Abstract
We report here a novel strategy to redirect oncolytic adenoviruses to CD123 by carry a soluble coxsackie-adenovirus receptor (sCAR)-IL3 expression cassette in the viral genome to form Ad.IL3, which sustainably infected acute myeloid leukemia (AML) cells through CD123. Ad.IL3 was further engineered to harbor gene encoding manganese superoxide dismutase (MnSOD) or mannose-binding plant lectin Pinellia pedatisecta agglutinin (PPA), forming Ad.IL3-MnSOD and Ad.IL3-PPA. As compared with Ad.IL3 or Ad.sp-E1A control, Ad.IL3-MnSOD and Ad.IL3-PPA significantly suppressed in vitro proliferation of HL60 and KG-1 cells. Elevated apoptosis was detected in HL60 and KG-1 cells treated with either Ad.IL3-MnSOD or Ad.IL3-PPA. The caspase-9–caspase-7 pathway was determined to be activated by Ad.IL3-MnSOD as well as by Ad.IL3-PPA in HL60 cells. In an HL60/Luc xenograft nonobese diabetic/severe-combined immunodeficiency mice model, Ad.IL3-MnSOD and Ad.IL3-PPA suppressed cancer cell growth as compared with Ad.IL3. A significant difference of cancer cell burden was detected between Ad.IL3 and Ad.IL3-PPA groups at day 9 after treatment. Furthermore, Ad.IL3-MnSOD significantly prolonged mouse survival as compared with Ad.sp-E1A. These findings demonstrated that Ad.IL3-gene could serve as a novel agent for AML therapy. Harboring sCAR-ligand expression cassette in the viral genome may provide a universal method to redirect oncolytic adenoviruses to various membrane receptors on cancer cells resisting serotype 5 adenovirus infection.
Introduction
Acute myeloid leukemia (AML) is a cancer of myeloid lineage of cells marked by accumulation of immature, abnormal hematopoietic cells. Poor prognosis, chemoresistance and relapse are frequent in AML patients.1 Because conventional chemotherapies were frequently acutely toxic, nonselective and resulting in resistance, alternative therapeutic approaches with specific targeting capacities are needed to complement currently used chemotherapy protocols. Oncolytic adenoviruses, or conditionally replicating adenoviruses, represent a promising strategy for cancer therapy because of their lytic replication, efficient gene transfer and low pathogenicity.2, 3, 4, 5 The selectivity of oncolytic adenoviruses was achieved by controlling the expression of genes involved in viral replication with tumor-specific promoters,6, 7, 8 or deletion of viral genes encoding proteins that help to complete the viral lytic cycle in normal cells.9, 10 Oncolytic adenoviruses armed with anticancer genes usually elicited significantly better therapeutic effects than viruses alone.2 The most commonly used adenoviral vector in gene therapy is serotype 5 (Ad5), which uses coxsackie-adenovirus receptor (CAR) as the primary receptor for viral internalization.11, 12 However, leukemia cells only express low levels of CAR, resulting in resistance to Ad5 infection.13 Previously, an oncolytic adenovirus with Ad5/F35 chimeric fibers efficiently infected leukemia cells through CD46 and elicited selective cytotoxicity both in vitro and in vivo.14, 15 These studies suggested that Ad5 oncolytic adenoviruses could be further engineered to retarget leukemia cells through cell surface molecules.
To date, a variety of cell surface molecules such as CD25,16 CD32,16 CD44,17 CD47,18, 19 CD96,20 CD123 (refs 21, 22, 23) and C-type lectin-like molecule-1 (refs 24, 25) have been identified to be differentially expressed on AML blasts as well as leukemia stem cells, which initiate and sustain AML cellular hierarchy, as compared with their normal hematopoietic counterparts. CD123, the IL-3 receptor α-subunit that together with CD131 (βc) promotes cell survival and proliferation upon the binding of IL-3,26 has been reported to be overexpressed on AML cells, including AML blasts, progenitors and leukemia stem cells, in comparison with hematopoietic stem cells.22, 23, 27 Moreover, the overexpression of CD123 has been clinically correlated with a lower survival rate in AML patients.28 Previously, IL-3 fused with diphtheria toxin has been shown to induce cytotoxicity to both AML leukemia stem cells and blasts.29, 30 More recently, a monoclonal antibody against CD123 impaired AML in mice by inhibiting the homing and self-renewal of AML LSC cells, as well as activating the host innate immune response.31 Therefore, CD123 provides a promising cell surface target for AML patients with high CD123 expression.
