfaces promoted platelet production, dermatan sulfate and heparan sulfate surfaces were more favorable and produced significantly higher numbers of platelets compared to both the controls [Fig. 7(A)] by the end of the culture. This difference in the activity between GAGs may be attributable to the differences in the degree of sulfation41 and the sulfation pattern.26 Both of these parameters are believed to influence the biological activity of GAGs. The number of platelets generated in this study correlated closely with the number of apoptotic Mks on each surface [Fig. 7(B)] suggesting that Mk apoptosis and platelet production are two intricately linked processes.
The projected platelet production under the current culture conditions when extrapolated to account for an entire cord blood unit was in the range of 108 platelets. One possibility to further enhance the production of platelets could be to divide the culture into two distinct phases: an initial CD34+ expansion phase,22 followed by differentiation of the expanded CD34+ population to megakaryocytes and platelets. This concept was used by Matsanuga et al.7 who reported large-scale generation of platelets. In addition, incorporation of additional cytokines that have a synergistic effect with TPO might enhance the overall yield of Mks and platelets.42 This study utilized TPO as the sole cytokine to better understand the effect of immobilized GAGs on the process of megakaryopoiesis. Future studies should focus on optimizing the culture system and demonstrating the effect of immobilized GAGs in the presence of a combination of cytokines. Finally, scaling up the existing culture system to a three-dimensional perfusion bioreactor culture setting43 using GAG derivatized chitosan scaffolds might assist in increasing the overall yield of Mks and platelets to clinically feasible levels. Perfusion culture would produce a double benefit by improving both nutrient delivery to the cells and proplatelet fragmentation to platelets.
In conclusion, the results from this study indicate that covalently immobilized GAGs enhance Mk expansion and platelet production by reducing early apoptosis and maintaining high cell viability. Thus, immobilized GAGs are promising tools for developing systems for in vitro Mk expansion and platelet production.
Acknowledgments
Contract grant sponsor: National Institutes of Health; contract grant number: RO1DK58711
REFERENCES
- 1.Rubinstein P, Rosenfield R, Adamson J, Stevens C. Stored placental blood for unrelated bone marrow reconstitution. Blood. 1993;81:1679–1690. [PubMed] [Google Scholar]
- 2.Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio A, Berkowitz R, Cabbad M, Dobrila N, Taylor P, Rosenfield R, Stevens C. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 3.Wallace E, Churchill W, Surgenor D, An J, Cho G, McGurk S, Murphy L. Collection and transfusion of blood and blood components in the United States, 1992. Transfusion. 1995;35:802–812. doi: 10.1046/j.1537-2995.1995.351096026360.x. [DOI] [PubMed] [Google Scholar]
- 4.McCullough J. Current issues with platelet transfusion in patients with cancer. Semin Hematol. 2000;37(2 Suppl 4):3–10. doi: 10.1016/s0037-1963(00)90047-7. [DOI] [PubMed] [Google Scholar]
- 5.Webb I, Anderson K. Risks, costs, and alternatives to platelet transfusions. Leuk Lymphoma. 1999;34:71–84. doi: 10.3109/10428199909083382. [DOI] [PubMed] [Google Scholar]
- 6.Bertolini F, Battaglia M, Pedrazzoli P, Da Prada G, Lanza A, Soligo D, Caneva L, Sarina B, Murphy S, Thomas T, della Cuna G. Megakaryocytic progenitors can be generated ex vivo and safely administered to autologous peripheral blood progenitor cell transplant recipients. Blood. 1997;89:2679–2688. [PubMed] [Google Scholar]
- 7.Matsunaga T, Tanaka I, Kobune M, Kawano Y, Tanaka M, Kuribayashi K, Iyama S, Sato T, Sato Y, Takimoto R, Takayama T, Kato J, Ninomiya T, Hamada H, Niitsu Y. Ex vivo large-scale generation of human platelets from cord blood CD34+ cells. Stem Cells. 2006;24:2877–2887. doi: 10.1634/stemcells.2006-0309. [DOI] [PubMed] [Google Scholar]
- 8.de Sauvage F, Hass P, Spencer S, Malloy B, Gurney A, Spencer S, Darbonne W, Henzel W, Wong S, Kuang W. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994;369:533–538. doi: 10.1038/369533a0. [DOI] [PubMed] [Google Scholar]
