Abstract
OBJECTIVES:
Fatty infiltration (FI) in the pancreas is positively correlated with high body mass index (BMI) or obesity, and the prevalence of diabetes mellitus (DM), which are well-known risk factors of pancreatic cancer. However, the association of FI in the pancreas with pancreatic cancer is unclear. Recently, we have shown that Syrian golden hamsters feature FI of the pancreas, the severity of which increases along with the progression of carcinogenesis induced by a chemical carcinogen. To translate the results to a clinical setting, we investigated whether FI in the pancreas is associated with pancreatic cancer in a series of patients who had undergone pancreatoduodenectomy.
METHODS:
In the series, we identified 102 cases with pancreatic ductal adenocarcinoma (PDAC) and 85 controls with cancers except for PDAC. The degree of FI was evaluated histopathologically from the area occupied by adipocytes in pancreas sections, and was compared between the cases and controls.
RESULTS:
The degree of FI in the pancreas was significantly higher in cases than in controls (median 26 vs. 15%, P<0.001) and positively associated with PDAC, even after adjustment for BMI, prevalence of DM and other confounding factors (odds ratio (OR), 6.1; P<0.001). BMI was identified as the most significantly associated factor with FI in the pancreas.
CONCLUSIONS:
There is a positive correlation between FI in the pancreas and pancreatic cancer.
INTRODUCTION
Pancreatic cancer is one of the most lethal human cancers with a 5-year survival rate of <5% in both Japan and the United States.1 Thus, the development of useful predictive markers for individuals with a high risk of pancreatic cancer would be of great help in detecting pancreatic cancer at its early stages, and might contribute to a significant reduction of mortality. Epidemiological studies have shown that a family history of pancreatic cancer, cigarette smoking, age, obesity, and diseases such as chronic pancreatitis and diabetes mellitus (DM) increase the risk of pancreatic cancer.2, 3, 4 A few pathologic studies of patients with pancreatic cancer have demonstrated fatty infiltraton (FI) in the pancreas parenchyma.5, 6 FI in the pancreas is positively correlated with age, body mass index (BMI), and a history of DM.7, 8, 9
Recently, we have shown that in Syrian golden hamsters, which exhibit a substantial age-related increase of hypertriglyceridemia and FI in the pancreas, there is further progression of pancreatic FI and carcinogenesis upon treatment with a carcinogen, N-nitrosobis (2-oxopropyl) amine (BOP), while the animals are fed a high-fat diet (HFD).10 Therefore, we hypothesized that FI in the pancreas accompanied by hypertriglyceridemia might be associated with pancreatic cancer in both humans and experimental animals.
In the present case–control study, we examined whether FI in the pancreas is associated with pancreatic ductal adenocarcinoma (PDAC) in humans, independently of several other suggested risk factors for pancreatic cancer, such as obesity and DM.
METHODS
Patients and samples
Between January 2004 and December 2010, 367 patients underwent pancreatoduodenectomy for PDAC at the National Cancer Center Hospital, Japan. Among them, 102 were considered to be appropriate for the present study on the basis of the criteria detailed later. As controls, we used non-cancerous pancreas tissues from 85 patients who had undergone pancreatoduodenectomy for cancer, except for PDAC; these included 46 patients with distal bile duct cancer, 33 with cancer of the ampulla of Vater, 4 with gallbladder cancer, and 2 with duodenal cancer. DM was clinically diagnosed at the referring hospitals, using criteria of fasting blood glucose level ≥126 mg/dl and HbA1c ≥6.1%, before the patients visited our hospital to resect pancreatic cancer. BMI was calculated when the patients were admitted to our hospital. The use of each individual's material for analysis in the present study was approved by the Ethics Review Committee of the National Cancer Center (2010-088). The materials are from patients who had given general consent for the research use of their leftover samples, and all clinical investigations were conducted in accordance with the principles of the Declaration of Helsinki.