We report here a novel strategy for oncolytic adenovirus-mediated AML therapy, in which oncolytic adenoviruses were genetically modified to carry a soluble CAR (sCAR)-IL3 expression cassette. This modification resulted in the expression of sCAR-IL3 in both packaging cells and leukemia cells, which subsequently decorated the viral fibers and redirected oncolytic adenoviruses to CD123+ leukemia cells. Moreover, the sCAR-IL3 expressing oncolytic adenoviruses were further armed with gene encoding manganese superoxide dismutase (MnSOD) or mannose-binding plant lectin Pinellia pedatisecta agglutinin (PPA), and achieved significant antileukemia effects both in vitro and in vivo.
Materials and methods
Cells
Human leukemia cell lines HL60 and KG-1 were obtained from American Type Culture Collection (ATCC, Rockville, MD, USA). Cells were routinely cultured in RPMI1640 (Hyclone Laboratories, Logan, UT, USA) supplemented with 10% fetal bovine serum (Hyclone Laboratories). The human embryonic kidney cell line HEK293 was obtained from Microbix Biosystems (Toronto, ON, Canada) and cultured in Dulbecco's modified Eagle's medium (Gibco-BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum. All cells were kept at 37 °C in a 95% air and 5% CO2 humidified incubator.
Preparation of recombinant proteins
The plasmid pET-28a-sCARL was constructed previously.32 To obtain the fusion gene sCAR-IL3, the IL-3 gene (GenBank accession number BC066272) without the leading sequence was amplified by polymerase chain reaction using the primers 5′-TCAGCGGCCGCCGCTCCCATGACCCAGACAACGT-3′ and 5′-TCAGCGGCCGCAAGCTTTCAAAAGATCGAGAGAAAGTC-3′ from a plasmid containing an IL-3 gene (Wuhan Sanying Biotechnology Company, Wuhan, China). The 5′-TCAGCGGCCGCCGCTCCCATGACCCAGACAACGT-3′ and 5′-TCAGCGGCCGCAAGCTTTCACTGTTGAGCCTGCGCATT-3′ primers were used to clone a region encoding the IL-3 [Δ125-133]. The IL-3 [Δ125-133, K116W] was amplified with the primers 5′-TCAGCGGCCGCCGCTCCCATGACCCAGACAACGT-3′ and 5′-TCAGCGGCCGCAAGCTTTCACTGTTGAGCCTGCGCATTCTCAAGGGTCCACAGATAGAA-3′. The polymerase chain reaction products were then inserted into the pMD-18T simple vector to generate pMD-18T-IL3 variants, and the NotI–NotI fragments from pMD-18T-IL3 variants were inserted into the pET-28a-sCARL plasmid to generate plasmid pET-28a-sCAR-IL3. The sequences of sCAR-IL3 flanked by HindIII restriction sites were then amplified by polymerase chain reaction from pET-28a-sCAR-IL3 and inserted into the corresponding site of pQE30 (Qiagen, Hilden, Germany) to generate pQE30-sCAR-IL3. His-tagged sCAR-IL3 variants were expressed in Escherichia coli M15 transformed with pQE30-sCAR-IL3.
