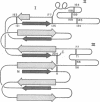
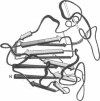
Abstract
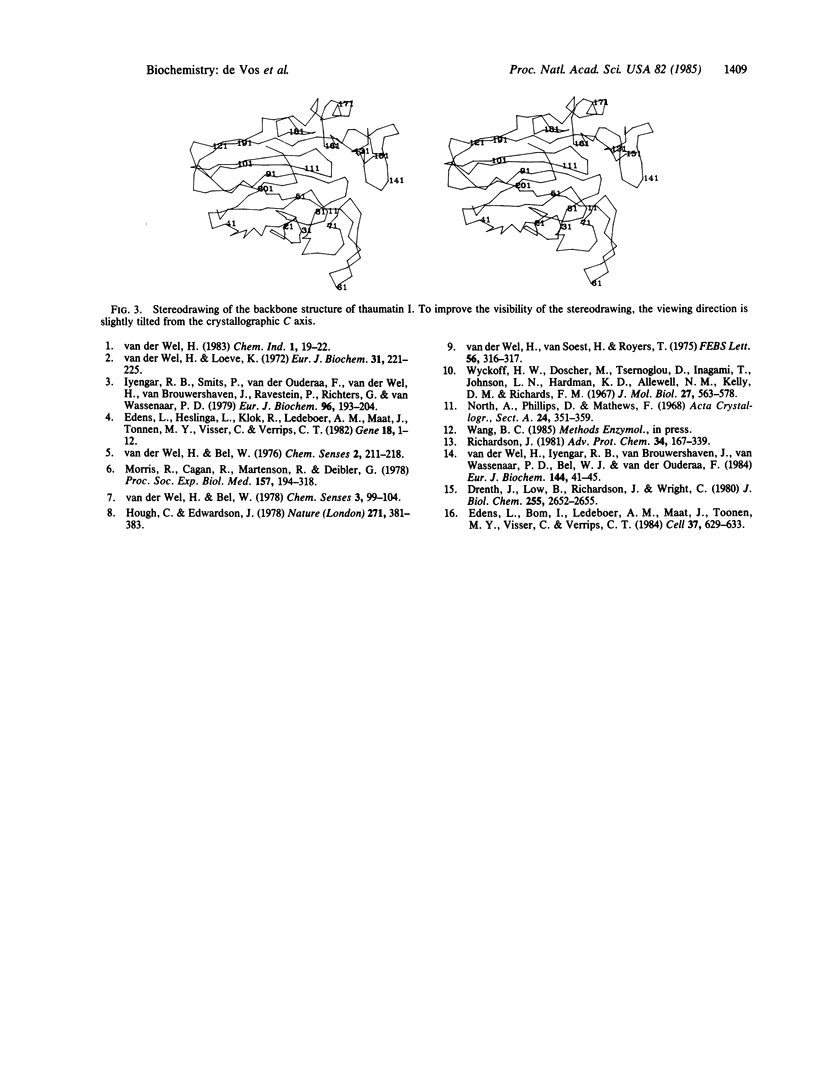
Thaumatin and monellin are the two sweetest compounds known to man--about 100,000 times sweeter than sugar on a molar basis and 3000 times on a weight basis. These proteins represent a unique class of proteins that are taste-active. We report the three-dimensional structure of thaumatin I at 3.1 A resolution.
