Abstract
Abstract Inhibitors of Apoptosis (IAPs) are a family of proteins with various biological functions including regulation of innate immunity and inflammation, cell proliferation, cell migration and apoptosis. They are characterized by the presence of at least one N-terminal baculoviral IAP repeat (BIR) domain involved in protein-protein interaction. Most of them also contain a C-terminal RING domain conferring an E3-ubiquitin ligase activity. In drosophila, IAPs are essential to ensure cell survival, preventing the uncontrolled activation of the apoptotic protease caspases. In mammals, IAPs can also regulate apoptosis through controlling caspase activity and caspase-activating platform formation. Mammalian IAPs, mainly X-linked IAP (XIAP) and cellular IAPs (cIAPs) appeared to be important determinants of the response of cells to endogenous or exogenous cellular injuries, able to convert the survival signal into a cell death-inducing signal. This review highlights the role of IAP in regulating apoptosis in Drosophila and Mammals.
Keywords: KeywordsDIAP1, XIAP, cIAPs, apoptosis, caspases, RIP, apoptosome, IAP antagonists
1. Introduction
Apoptosis is a highly evolutionary conserved mechanism of cell death triggered by a large range of extracellular or intracellular stimuli including developmental signals, environmental and intracellular stress. This is a genetically controlled process, playing a major role in normal development and tissue homeostasis. It is considered as a potent mechanism of tumour protection, ensuring the selective removal of supernumerary, undesirable or damaged cells. It is also essential in immune response and constitutes an efficient strategy of antiviral defence [1]. Viruses have developed strategies in order to overcome this protection mechanism, allowing the replication and the spreading of virus. A genetic screen aiming to identify viral proteins that evade virus-induced cell death in host cells revealed Inhibitors of Apoptosis (IAPs) as potent inhibitors of apoptosis in insect cells [2]. IAP homologs were then identified in yeasts, nematode, insects, fishes and mammals, sharing structural features [3]. Only a subset of IAPs functions as apoptosis regulators, many of them display non-apoptotic functions [4,5]. For example, Drosophila IAP2 (DIAP2) and mammalian cellular IAPs (cIAPs) are important intermediates in Tumour Necrosis Factor (TNF) Receptor (TNFR) superfamily and Nuclear Factor-kappaB (NF-κB) activating signalling pathways (for review, see [6,7,8]), regulating cell differentiation [9], cell motility [10], pro-inflammatory and immune response [5]; cIAP1 regulates cell proliferation through its capacity to control the activity of transcription factors such as c-myc [11] and E2F1 [12]; survivin regulates cell division by controlling cytodieresis [13]. This review focuses on the role of IAPs in the regulation of apoptosis, in insect and mammalian cells.
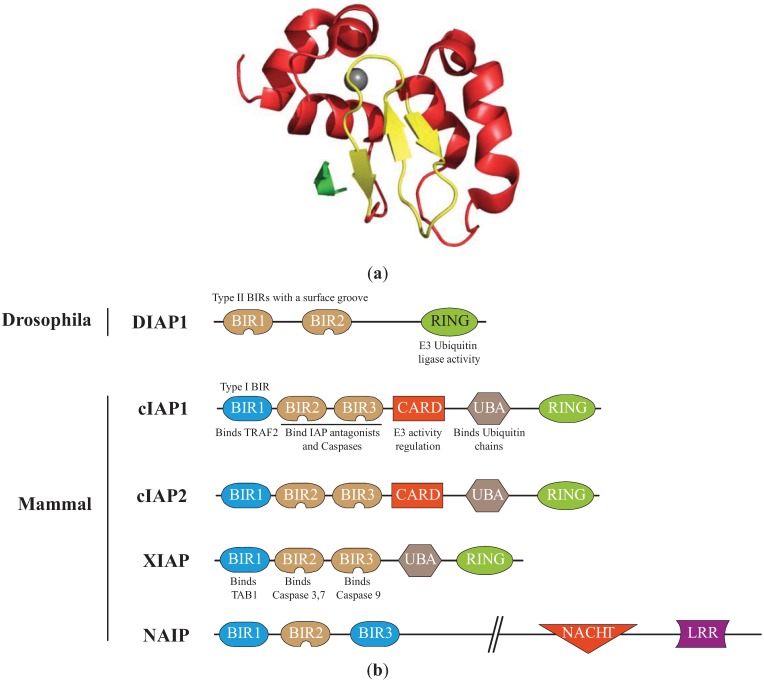
2. Structural Features of IAPs (Figure 1)
Figure 1.
Structure of the Inhibitors of apoptosis. (a) Structure of the cellular IAP1 (cIAP1)-baculoviral IAP repeat (BIR)3 bound to the caspase-9 N-terminal peptide [30]. BIR3 is organized in four α-helices (red) and 3 β-strand sheets (yellow) maintained by zinc ion (grey). The interaction involved the surface hydrophobic groove of cIAP1 and the N-terminal peptide (ATPFQ) of the caspase 9 sub-unit (Constructed using the PyMOL Molecular Graphics System). (b)Representation of IAPs involved in the regulation of apoptosis. The type I baculoviral IAP repeats (BIR, blue) of cIAPs and X-linked IAP (XIAP) can bind to cell signalling intermediates TNFR associated factor 2 (TRAF2) and Transforming Growth Factor beta-activated kinase 1-binding protein 1 (TAB1), respectively. The type II BIRs (brown) contain a surface hydrophobic groove allowing the interaction with IBM found in caspase sub-units and IAP antagonists. The ubiquitin Associated (UBA) domain binds ubiquitin chains. The caspase recruitment domain (CARD) is a module of regulation of the RING E3-ubiquitin ligase activity. The RING domain confers to IAPs an E3-ubiquitin ligase activity. NACHT (domain present in NAIP, CIITA, HETE and TP1). LRR: Leucine Rich Repeat.
The IAP family is defined by the presence of one to three tandem specific motifs of about 70 residues named Baculovirus Iap Repeat (BIR) located at the N-terminal end of the protein [14] (Figure 1). The core component of the BIRs is a consensus Cys/His motif (GX2YX4DX3CX2CX6WX9HX6–10C) that coordinates a single zinc ion [14,15]. It is organized in series of short α-helices with intervening β-sheets, forming a specific folded structure stabilized by the Zinc (Figure 1). BIRs are protein interacting modules with specific distinct binding properties [16]. Most of them (referred to as type II BIR) form a surface hydrophobic groove that binds a conserved tetrapeptide motif called IAP Binding Motifs (IBMs, A(K, T, V, I)(P, A, E)(F, E, I, S, Y)). IBMs were found in the extreme N-terminus of sub-units of some caspases and in IAP antagonists [17,18]. The N-terminal exposure of IBM is required for the recognition and binding by IAPs. Thus, BIR hydrophobic groove can only bind processed, activated caspases. Type II BIRs are essential for the anti-apoptotic properties of IAPs. Type I BIRs do not bind IBM but can interact by distinct mode to another set of proteins mainly involved in cell signalling pathways [19,20,21]. The second conserved motif important for the anti-apoptotic activity of IAPs is the C-terminal RING (really interesting new gene) zinc-finger that displays an E3-ubiquitin ligase activity. Thanks to this domain, IAPs can catalyse the covalent conjugation, to a lysine of target partner proteins, of NEDD8 (neural precursor cell expressed developmentally downregulated protein 8) [22,23] or a single ubiquitin (Ub) molecule (monoubiquination) or polyubiquitin chains formed by the binding of the C-terminal Glycine of Ub to the Lysine (mainly K11, K48 or K63) residue of another. K48-linked chains usually target the protein for 26S proteasomal degradation, while mono-ubiquitin or K63 or K11-linked ubiquitin chains modulate the activity or the cellular distribution of target proteins. IAPs can also mediate their own ubiquitination [24]. Furthermore, the RING enables homo- or heterodimerization of IAPs that regulates their stability and possibly their activity [24,25,26]. In addition to the BIRs and RING, other conserved protein domains can be found in IAPs including a central caspase recruitment domain (CARD) that regulates E3-ubiquitin ligase activity [27], a Ub associated (UBA) domain that can recognize mono- and poly-Ub chains and that allows the recruitment of IAP in protein complexes (Figure 1) [28,29].
3. Apoptotic Signalling Pathways
Apoptosis is a highly evolutionary conserved process. Both vertebrate and invertebrate apoptotic pathways are mediated by a sequential activation of cysteine proteases from caspase family which are responsible for characteristic morphological and biochemical changes [31]. Caspases are expressed as inactive-zymogens consisting of one prodomain and two active sub-units (a small and a large one). They are sub-divided into initiator and effector caspases, depending of the length of the pro-domain and the mechanism of activation. The initiator caspases possess a long pro-domain allowing their recruitment by adaptors into caspase-activating complexes. These molecular platforms provide proximity required for caspase homodimerisation and self-activation. They activate the effector caspases by a proteolytic cleavage allowing the assembly of two small and two large sub-units into an active tetramer. Effector caspases cleave broad spectrum of cellular proteins leading to cell dismantlement. Members of Caspase family are also involved in non-apoptotic processes such as inflammatory response, cell proliferation and differentiation [31].
4. Regulation of Apoptosis by IAPs in Invertebrates
Insect models such drosophila melanogaster and spodoptera frugiperda provide powerful genetic tools to dissect cell death pathways. Apoptosis is essential to eliminate damaged and unwanted cells, but it also controls organ morphogenesis during drosophila embryogenesis [32]. A genetic screen in cell death defective drosophila embryos identified IAP genes that regulate developmental programmed cell death [33,34,35,36,37].
4.1. Drosophila Apoptotic Signalling Pathway
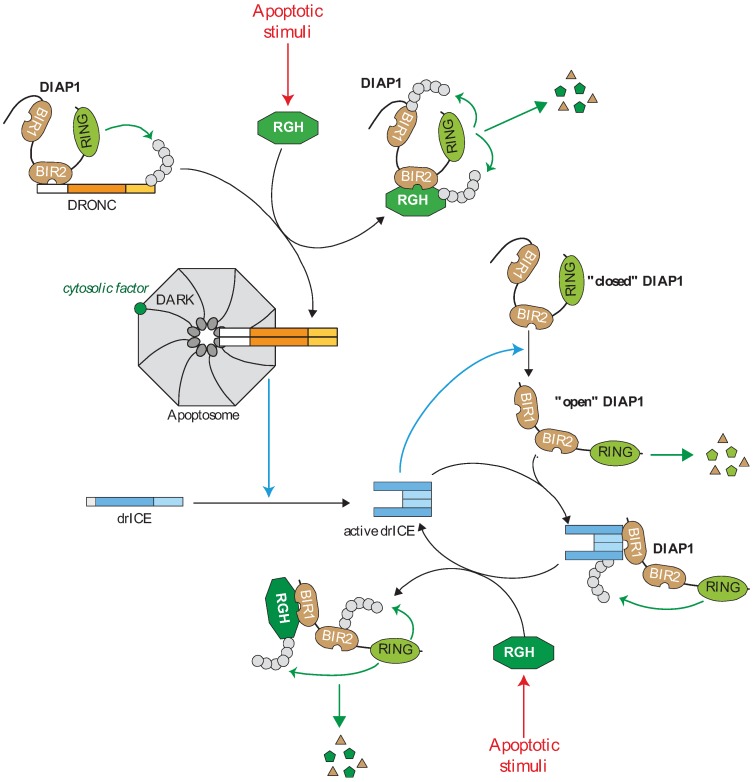
In drosophila, the apoptotic initiator caspase DRONC (DROsophila Nedd-2-like Caspase) is involved in almost all forms of apoptosis [38,39,40,41,42,43]. It is activated by dimerization through the recruitment by the Apaf-1 (apoptotic protease activating factor 1) ortholog DARK (Drosophila Apaf-1 related killer) at the caspase-activating platform apoptosome [40,41,42,44,45]. Unlike mammalian models, cytosolic cytochrome c seems dispensable for the in vitro apoptosome assembly [45,46,47], although the requirement for a cytosolic factor has been demonstrated [48]. Once activated, DRONC activates the effector caspase drICE (drosophila melanogaster Interleukin-1-converting enzyme/Ced-3 related protease) and DCP-1 (death caspase-1) [44,49,50] (Figure 2). Caspases and DARK are constitutively expressed. In the absence of apoptotic inducers, the cell death machinery is frozen by the presence of important regulatory mechanisms. Among them, IAPs prevent unexpected assembly of apoptosome and caspase cascade activation [3] (Figure 2).