Pathological examination
PDACs were examined pathologically and classified according to the World Health Organization classification and TNM classification.11, 12 Surgically resected specimens were fixed in 10% formalin, and the pancreas heads were cut horizontally into serial slices 5 mm thick. In order to evaluate FI appropriately, we conducted a preliminary study to select a target area of pancreas parenchyma in 16 cases of PDAC. As FI is easily affected by any type of pancreatitis associated with cancer infiltration, including obstructive pancreatitis, we selected the FI area for measurement, avoiding any primary and/or secondary effect caused by cancer infiltration (Supplementary Figure S1 online). Thus, pancreatitis patients were ruled out from the FI evaluation. First, we selected anterior and cranial areas of the pancreas near the duodenum that correspond to the dorsal pancreas during organogenesis. Second, we chose areas of the pancreas near the ampulla of Vater if the former area was affected by cancer infiltration. If both of these areas were affected by cancer infiltration, such cases were excluded from the study. Thus, we selected one section containing non-tumorous pancreatic tissue and confirmed whether it fulfilled the above conditions. Then, FI areas were measured quantitatively as the percentage of area infiltrated by adipocytes relative to the total area on the section was calculated using the WinROOF image analysis software package (Mitani Corp, Tokyo, Japan). The reproducibility of this quantitation method was checked preliminarily by comparing the FI area of one section with another section derived from tissue immediately adjacent to the former. The difference between the two FI area values measured in 16 pairs of sections was 5.6% on average.
Serum sample collection and assays
Peripheral blood was collected from each patient at the time of the hospital visit prior to treatment, and blood sugar, HbA1c, and serum levels of total cholesterol (TC), high-density lipoprotein (HDL), amylase, CEA, and CA19-9 were measured by participants at the National Cancer Center Hospital. For further examination, serum provided by the National Cancer Center Biobank, Japan, was stored at −20°C. Serum adiponectin, leptin and insulin growth factor-I (IGF-I) (R&D Systems, Inc., Minneapolis, MN, USA), apolipoprotein A-II (apoA-II) (Assay pro, St Charles, MO, USA), insulin (Millipore, Billerica, MA, USA), and serum amyloid A (SAA; Invitrogen, Camarillo, CA, USA) were measured using enzyme-linked immunosorbent assay kits in accordance with the manufacturers' instructions. The levels of serum triglycerides (TGs), HDL, and gamma-glutamyltransferase (GGT) were analyzed using the FUJI Dri-Chem system (Fuji Film, Tokyo, Japan).
Statistical analysis
The cases and controls were classified into three subgroups, <10%, 10–20%, and ≥20%, according to the area of FI. The cutoff points of 10 and 20 were nearly equal to the tertile cutoff points in the controls, namely 9.5 and 20.4. An unconditional logistic regression model was used to estimate odds ratios (ORs) and their 95% confidence intervals (CIs) of PDAC according to the three categories of FI in the pancreas, the lowest value being used as a reference. Two-sided P values <0.05 were considered to indicate statistical significance. All statistical analyses were carried out using the Statistical Analysis System (SAS), version 9.1 software package (SAS Institute, Cary, NC, USA) by a statistician (T.Y.).
RESULTS
Patient characteristics
Among 102 cases, one was classified as stage IB, 27 as stage IIA, 65 as stage IIB, and 9 as stage IV. The characteristics of the case and control patients are summarized in Table 1. Controls were older than cases (P=0.001), and there was a male predominance in both groups. The known risk factors for PDAC were compared between cases and controls. The prevalence of DM (P=0.03) and family history of pancreatic cancer (P=0.007) in cases was higher than in controls. The values of blood sugar (P=0.002) and HbA1c (P<0.001) in cases were also significantly higher than in the controls. The serum apoA-II level was shown to be lower in cases than in controls (P=0.02), as reported previously, in comparison with healthy subjects. CEA (P=0.04) and CA19-9 (P<0.001), serum tumor markers for PDAC, were also significantly higher in cases than in controls. Meanwhile, serum levels of GGT (P<0.001), which are associated with liver and biliary disorders, were higher in controls than in cases.