Full text
PDF
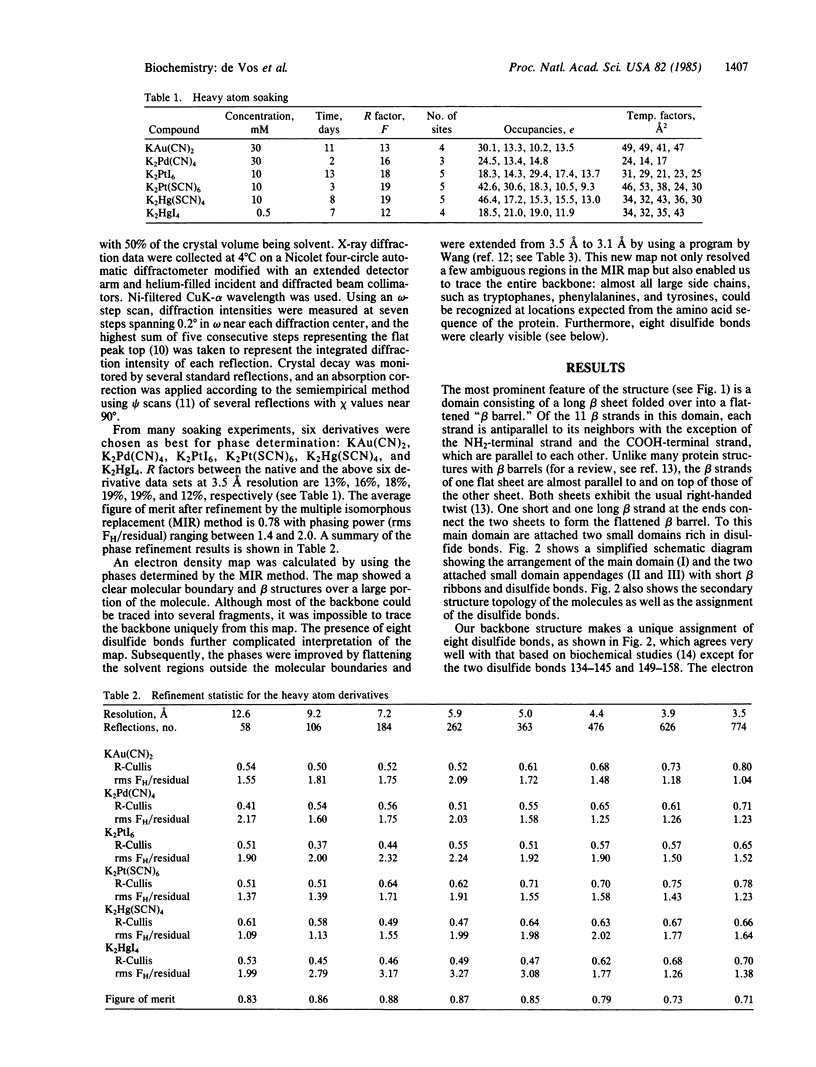
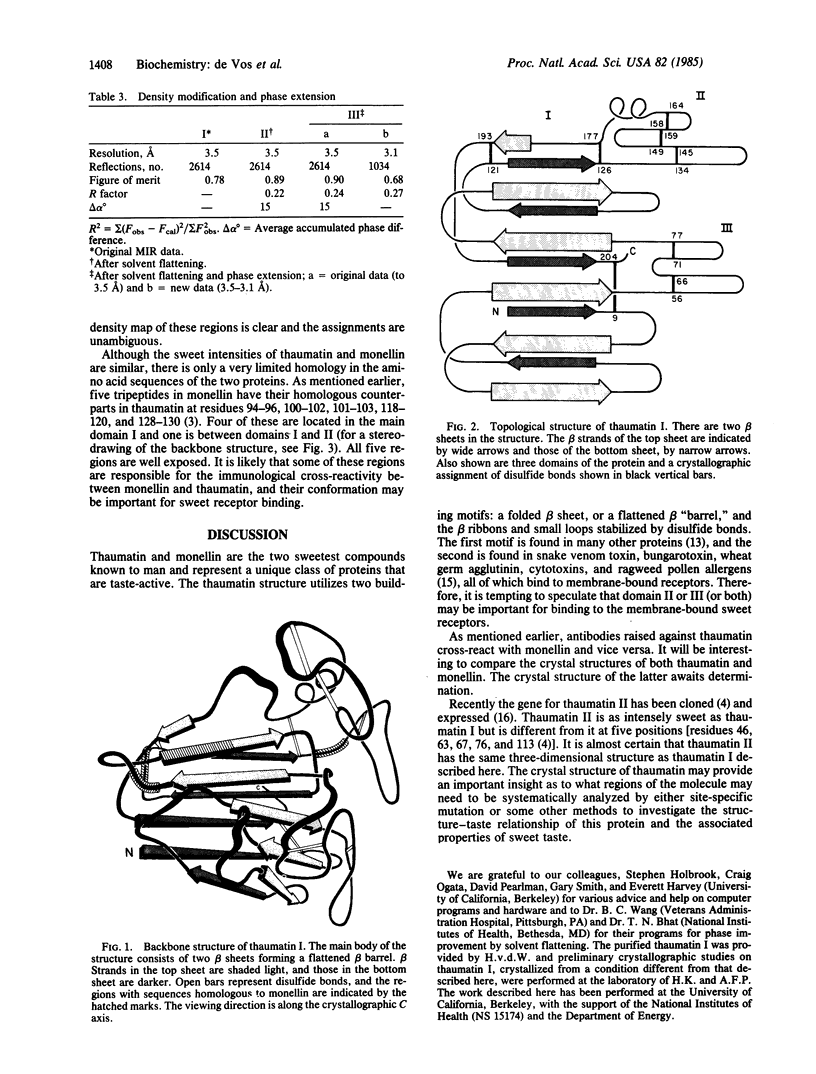
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Drenth J., Low B. W., Richardson J. S., Wright C. S. The toxin-agglutinin fold. A new group of small protein structures organized around a four-disulfide core. J Biol Chem. 1980 Apr 10;255(7):2652–2655. [PubMed] [Google Scholar]
- Edens L., Bom I., Ledeboer A. M., Maat J., Toonen M. Y., Visser C., Verrips C. T. Synthesis and processing of the plant protein thaumatin in yeast. Cell. 1984 Jun;37(2):629–633. doi: 10.1016/0092-8674(84)90394-5. [DOI] [PubMed] [Google Scholar]
- Edens L., Heslinga L., Klok R., Ledeboer A. M., Maat J., Toonen M. Y., Visser C., Verrips C. T. Cloning of cDNA encoding the sweet-tasting plant protein thaumatin and its expression in Escherichia coli. Gene. 1982 Apr;18(1):1–12. doi: 10.1016/0378-1119(82)90050-6. [DOI] [PubMed] [Google Scholar]
- Hough C. A., Edwardson J. A. Antibodies to thaumatin as a model of the sweet taste receptor. Nature. 1978 Jan 26;271(5643):381–383. doi: 10.1038/271381a0. [DOI] [PubMed] [Google Scholar]
- Iyengar R. B., Smits P., van der Ouderaa F., van der Wel H., van Brouwershaven J., Ravestein P., Richters G., van Wassenaar P. D. The complete amino-acid sequence of the sweet protein thaumatin I. Eur J Biochem. 1979 May 2;96(1):193–204. doi: 10.1111/j.1432-1033.1979.tb13029.x. [DOI] [PubMed] [Google Scholar]