Figure 2.
Regulation of the caspase cascade by IAPs in drosophila. In living cells, the caspase activating cascade is maintained in check by a direct interaction of caspases with the Drosophila IAP1 (DIAP1). The DIAP1 BIR2 binds to the prodomain of the apoptotic initiator DROsophila Nedd-2-like Caspase (DRONC) and the RING induces DRONC ubiquitination preventing apoptosome assembly. DIAP1 is expressed in “closed conformation” in which the N-terminal sequence hides BIR1 surface groove. Effector caspase mediates the cleavage of the N-extremity of DIAP1 that releases the BIR1 domain which in turn interacts with the IBM exposed on the active form of effector drosophila melanogaster Interleukin-1-converting enzyme/Ced-3 related protease (drICE). DIAP1 inhibits drICE activity through a degradative or non-degradative ubiquitination or neddylation. The “open” form of DIAP1 is highly unstable and rapidly degraded by the N-end-rule-associated degradation machinery. Apoptotic stimuli induce the expression of the IBM-carrying IAP antagonists Reaper, Grim or Hid (RGH) wich strongly bind and neutralize DIAP1 through IBM-BIRs interaction. The IAP antagonist-DIAP1 interaction promotes DIAP1-autoubiquitination and degradation.
4.2. Drosophila IAPs as Caspase Inhibitors
The drosophila genome encodes at least four members of IAP family: drosophila IAP1 (DIAP1), drosophila IAP2 (DIAP2), DETERIN and drosophila BIR repeat-containing ubiquitin-conjugating (dBRUCE) [3]. DIAP1 (Figure 1), referred as a “gatekeeper of death” [3], is essential to ensure cell survival, neutralization of DIAP1 being necessary and sufficient to trigger apoptosis [33,40,44]. Loss-of-function mutations in DIAP1encoding gene (thread) lead to early embryonic death with a massive caspase-dependent apoptosis [33,51,52]. Diap1-deficiency-induced cell death is rescued by the inactivation of DRONC or DARK [40]. Moreover, DIAP1 inhibits apoptosis induced by the expression of either the initiator DRONC or the executor drICE caspase [38,39,53], suggesting that DIAP1 can control canonical apoptotic pathway at different steps (Figure 2).
DIAP1 contains two tandem BIRs and one RING domain providing an E3-ubiquitin ligase activity (Figure 1b). It can physically interact with both DRONC and drICE and, thanks to the RING, induces their ubiquitination [38,39,41,53,54,55]. The BIR2 DIAP1 binds a 12-residue linker region located in the prodomain of DRONC [38,39,41,55,56]. Although essential, binding is not sufficient for DRONC regulation in vivo since diap1 mutant able to bind DRONC but lacking E3-ubiquitin ligase activity are inefficient to prevent apoptosis [54]. The consequence of DIAP1-mediated DRONC ubiquitination is still unclear. It has been suggested that ubiquitination leads to proteasome-mediated depletion of DRONC, preventing its accumulation in living cells [44,57]. However, a more recent report demonstrated that DIAP1-mediated ubiquitination of full length DRONC inhibits its activation and processing through a non-degradative mechanism [58]. The level of activation of DRONC is correlated with the amount of active apoptosome formed by DRONC and the adaptor DARK. A feedback regulation of the expression of both apoptosome components has been described [57]. The adaptor DARK can decrease the level of DRONC protein expression and conversely, DRONC lowers DARK protein level by a proteolytic cleavage. The ubiquitin ligase activity of DIAP1 is required for this process, suggesting that DIAP1 also regulates apoptosome assembly [57].
Unlike DRONC, only the active forms of effector caspases bind DIAP1 [53,56]. The mechanisms of binding have been extensively investigated and involve the surface groove of DIAP1 BIR1 domain that specifically recognizes the IBM found on the N-terminus of the large sub-unit of executive caspases, which becomes exposed after activating proteolytic cleavage [39,53,56,59]. In native form, DIAP1 stays in a closed conformation [60,61] in which its N-terminal sequence binds and occupies the BIR1 surface groove, preventing DIAP1-drICE interaction [61]. Auto-inhibition of DIAP1 is disabled by drICE caspase-mediated cleavage of DIAP1 after Asp20, that releases the BIR1 surface groove and renders fully competent DIAP1 to bind and inhibit active drICE [60,61,62,63]. Such feedback inhibition could allow regulation of the level of caspase activation in apoptotic or non-apoptotic process. Several mechanisms have been proposed to explain the DIAP1-mediated caspase inhibition including degradative and non-degradative ubiquitination [64] and neddylation [22]. The caspase-mediated cleavage at position 20 renders DIAPs highly unstable, exposing an Asn at N-terminal position, which is an acceptor site for the N-end-rule-associated degradation machinery [60,63,65].
Thus, drosophila cell survival is ensured by a highly regulated control of the stability of DIAP1 and associated caspases. Although DIAP2 and dBRUCE are also able to bind and control the activity of caspases, their role in apoptosis seem much more limited. A diap2 mutation mainly affects innate immunity because of the capacity of DIAP2 to control the non-apoptotic caspase DREDD (Death related ced-3/Nedd2-like protein) [66,67] and dBruce mutation causes male sterility because of its ability to regulate the caspases required for spermatogenesis process [68].
4.3. Drosophila IAP Antagonists from Reaper Family
Drosophila apoptosis requires the neutralization or destruction of DIAP1, allowing the DARK-mediated DRONC activation. A genetic analysis of defective mutant for developmental cell death revealed the requirement of reaper (rpr), head involution defective (hid) and grim in apoptosis induction [33,34,35,36,37]. These proteins share a N-terminal IBM recognized by the IBM groove of DIAP1 BIR1 and BIR2, and prevent DIAP1 to bind and neutralize caspases [33,37,51,55,69,70,71]. Along with Sickle and Jafrac2, two other IBM-containing proteins [72,73,74,75], they are referred as IAP antagonists. IAP antagonist-DIAP1 interaction also promotes DIAP1-auto-ubiquitination and degradation [71,74,76,77,78]. Overexpression of IAP antagonists can induce apoptosis in several insect and non-insect cells. IAP antagonists cooperate to connect cell death stimuli with apoptotic signalling pathways. Reaper, Grim, Hid and Sickle are transcriptionally up-regulated in response to different apoptotic stimuli including developmental signals and DNA damages [73], and Jafrac2 is activated by apoptotic stimuli induced-removal of the N-terminal endoplasmic reticulum (ER) signal peptide that leads to the exposition of IBM [72].
4.4. Viral Anti-Apoptotic IAPs
Insect viruses including baculoviruses, iridoviruses, entomopoxviruses encode members of IAPs with anti-apoptotic properties, preventing death of infected cells and promoting virus multiplication [2,79,80]. Viral IAPs inhibit the activation of initiator caspases but seem inefficient to block downstream effector caspases [81]. The analysis of binding specificity of viral IAPs suggested that they inhibit apoptosis through their capacity to bind and neutralize IBM-containing IAP antagonists through an IBM-BIR interaction [36,80,82].
5. Regulation of Apoptosis by IAP in Mammals
5.1. Mammalian IAPs
The human genome encodes 8 members of IAP family involved in innate immune response, cell division, cell proliferation and cell death pathways [6]. Among human IAPs, X-linked IAP (XIAP) and cellular IAP1 and 2 (cIAP1/2) share several properties with DIAP1 including the capacity to bind caspases and IAP antagonists through the BIR, and the ability of self ubiquitination and ubiquitination and Neddylation of caspases via the RING domain. cIAP1, cIAP2 and XIAP own three tandem BIR domains, one UBA and one C-term RING domain (Figure 1). In addition, cIAPs contain a central CARD domain. The BIR1 is a type I BIR (unable to bind IBM) required for cell signalling activity. The XIAP BIR1 binds the adaptor TAB1 (Transforming Growth Factor beta-activated kinase 1 (TAK1)-binding protein 1) [19]. TAB1 is involved in transforming growth factor β (TGF-β) and bone morphogenetic protein (BMP) signalling pathways, and binds the kinase TAK1 (TGF-β-activated kinase 1) controlling NF-κB and MAP (mitogen-activated protein kinase) activating signaling pathways. The cIAP BIR1 binds the adaptor TRAF2 (TNFR associated factor 2) which bridges receptors from TNFR superfamily to downstream signalling pathways [20,25,83]. The BIR2 and BIR3 are type II BIRs able to bind IBM motifs exposed in active tetrameric Caspases and IAP antagonists [17,84,85]. The deletion or down-regulation of cIAPs or XIAP does not usually trigger apoptotis but sensitizes cells to extracellular or intracellular apoptotic inducers. XIAP and cIAPs demonstrate overlapping activities, which render difficult their functional analysis. Knocking out (KO) of single iap gene in mouse does not lead to obvious developmental abnormalities [86,87], however, a combined deletion of ciap1 with ciap2 or xiap in mice resulted in mid-embryonic lethality due to cardiovascular failure [88]. The main activity of cIAP1 and cIAP2 likely involves their ability to regulate the NF-κB activating signalling pathway in innate immune responses (reviewed by [6]). Although XIAP also displays some signalling activities in TGF-β/BMP and NF-κB signalling pathways [19], it is considered as the most potent mammalian IAP apoptotic regulator, able to directly inhibit caspase activity [84].