Table 1. Selected characteristics of study subjects.
| Characteristic | Cases (n=102) | Controls (n=85) | Pa |
|---|---|---|---|
| Categorical variables, n (%) | |||
| FI in the pancreas≥20% | 64 (62.7) | 30 (35.2) | <0.001 |
| Male | 60 (58.8) | 60 (70.5) | 0.12 |
| Ever smoking | 53 (51.9) | 42 (49.4) | 0.77 |
| Frequent drinking (5–7 times/week) | 34 (33.3) | 31 (36.9) | 0.85 |
| DM | 30 (29.4) | 14 (16.4) | 0.03 |
| Hypertension | 36 (35.2) | 27 (31.7) | 0.64 |
| Hyperlipidemia | 7 (6.8) | 10 (11.7) | 0.30 |
| Family history of PC | 11 (10.7) | 1 (1.1) | 0.007 |
| Continuous variables, median (IQR) | |||
| FI in the pancreas, % | 25.8 (14.2–40.9) | 15.0 (7.7–24.8) | <0.001 |
| Age, years | 63.5 (56–69) | 68.0 (63–73) | 0.001 |
| BMI, kg/m2 | 22.4 (20.3–24.3) | 22.7 (20.7–24.2) | 0.95 |
| Blood sugar, mg/dl | 114.0 (100–141) | 106.0 (93–119) | 0.002 |
| HbA1c, % | 5.5 (5.1–6.4) | 5.1 (4.7–5.5) | <0.001 |
| TC, mg/dl | 189.0 (162–221) | 195.0 (169–227) | 0.31 |
| HDL, mg/dl | 52.0 (43–62) | 52.0 (42–67) | 0.47 |
| TG, mg/dl | 149.0 (109–209) | 155.0 (117–210) | 0.38 |
| Apo A-II, μg/ml | 219.3 (136.7–397.5) | 327.3 (174.0–444.4) | 0.02 |
| Adiponectin, μg/ml | 5.4 (3.0–9.6) | 6.3 (3.2–12.3) | 0.37 |
| Leptin, ng/ml | 3.2 (2.2–4.6) | 3.1 (2.4–3.8) | 0.57 |
| Insulin, mU/l | 3.5 (2.5–5.8) | 3.7 (2.8–6.4) | 0.41 |
| IGF-I, ng/ml | 69.7 (53.2–93.5) | 74.1 (54.4–96.4) | 0.69 |
| Amylase, IU/l | 107.0 (75–182) | 105.0 (83–141) | 0.55 |
| CEA, ng/ml | 2.6 (1.6–4.1) | 2.0 (1.3–3.4) | 0.04 |
| CA19-9, U/ml | 96.0 (46–400) | 30.0 (16–121) | <0.001 |
| SAA, μg/ml | 22.1 (8.93–54.3) | 35.8 (12.7–89.8) | 0.06 |
| GGT, ng/ml | 105.0 (33–311) | 339.0 (101–673) | <0.001 |
Apo A-II, apolipoprotein A-II; BMI, body mass index; DM, diabetes mellitus; FI, fatty infiltration; GGT, gamma-glutamyltransferase; HDL, high density lipoprotein; IGF-I, insulin growth factor-I; IQR, interquartile range; PC, pancreatic cancer; SAA, serum amyloid A; TC, total cholesterol; TG, triglyceride.
Based on the Fisher's exact test for percentage difference and the Wilcoxon rank-sum test for median difference.