Construction of recombinant adenovirus vectors
The plasmid pAd.sp-E1A was constructed previously.8 The sCAR-IL3 expression cassette was inserted into pAd.sp-E1A to generate pAd.sp-E1A-sCAR-IL3. The NheI–AleI region in plasmid pAd-ΔE1B-MnSOD or pAd-ΔE1B-PPA was replaced by the NheI–AleI fragments from pAd.sp-E1A-sCAR-IL3 to generate pAd.sp-E1A-sCAR-IL3-ΔE1B-MnSOD or pAd.sp-E1A-sCAR-IL3-ΔE1B-PPA. Plasmids pAd.sp-E1A-sCAR-IL3, pAd.sp-E1A-sCAR-IL3-ΔE1B-MnSOD or pAd.sp-E1A-sCAR-IL3-ΔE1B-PPA were then co-transfected with an adenoviral packaging plasmid pBHGE3 (Microbix Biosystems) into HEK293 cells to produce oncolytic adenovirus Ad.sp-E1A-sCAR-IL3 (Ad.IL3), Ad.sp-E1A-sCAR-IL3-ΔE1B-MnSOD (Ad.IL3-MnSOD) and Ad.sp-E1A-sCAR-IL3-ΔE1B-PPA (Ad.IL3-PPA) through homologous recombination. The recombinant adenoviruses were isolated through plaque purification in HEK293 cells, and a large-scale purification of adenoviruses was performed through cesium chloride density gradient centrifugation. The viral titers were determined by TCID50 (median tissue culture infective dose) assay in HEK293 cells.
Cell viability assay
Leukemia cells were plated on 96-well plates at 2 × 104 per well. Cells were then infected with viruses at the indicated multiplicity of infections (MOIs). Phosphate-buffered saline (PBS) was used as a control. The cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
For blocking assays, KG-1 cells were plated on 96-well plates at 2 × 104 per well. Cells were then treated with Ad.IL3 at the indicated MOIs in combination with recombinant human IL-3Rα (R&D Systems, Minneapolis, MN, USA) at concentrations indicated. Cell viability was then determined by MTT assay after 96 h.
Western blotting analysis
The cell extracts were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted onto nitrocellulose membranes. The membranes were then blocked with Tris-buffered saline and Tween-20 contaning 5% of bovine serum albumin at room temperature for 2 h and incubated with corresponding antibodies overnight at 4 °C. The membranes were washed and incubated with appropriate dilution of IRDye 800 donkey anti-mouse IgG or IRDye 700 donkey anti-rabbit IgG (LI-COR Inc., Lincoln, NA, USA) for 1 h at room temperature. After washing with Tris-buffered saline, the membranes were then analyzed by an Odyssey Infrared Imaging System (LI-COR Inc.). Rabbit anti-poly (ADP-ribose) polymerase antibody, goat anti-CAR antibody, mouse anti-6-his antibody were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Mouse anti-E1A antibody was purchased from Abcam (Cambridge, UK). Goat anti-hIL-3 antibody was purchased from R&D Systems. Rabbit anti-MnSOD antibody was purchased from Epitomics (Burlingame, CA, USA). Rabbit anti-GAPDH antibody, rabbit anti-Bcl-2 antibody, rabbit anti-Bax antibody, rabbit anti-caspase-7 antibody and rabbit anti-caspase-9 antibody were purchased from Cell Signaling Technology Inc. (Danvers, MA, USA).
Xenograft of leukemia cells into mice and in vivo treatment
Male nonobese diabetic/severe-combined immunodeficiency mice at 4–5 weeks of age were used for leukemia xenograft. HL-60/Luc cells at 6 × 106 cells per mouse were injected subcutaneously into the mice on the back. When leukemia burdens reached about 1 × 105 photons/s, mice were randomly grouped and in situ injected with 5 × 108 plaque-forming units of oncolytic adenoviruses each time for totally four injections. Every 4–5 days after treatment, mice were injected with D-luciferin, and bioluminescence was recorded under a Caliper IVIS kinetics (Caliper Life Sciences, Hopkinton, MA, USA). Regions of interest were assigned through the IVIS software (Caliper Life Sciences) and reported as area flux (photons/s), defined by the radiance (photons/s/cm2/steradian). Mice from each group were humanely killed and tumors were harvested 7 days after treatment for transmission electronic microscope analysis under a JEOL 100CX transmission electron microscope (JEOL, Akishima, Japan).
Ethnic statement
All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of Zhejiang Chinese Medical University, Zhejiang, China.