5.2. Mammalian Apoptotic Signalling Pathways
Mammalian cells contain four apoptotic initiator caspases (caspase-2, -8, -9 and -10) solicited by different stimuli. The closest DRONC homolog is caspase-9 involved in a mitochondria-dependent apoptotic pathway, so-called intrinsic pathway [89,90]. It is activated in response to a large range of intracellular or extracellular stimuli which trigger a Bcl-2 (B-cell lymphoma-2) family member-dependent mitochondrial outer membrane permeabilization, resulting in the release of pro-apoptotic molecules including cytochrome-c and the IAP antagonists Smac/Diablo (second mitochondria-derived activator of caspases/direct IAP-binding protein with low pI) and Omi/HtrA2 (Omi stress-regulated endoprotease/High temperature requirement protein A2) [91,92]. Once cytoplasmic, cytochrome-c triggers the oligomerization of the adaptor Apaf-1 (Apoptotic peptidase activating factor 1) which recruits pro-caspase-9 allowing its activation at the apoptosome (Figure 3) [89]. Caspase-8 and -10 are activated in response to the engagement of death receptor from TNFR superfamily. Stimulation of Fas (DR2, CD95) or Trail (TNF-related apoptosis-inducing ligand) Receptor I or II (DR4 and DR5) induces the recruitment of the adaptor FADD (Fas-associated death domain protein), which then recruits and activates pro-caspase-8 or -10 in a receptor-associated platform named DISC (death-inducing signalling complex) [90]. FADD can also induced caspase-8 and -10 activation in cytoplasmic platforms such as Complexes-II or Ripoptosome [93,94,95]. TNFR1 stimulation induces the assembly of membrane associated oligomeric complex which transduces survival or pro-inflammatory signal. When survival pathways are blocked, a secondary cytoplasmic caspase-activating complex named Complex-II is formed, composed, in addition to the adaptor and the caspase, of the adaptor TRADD (TNFR1-associated Death domain) or the kinase RIP1 (Receptor interacting protein 1) [90]. Ripoptosome also contains the kinase RIP1 and is assembled in response to genotoxic stress, Tweak engagement or Toll-like receptor 3 stimulation [94,95]. Caspase-2 is recruited and activated in response to DNA damages by the adaptor RAIDD (receptor-interacting protein-associated ICH-1/CED-3 homologous protein with a DD) in a soluble platform named PIDDosome that contains the protein PIDD (p53-induced protein with death domain) [90]. Caspase-2 can also be activated in response of ER stress or bacterial toxin but the exact mechanisms of its activation is not well established [96,97].
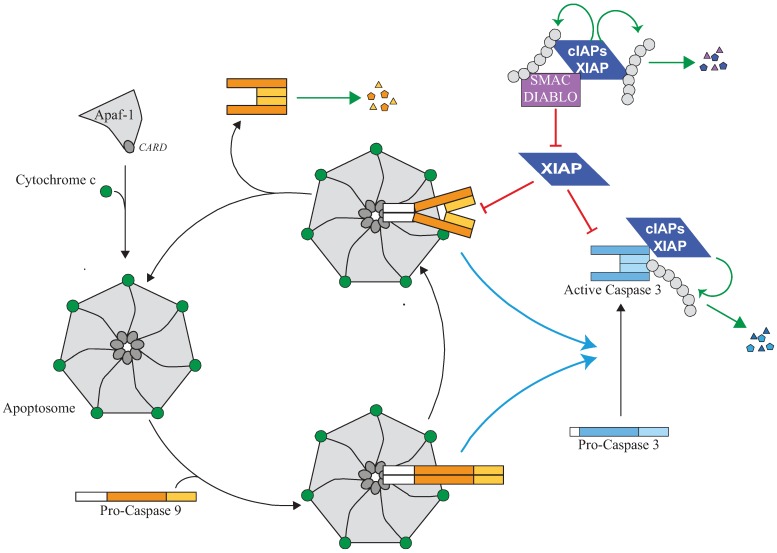
Figure 3.
Regulation of the apoptosome and caspase activity by IAPs. The release of cytochrome-c from mitochondria which occurs during intrinsic pathway of apoptosis triggers an ATP-dependent conformational change and oligomerisation of the adaptor apoptotic protease activating factor 1 (Apaf-1) in a heptameric complex apoptosome. Apaf-1 then recruits Caspase-9 via its pro-domain through a homotypic CARD-CARD interaction. Caspase-9 is activated by homodimerisation and promotes the activating cleavage of effector caspase-3 leading to apoptosis. Caspase-9 undergoes autocatalytic processing and is then quickly disconnected from the apoptosome which is free to recruit a new pro-caspase-9. XIAP can control caspase activating pathway at several steps. First, XIAP is present in the apoptosome where it directly binds processed caspase-9 and inhibits its activity. The inhibition of caspase-9 by XIAP could stabilize the caspase-9 apoptosome complex and block the cycle of caspase-9 activation. Second, XIAP can directly bind and inhibit active effector caspase-3. XIAP can inhibit caspase activity by hindering substrate accessibility or hiding the protease catalytic residue, and/or by promoting ubiquitination or neddylation. Although unable to inhibit their activity, cIAP1 can bind to processed caspases and promote their ubiquitination. An IBM-dependent binding of IAP antagonists such as Smac/Diablo prevents XIAP-mediated caspase inhibition while cIAPs could interfere with neutralizing binding XIAP-Smac/Diablo.
All of these apoptotic pathways converge to the proteolytic activation of effector caspase-3 and -7.
5.3. Regulation of Apoptosome and Caspase-9 Activity by IAPs
The core component of Apoptosome is the adaptor Apaf-1. Binding of cytochrome-c triggers an ATP-dependent conformational change and oligomerisation of Apaf-1 in a heptameric complex in which the CARD domain of Apaf-1 forms a central ring structure that recruits pro-caspase-9 through the CARD found in its prodomain (Figure 3) (reviewed in [89]). This provides proximity required for oligomerization and self-activation of caspase-9. Once activated, caspase-9 undergoes autocatalytic processing [98] and activates effector caspases. It is then quickly disconnected from the apoptosome and inactivated, and possibly replaced in the apoptosome by a new pro-caspase-9 [98] (Figure 3). The presence of caspase inhibitors significantly reduces the dissociation rate of caspase-9 from the apoptosome blocking the cycle of activation of caspase [99]. Thus, caspase-9 activation is a dynamic, highly regulated process. XIAP likely takes part to these mechanisms of regulation of apoptosome activity (Figure 3). It is normally present in the apoptosome complex [17,100]. It does not influence pro-caspase-9 auto-processing but inhibits the activity of processed caspase-9 and then the activation of effector caspases [17,100]. It can directly bind and inhibit the activity of processed-caspases-9 by a two-site binding mechanism [84]. First, the surface groove of BIR3 binds the IBM, exposed at the N-terminus of the active small sub-unit after caspase-9 processing [17]. Second, the C-terminal extremity of BIR3 binds the dimer interface of caspase-9, preventing caspase-9 dimerization and hiding the catalytic residue [17,101]. Thus, XIAP could control apoptosome by inhibiting the activity of caspase-9 and by interfering with the cycle of activation of caspase-9 (Figure 3). Interestingly, a feedback regulation of apoptosome by caspase-3, which amplifies apoptotic process, has been described. Caspase-9 contains two caspase-3 cleavage sites. The first one produces the two active sub-units, and the second removes the IBM of the small active sub-unit, and then release caspase-9 from XIAP regulation [102]. Apoptosome activity is also regulated by the amount of cytochrome-c, Apaf-1 and pro-caspase-9 available [89]. Interestingly, the capacity of XIAP to control capase-9 activity appears to be directly correlated with the level of Apaf-1 and apoptosome activity. For example, XIAP effectively regulates sensitivity to apoptotic stimuli in cells expressing a low level of Apaf-1 such as terminally differentiated neuronal cells and cardiomyocytes [103,104].
Among mammalian IAPs, NAIP has also been detected in the apoptosome [105]. NAIP is an atypical IAP since it owns, in addition to the BIRs, a central NACHT (domain present in NAIP, CIITA, HET E and TP1) and a C-terminus LRR (Leucine Rich repeat) region [106] (Figure 1). Both domains are characteristic of NLR (NOD (nucleotide binding and oligomerization domain)-like receptor) family involved in intracellular recognition of microbial production and in the formation of the inflammatory caspase activation complex [107]. In contrast to other IAPs, NAIP can interact with pro-caspase-9. It inhibits its autocatalytic processing and the activation of effector caspase. This interaction involves the BIR3 domain of NAIP and is IBM-independent [105,108].
5.4. Regulation of Effector Caspases by IAPs
XIAP can also directly bind and inhibit effector caspases-3 and -7 (Figure 3) [109,110,111]. However, this activity does not seem essential for the anti-apoptotic function of XIAP since an expression of XIAP mutant that has lost its ability to inhibit caspase-3 activity retained full ability to block ultra-violet-induced apoptosis [111].XIAP BIR2 IBM-binding groove binds the IBM of the large sub-unit of tetramer active effector caspases, and the linker region upstream of BIR 2 hinders the substrate binding pocket of caspase-3 and -7, preventing substrate accessibility [59,111,112,113,114]. Although the E3-ubiquitin ligase activity of XIAP seems dispensable, the analysis of cells from XIAP∆RING transgenic mice revealed the influence of the RING-dependent post-traductional modifications on XIAP-mediated caspase-3 inhibition [115]. Expression of the RING-deletion XIAP mutant did not compensate the deficiency of XIAP and increased caspase-3 activity and apoptosis in stem cells and thymocytes [115]. XIAP is able to induce the K48 ubiquitination of active caspases leading to their degradation [116], and the neddylation of caspase-7 inhibiting its activity [22].
cIAPs can interact with caspase-3 and caspase-7 in an IBM-dependent manner [59]. Moreover, cIAP1 seems able to interact with the pro-domain of caspases, independently of IBM [117]. Although unable to inhibit enzymatic activity of caspases [114], cIAPs can regulate the stability of active tetrameric caspases through a UPS (Ub Proteasome system)-dependent mechanism [59,117]. cIAP2 is able, at least in vitro, to induce a non degradative mono-ubiquitination of caspase-3 and -7 [118], suggesting a UPS-independent, Ub-dependent mechanisms of regulation.
5.5. Regulation of RIP1-Containing Caspase-Activating Platform Assembly
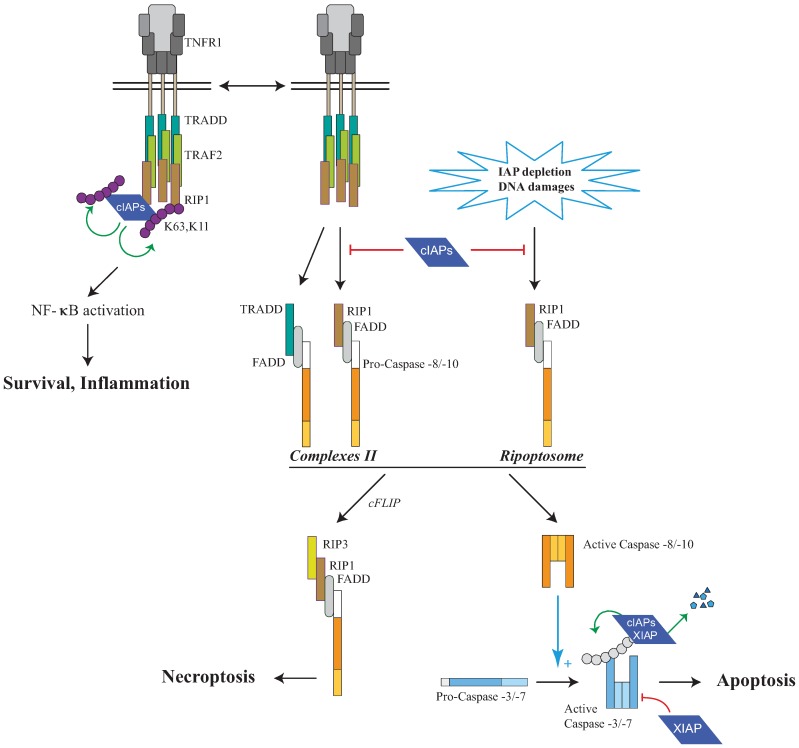
The serine/threonine kinases from RIP family are mediators in adaptive response to cellular stress caused by pathogen infections, inflammation or genotoxic stress (reviewed in [119]. They are important determinants of the response of cells, able to transduce survival or differentiation signals and to activate cell death pathways. RIP1 is a component of TNF-signalling pathway. It owns the homotypic interacting module death domain (DD) found in the death receptor Fas, TRAIL-R1 and TRAIL-R2, in the TNF signaling pathway adaptor TRADD, and in FADD and RAIDD, adaptors in caspase-8 and caspase-2 activating platforms, respectively. RIP1 is recruited via TRADD to TNFR1, and then is subjected to post-translational modifications including ubiquitination which determines its molecular function. When conjugated to K63-linked ubiquitin chains, RIP1 serves as a recognition signal for the recruitment of signaling complex leading to downstream activation of MAPK and NF-κB [120,121,122,123]. When ubiquitination is reduced, RIP1, through its kinase activity, promotes the assembly of the caspase-activating platform such as complex II that leads to caspase activation and cell death [121,123]. Such cytoplasmic RIP1-containing caspase-activating platform can also be formed independently of death receptor and is named RIPoptosome [94,95]. RIP1-containing complex can elicit apoptotic response, or caspase-independent cell death referred to as necroptosis, depending on the presence of RIP3, cellular FLICE-inhibitory protein (cFLIP) and the generation of reactive oxygen species [124].