Association of FI in the pancreas with PDAC
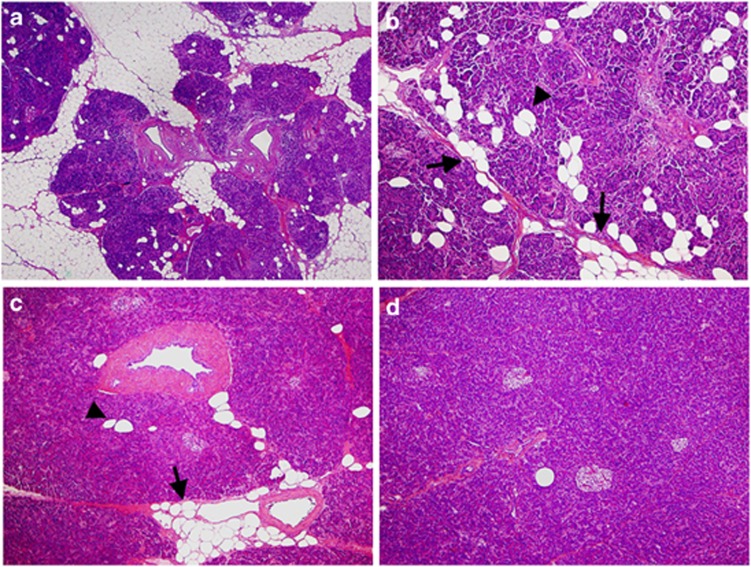
In the human pancreas, adipocytes were observed to accumulate in the area between pancreatic lobules (interlobular fat), especially around great vessels, or to be scattered in the lobules (intralobular fat), as shown in Figure 1. The distribution pattern of FI in some patients was similar to that observed in hamster pancreas.10 In this study, FI in the pancreas was defined as the sum of the areas showing any types of FI in the pancreas parenchyma. Table 1 shows that the area of FI in the pancreas was significantly greater in cases than in controls (median 26 vs. 15%, P<0.001). Types of differentiation and stages of PDACs were not associated with the degree of FI (data not shown).
Figure 1.
Histology of the human pancreas with fatty infiltration. (a, b) Pancreas tissue with moderate to severe FI. Most of the pancreas parenchyma has been replaced by adipocytes, and the remaining pancreas lobules resemble islets surrounded by a fatty lake. Most adipocytes have accumulated interlobularly (arrow in b), but some are scattered within the lobules (arrowhead in b). (c) Pancreas tissue with mild FI. Adipocytes have accumulated around arterioles (arrow), and several adipocytes are scattered within the lobules (arrowhead). (d) Pancreas tissue with minimal FI. Super-low magnification in a, and low magnification in (b to d).
Table 2 shows the association between the area of FI in the pancreas and PDAC. A significantly higher OR for PDAC was observed according to the area of FI in the pancreas (P<0.001). Adjusted for sex, age, BMI, history of DM, and family history of pancreatic cancer, confounding factors for pancreatic cancer, ORs for PDAC showed an increasing trend according to the area of FI (P<0.001). Even when patients with a BMI >25 kg/m2, a history of DM, and a family history of pancreatic cancer were excluded, positive associations between the degree of FI in the pancreas and PDAC were observed (P<0.001 overall).
Table 2. Association of the degree of FI in the pancreas with pancreatic ductal adenocarcinoma.
|
FI in the pancreas |
|||||||
|---|---|---|---|---|---|---|---|
|
<10% |
≥10%, <20% |
≥20% |
|||||
| Population | OR | 95% CI | OR | 95% CI | OR | 95% CI | Pa |
| All subjects | |||||||
| Cases/controls | 17/30 | 21/25 | 64/30 | ||||
| Crude estimate | 1 | Reference | 1.4 | (0.6–3.4) | 3.7 | (1.8–7.8) | <0.001 |
| Adjusted estimateb | 1 | Reference | 2.3 | (0.8–6.2) | 6.1 | (2.4–15.2) | <0.001 |
| Excluding those with BMI of ≥25 kg/m2 | |||||||
| Cases/controls | 17/28 | 18/22 | 49/24 | ||||
| Adjusted estimatec | 1 | Reference | 2.1 | (0.7–5.9) | 6.3 | (2.4–16.5) | <0.001 |
| Excluding those with past history of DM | |||||||
| Cases/controls | 14/28 | 18/21 | 40/22 | ||||
| Adjusted estimated | 1 | Reference | 3.1 | (1.0–9.3) | 7.5 | (2.6–21.3) | <0.001 |
| Excluding those with family history of PC | |||||||
| Cases/controls | 15/29 | 17/25 | 59/30 | ||||
| Adjusted estimatee | 1 | Reference | 2.0 | (0.7–5.5) | 5.4 | (2.2–13.6) | <0.001 |
BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; FI, fatty infiltration; OR, odds ratio; PC, pancreatic cancer.