Statistical analysis
Differences among the treatment groups were assessed by Student's t-test. P<0.05 was considered significant.
Results
Recombinant sCAR-IL3 variants increased Ad-EGFP infection of KG-1 leukemia cells
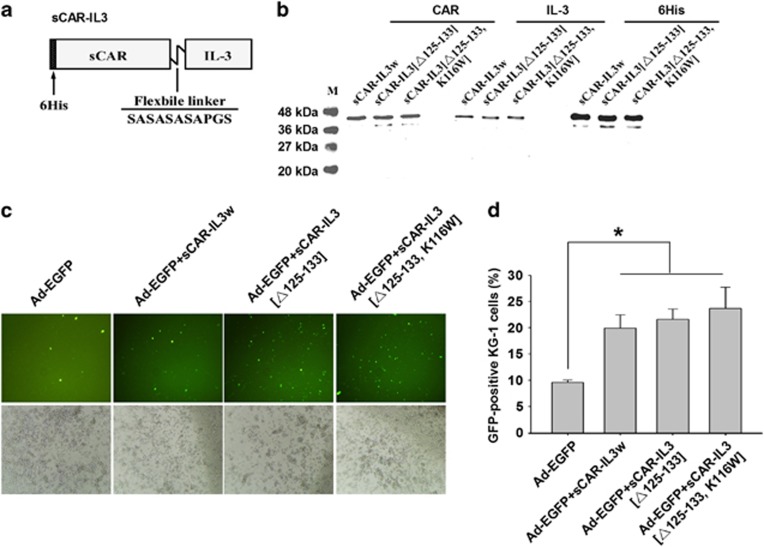
Recombinant sCAR-IL3 variants contain a 6-his-tag, a human coxsackie-adenovirus receptor ectodomain (sCAR), a short flexible linker and IL-3 variants (Figure 1a). The IL-3 variants were designed based on a previous study that IL-3 [K116W] and IL-3 [Δ125-133] enhanced binding and cytotoxicity of diphtheria toxin-IL3 fusion proteins to leukemia cells.33 Western blot analysis was performed to verify the production of these proteins. As shown in Figure 1b, the presence of the 6-his-tag, IL-3 and sCAR was detected. To test the activity of sCAR-IL3 variants, KG-1 cells showing 100% CD34+ and 82% CD123+ (Supplementary Figure S1) were treated with Ad-EGFP combined with sCAR-IL3 variants. As determined by both fluorescent microscopy (Figure 1c) and flow cytometry (Figure 1d), sCAR-IL3 variants significantly increased the Ad-EGFP infection of KG-1 cells. However, the infection of Ad-EGFP to K562 leukemia cells, which do not express CD123, was not altered by sCAR-IL3 variants (Supplementary Figure S2). Furthermore, as shown in Figure 1d, sCAR-IL3 [Δ125-133, K116W] and sCAR-IL3 [Δ125-133] variants did not significantly increase the Ad-EGFP infection of KG-1 cells compared with sCAR-IL3 wild type.
Figure 1.
Fusion protein sCAR-IL3 variants facilitated Ad5 infection to KG-1 leukemia cells. (a) Schematic structure of the sCAR-IL3 fusion proteins. The recombinant protein consists of a 6-his-tag, an extracellular domain of CAR with 239 amino acids, a flexible linker (SASASASAPGS) and IL-3 variants (wild-type IL-3, IL-3 [Δ125-133, K116W] or IL-3 [Δ125-133]). (b) The production of recombinant sCAR-IL3 variants. The sCAR-IL3 variants were purified through an Ni-NTA-Sepharose column, and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by western blotting analysis with a goat anti-CAR antibody, a goat anti-hIL-3 antibody and a mouse anti-6-his antibody. (c) KG-1 cells at 2.5 × 105 cells per well were treated with 4 × 108 viral particles (v.p.) of Ad-EGFP premixed with 4 μg of sCAR-IL3w, sCAR-IL3 [Δ125-133, K116W] or sCAR-IL3 [Δ125-133]. Cells treated with Ad-EGFP added with PBS served as the control. After 2 days, green fluorescent protein (GFP)-positive cells were analyzed under a fluorescence microscope. Shown is a representative experiment from three separate experiments. (d) KG-1 cells were analyzed under a flow cytometer for the percent of GFP-positive cells. Values are expressed as mean±s.e.m. from six separate experiments (*P<0.05).