The recent analysis of ciap1/xiap and ciap1/ciap2 double knockout mice demonstrated the importance of IAPs in the regulation of RIP1-dependent cell death. Indeed, the deletion of ciap1 plus ciap2 or xiap leads to the embryonic lethality, which is rescued or delayed by hemizygosity for rip1 [88].RIP proteins are ubiquitination targets of cIAPs which can mediate the conjugation of K11, K48 and K63 and linear Ub chains [94,121,122,125,126,127,128]. Upon TNFR1 stimulation, cIAP1 induced self-ubiqitination and K11 and K63 poly-ubiquitination of RIP-1 required for the activation of the NF-κB signaling pathway [121,122,127,129]. In the absence of cIAPs, RIP1, in a non-ubiquitinated form, is unable to transduce survival pathway, and is recruited to a secondary cytoplasmic cell platform (complex-II) resulting to cell death (Figure 4). cIAP1 also prevents the assembly of secondary RIP1-containing cell death complexes after TRAILR, or CD95 stimulation [126]. Independently of the stimulation of TNFR members, the Ripoptosome formed in response to genotoxic stress, Tweak engagement or Toll-like receptor 3 stimulation is also negatively regulated by cIAP1, cIAP2 and XIAP [94,95] (Figure 4). cIAP1 could promote proteasomal-mediated degradation of component of RIPoptosome [94]. Ubiquitination of RIP1 could also inhibit the RIP1 kinase activity required for cell death complex assembly.
Figure 4.
Regulation of RIP1-containing platforms by IAPs. Tumor Necrosis Factor Receptor 1 (TNFR1) stimulation induces the recruitment, to the receptor, of cIAPs and Receptor interacting protein 1 (RIP1) via the adaptors TNFR1-associated Death domain (TRADD) and TNFR associated factor (TRAF2). cIAPs trigger K63 self-ubiquitination and K11 and K63-ubiquitination of RIP1 leading to NF-κB activation and survival. In the absence of cIAPs, a secondary RIP1-containing cytoplasmic complex is formed (complex II) leading to cell death. A cytoplasmic RIP-1-containing complex named RIPoptosome can also be assembled, in the absence of Death Receptor stimulation, after DNA-damage-mediated IAP depletion or the use of synthetic IAP antagonists known to induce IAP degradation. RIP1-containing platform can lead to either caspase-8 or -10 activation and apoptosis, or caspase-independent cell death referred to as Necroptosis. Initiator caspases-8 or -10 induce the activating proteolytic processing of effector caspase-3 or -7 responsible for apoptosis. IAPs can control cell death at different levels: (1) cIAPs can induce the K63 and K11 ubiquitination of RIP1 allowing NF-κB activation and preventing the formation of complex II or RIPoptosome; (2) cIAPs and XIAP can induce K48-ubiquitination of RIP1 leading to its proteasomal degradation. (3) XIAP can directly inhibit the activity of processed effector caspase-3 or -7; (4) cIAPs and XIAP can induce ubiquitination and proteasome-mediated degradation of processed forms of caspase-3 or -7.
5.6. Mammalian Endogenous IAP Antagonists
Mammalian cells express large number of proteins that bear a potential IBM motif but their ability to bind and regulate IAPs and apoptosis remains to be investigated [23,130]. Most of them are expressed as a precursor into the mitochondrial. The best characterized are Smac/Diablo and HtrA2 [91,92]. They are released into the cytosol during apoptosis, after matured processing that removes the N-terminal mitochondrial import signal and then exposed the IBM to the N-extremity of the protein. Once cytosolic, they bind the IBM groove of IAPs, preventing them from binding caspases. Smac/Diablo can bind to both BIR3 and BIR2 domains and antagonizes XIAP inhibition of caspase-9 and caspase-3 [131,132]. Smac/Diablo can also interact with cIAP1 and cIAP2. It is generally admitted that Smac/Diablo could abrogate XIAP-mediated caspase inhibition while cIAPs could prevent Smac/Diablo from neutralizing XIAP (Figure 3). IAPs might also mediate the ubiquitination and degradation of these proteins [133,134]. Conversely, Smac/Diablo can promote auto-ubiquination and degradation of cIAPs [28,135]. Omi/HtrA2 is a serine protease that binds and inactivates cIAPs and XIAP by an irreversible proteolytic cleavage [136,137,138]. The transcriptional expression of Omi/HtrA2 is controlled by p53 and then up-regulated after DNA damages [138]. Another mitochondrial protein translocated into the cytosol in response to apoptotic stimuli and that can antagonize IAP is the septin-like protein ARTS [139]. Although it does not harbour conventional IBM, it efficiently binds XIAP and induces its ubiquitin/proteasomal-dependent downregulation [140,141]. Inactivation of ARTS in mice leads to an increased incidence of leukemia/lymphoma and hematopoietic stem cells and progenitors are significantly more resistant to apoptotic stimuli. This phenotype is partly reversed by the inactivation of XIAP [142], demonstrating the importance of ARTS-mediated XIAP regulation in hematopoietic compartment.
As Jafrac2 in drosophila [72], GSPT1/eRF3 (G1 to S phase transition protein/eukaryotic Release Factor 3) is an IBM-containing ER protein. The IBM motif is exposed after ER-stress that induces the removal of the N-terminal endoplasmic reticulum signal peptide. GSPT1/eRF3-cIAPs interaction selectively stimulates cIAP1 auto-ubiquitination and degradation [143].
In contrast to drosophila, mammalian IAP antagonists seem dispensable for apoptosis induction. In most cases, deletion or neutralisation of IAPs did not result in apoptosis, except in some tumour cells in which depletion of IAPs triggered a spontaneous assembly of Ripoptosome [94,95] or resulted to the production of TNFα, which triggers cell death via an autocrine pathway [121,144,145,146,147,148]. Apoptotic stimuli such as glucocorticoid or DNA damaging agents can induce the auto-ubiquitination and degradation of cIAPs [149] which could result to RIPoptosome assembly [94] but the role of IAP antagonists in these processes was not determined. The physiological functions of IBM-bearing proteins are not established. The role and the importance of most of these molecules in the regulation of IAPs is not much documented and more investigations will be required for determining their level of action in the regulation of apoptosis or in the regulation of other functions of IAPs such as innate immunity in mammals. One other possibility is that IAPs, through their E3-ubiquitin ligase activity could catalyse the destruction of IBM-containing proteins.
6. Conclusion
Although IAPs were first described as apoptotic regulators, the importance of this function in mammals has long been discussed. However, the analysis of cells derived from IAPs or IAP antagonist-deleted mice revealed the importance of IAPs in adaptive response of cells to specific cellular or environmental injuries [4]. For example, XIAP and NAIP have been involved in the adaptive response of neuronal cells to hypoxic-ischemia injury [150,151,152]. Furthermore, abnormalities in IAP expression have been observed in diseases linked to a deregulation of cell death pathways. A reduced expression of XIAP has been found in neurodegerative disorders such as Huntington’s and Wilson’s disease [153,154]. Inversely, overexpression of IAPs has been detected in number of tumour samples and correlated with bad prognosis or poor response to chemotherapy [155]. The importance of IAPs in adaptive response to cellular stress was strengthened by the discovery of the presence of IAP-translational and transcriptional regulation-mechanisms which keep high the level of expression of IAPs under stressful conditions such as hypoxia, anoxia, serum deprivation, reticular or genotoxic stress [156,157,158,159]. However, the mechanisms regulating IAP activity remain poorly understood. A number of mammalian IBM-bearing proteins, potential IAP regulators remain to be characterised and post-translational regulations such as phosphorylation, oligomerization and subcellular localization are poorly documented.
The knowledge of the mechanisms of interaction of XIAP with caspases and IAP antagonists provided very potent tools for the design of synthetic IAP antagonists named Smac mimetics (SMs) aiming to inhibit the anti-apoptotic function of IAPs. These molecules are currently under clinical evaluation and give promising results in treating cancer, in association with conventional therapy or death receptor agonists (for review, see [160]). They were also helpful tools for the investigation of IAP functions. SMs appeared to be potent inhibitors of cIAPs, mediating their auto-ubiquitination and rapid proteasomal-mediated degradation [144,145,161]. SMs considerably alter the NF-κB activating signalling pathway, stimulating the production of pro-inflammatory cytokines including TNF-α [121,144,145,146]. Thus, SMs highlighted the important role of cIAPs in the regulation of NF-κB activation and innate immunity. The consequences of SMs on immune system in vivo, and the use of cIAPs as potential therapeutic targets for inflammatory or immune disorders are still important questions that need to be addressed.