Statistical tests for trend (two-sided) were assessed by assigning ordinal values to the degree of FI in the pancreas.
Adjusted for sex, age (≤60, 61–70 and >70), BMI (<25, ≥25), past history of DM (yes or no) and family history of PC (yes or no).
Adjusted for sex, age (≤60, 61–70 and >70), past history of DM (yes or no), and family history of PC (yes or no).
Adjusted for sex, age (≤60, 61–70 and >70), BMI (<25, ≥25), and family history of PC (yes or no).
Adjusted for sex, age (≤60, 61–70 and >70), BMI (<25, ≥25), and past history of DM (yes or no).
The factors associated with FI
The characteristics of the study participants were examined in relation to the degree of FI of the pancreas in controls and cases and are shown in Supplementary Tables 1 and 2, respectively. BMI and age were positively correlated with the area of FI of the pancreas in both cases and controls. In control patients, the serum TG and amylase values were also positively correlated with the area of FI in the pancreas. Meanwhile, the levels of the serum insulin, HbA1c and blood sugar in case patients were positively correlated with the area of FI in the pancreas. To further investigate an association with FI in the pancreas, we conducted a multivariable linear regression analysis in each group, in which the above variables (BMI, serum TG, and amylase for controls; BMI, serum insulin, HbA1c, and blood sugar for cases), as well as age and sex, were included in one model. After mutual adjustment, a statistically significant association was noted only for BMI (controls, P=0.001; cases, P=0.01).
DISCUSSION
Based on epidemiological observation of human pancreatic cancers, FI in the pancreas was suggested to associate with PDAC, independently of known risk factors such as obesity and DM (Supplementary Figure S2). Although we identified BMI, a measurement of obesity, as the most significantly associated factor among several factors related to FI in the pancreas, FI in the pancreas was likely to increase the risk of pancreatic cancer beyond the effect of obesity alone. Some previous studies have evaluated pancreatic FI in humans using diagnostic modalities such as ultrasound, magnetic resonance imaging, or magnetic resonance spectroscopy.9, 13, 14, 15, 16 FI in the pancreas has been suggested to promote dissemination and lethality of PDAC and to increase the risk of postoperative pancreatic fistula.17, 18 Here we demonstrated that the area of FI in histopathological sections of PDAC resected can be used as a quantitative indicator of the degree of FI. This is the first report to indicate an association between the area of FI and the development of PDAC.
Although mechanistic insights into how PDAC could develop from such an adipocyte-rich microenvironment are not clear, recent evidence suggests that ectopic fat accumulation produces certain adipocytokines that induce cell proliferation.19, 20 Serum adipocytokine levels were not clearly correlated with the area of FI in the present study, but the level of leptin expression was high in the pancreas of BOP-treated hamsters fed a HFD.10 Thus, local release of adipocytokines from adipocytes in an adipocyte-rich microenvironment appeared to be correlated with PDAC development.
In the present study, serum insulin levels in cases were positively correlated with FI in the pancreas. It has also been reported that HOMA-IR is strongly correlated with FI of the pancreas except in subjects with a history of DM, pancreatic diseases and liver diseases.9 In an in vitro setting, it has been shown that glucose-dependent insulinotropic polypeptide activates lipoprotein lipase, leading to TG accumulation in differentiated 3T3-L1 adipocytes in the presence of insulin.21 Therefore, it is conceivable that induction of high glucose and insulin levels by hyperphagia could be associated with FI through activation of lipoprotein lipase in the pancreas. Conversely, it has also been suggested that increased pancreatic FI is related to β-cell dysfunction in the absence of type 2 DM,22 and that this can lead to subsequent development of type 2 DM.23, 24 The hyperinsulinemia seen in human obesity, including the early phase of type 2 DM, may be closely related to FI in the pancreas.