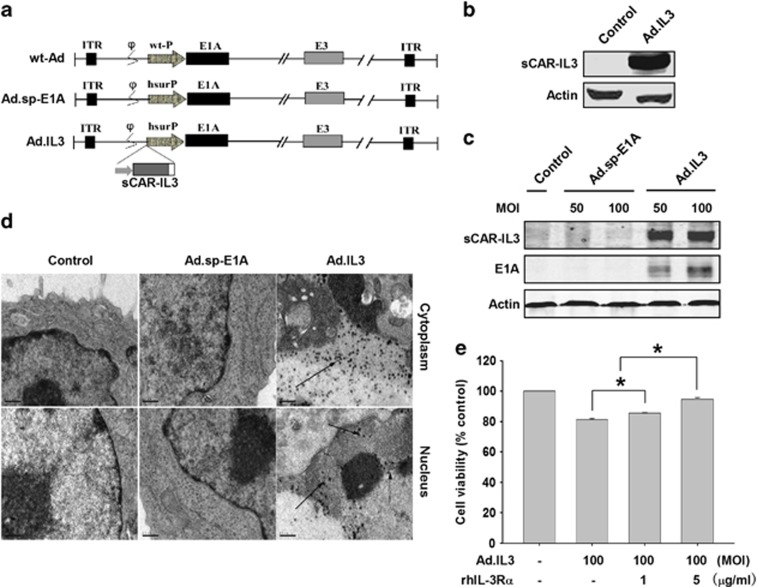
Oncolytic adenoviruses carrying sCAR-IL3 expression cassettes efficiently infected KG-1 leukemia cells through CD123
Owing to the relatively low activity of the recombinant sCAR-IL3 as shown in Figure 1d, we further engineered a previously reported oncolytic adenovirus Ad.sp-E1A8 to harbor a cytomegalovirus promoter-controlled sCAR-IL3 [Δ125-133, K116W] expression cassette, forming a novel oncolytic adenovirus Ad.IL3 (Figure 2a). HEK293 cells hosting Ad.IL3 packaging were determined to express sCAR-IL3 (Figure 2b), indicating the expression of sCAR-IL3 in packaging cells, which could in turn noncovalently decorate the viral fibers during viral production. The infectious capability of Ad.IL3 to KG-1 cells was determined by western blot for viral E1A and sCAR-IL3 expression (Figure 2c). Using transmission electron microscopy, viral particles were detected in the cytoplasm and nucleus of KG-1 cells treated with Ad.IL3, but not Ad.sp-E1A and PBS (Figure 2d). Data indicated the successful infection of Ad.IL3 to KG-1 cells. To determine that Ad.IL3 infected KG-1 cells through CD123, a recombinant CD123 (rhIL-3Rα) was combined with Ad.IL3 to treat cells, followed by MTT assay for cell viability. As shown in Figure 2e, rhIL-3Rα significantly counteracted with the Ad.IL3-induced proliferation inhibition in a dose-dependent manner, indicating that Ad.IL3 used CD123 as the cell membrane receptor for efficient infection, and strongly suggested that sCAR-IL3 was decorated on the viral surface.
Figure 2.