Acknowledgments
We thank Jennifer Cultot for reading the manuscript. Our work is supported by grants from the «Comité de Côte d’Or de la Ligue contre le Cancer», from the «Association pour la recherche sur le Cancer (ARC)» and from the «Conseil Régional de Bourgogne». JB received a fellowships from the “Ministère de l’Enseignement Supérieur et de la Recherche” of France.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Clarke T.E., Clem R.J. Insect defenses against virus infection: The role of apoptosis. Int. Rev. Immunol. 2003;22:401–424. doi: 10.1080/08830180305215. [DOI] [PubMed] [Google Scholar]
- 2.Crook N.E., Clem R.J., Miller L.K. An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J. Virol. 1993;67:2168–2174. doi: 10.1128/jvi.67.4.2168-2174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orme M., Meier P. Inhibitor of apoptosis proteins in Drosophila: Gatekeepers of death. Apoptosis. 2009;14:950–960. doi: 10.1007/s10495-009-0358-2. [DOI] [PubMed] [Google Scholar]
- 4.Marivin A., Berthelet J., Plenchette S., Dubrez L. The inhibitor of apoptosis (IAPs) in adaptive response to cellular stress. Cells. 2012;1:711–737. doi: 10.3390/cells1040711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beug S.T., Cheung H.H., Lacasse E.C., Korneluk R.G. Modulation of immune signalling by inhibitors of apoptosis. Trends Immunol. 2012;33:535–545. doi: 10.1016/j.it.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Gyrd-Hansen M., Meier P. IAPs: From caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat. Rev. Cancer. 2010;10:561–574. doi: 10.1038/nrc2889. [DOI] [PubMed] [Google Scholar]
- 7.Wertz I.E., Dixit V.M. Regulation of death receptor signaling by the ubiquitin system. Cell Death Differ. 2010;17:14–24. doi: 10.1038/cdd.2009.168. [DOI] [PubMed] [Google Scholar]
- 8.Silke J., Brink R. Regulation of TNFRSF and innate immune signalling complexes by TRAFs and cIAPs. Cell Death Differ. 2010;17:35–45. doi: 10.1038/cdd.2009.114. [DOI] [PubMed] [Google Scholar]
- 9.Dupoux A., Cartier J., Cathelin S., Filomenko R., Solary E., Dubrez-Daloz L. cIAP1-dependent TRAF2 degradation regulates the differentiation of monocytes into macrophages and their response to CD40 ligand. Blood. 2009;113:175–185. doi: 10.1182/blood-2008-02-137919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dogan T., Harms G.S., Hekman M., Karreman C., Oberoi T.K., Alnemri E.S., Rapp U.R., Rajalingam K. X-Linked and cellular IAPs modulate the stability of C-RAF kinase and cell motility. Nat. Cell Biol. 2008;10:1447–1455. doi: 10.1038/ncb1804. [DOI] [PubMed] [Google Scholar]
- 11.Xu L., Zhu J., Hu X., Zhu H., Kim H.T., LaBaer J., Goldberg A., Yuan J. c-IAP1 cooperates with Myc by acting as a ubiquitin ligase for Mad1. Mol. Cell. 2007;28:914–922. doi: 10.1016/j.molcel.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 12.Cartier J., Berthelet J., Marivin A., Gemble S., Edmond V., Plenchette S., Lagrange B., Hammann A., Dupoux A., Delva L., et al. Cellular Inhibitor of Apoptosis Protein-1 (cIAP1) Can Regulate E2F1 transcription factor-mediated control of cyclin transcription. J. Biol. Chem. 2011;286:26406–26417. doi: 10.1074/jbc.M110.191239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lens S.M., Vader G., Medema R.H. The case for Survivin as mitotic regulator. Curr. Opin. Cell Biol. 2006;18:616–622. doi: 10.1016/j.ceb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Birnbaum M.J., Clem R.J., Miller L.K. An apoptosis-inhibiting gene from a nuclear polyhedrosis virus encoding a polypeptide with Cys/His sequence motifs. J. Virol. 1994;68:2521–2528. doi: 10.1128/jvi.68.4.2521-2528.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinds M.G., Norton R.S., Vaux D.L., Day C.L. Solution structure of a baculoviral inhibitor of apoptosis (IAP) repeat. Nat. Struct. Biol. 1999;6:648–651. doi: 10.1038/10701. [DOI] [PubMed] [Google Scholar]
- 16.Eckelman B.P., Drag M., Snipas S.J., Salvesen G.S. The mechanism of peptide-binding specificity of IAP BIR domains. Cell Death Differ. 2008;15:920–928. doi: 10.1038/cdd.2008.6. [DOI] [PubMed] [Google Scholar]
- 17.Srinivasula S.M., Hegde R., Saleh A., Datta P., Shiozaki E., Chai J., Lee R.A., Robbins P.D., Fernandes-Alnemri T., Shi Y., Alnemri E.S. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature. 2001;410:112–116. doi: 10.1038/35065125. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z., Sun C., Olejniczak E.T., Meadows R.P., Betz S.F., Oost T., Herrmann J., Wu J.C., Fesik S.W. Structural basis for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature. 2000;408:1004–1008. doi: 10.1038/35050006. [DOI] [PubMed] [Google Scholar]
- 19.Lu M., Lin S.C., Huang Y., Kang Y.J., Rich R., Lo Y.C., Myszka D., Han J., Wu H. XIAP induces NF-kappaB activation via the BIR1/TAB1 interaction and BIR1 dimerization. Mol. Cell. 2007;26:689–702. doi: 10.1016/j.molcel.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samuel T., Welsh K., Lober T., Togo S.H., Zapata J.M., Reed J.C. Distinct BIR domains of cIAP1 mediate binding to and ubiquitination of tumor necrosis factor receptor-associated factor 2 and second mitochondrial activator of caspases. J. Biol. Chem. 2006;281:1080–1090. doi: 10.1074/jbc.M509381200. [DOI] [PubMed] [Google Scholar]
- 21.Mace P.D., Smits C., Vaux D.L., Silke J., Day C.L. Asymmetric recruitment of cIAPs by TRAF2. J. Mol. Biol. 2010;400:8–15. doi: 10.1016/j.jmb.2010.04.055. [DOI] [PubMed] [Google Scholar]
- 22.Broemer M., Tenev T., Rigbolt K.T., Hempel S., Blagoev B., Silke J., Ditzel M., Meier P. Systematic in vivo RNAi analysis identifies IAPs as NEDD8-E3 ligases. Mol. Cell. 2010;40:810–822. doi: 10.1016/j.molcel.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Zhuang M., Guan S., Wang H., Burlingame A.L., Wells J.A. Substrates of IAP Ubiquitin ligases identified with a designed orthogonal E3 ligase, the NEDDylator. Mol. Cell. 2013;49:273–282. doi: 10.1016/j.molcel.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silke J., Kratina T., Chu D., Ekert P.G., Day C.L., Pakusch M., Huang D.C., Vaux D.L. Determination of cell survival by RING-mediated regulation of inhibitor of apoptosis (IAP) protein abundance. Proc. Natl. Acad. Sci. USA. 2005;102:16182–16187. doi: 10.1073/pnas.0502828102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mace P.D., Linke K., Feltham R., Schumacher F.R., Smith C.A., Vaux D.L., Silke J., Day C.L. Structures of the cIAP2 RING domain reveal conformational changes associated with ubiquitin-conjugating enzyme (E2) recruitment. J. Biol. Chem. 2008;283:31633–31640. doi: 10.1074/jbc.M804753200. [DOI] [PubMed] [Google Scholar]
- 26.Rajalingam K., Sharma M., Paland N., Hurwitz R., Thieck O., Oswald M., Machuy N., Rudel T. IAP-IAP complexes required for apoptosis resistance of C. trachomatis-infected cells. PLoS Pathog. 2006;2:e114. doi: 10.1371/journal.ppat.0020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez J., John S.W., Tenev T., Rautureau G.J., Hinds M.G., Francalanci F., Wilson R., Broemer M., Santoro M.M., Day C.L., et al. CARD-Mediated autoinhibition of cIAP1’s E3 ligase activity suppresses cell proliferation and migration. Mol. Cell. 2011;42:569–583. doi: 10.1016/j.molcel.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Blankenship J.W., Varfolomeev E., Goncharov T., Fedorova A.V., Kirkpatrick D.S., Izrael-Tomasevic A., Phu L., Arnott D., Aghajan M., Zobel K., et al. Ubiquitin binding modulates IAP antagonist-stimulated proteasomal degradation of c-IAP1 and c-IAP2(1) Biochem. J. 2009;417:149–160. doi: 10.1042/BJ20081885. [DOI] [PubMed] [Google Scholar]
- 29.Gyrd-Hansen M., Darding M., Miasari M., Santoro M.M., Zender L., Xue W., Tenev T., da Fonseca P.C., Zvelebil M., Bujnicki J.M., et al. IAPs contain an evolutionarily conserved ubiquitin-binding domain that regulates NF-kappaB as well as cell survival and oncogenesis. Nat. Cell Biol. 2008;10:1309–1317. doi: 10.1038/ncb1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulathila R., Vash B., Sage D., Cornell-Kennon S., Wright K., Koehn J., Stams T., Clark K., Price A. The structure of the BIR3 domain of cIAP1 in complex with the N-terminal peptides of SMAC and caspase-9. Acta Crystallogr. D Biol. Crystallogr. 2009;65:58–66. doi: 10.1107/S0907444908039243. [DOI] [PubMed] [Google Scholar]
- 31.Chowdhury I., Tharakan B., Bhat G.K. Caspases—An update. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008;151:10–27. doi: 10.1016/j.cbpb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Ryoo H.D., Baehrecke E.H. Distinct death mechanisms in Drosophila development. Curr. Opin. Cell Biol. 2010;22:889–895. doi: 10.1016/j.ceb.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S.L., Hawkins C.J., Yoo S.J., Muller H.A., Hay B.A. The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by HID. Cell. 1999;98:453–463. doi: 10.1016/S0092-8674(00)81974-1. [DOI] [PubMed] [Google Scholar]
- 34.White K., Grether M.E., Abrams J.M., Young L., Farrell K., Steller H. Genetic control of programmed cell death in Drosophila. Science. 1994;264:677–683. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]
- 35.Grether M.E., Abrams J.M., Agapite J., White K., Steller H. The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev. 1995;9:1694–1708. doi: 10.1101/gad.9.14.1694. [DOI] [PubMed] [Google Scholar]
- 36.Vucic D., Kaiser W.J., Harvey A.J., Miller L.K. Inhibition of reaper-induced apoptosis by interaction with inhibitor of apoptosis proteins (IAPs) Proc. Natl. Acad. Sci. USA. 1997;94:10183–10188. doi: 10.1073/pnas.94.19.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goyal L., McCall K., Agapite J., Hartwieg E., Steller H. Induction of apoptosis by Drosophila reaper, hid and grim through inhibition of IAP function. EMBO J. 2000;19:589–597. doi: 10.1093/emboj/19.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meier P., Silke J., Leevers S.J., Evan G.I. The Drosophila caspase DRONC is regulated by DIAP1. EMBO J. 2000;19:598–611. doi: 10.1093/emboj/19.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hawkins C.J., Yoo S.J., Peterson E.P., Wang S.L., Vernooy S.Y., Hay B.A. The Drosophila caspase DRONC cleaves following glutamate or aspartate and is regulated by DIAP1, HID, and GRIM. J. Biol. Chem. 2000;275:27084–27093. doi: 10.1074/jbc.M000869200. [DOI] [PubMed] [Google Scholar]
- 40.Igaki T., Yamamoto-Goto Y., Tokushige N., Kanda H., Miura M. Down-Regulation of DIAP1 triggers a novel Drosophila cell death pathway mediated by Dark and DRONC. J. Biol. Chem. 2002;277:23103–23106. doi: 10.1074/jbc.C200222200. [DOI] [PubMed] [Google Scholar]