- 9.Lok S, Kaushansky K, Holly R, Kuijper J, Lofton-Day C, Oort P, Grant F, Heipel M, Burkhead S, Kramer J. Cloning and expression of murine thrombopoietin cDNA and stimulation of platelet production in vivo. Nature. 1994;369:565–568. doi: 10.1038/369565a0. [DOI] [PubMed] [Google Scholar]
- 10.Schipper L, Brand A, Reniers N, Melief C, Willemze R, Fibbe W. Effects of thrombopoietin on the proliferation and differentiation of primitive and mature haemopoietic progenitor cells in cord blood. Br J Haematol. 1998;101:425–435. doi: 10.1046/j.1365-2141.1998.00737.x. [DOI] [PubMed] [Google Scholar]
- 11.Choi E, Hokom M, Bartley T, Li Y, Ohashi H, Kato T, Nichol J, Skrine J, Knudten A, Chen J. Recombinant human megakaryocyte growth and development factor (rHuMGDF), a ligand for c-Mpl, produces functional human platelets in vitro. Stem Cells. 1995;13:317–322. doi: 10.1002/stem.5530130313. [DOI] [PubMed] [Google Scholar]
- 12.Oda A, Miyakawa Y, Druker B, Ozaki K, Yabusaki K, Shirasawa Y, Handa M, Kato T, Miyazaki H, Shimosaka A, Ikeda Y. Thrombopoietin primes human platelet aggregation induced by shear stress and by multiple agonists. Blood. 1996;87:4664–4670. [PubMed] [Google Scholar]
- 13.Patel S, Hartwig J, Italiano JJ. The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest. 2005;115:3348–3354. doi: 10.1172/JCI26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Botton S, Sabri S, Daugas E, Zermati Y, Guidotti J, Hermine O, Kroemer G, Vainchenker W, Debili N. Platelet formation is the consequence of caspase activation within megakaryocytes. Blood. 2002;100:1310–1317. doi: 10.1182/blood-2002-03-0686. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Kuter D. The end is just the beginning: Megakaryocyte apoptosis and platelet release. Int J Hematol. 2001;74:365–374. doi: 10.1007/BF02982078. [DOI] [PubMed] [Google Scholar]
- 16.Junt T, Schulze H, Chen Z, Massberg S, Goerge T, Krueger A, Wagner D, Graf T, Italiano JJ, Shivdasani R, von Andrian U. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317:1767–1770. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 17.Bruno E, Luikart S, Long M, Hoffman R. Marrow-derived heparan sulfate proteoglycan mediates the adhesion of hematopoi etic progenitor cells to cytokines. Exp Hematol. 1995;23:1212–1217. [PubMed] [Google Scholar]
- 18.Gordon M, Riley G, Watt S, Greaves M. Compartmentalization of a haematopoietic growth factor (GM-CSF) by glycosaminoglycans in the bone marrow microenvironment. Nature. 1987;326:403–405. doi: 10.1038/326403a0. [DOI] [PubMed] [Google Scholar]
- 19.Gupta P, McCarthy J, Verfaillie C. Stromal fibroblast heparan sul-fate is required for cytokine-mediated ex vivo maintenance of human long-term culture-initiating cells. Blood. 1996;87:3229–3236. [PubMed] [Google Scholar]
- 20.Gupta P, Oegema TJ, Brazil J, Dudek A, Slungaard A, Verfaillie C. Structurally specific heparan sulfates support primitive human hematopoiesis by formation of a multimolecular stem cell niche. Blood. 1998;92:4641–4651. [PubMed] [Google Scholar]
- 21.Gupta P, Oegema TJ, Brazil J, Dudek A, Slungaard A, Verfaillie C. Human LTC-IC can be maintained for at least 5 weeks in vitro when interleukin-3 and a single chemokine are combined with O-sulfated heparan sulfates: Requirement for optimal binding interactions of heparan sulfate with early-acting cytokines and matrix proteins. Blood. 2000;95:147–155. [PubMed] [Google Scholar]
- 22.Madihally S, Flake A, Matthew H. Maintenance of CD34 expression during proliferation of CD34+ cord blood cells on glycosaminoglycan surfaces. Stem Cells. 1999;17:295–305. doi: 10.1002/stem.170295. [DOI] [PubMed] [Google Scholar]
- 23.Roberts R, Gallagher J, Spooncer E, Allen T, Bloomfield F, Dexter T. Heparan sulphate bound growth factors: A mechanism for stromal cell mediated haemopoiesis. Nature. 1988;332:376–378. doi: 10.1038/332376a0. [DOI] [PubMed] [Google Scholar]
- 24.Spooncer E, Gallagher J, Krizsa F, Dexter T. Regulation of haemopoiesis in long-term bone marrow cultures. IV. Glycosaminoglycan synthesis and the stimulation of haemopoiesis by beta-D-xylosides. J Cell Biol. 1983;96:510–514. doi: 10.1083/jcb.96.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Netelenbos T, van den Born J, Kessler F, Zweegman S, Huijgens P, Drager A. In vitro model for hematopoietic progenitor cell homing reveals endothelial heparan sulfate proteoglycans as direct adhesive ligands. J Leukoc Biol. 2003;74:1035–1044. doi: 10.1189/jlb.1202593. [DOI] [PubMed] [Google Scholar]