Characterization and production of oncolytic adenovirus Ad.IL3. (a) Schematic structure of wild-type adenovirus (wt-Ad), Ad.sp-E1A and Ad.IL3. As shown in Ad.sp-E1A and Ad.IL3, the viral E1A promoter was replaced by a human survivin promoter (hSurP). Ad.IL3 contains a sCAR-IL3 expression cassette. ITR, inverted terminal repeat; wt-P, wild-type promoter. (b) The expression of sCAR-IL3 in HEK293 cells during viral packaging was detected by western blot analysis with an anti-hIL-3 antibody. Actin was used as the loading control. (c) KG-1 cells were treated with Ad.sp-E1A, Ad.IL3 at 100 MOI for 72 h. The viral particles were observed under a transmission electron microscope ( × 17 500, bars=0.5 μm). (d) Analysis of E1A and sCAR-IL-3 expression in KG-1 cells infected by Ad.IL-3. KG-1 cells were treated with Ad.sp-E1A or Ad.IL3 at MOIs indicated for 72 h. Cell lysates were then subjected to western blot analysis with an anti-hIL-3 antibody or anti-E1A antibody. Actin was used as a loading control. (e) KG-1 cells were treated with Ad.IL3 combined with recombinant human IL-3Rα at dosages indicated. Cell viability was then determined by MTT assay after 96 h. Values were calculated as percent of PBS control and presented as mean±s.e.m. (*P<0.05).
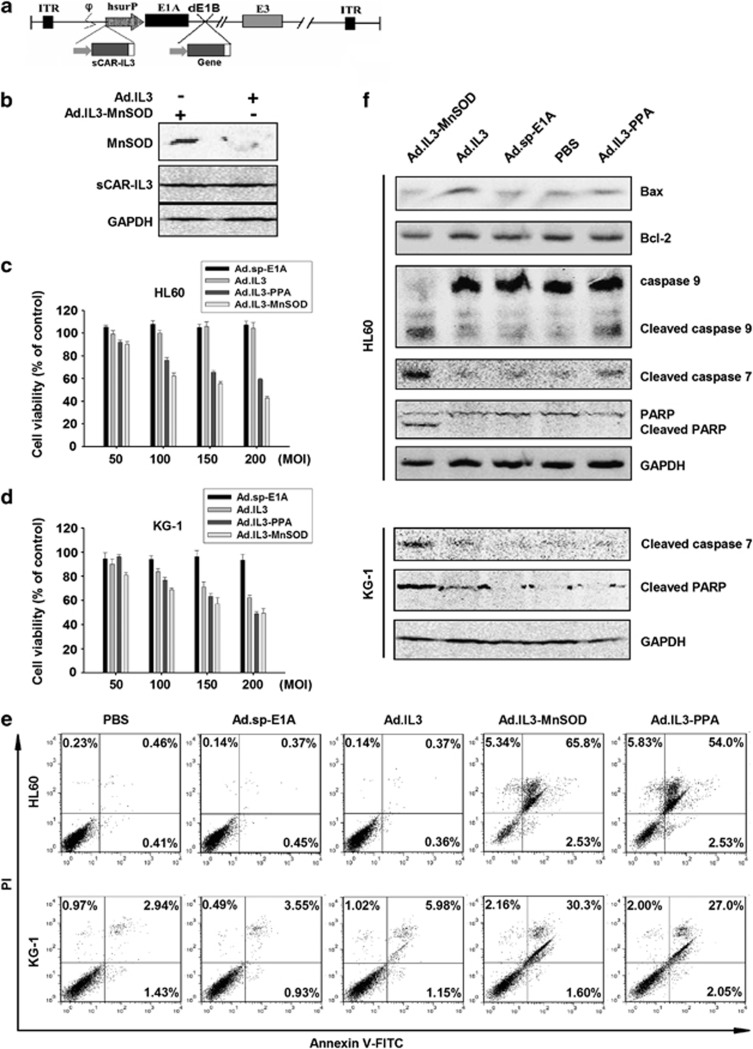
Ad.IL3 armed with anticancer genes elicited higher cytotoxicity to AML cell lines
To further elevate the antiproliferative effect of Ad.IL3, viral E1B was further deleted from Ad.IL3, and the resulting restriction site was used to harbor anticancer genes, forming oncolytic adenovirus Ad.IL3-gene. The schematic structure of the viral genome of Ad.IL3-gene was shown in Figure 3a. Previously, oncolytic adenovirus armed with gene encoding MnSOD induced apoptosis in various solid tumor cell lines, and suppressed xenograft colon cancer growth in vivo.34 However, the antileukemia effect of MnSOD has not been determined. Furthermore, we previously found that exogenous expression of mannose-binding plant lectin PPA through gene delivery induced cancer cell death by using the methylosome containing methylosome protein 50 and protein arginine methyltransferase 5 as a target.35 The in vivo anticancer effect of PPA gene delivery remains unknown. Therefore, expression cassettes of MnSOD and PPA were inserted into Ad.IL3 to form Ad.IL3-MnSOD and Ad.IL3-PPA. KG-1 cells infected with Ad.IL3 or Ad.IL3-MnSOD were lysed, and the expression of MnSOD and sCAR-IL3 was examined by western blot analysis. As shown in Figure 2b, cells infected with Ad.IL3-MnSOD expressed a significantly higher level of MnSOD, as compared with Ad.IL3-treated cells, demonstrating that Ad.IL3-MnSOD successfully forced the expression of MnSOD. Data indicate that oncolytic adenovirus Ad.IL3-gene could deliver anticancer genes to leukemia cells through CD123.