- Morris R. W., Cagan R. H., Martenson R. E., Deibler G. Methylation of the lysine residues of monellin. Proc Soc Exp Biol Med. 1978 Feb;157(2):194–199. doi: 10.3181/00379727-157-40019. [DOI] [PubMed] [Google Scholar]
- Richardson J. S. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Wyckoff H. W., Doscher M., Tsernoglou D., Inagami T., Johnson L. N., Hardman K. D., Allewell N. M., Kelly D. M., Richards F. M. Design of a diffractometer and flow cell system for X-ray analysis of crystalline proteins with applications to the crystal chemistry of ribonuclease-S. J Mol Biol. 1967 Aug 14;27(3):563–578. doi: 10.1016/0022-2836(67)90059-9. [DOI] [PubMed] [Google Scholar]
- van der Wel H., Iyengar R. B., van Brouwershaven J., van Wassenaar P. D., Bel W. J., van der Ouderaa F. J. Assignment of the disulphide bonds in the sweet-tasting protein thaumatin I. Eur J Biochem. 1984 Oct 1;144(1):41–45. doi: 10.1111/j.1432-1033.1984.tb08428.x. [DOI] [PubMed] [Google Scholar]
- van der Wel H., Loeve K. Isolation and characterization of thaumatin I and II, the sweet-tasting proteins from Thaumatococcus daniellii Benth. Eur J Biochem. 1972 Dec 4;31(2):221–225. doi: 10.1111/j.1432-1033.1972.tb02522.x. [DOI] [PubMed] [Google Scholar]
- van der Wel H., van Soest T. C., Royers E. C. Crystallization and crystal data of thaumatin I, a sweet-tasting protein from Thaumatococcus daniellii benth. FEBS Lett. 1975 Aug 15;56(2):316–317. doi: 10.1016/0014-5793(75)81117-3. [DOI] [PubMed] [Google Scholar]