- 26.Kashiwakura I, Teramachi T, Kakizaki I, Takagi Y, Takahashi T, Takagaki K. The effects of glycosaminoglycans on thrombopoietin-induced megakaryocytopoiesis. Haematologica. 2006;91:445–451. [PubMed] [Google Scholar]
- 27.Teramachi T, Kashiwakura I, Takahashi T, Takagi Y. Effects of glycosaminoglycans on the in vitro colony formation of CD34+ megakaryocytic progenitor cells in human placental/umbilical cord blood. Yakugaku Zasshi. 2001;121:691–699. doi: 10.1248/yakushi.121.691. [DOI] [PubMed] [Google Scholar]
- 28.Chen Q, Shen Z, Han Z. Heparin enhances the stimulating effect of thrombopoietin on megakaryocytopoiesis. Zhonghua Xue Ye Xue Za Zhi. 1999;20:462–464. [PubMed] [Google Scholar]
- 29.Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20:1133–1142. doi: 10.1016/s0142-9612(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 30.VandeVord PJ, Matthew HW, DeSilva SP, Mayton L, Wu B, Wooley PH. Evaluation of the biocompatibility of a chitosan scaffold in mice. J Biomed Mater Res. 2002;59:585–590. doi: 10.1002/jbm.1270. [DOI] [PubMed] [Google Scholar]
- 31.Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54:626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kroll MH, Schafer AI. Biochemical mechanisms of platelet activation. Blood. 1989;74:1181–1195. [PubMed] [Google Scholar]
- 33.Chou TC, Fu E, Wu CJ, Yeh JH. Chitosan enhances platelet adhesion and aggregation. Biochem Biophys Res Commun. 2003;302:480–483. doi: 10.1016/s0006-291x(03)00173-6. [DOI] [PubMed] [Google Scholar]
- 34.Hartwig JH. Mechanisms of actin rearrangements mediating platelet activation. J Cell Biol. 1992;118:1421–1442. doi: 10.1083/jcb.118.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattia G, Vulcano F, Milazzo L, Barca A, Macioce G, Giampaolo A, Hassan HJ. Different ploidy levels of megakaryocytes generated from peripheral or cord blood CD34+ cells are correlated with different levels of platelet release. Blood. 2002;99:888–897. doi: 10.1182/blood.v99.3.888. [DOI] [PubMed] [Google Scholar]
- 36.Majka M, Janowska-Wieczorek A, Ratajczak J, Ehrenman K, Pietrzkowski Z, Kowalska M, Gewirtz A, Emerson S, Ratajczak M. Numerous growth factors, cytokines, and chemokines are secreted by human CD34+ cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood. 2001;97:3075–3085. doi: 10.1182/blood.v97.10.3075. [DOI] [PubMed] [Google Scholar]
- 37.Han Z, Bellucci S, Shen Z, Maffrand J, Pascal M, Petitou M, Lormeau J, Caen J. Glycosaminoglycans enhance megakaryocytopoiesis by modifying the activities of hematopoietic growth regulators. J Cell Physiol. 1996;168:97–104. doi: 10.1002/(SICI)1097-4652(199607)168:1<97::AID-JCP12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 38.Debili N, Wendling F, Katz A, Guichard J, Breton-Gorius J, Hunt P, Vainchenker W. The Mpl-ligand or thrombopoietin or megakaryocyte growth and differentiative factor has both direct proliferative and differentiative activities on human megakaryocyte progenitors. Blood. 1995;86:2516–2525. [PubMed] [Google Scholar]
- 39.Guerriero R, Testa U, Gabbianelli M, Mattia G, Montesoro E, Macioce G, Pace A, Ziegler B, Hassan HJ, Peschle C. Unilineage megakaryocytic proliferation and differentiation of purified hematopoietic progenitors in serum-free liquid culture. Blood. 1995;86:3725–3736. [PubMed] [Google Scholar]
- 40.Zauli G, Vitale M, Falcieri E, Gibellini D, Bassini A, Celeghini C, Columbaro M, Capitani S. In vitro senescence and apoptotic cell death of human megakaryocytes. Blood. 1997;90:2234–2243. [PubMed] [Google Scholar]
- 41.Aviezer D, Levy E, Safran M, Svahn C, Buddecke E, Schmidt A, David G, Vlodavsky I, Yayon A. Differential structural requirements of heparin and heparan sulfate proteoglycans that promote binding of basic fibroblast growth factor to its receptor. J Biol Chem. 1994;269:114–121. [PubMed] [Google Scholar]
- 42.Williams JL, Pipia GG, Datta NS, Long MW. Thrombopoietin requires additional megakaryocyte-active cytokines for optimal ex vivo expansion of megakaryocyte precursor cells. Blood. 1998;91:4118–4126. [PubMed] [Google Scholar]
- 43.Cho CH, Eliason JF, Matthew HW. Application of porous glycosaminoglycan-based scaffolds for expansion of human cord blood stem cells in perfusion culture. J Biomed Mater Res A. 2008;86:98–107. doi: 10.1002/jbm.a.31614. [DOI] [PubMed] [Google Scholar]