- 41.Quinn L.M., Dorstyn L., Mills K., Colussi P.A., Chen P., Coombe M., Abrams J., Kumar S., Richardson H. An essential role for the caspase dronc in developmentally programmed cell death in Drosophila. J. Biol. Chem. 2000;275:40416–40424. doi: 10.1074/jbc.M002935200. [DOI] [PubMed] [Google Scholar]
- 42.Chew S.K., Akdemir F., Chen P., Lu W.J., Mills K., Daish T., Kumar S., Rodriguez A., Abrams J.M. The apical caspase dronc governs programmed and unprogrammed cell death in Drosophila. Dev. Cell. 2004;7:897–907. doi: 10.1016/j.devcel.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 43.Daish T.J., Mills K., Kumar S. Drosophila caspase DRONC is required for specific developmental cell death pathways and stress-induced apoptosis. Dev. Cell. 2004;7:909–915. doi: 10.1016/j.devcel.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 44.Muro I., Hay B.A., Clem R.J. The Drosophila DIAP1 protein is required to prevent accumulation of a continuously generated, processed form of the apical caspase DRONC. J. Biol. Chem. 2002;277:49644–49650. doi: 10.1074/jbc.M203464200. [DOI] [PubMed] [Google Scholar]
- 45.Yuan S., Yu X., Topf M., Dorstyn L., Kumar S., Ludtke S.J., Akey C.W. Structure of the Drosophila apoptosome at 6.9 a resolution. Structure. 2011;19:128–140. doi: 10.1016/j.str.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu X., Wang L., Acehan D., Wang X., Akey C.W. Three-Dimensional structure of a double apoptosome formed by the Drosophila Apaf-1 related killer. J. Mol. Biol. 2006;355:577–589. doi: 10.1016/j.jmb.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 47.Dorstyn L., Kumar S. A cytochrome c-free fly apoptosome. Cell Death Differ. 2006;13:1049–1051. doi: 10.1038/sj.cdd.4401918. [DOI] [PubMed] [Google Scholar]
- 48.Dorstyn L., Kumar S. A biochemical analysis of the activation of the Drosophila caspase DRONC. Cell Death Differ. 2008;15:461–470. doi: 10.1038/sj.cdd.4402288. [DOI] [PubMed] [Google Scholar]
- 49.Kilpatrick Z.E., Cakouros D., Kumar S. Ecdysone-Mediated up-regulation of the effector caspase DRICE is required for hormone-dependent apoptosis in Drosophila cells. J. Biol. Chem. 2005;280:11981–11986. doi: 10.1074/jbc.M413971200. [DOI] [PubMed] [Google Scholar]
- 50.Xu D., Wang Y., Willecke R., Chen Z., Ding T., Bergmann A. The effector caspases drICE and dcp-1 have partially overlapping functions in the apoptotic pathway in Drosophila. Cell Death Differ. 2006;13:1697–1706. doi: 10.1038/sj.cdd.4401920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lisi S., Mazzon I., White K. Diverse domains of THREAD/DIAP1 are required to inhibit apoptosis induced by REAPER and HID in Drosophila. Genetics. 2000;154:669–678. doi: 10.1093/genetics/154.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chandraratna D., Lawrence N., Welchman D.P., Sanson B. An in vivo model of apoptosis: Linking cell behaviours and caspase substrates in embryos lacking DIAP1. J. Cell Sci. 2007;120:2594–2608. doi: 10.1242/jcs.03472. [DOI] [PubMed] [Google Scholar]
- 53.Kaiser W.J., Vucic D., Miller L.K. The Drosophila inhibitor of apoptosis D-IAP1 suppresses cell death induced by the caspase drICE. FEBS Lett. 1998;440:243–248. doi: 10.1016/S0014-5793(98)01465-3. [DOI] [PubMed] [Google Scholar]
- 54.Wilson R., Goyal L., Ditzel M., Zachariou A., Baker D.A., Agapite J., Steller H., Meier P. The DIAP1 RING finger mediates ubiquitination of Dronc and is indispensable for regulating apoptosis. Nat. Cell Biol. 2002;4:445–450. doi: 10.1038/ncb799. [DOI] [PubMed] [Google Scholar]
- 55.Chai J., Yan N., Huh J.R., Wu J.W., Li W., Hay B.A., Shi Y. Molecular mechanism of Reaper-Grim-Hid-mediated suppression of DIAP1-dependent Dronc ubiquitination. Nat. Struct. Biol. 2003;10:892–898. doi: 10.1038/nsb989. [DOI] [PubMed] [Google Scholar]
- 56.Zachariou A., Tenev T., Goyal L., Agapite J., Steller H., Meier P. IAP-Antagonists exhibit non-redundant modes of action through differential DIAP1 binding. EMBO J. 2003;22:6642–6652. doi: 10.1093/emboj/cdg617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shapiro P.J., Hsu H.H., Jung H., Robbins E.S., Ryoo H.D. Regulation of the Drosophila apoptosome through feedback inhibition. Nat. Cell Biol. 2008;10:1440–1446. doi: 10.1038/ncb1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee T.V., Fan Y., Wang S., Srivastava M., Broemer M., Meier P., Bergmann A. Drosophila IAP1-mediated ubiquitylation controls activation of the initiator caspase DRONC independent of protein degradation. PLoS Genet. 2011;7:e1002261. doi: 10.1371/journal.pgen.1002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tenev T., Zachariou A., Wilson R., Ditzel M., Meier P. IAPs are functionally non-equivalent and regulate effector caspases through distinct mechanisms. Nat. Cell Biol. 2005;7:70–77. doi: 10.1038/ncb1204. [DOI] [PubMed] [Google Scholar]
- 60.Tenev T., Ditzel M., Zachariou A., Meier P. The antiapoptotic activity of insect IAPs requires activation by an evolutionarily conserved mechanism. Cell Death Differ. 2007;14:1191–1201. doi: 10.1038/sj.cdd.4402118. [DOI] [PubMed] [Google Scholar]
- 61.Li X., Wang J., Shi Y. Structural mechanisms of DIAP1 auto-inhibition and DIAP1-mediated inhibition of drICE. Nat. Commun. 2011;2:408. doi: 10.1038/ncomms1418. [DOI] [PubMed] [Google Scholar]
- 62.Yokokura T., Dresnek D., Huseinovic N., Lisi S., Abdelwahid E., Bangs P., White K. Dissection of DIAP1 functional domains via a mutant replacement strategy. J. Biol. Chem. 2004;279:52603–52612. doi: 10.1074/jbc.M409691200. [DOI] [PubMed] [Google Scholar]
- 63.Ditzel M., Wilson R., Tenev T., Zachariou A., Paul A., Deas E., Meier P. Degradation of DIAP1 by the N-end rule pathway is essential for regulating apoptosis. Nat. Cell Biol. 2003;5:467–473. doi: 10.1038/ncb984. [DOI] [PubMed] [Google Scholar]
- 64.Ditzel M., Broemer M., Tenev T., Bolduc C., Lee T.V., Rigbolt K.T., Elliott R., Zvelebil M., Blagoev B., Bergmann A., et al. Inactivation of effector caspases through nondegradative polyubiquitylation. Mol. Cell. 2008;32:540–553. doi: 10.1016/j.molcel.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herman-Bachinsky Y., Ryoo H.D., Ciechanover A., Gonen H. Regulation of the Drosophila ubiquitin ligase DIAP1 is mediated via several distinct ubiquitin system pathways. Cell Death Differ. 2007;14:861–871. doi: 10.1038/sj.cdd.4402079. [DOI] [PubMed] [Google Scholar]
- 66.Huh J.R., Foe I., Muro I., Chen C.H., Seol J.H., Yoo S.J., Guo M., Park J.M., Hay B.A. The Drosophila inhibitor of apoptosis (IAP) DIAP2 is dispensable for cell survival, required for the innate immune response to gram-negative bacterial infection, and can be negatively regulated by the reaper/hid/grim family of IAP-binding apoptosis inducers. J. Biol. Chem. 2007;282:2056–2068. doi: 10.1074/jbc.M608051200. [DOI] [PubMed] [Google Scholar]
- 67.Meinander A., Runchel C., Tenev T., Chen L., Kim C.H., Ribeiro P.S., Broemer M., Leulier F., Zvelebil M., Silverman N., et al. Ubiquitylation of the initiator caspase DREDD is required for innate immune signalling. EMBO J. 2012;31:2770–2783. doi: 10.1038/emboj.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaplan Y., Gibbs-Bar L., Kalifa Y., Feinstein-Rotkopf Y., Arama E. Gradients of a ubiquitin E3 ligase inhibitor and a caspase inhibitor determine differentiation or death in spermatids. Dev. Cell. 2010;19:160–173. doi: 10.1016/j.devcel.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 69.Vucic D., Kaiser W.J., Miller L.K. Inhibitor of apoptosis proteins physically interact with and block apoptosis induced by Drosophila proteins HID and GRIM. Mol. Cell Biol. 1998;18:3300–3309. doi: 10.1128/mcb.18.6.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu J.W., Cocina A.E., Chai J., Hay B.A., Shi Y. Structural analysis of a functional DIAP1 fragment bound to grim and hid peptides. Mol. Cell. 2001;8:95–104. doi: 10.1016/S1097-2765(01)00282-9. [DOI] [PubMed] [Google Scholar]
- 71.Yoo S.J., Huh J.R., Muro I., Yu H., Wang L., Wang S.L., Feldman R.M., Clem R.J., Muller H.A., Hay B.A. Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nat. Cell Biol. 2002;4:416–424. doi: 10.1038/ncb793. [DOI] [PubMed] [Google Scholar]
- 72.Tenev T., Zachariou A., Wilson R., Paul A., Meier P. Jafrac2 is an IAP antagonist that promotes cell death by liberating Dronc from DIAP1. EMBO J. 2002;21:5118–5129. doi: 10.1093/emboj/cdf530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Christich A., Kauppila S., Chen P., Sogame N., Ho S.I., Abrams J.M. The damage-responsive Drosophila gene sickle encodes a novel IAP binding protein similar to but distinct from reaper, grim, and hid. Curr. Biol. 2002;12:137–140. doi: 10.1016/S0960-9822(01)00658-3. [DOI] [PubMed] [Google Scholar]
- 74.Wing J.P., Karres J.S., Ogdahl J.L., Zhou L., Schwartz L.M., Nambu J.R. Drosophila sickle is a novel grim-reaper cell death activator. Curr. Biol. 2002;12:131–135. doi: 10.1016/S0960-9822(01)00664-9. [DOI] [PubMed] [Google Scholar]
- 75.Srinivasula S.M., Datta P., Kobayashi M., Wu J.W., Fujioka M., Hegde R., Zhang Z., Mukattash R., Fernandes-Alnemri T., Shi Y., et al. Sickle, a novel Drosophila death gene in the reaper/hid/grim region, encodes an IAP-inhibitory protein. Curr. Biol. 2002;12:125–130. doi: 10.1016/S0960-9822(01)00657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hays R., Wickline L., Cagan R. Morgue mediates apoptosis in the Drosophila melanogaster retina by promoting degradation of DIAP1. Nat. Cell Biol. 2002;4:425–431. doi: 10.1038/ncb794. [DOI] [PubMed] [Google Scholar]
- 77.Holley C.L., Olson M.R., Colon-Ramos D.A., Kornbluth S. Reaper eliminates IAP proteins through stimulated IAP degradation and generalized translational inhibition. Nat. Cell Biol. 2002;4:439–444. doi: 10.1038/ncb798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ryoo H.D., Bergmann A., Gonen H., Ciechanover A., Steller H. Regulation of Drosophila IAP1 degradation and apoptosis by reaper and ubcD1. Nat. Cell Biol. 2002;4:432–438. doi: 10.1038/ncb795. [DOI] [PubMed] [Google Scholar]
