Abstract
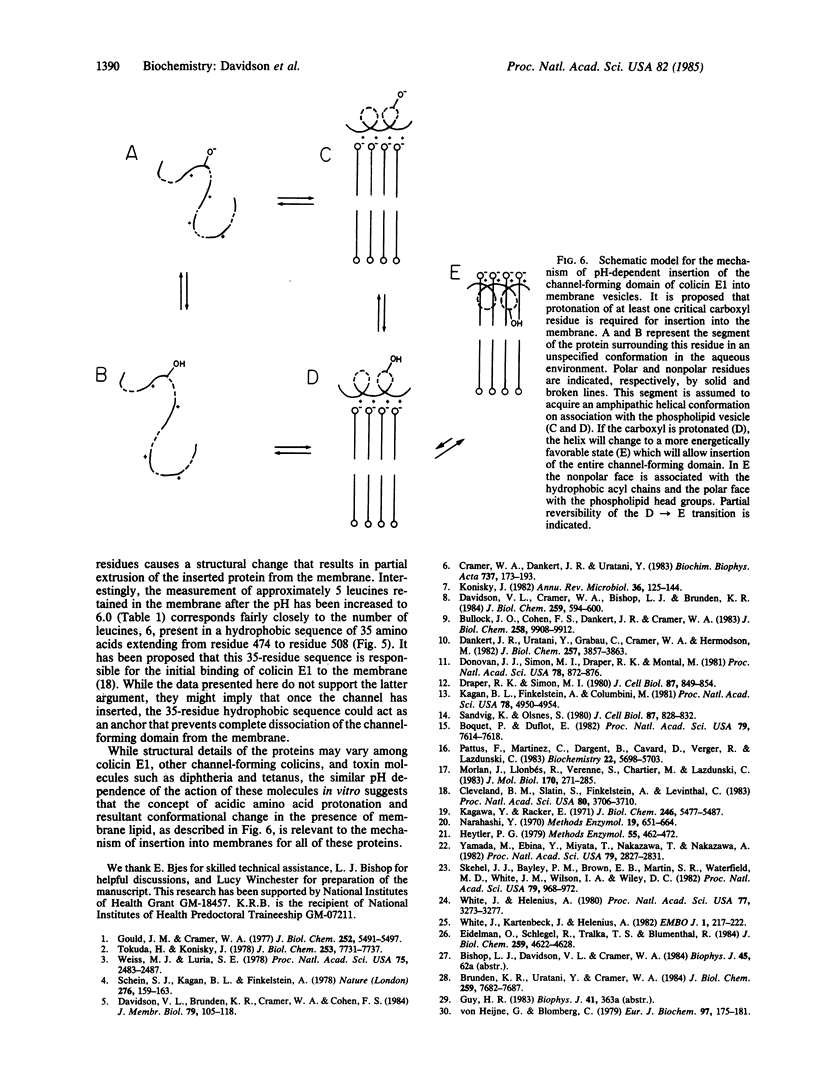
The channel-forming activity of colicin E1 in artificial membranes is known to increase at low pH values and to have a maximum near pH 4 in such membrane vesicles. The present work demonstrates that this pH dependence of activity can be attributed to membrane binding. Maximal binding of colicin E1 and a more slowly binding channel-forming carboxyl-terminal tryptic peptide occurred at acidic pH values, with the effective pK values for binding equal to 4.6 and less than 4.0, respectively. The binding did not require imposition of a transmembrane potential. Insertion of the tryptic peptide into the membrane was shown by retention of bound [3H]leucine-labeled peptide by vesicles after digestion with protease, as well as by retention of the peptide in salt-washed vesicles. The retention after protease treatment was also used to estimate the amount of carboxyl-terminal peptide inserted into the membrane. Approximately 12 of the 21 leucines present in the carboxyl-terminal peptide were retained after Pronase treatment at pH less than 4. Reversibility of the insertion at low pH values was seen after an alkaline shift of pH to 6.0, resulting in a decrease of the protease-inaccessible fraction of the bound protein. A model is presented describing a mechanism in which protonation of one or more carboxyl residues is necessary for effective binding and insertion into the membrane by the channel-forming domain of colicin E1. This model may also be relevant to the mechanism of membrane insertion by certain toxins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boquet P., Duflot E. Tetanus toxin fragment forms channels in lipid vesicles at low pH. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7614–7618. doi: 10.1073/pnas.79.24.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunden K. R., Uratani Y., Cramer W. A. Dependence of the conformation of a colicin E1 channel-forming peptide on acidic pH and solvent polarity. J Biol Chem. 1984 Jun 25;259(12):7682–7687. [PubMed] [Google Scholar]
- Bullock J. O., Cohen F. S., Dankert J. R., Cramer W. A. Comparison of the macroscopic and single channel conductance properties of colicin E1 and its COOH-terminal tryptic peptide. J Biol Chem. 1983 Aug 25;258(16):9908–9912. [PubMed] [Google Scholar]
- Cleveland M. V., Slatin S., Finkelstein A., Levinthal C. Structure-function relationships for a voltage-dependent ion channel: properties of COOH-terminal fragments of colicin E1. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3706–3710. doi: 10.1073/pnas.80.12.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer W. A., Dankert J. R., Uratani Y. The membrane channel-forming bacteriocidal protein, colicin El. Biochim Biophys Acta. 1983 Mar 21;737(1):173–193. doi: 10.1016/0304-4157(83)90016-3. [DOI] [PubMed] [Google Scholar]
- Dankert J. R., Uratani Y., Grabau C., Cramer W. A., Hermodson M. On a domain structure of colicin E1. A COOH-terminal peptide fragment active in membrane depolarization. J Biol Chem. 1982 Apr 10;257(7):3857–3863. [PubMed] [Google Scholar]
- Davidson V. L., Brunden K. R., Cramer W. A., Cohen F. S. Studies on the mechanism of action of channel-forming colicins using artificial membranes. J Membr Biol. 1984;79(2):105–118. doi: 10.1007/BF01872115. [DOI] [PubMed] [Google Scholar]
- Davidson V. L., Cramer W. A., Bishop L. J., Brunden K. R. Dependence of the activity of colicin E1 in artificial membrane vesicles on pH, membrane potential, and vesicle size. J Biol Chem. 1984 Jan 10;259(1):594–600. [PubMed] [Google Scholar]