Several possible mechanisms underlying the development of FI in the pancreas can be speculated. It has been shown in experimental animal models that FI can be induced in the pancreas by obstruction of the pancreatic duct or vasculature.25, 26 Smits and van Geenen27 have showed that FI or non-alcoholic fatty pancreas disease represents fat accumulation induced by obesity and metabolic syndrome, while fatty replacement represents replacement of adipocytes induced by death of acinar cells. We agree with their statements that pancreatic fat accumulation is mainly induced by these two factors. In this study, pancreatic FI in cases represents any type of fat accumulation caused by any type of etiology. It has been reported that lipotoxicity caused by a high TG content induces inflammatory responses and necrosis in pancreatic acinar cells in vitro.28, 29 It has also been shown that c-Myc activity is required for growth and maturation of the exocrine pancreas and for the transdifferentiation of acinar cells into adipocytes in mice.30 Thus, pancreas containing scattered adipocytes might be more sensitive to acinar cell damage due to lipotoxity and other genetic factors, and scattered FI may reflect the acinar cell death or transdifferentiation after the damage.
Some limitations could be pointed out in this study. The major limitation is that it lacked normal healthy controls because pancreatic sections could be obtained only from patients who had undergone pancreatoduodenectomy. A second limitation is that we could not measure FI in more than one pancreatic section, as areas for measuring FI were limited and small because the areas of tumor and secondary inflammation were avoided. Therefore, a future study using a non-invasive method will be required to evaluate FI in a large area/volume of pancreas from healthy and case subjects. Previously, we have reported a case of PDAC that was associated with marked FI in the pancreas, as seen on computed tomography images.31 Computed tomography imaging of the pancreas would be a useful approach for accurate evaluation and follow-up of pancreatic FI in normal subjects, as well as in cohort studies. The third limitation is that we did not exclude the areas of pancreas with PanINs from the sections for measuring FI because it is known that PanINs are sometimes found in pancreatic tissue of the elderly, and also that a large number of PanINs with various grades are found in the pancreas of the patients with PDAC. Therefore, it is extremely difficult to measure FI in the pancreas tissue without PanINs, especially in the limited area for measuring FI. The fourth limitation is that BMI could be underestimated in the cases, because weight loss is a very common symptom of patients suffering from pancreatic cancer even though most cases were classified as stage IIA or IIB. The fifth limitation is that there is no validation study. To confirm the observation in the present study, the same study should be repeated with the same methods in another center (hospital/institution). The final limitation is that we cannot distinguish whether FI was a risk factor or a consequence of the cancer. The only way to demonstrate that FI is a risk factor for PDAC is to perform a prospective cohort study to observe whether individuals with fatty pancreas could develop PDAC. For this purpose, we are now trying to establish the methods to evaluate FI in a large area/volume of pancreas by non-invasive method, using computed tomography and magnetic resonance imaging. In addition, studies on pancreatic carcinogenesis using animal models of fatty pancreas would be helpful to elucidate underlying mechanisms.
In conclusion, there is a positive correlation between FI in the pancreas and pancreatic cancer. The development of effective detection methods and/or markers of FI, especially “fatty pancreas” with severe FI, is warranted for mass screening of individuals at high risk of pancreatic cancer at health examinations.
Study Highlights

Acknowledgments
The National Cancer Center Biobank is supported by the National Cancer Center Research and Development Fund, Japan.
Guarantor of the article: Hitoshi Nakagama, MD, DMSc.
Specific author contributions: Mika Hori contributed to the design of the study, acquisition, analysis and interpretation of data, writing and drafting of the manuscript; Mami Takahashi contributed to the conception of the study, development of methodology and data analysis and revision of the manuscript; Nobuyoshi Hiraoka contributed to the histopathological analysis and revision of the manuscript; Taiki Yamaji contributed to the statistical analysis and revision of the manuscript; Michihiro Mutoh contributed to data analysis and revision of the manuscript; Rikako Ishigamori contributed to the histopathological analysis; Koh Furuta contributed to material supports in human serum analysis; Takuji Okusaka contributed to the clinical revision of the manuscript; Kazuaki Shimada contributed to the clinical revision of the manuscript; Tomoo Kosuge contributed to the clinical revision of the manuscript; Yae Kanai contributed to the histopathological analysis; Hitoshi Nakagama contributed to study supervision and revision of the manuscript.