- 79.Ince I.A., Westenberg M., Vlak J.M., Demirbag Z., Nalcacioglu R., van Oers M.M. Open reading frame 193R of Chilo iridescent virus encodes a functional inhibitor of apoptosis (IAP) Virology. 2008;376:124–131. doi: 10.1016/j.virol.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 80.Li Q., Liston P., Schokman N., Ho J.M., Moyer R.W. Amsacta moorei Entomopoxvirus inhibitor of apoptosis suppresses cell death by binding Grim and Hid. J. Virol. 2005;79:3684–3691. doi: 10.1128/JVI.79.6.3684-3691.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang Q., Deveraux Q.L., Maeda S., Salvesen G.S., Stennicke H.R., Hammock B.D., Reed J.C. Evolutionary conservation of apoptosis mechanisms: Lepidopteran and baculoviral inhibitor of apoptosis proteins are inhibitors of mammalian caspase-9. Proc. Natl. Acad. Sci. USA. 2000;97:1427–1432. doi: 10.1073/pnas.97.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wright C.W., Clem R.J. Sequence requirements for Hid binding and apoptosis regulation in the baculovirus inhibitor of apoptosis Op-IAP. Hid binds Op-IAP in a manner similar to Smac binding of XIAP. J. Biol. Chem. 2002;277:2454–2462. doi: 10.1074/jbc.M110500200. [DOI] [PubMed] [Google Scholar]
- 83.Varfolomeev E., Wayson S.M., Dixit V.M., Fairbrother W.J., Vucic D. The inhibitor of apoptosis protein fusion c-IAP2.MALT1 stimulates NF-kappaB activation independently of TRAF1 AND TRAF2. J. Biol. Chem. 2006;281:29022–29029. doi: 10.1074/jbc.M605116200. [DOI] [PubMed] [Google Scholar]
- 84.Eckelman B.P., Salvesen G.S., Scott F.L. Human inhibitor of apoptosis proteins: Why XIAP is the black sheep of the family. EMBO Rep. 2006;7:988–994. doi: 10.1038/sj.embor.7400795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deveraux Q.L., Leo E., Stennicke H.R., Welsh K., Salvesen G.S., Reed J.C. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J. 1999;18:5242–5251. doi: 10.1093/emboj/18.19.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Conze D.B., Albert L., Ferrick D.A., Goeddel D.V., Yeh W.C., Mak T., Ashwell J.D. Posttranscriptional downregulation of c-IAP2 by the ubiquitin protein ligase c-IAP1 in vivo. Mol. Cell Biol. 2005;25:3348–3356. doi: 10.1128/MCB.25.8.3348-3356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harlin H., Reffey S.B., Duckett C.S., Lindsten T., Thompson C.B. Characterization of XIAP-deficient mice. Mol. Cell Biol. 2001;21:3604–3608. doi: 10.1128/MCB.21.10.3604-3608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moulin M., Anderton H., Voss A.K., Thomas T., Wong W.W., Bankovacki A., Feltham R., Chau D., Cook W.D., Silke J., et al. IAPs limit activation of RIP kinases by TNF receptor 1 during development. EMBO J. 2012;31:1679–1691. doi: 10.1038/emboj.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wurstle M.L., Laussmann M.A., Rehm M. The central role of initiator caspase-9 in apoptosis signal transduction and the regulation of its activation and activity on the apoptosome. Exp. Cell Res. 2012;318:1213–1220. doi: 10.1016/j.yexcr.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 90.Mace P.D., Riedl S.J. Molecular cell death platforms and assemblies. Curr. Opin. Cell Biol. 2010;22:828–836. doi: 10.1016/j.ceb.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Silke J., Verhagen A.M., Ekert P.G., Vaux D.L. Sequence as well as functional similarity for DIABLO/Smac and Grim, Reaper and Hid? Cell Death Differ. 2000;7:1275. doi: 10.1038/sj.cdd.4400790. [DOI] [PubMed] [Google Scholar]
- 92.Hegde R., Srinivasula S.M., Zhang Z., Wassell R., Mukattash R., Cilenti L., DuBois G., Lazebnik Y., Zervos A.S., Fernandes-Alnemri T., et al. Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts inhibitor of apoptosis protein-caspase interaction. J. Biol. Chem. 2002;277:432–438. doi: 10.1074/jbc.M109721200. [DOI] [PubMed] [Google Scholar]
- 93.Micheau O., Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/S0092-8674(03)00521-X. [DOI] [PubMed] [Google Scholar]
- 94.Tenev T., Bianchi K., Darding M., Broemer M., Langlais C., Wallberg F., Zachariou A., Lopez J., MacFarlane M., Cain K., et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol. Cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 95.Feoktistova M., Geserick P., Kellert B., Dimitrova D.P., Langlais C., Hupe M., Cain K., MacFarlane M., Hacker G., Leverkus M. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Imre G., Heering J., Takeda A.N., Husmann M., Thiede B., zu Heringdorf D.M., Green D.R., van der Goot F.G., Sinha B., Dotsch V., et al. Caspase-2 is an initiator caspase responsible for pore-forming toxin-mediated apoptosis. EMBO J. 2012;31:2615–2628. doi: 10.1038/emboj.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Upton J.P., Austgen K., Nishino M., Coakley K.M., Hagen A., Han D., Papa F.R., Oakes S.A. Caspase-2 cleavage of BID is a critical apoptotic signal downstream of endoplasmic reticulum stress. Mol. Cell Biol. 2008;28:3943–3951. doi: 10.1128/MCB.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Malladi S., Challa-Malladi M., Fearnhead H.O., Bratton S.B. The Apaf-1*procaspase-9 apoptosome complex functions as a proteolytic-based molecular timer. EMBO J. 2009;28:1916–1925. doi: 10.1038/emboj.2009.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saikumar P., Mikhailova M., Pandeswara S.L. Regulation of caspase-9 activity by differential binding to the apoptosome complex. Front. Biosci. 2007;12:3343–3354. doi: 10.2741/2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bratton S.B., Walker G., Srinivasula S.M., Sun X.M., Butterworth M., Alnemri E.S., Cohen G.M. Recruitment, activation and retention of caspases-9 and -3 by Apaf-1 apoptosome and associated XIAP complexes. EMBO J. 2001;20:998–1009. doi: 10.1093/emboj/20.5.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shiozaki E.N., Chai J., Rigotti D.J., Riedl S.J., Li P., Srinivasula S.M., Alnemri E.S., Fairman R., Shi Y. Mechanism of XIAP-mediated inhibition of caspase-9. Mol. Cell. 2003;11:519–527. doi: 10.1016/S1097-2765(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 102.Zou H., Yang R., Hao J., Wang J., Sun C., Fesik S.W., Wu J.C., Tomaselli K.J., Armstrong R.C. Regulation of the Apaf-1/caspase-9 apoptosome by caspase-3 and XIAP. J. Biol. Chem. 2003;278:8091–8098. doi: 10.1074/jbc.M204783200. [DOI] [PubMed] [Google Scholar]
- 103.Wright K.M., Linhoff M.W., Potts P.R., Deshmukh M. Decreased apoptosome activity with neuronal differentiation sets the threshold for strict IAP regulation of apoptosis. J. Cell Biol. 2004;167:303–313. doi: 10.1083/jcb.200406073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Potts M.B., Vaughn A.E., McDonough H., Patterson C., Deshmukh M. Reduced Apaf-1 levels in cardiomyocytes engage strict regulation of apoptosis by endogenous XIAP. J. Cell Biol. 2005;171:925–930. doi: 10.1083/jcb.200504082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davoodi J., Lin L., Kelly J., Liston P., MacKenzie A.E. Neuronal apoptosis-inhibitory protein does not interact with Smac and requires ATP to bind caspase-9. J. Biol. Chem. 2004;279:40622–40628. doi: 10.1074/jbc.M405963200. [DOI] [PubMed] [Google Scholar]
- 106.Roy N., Mahadevan M.S., McLean M., Shutler G., Yaraghi Z., Farahani R., Baird S., Besner-Johnston A., Lefebvre C., Kang X., et al. The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell. 1995;80:167–178. doi: 10.1016/0092-8674(95)90461-1. [DOI] [PubMed] [Google Scholar]
- 107.Fritz J.H., Ferrero R.L., Philpott D.J., Girardin S.E. Nod-Like proteins in immunity, inflammation and disease. Nat. Immunol. 2006;7:1250–1257. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- 108.Davoodi J., Ghahremani M.H., Es-Haghi A., Mohammad-Gholi A., Mackenzie A. Neuronal apoptosis inhibitory protein, NAIP, is an inhibitor of procaspase-9. Int. J. Biochem. Cell Biol. 2010;42:958–964. doi: 10.1016/j.biocel.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 109.Deveraux Q.L., Takahashi R., Salvesen G.S., Reed J.C. X-Linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 110.Takahashi R., Deveraux Q., Tamm I., Welsh K., Assa-Munt N., Salvesen G.S., Reed J.C. A single BIR domain of XIAP sufficient for inhibiting caspases. J. Biol. Chem. 1998;273:7787–7790. doi: 10.1074/jbc.273.14.7787. [DOI] [PubMed] [Google Scholar]
- 111.Silke J., Ekert P.G., Day C.L., Hawkins C.J., Baca M., Chew J., Pakusch M., Verhagen A.M., Vaux D.L. Direct inhibition of caspase 3 is dispensable for the anti-apoptotic activity of XIAP. EMBO J. 2001;20:3114–3123. doi: 10.1093/emboj/20.12.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chai J., Shiozaki E., Srinivasula S.M., Wu Q., Datta P., Alnemri E.S., Shi Y. Structural basis of caspase-7 inhibition by XIAP. Cell. 2001;104:769–780. doi: 10.1016/S0092-8674(01)00272-0. [DOI] [PubMed] [Google Scholar]
- 113.Riedl S.J., Fuentes-Prior P., Renatus M., Kairies N., Krapp S., Huber R., Salvesen G.S., Bode W. Structural basis for the activation of human procaspase-7. Proc. Natl. Acad. Sci. USA. 2001;98:14790–14795. doi: 10.1073/pnas.221580098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eckelman B.P., Salvesen G.S. The human anti-apoptotic proteins cIAP1 and cIAP2 bind but do not inhibit caspases. J. Biol. Chem. 2006;281:3254–3260. doi: 10.1074/jbc.M510863200. [DOI] [PubMed] [Google Scholar]
- 115.Schile A.J., Garcia-Fernandez M., Steller H. Regulation of apoptosis by XIAP ubiquitin-ligase activity. Genes Dev. 2008;22:2256–2266. doi: 10.1101/gad.1663108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Suzuki Y., Nakabayashi Y., Takahashi R. Ubiquitin-Protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc. Natl. Acad. Sci. USA. 2001;98:8662–8667. doi: 10.1073/pnas.161506698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Choi Y.E., Butterworth M., Malladi S., Duckett C.S., Cohen G.M., Bratton S.B. The E3 ubiquitin ligase cIAP1 binds and ubiquitinates caspase-3 and -7 via unique mechanisms at distinct steps in their processing. J. Biol. Chem. 2009;284:12772–12782. doi: 10.1074/jbc.M807550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang H., Joazeiro C.A., Bonfoco E., Kamada S., Leverson J.D., Hunter T. The inhibitor of apoptosis, cIAP2, functions as a ubiquitin-protein ligase and promotes in vitro monoubiquitination of caspases 3 and 7. J. Biol. Chem. 2000;275:26661–26664. doi: 10.1074/jbc.C000199200. [DOI] [PubMed] [Google Scholar]