- Draper R. K., Simon M. I. The entry of diphtheria toxin into the mammalian cell cytoplasm: evidence for lysosomal involvement. J Cell Biol. 1980 Dec;87(3 Pt 1):849–854. doi: 10.1083/jcb.87.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidelman O., Schlegel R., Tralka T. S., Blumenthal R. pH-dependent fusion induced by vesicular stomatitis virus glycoprotein reconstituted into phospholipid vesicles. J Biol Chem. 1984 Apr 10;259(7):4622–4628. [PubMed] [Google Scholar]
- Gould J. M., Cramer W. A. Studies on the depolarization of the Escherichia coli cell membrane by colicin E1. J Biol Chem. 1977 Aug 10;252(15):5491–5497. [PubMed] [Google Scholar]
- Heytler P. G. Uncouplers of oxidative phosphorylation. Methods Enzymol. 1979;55:462–442. doi: 10.1016/0076-6879(79)55060-5. [DOI] [PubMed] [Google Scholar]
- Kagan B. L., Finkelstein A., Colombini M. Diphtheria toxin fragment forms large pores in phospholipid bilayer membranes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4950–4954. doi: 10.1073/pnas.78.8.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konisky J. Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol. 1982;36:125–144. doi: 10.1146/annurev.mi.36.100182.001013. [DOI] [PubMed] [Google Scholar]
- Morlon J., Lloubès R., Varenne S., Chartier M., Lazdunski C. Complete nucleotide sequence of the structural gene for colicin A, a gene translated at non-uniform rate. J Mol Biol. 1983 Oct 25;170(2):271–285. doi: 10.1016/s0022-2836(83)80148-x. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin entry into cells is facilitated by low pH. J Cell Biol. 1980 Dec;87(3 Pt 1):828–832. doi: 10.1083/jcb.87.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein S. J., Kagan B. L., Finkelstein A. Colicin K acts by forming voltage-dependent channels in phospholipid bilayer membranes. Nature. 1978 Nov 9;276(5684):159–163. doi: 10.1038/276159a0. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Bayley P. M., Brown E. B., Martin S. R., Waterfield M. D., White J. M., Wilson I. A., Wiley D. C. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc Natl Acad Sci U S A. 1982 Feb;79(4):968–972. doi: 10.1073/pnas.79.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda H., Konisky J. In vitro depolarization of Escherichia coli membrane vesicles by colicin Ia. J Biol Chem. 1978 Nov 10;253(21):7731–7737. [PubMed] [Google Scholar]
- Weiss M. J., Luria S. E. Reduction of membrane potential, an immediate effect of colicin K. Proc Natl Acad Sci U S A. 1978 May;75(5):2483–2487. doi: 10.1073/pnas.75.5.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Helenius A. pH-dependent fusion between the Semliki Forest virus membrane and liposomes. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3273–3277. doi: 10.1073/pnas.77.6.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Kartenbeck J., Helenius A. Membrane fusion activity of influenza virus. EMBO J. 1982;1(2):217–222. doi: 10.1002/j.1460-2075.1982.tb01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Ebina Y., Miyata T., Nakazawa T., Nakazawa A. Nucleotide sequence of the structural gene for colicin E1 and predicted structure of the protein. Proc Natl Acad Sci U S A. 1982 May;79(9):2827–2831. doi: 10.1073/pnas.79.9.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G., Blomberg C. Trans-membrane translocation of proteins. The direct transfer model. Eur J Biochem. 1979 Jun;97(1):175–181. doi: 10.1111/j.1432-1033.1979.tb13100.x. [DOI] [PubMed] [Google Scholar]