Financial support: This work was supported by: Grants-in-Aid for Cancer Research from the Ministry of Health, Labour, and Welfare of Japan and Management Expenses Grants from the Government to the National Cancer Center (21–2–1, 23-A-4); a grant of the Third-Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health, Labor, and Welfare of Japan; a grant of the Research Grant of the Princess Takamatsu Cancer Research Fund; Grants-in-Aid from the Foundation for Promotion of Cancer Research and the Pancreas Research Foundation of Japan. M. Hori was an Awardee of Research Resident Fellowships from the Foundation for Promotion of Cancer Research (Japan) and from the Third-Term Comprehensive 10-Year Strategy for Cancer Control during the course of the present research.
Potential competing interests: None.
Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Supplementary Material
References
- Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AV, Rodriguez C, Bernstein L, et al. Obesity, recreational physical activity, and risk of pancreatic cancer in a large U.S. Cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:459–466. doi: 10.1158/1055-9965.EPI-04-0583. [DOI] [PubMed] [Google Scholar]
- Huxley R, Ansary-Moghaddam A, Berrington de González A, et al. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301:2553–2562. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama N, Kamiyama H, Suminaga Y, et al. Pancreas head carcinoma with total fat replacement of the dorsal exocrine pancreas. J Gastroenterol. 2004;39:76–80. doi: 10.1007/s00535-003-1250-4. [DOI] [PubMed] [Google Scholar]
- Makay O, Kazimi M, Aydin U, et al. Fat replacement of the malignant pancreatic tissue after neoadjuvant therapy. Int J Clin Oncol. 2010;15:88–92. doi: 10.1007/s10147-009-0001-9. [DOI] [PubMed] [Google Scholar]
- Walters MN. Adipose atrophy of the exocrine pancreas. J Pathol Bacteriol. 1966;92:547–557. doi: 10.1002/path.1700920232. [DOI] [PubMed] [Google Scholar]
- Rosso E, Casnedi S, Pessaux P, et al. The role of "fatty pancreas" and of BMI in the occurrence of pancreatic fistula after pancreaticoduodenectomy. J Gastrointest Surg. 2009;13:1845–1851. doi: 10.1007/s11605-009-0974-8. [DOI] [PubMed] [Google Scholar]
- Lee JS, Kim SH, Jun DW, et al. Clinical implications of fatty pancreas: correlations between fatty pancreas and metabolic syndrome. World J Gastroenterol. 2009;15:1869–1875. doi: 10.3748/wjg.15.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori M, Kitahashi T, Imai T, et al. Enhancement of carcinogenesis and fatty infiltration in the pancreas in N-nitrosobis(2-oxopropyl) amine-treated hamsters by high fat diet. Pancreas. 2011;40:1234–1240. doi: 10.1097/MPA.0b013e318220e742. [DOI] [PubMed] [Google Scholar]
- Hruban RH, Boffetta P, Hiraoka N, et al. Ductal adenocarcinoma of the pancreasIn: Bosman FT, Carneiro F, Hruban RH, Theise ND (eds).WHO Classification of Tumours of the Digestive System4th edn. World Health Organization Classification of TumoursIARC: Lyon, France; 2010281–291. [Google Scholar]
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. Willey-Blackewell: Oxford, UK; 2009. [Google Scholar]
- Kovanlikaya A, Mittelman SD, Ward A, et al. Obesity and fat quantification in lean tissues using three-point Dixon MR imaging. Pediatr Radiol. 2005;35:601–607. doi: 10.1007/s00247-005-1413-y. [DOI] [PubMed] [Google Scholar]
- Schwenzer NF, Machann J, Martirosian P, et al. Quantification of pancreatic lipomatosis and liver steatosis by MRI: comparison of in/opposed-phase and spectral-spatial excitation techniques. Invest Radiol. 2008;43:330–337. doi: 10.1097/RLI.0b013e31816a88c6. [DOI] [PubMed] [Google Scholar]