- 119.Declercq W., Vanden Berghe T., Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009;138:229–232. doi: 10.1016/j.cell.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 120.Haas T.L., Emmerich C.H., Gerlach B., Schmukle A.C., Cordier S.M., Rieser E., Feltham R., Vince J., Warnken U., Wenger T., et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol. Cell. 2009;36:831–844. doi: 10.1016/j.molcel.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 121.Bertrand M.J., Milutinovic S., Dickson K.M., Ho W.C., Boudreault A., Durkin J., Gillard J.W., Jaquith J.B., Morris S.J., Barker P.A. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 122.Dynek J.N., Goncharov T., Dueber E.C., Fedorova A.V., Izrael-Tomasevic A., Phu L., Helgason E., Fairbrother W.J., Deshayes K., Kirkpatrick D.S., et al. c-IAP1 and UbcH5 promote K11-linked polyubiquitination of RIP1 in TNF signalling. Embo J. 2010;29:4198–4209. doi: 10.1038/emboj.2010.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.O’Donnell M.A., Legarda-Addison D., Skountzos P., Yeh W.C., Ting A.T. Ubiquitination of RIP1 regulates an NF-kappaB-independent cell-death switch in TNF signaling. Curr. Biol. 2007;17:418–424. doi: 10.1016/j.cub.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vandenabeele P., Galluzzi L., Vanden Berghe T., Kroemer G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 125.Park S.M., Yoon J.B., Lee T.H. Receptor interacting protein is ubiquitinated by cellular inhibitor of apoptosis proteins (c-IAP1 and c-IAP2) in vitro. FEBS Lett. 2004;566:151–156. doi: 10.1016/j.febslet.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 126.Geserick P., Hupe M., Moulin M., Wong W.W., Feoktistova M., Kellert B., Gollnick H., Silke J., Leverkus M. Cellular IAPs inhibit a cryptic CD95-induced cell death by limiting RIP1 kinase recruitment. J. Cell Biol. 2009;187:1037–1054. doi: 10.1083/jcb.200904158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Varfolomeev E., Goncharov T., Fedorova A.V., Dynek J.N., Zobel K., Deshayes K., Fairbrother W.J., Vucic D. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J. Biol. Chem. 2008;283:24295–24299. doi: 10.1074/jbc.C800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bertrand M.J., Lippens S., Staes A., Gilbert B., Roelandt R., de Medts J., Gevaert K., Declercq W., Vandenabeele P. cIAP1/2 are direct E3 ligases conjugating diverse types of ubiquitin chains to receptor interacting proteins kinases 1 to 4 (RIP1–4) PLoS One. 2011;6:e22356. doi: 10.1371/journal.pone.0022356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vanlangenakker N., Vanden Berghe T., Bogaert P., Laukens B., Zobel K., Deshayes K., Vucic D., Fulda S., Vandenabeele P., Bertrand M.J. cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death Differ. 2011;18:656–665. doi: 10.1038/cdd.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Verhagen A.M., Kratina T.K., Hawkins C.J., Silke J., Ekert P.G., Vaux D.L. Identification of mammalian mitochondrial proteins that interact with IAPs via N-terminal IAP binding motifs. Cell Death Differ. 2007;14:348–357. doi: 10.1038/sj.cdd.4402001. [DOI] [PubMed] [Google Scholar]
- 131.Du C., Fang M., Li Y., Li L., Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/S0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 132.Huang Y., Rich R.L., Myszka D.G., Wu H. Requirement of both the second and third BIR domains for the relief of X-linked inhibitor of apoptosis protein (XIAP)-mediated caspase inhibition by Smac. J. Biol. Chem. 2003;278:49517–49522. doi: 10.1074/jbc.M310061200. [DOI] [PubMed] [Google Scholar]
- 133.Morizane Y., Honda R., Fukami K., Yasuda H. X-Linked inhibitor of apoptosis functions as ubiquitin ligase toward mature caspase-9 and cytosolic Smac/DIABLO. J. Biochem. 2005;137:125–132. doi: 10.1093/jb/mvi029. [DOI] [PubMed] [Google Scholar]
- 134.Hu S., Yang X. Cellular inhibitor of apoptosis 1 and 2 are ubiquitin ligases for the apoptosis inducer Smac/DIABLO. J. Biol. Chem. 2003;278:10055–10060. doi: 10.1074/jbc.M207197200. [DOI] [PubMed] [Google Scholar]
- 135.Yang Q.H., Du C. Smac/DIABLO selectively reduces the levels of c-IAP1 and c-IAP2 but not that of XIAP and livin in HeLa cells. J. Biol. Chem. 2004;279:16963–16970. doi: 10.1074/jbc.M401253200. [DOI] [PubMed] [Google Scholar]
- 136.Verhagen A.M., Silke J., Ekert P.G., Pakusch M., Kaufmann H., Connolly L.M., Day C.L., Tikoo A., Burke R., Wrobel C., et al. HtrA2 promotes cell death through its serine protease activity and its ability to antagonize inhibitor of apoptosis proteins. J. Biol. Chem. 2002;277:445–454. doi: 10.1074/jbc.M109891200. [DOI] [PubMed] [Google Scholar]
- 137.Yang Q.H., Church-Hajduk R., Ren J., Newton M.L., Du C. Omi/HtrA2 catalytic cleavage of inhibitor of apoptosis (IAP) irreversibly inactivates IAPs and facilitates caspase activity in apoptosis. Genes Dev. 2003;17:1487–1496. doi: 10.1101/gad.1097903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jin S., Kalkum M., Overholtzer M., Stoffel A., Chait B.T., Levine A.J. CIAP1 and the serine protease HTRA2 are involved in a novel p53-dependent apoptosis pathway in mammals. Genes Dev. 2003;17:359–367. doi: 10.1101/gad.1047003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gottfried Y., Rotem A., Lotan R., Steller H., Larisch S. The mitochondrial ARTS protein promotes apoptosis through targeting XIAP. EMBO J. 2004;23:1627–1635. doi: 10.1038/sj.emboj.7600155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bornstein B., Gottfried Y., Edison N., Shekhtman A., Lev T., Glaser F., Larisch S. ARTS binds to a distinct domain in XIAP-BIR3 and promotes apoptosis by a mechanism that is different from other IAP-antagonists. Apoptosis. 2011;16:869–881. doi: 10.1007/s10495-011-0622-0. [DOI] [PubMed] [Google Scholar]
- 141.Garrison J.B., Correa R.G., Gerlic M., Yip K.W., Krieg A., Tamble C.M., Shi R., Welsh K., Duggineni S., Huang Z., et al. ARTS and Siah collaborate in a pathway for XIAP degradation. Mol. Cell. 2011;41:107–116. doi: 10.1016/j.molcel.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Garcia-Fernandez M., Kissel H., Brown S., Gorenc T., Schile A.J., Rafii S., Larisch S., Steller H. Sept4/ARTS is required for stem cell apoptosis and tumor suppression. Genes Dev. 2010;24:2282–2293. doi: 10.1101/gad.1970110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hegde R., Srinivasula S.M., Datta P., Madesh M., Wassell R., Zhang Z., Cheong N., Nejmeh J., Fernandes-Alnemri T., Hoshino S., et al. The polypeptide chain-releasing factor GSPT1/eRF3 is proteolytically processed into an IAP-binding protein. J. Biol. Chem. 2003;278:38699–38706. doi: 10.1074/jbc.M303179200. [DOI] [PubMed] [Google Scholar]
- 144.Varfolomeev E., Blankenship J.W., Wayson S.M., Fedorova A.V., Kayagaki N., Garg P., Zobel K., Dynek J.N., Elliott L.O., Wallweber H.J., et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 145.Vince J.E., Wong W.W., Khan N., Feltham R., Chau D., Ahmed A.U., Benetatos C.A., Chunduru S.K., Condon S.M., McKinlay M., et al. IAP Antagonists Target cIAP1 to Induce TNFalpha-Dependent Apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 146.Petersen S.L., Wang L., Yalcin-Chin A., Li L., Peyton M., Minna J., Harran P., Wang X. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gaither A., Porter D., Yao Y., Borawski J., Yang G., Donovan J., Sage D., Slisz J., Tran M., Straub C., et al. A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-alpha signaling. Cancer Res. 2007;67:11493–11498. doi: 10.1158/0008-5472.CAN-07-5173. [DOI] [PubMed] [Google Scholar]
- 148.Vince J.E., Chau D., Callus B., Wong W.W., Hawkins C.J., Schneider P., McKinlay M., Benetatos C.A., Condon S.M., Chunduru S.K., et al. TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1-TRAF2 complex to sensitize tumor cells to TNFalpha. J. Cell Biol. 2008;182:171–184. doi: 10.1083/jcb.200801010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang Y., Fang S., Jensen J.P., Weissman A.M., Ashwell J.D. Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science. 2000;288:874–877. doi: 10.1126/science.288.5467.874. [DOI] [PubMed] [Google Scholar]
- 150.West T., Stump M., Lodygensky G., Neil J.J., Deshmukh M., Holtzman D.M. Lack of X-linked inhibitor of apoptosis protein leads to increased apoptosis and tissue loss following neonatal brain injury. ASN Neuro. 2009;1:e00004. doi: 10.1042/AN20090005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Russell J.C., Whiting H., Szuflita N., Hossain M.A. Nuclear translocation of X-linked inhibitor of apoptosis (XIAP) determines cell fate after hypoxia ischemia in neonatal brain. J. Neurochem. 2008;106:1357–1370. doi: 10.1111/j.1471-4159.2008.05482.x. [DOI] [PubMed] [Google Scholar]
- 152.Holcik M., Thompson C.S., Yaraghi Z., Lefebvre C.A., MacKenzie A.E., Korneluk R.G. The hippocampal neurons of neuronal apoptosis inhibitory protein 1 (NAIP1)-deleted mice display increased vulnerability to kainic acid-induced injury. Proc. Natl. Acad. Sci. USA. 2000;97:2286–2290. doi: 10.1073/pnas.040469797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Goffredo D., Rigamonti D., Zuccato C., Tartari M., Valenza M., Cattaneo E. Prevention of cytosolic IAPs degradation: A potential pharmacological target in Huntington's Disease. Pharmacol. Res. 2005;52:140–150. doi: 10.1016/j.phrs.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 154.Weiss K.H., Runz H., Noe B., Gotthardt D.N., Merle U., Ferenci P., Stremmel W., Fullekrug J. Genetic analysis of BIRC4/XIAP as a putative modifier gene of Wilson disease. J. Inherit. Metab. Dis. 2010 doi: 10.1007/s10545-010-9123-5. [DOI] [PubMed] [Google Scholar]
- 155.Lau R., Pratt M.A. The opposing roles of cellular inhibitor of apoptosis proteins in cancer. ISRN Oncol. 2012;2012:928120. doi: 10.5402/2012/928120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Warnakulasuriyarachchi D., Cerquozzi S., Cheung H.H., Holcik M. Translational induction of the inhibitor of apoptosis protein HIAP2 during endoplasmic reticulum stress attenuates cell death and is mediated via an inducible internal ribosome entry site element. J. Biol. Chem. 2004;279:17148–17157. doi: 10.1074/jbc.M308737200. [DOI] [PubMed] [Google Scholar]
- 157.Van Eden M.E., Byrd M.P., Sherrill K.W., Lloyd R.E. Translation of cellular inhibitor of apoptosis protein 1 (c-IAP1) mRNA is IRES mediated and regulated during cell stress. RNA. 2004;10:469–481. doi: 10.1261/rna.5156804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Holcik M., Lefebvre C., Yeh C., Chow T., Korneluk R.G. A new internal-ribosome-entry-site motif potentiates XIAP-mediated cytoprotection. Nat. Cell Biol. 1999;1:190–192. doi: 10.1038/11109. [DOI] [PubMed] [Google Scholar]
- 159.Holcik M., Yeh C., Korneluk R.G., Chow T. Translational upregulation of X-linked inhibitor of apoptosis (XIAP) increases resistance to radiation induced cell death. Oncogene. 2000;19:4174–4177. doi: 10.1038/sj.onc.1203765. [DOI] [PubMed] [Google Scholar]
- 160.Fulda S., Vucic D. Targeting IAP proteins for therapeutic intervention in cancer. Nat. Rev. Drug. Discov. 2012;11:109–124. doi: 10.1038/nrd3627. [DOI] [PubMed] [Google Scholar]
- 161.Darding M., Feltham R., Tenev T., Bianchi K., Benetatos C., Silke J., Meier P. Molecular determinants of Smac mimetic induced degradation of cIAP1 and cIAP2. Cell Death Differ. 2011;18:1376–1386. doi: 10.1038/cdd.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]