- Lingvay I, Esser V, Legendre JL, et al. Noninvasive quantification of pancreatic fat in humans. J Clin Endocrinol Metab. 2009;94:4070–4076. doi: 10.1210/jc.2009-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Jang JY, Lim CS, et al. Measurement of pancreatic fat by magnetic resonance imaging: predicting the occurrence of pancreatic fistula after pancreatoduodenectomy. Ann Surg. 2010;251:932–936. doi: 10.1097/SLA.0b013e3181d65483. [DOI] [PubMed] [Google Scholar]
- Mathur A, Zyromski NJ, Pitt HA, et al. Pancreatic steatosis promotes dissemination and lethality of pancreatic cancer. J Am Coll Surg. 2009;208:989–994. doi: 10.1016/j.jamcollsurg.2008.12.026. [DOI] [PubMed] [Google Scholar]
- Mathur A, Pitt HA, Marine M, et al. Fatty pancreas: a factor in postoperative pancreatic fistula. Ann Surg. 2007;246:1058–1064. doi: 10.1097/SLA.0b013e31814a6906. [DOI] [PubMed] [Google Scholar]
- Okuya S, Tanabe K, Tanizawa Y, et al. Leptin increases the viability of isolated rat pancreatic islets by suppressing apoptosis. Endocrinology. 2001;142:4827–4830. doi: 10.1210/endo.142.11.8494. [DOI] [PubMed] [Google Scholar]
- Hardwick JC, Van Den Brink GR, Offerhaus GJ, et al. Leptin is a growth factor for colonic epithelial cells. Gastroenterology. 2001;121:79–90. doi: 10.1053/gast.2001.25490. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Nian C, Mclntosh CH. Activation of lipoprotein lipase by glucose-dependent insulinotropic polypeptide in adipocytes. A role for a protein kinase B, LKB1, and AMP-activated protein kinase cascade. J Biol Chem. 2007;282:8557–8567. doi: 10.1074/jbc.M609088200. [DOI] [PubMed] [Google Scholar]
- Tushuizen ME, Bunck MC, Pouwels PJ, et al. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care. 2007;30:2916–2921. doi: 10.2337/dc07-0326. [DOI] [PubMed] [Google Scholar]
- Van Herpen NA, Schrauwen-Hinderling VB. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol Behav. 2008;94:231–241. doi: 10.1016/j.physbeh.2007.11.049. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Uchida T, Tsuchiya R, Harada N, et al. Ischemic changes in the pancreas of Watanabe heritable hyper-lipidemic (WHHL) rabbits. Int J Pancreatol. 1988;3:261–272. doi: 10.1007/BF02788455. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Abe K, Anbo Y, et al. Changes in the mouse exocrine pancreas after pancreatic duct ligation: a qualitative and quantitative histological study. Arch Histol Cytol. 1995;58:365–374. doi: 10.1679/aohc.58.365. [DOI] [PubMed] [Google Scholar]
- Smits MM, van Geenen EJ. The clinical significance of pancreatic steatosis. Nat Rev Gastroenterol Hepatol. 2011;8:169–177. doi: 10.1038/nrgastro.2011.4. [DOI] [PubMed] [Google Scholar]
- Navina S, Acharya C, DeLany JP, et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med. 2011;3:107–110. doi: 10.1126/scitranslmed.3002573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnick KE, Collins SC, Londos C, et al. Pancreatic ectopic fat is characterized by adipocyte infiltration and altered lipid composition. Obesity. 2008;16:522–530. doi: 10.1038/oby.2007.110. [DOI] [PubMed] [Google Scholar]
- Bonal C, Thorel F, Ait-Lounis A, et al. Pancreatic inactivation of c-Myc decreases acinar mass and transdifferentiates acinar cells into adipocytes in mice. Gastroenterology. 2009;136:309–319. doi: 10.1053/j.gastro.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Hori M, Onaya H, Takahashi M, et al. Invasive ductal carcinoma developing in pancreas with severe fatty infiltration. Pancreas. 2012;41:1137–1139. doi: 10.1097/MPA.0b013e318252ea08. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.