Figure 3.
The in vitro antiproliferative effect of Ad.IL3-gene. (a) Schematic structure of Ad.IL3-gene. Viral E1B was deleted (dE1B), and the resulting restriction site was used to harbor foreign genes. (b) The expression of MnSOD and sCAR-IL3 in cells infected with Ad.IL3-MnSOD. (c) HL60 cells or (d) KG-1 cells were treated with Ad.sp-E1A, Ad.IL3, Ad.IL3-MnSOD or Ad.IL3-PPA at MOIs indicated for 72 h. Cell viability was analyzed by MTT assay. Values were calculated as the percent of PBS control and presented as mean±s.e.m. (e) HL60 and KG-1 cells treated with 200 MOI of Ad.sp-E1A, Ad.IL3, Ad.IL3-MnSOD or Ad.IL3-PPA, as well as PBS for 72 h, and then stained with Annexin V-FITC and PI, followed by flow cytometry analysis. (f) HL60 or KG-1 cells treated with 200 MOI of Ad.sp-E1A, Ad.IL3, Ad.IL3-MnSOD or Ad.IL3-PPA, as well as PBS for 48 h. Cell lysates were examined for poly (ADP-ribose) polymerase, caspase-9, caspase-7, Bcl-2 and Bax through western blot analysis. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as the loading control.
Ad.IL3-PPA and Ad.IL3-MnSOD induced apoptosis in AML cells
To evaluate the cytotoxic effect of Ad.IL3-gene, AML cell lines HL60 and KG-1 were treated with Ad.sp-E1A, Ad.IL3, Ad.IL3-PPA and Ad.IL3-MnSOD at MOIs indicated, as well as PBS control, followed by MTT assay. As shown in Figures 3c and d, Ad.IL3-PPA and Ad.IL3-MnSOD significantly suppressed HL60 and KG-1 in vitro proliferation, as compared with either Ad.sp-E1A or Ad.IL3. Furthermore, Ad.IL3 significantly induced an in vitro antiproliferative effect on KG-1, but not HL60. The suppressive effect of Ad.IL3, Ad.IL3-PPA and Ad.IL3-MnSOD showed dose-dependent manner. To investigate the mechanism of Ad.IL3-gene-induced cytotoxicity of leukemia cells, HL60 and KG-1 were treated with PBS, Ad.IL3, Ad.IL3-PPA or Ad.IL3-MnSOD, followed by staining with Annexin V-FITC and propidium iodide (PI), a common method for apoptotic cell detection, and analyzed under a flow cytometer. Figure 3e showed that Ad.IL3-PPA and Ad.IL3-MnSOD induced a significantly higher percent of Annexin V+/PI− and Annexin V+/PI+ cells, as compared with PBS, Ad.sp-E1A and Ad.IL3 treatments. Results indicate that Ad.IL3-PPA and Ad.IL3-MnSOD elevated apoptosis of HL60 and KG-1 leukemia cells.
To further analyze the apoptosis induced by Ad.IL3-PPA and Ad.IL3-MnSOD, HL60 or KG-1 cells treated with PBS, Ad.sp-E1A, Ad.IL3, Ad.IL3-PPA or Ad.IL3-MnSOD were lysed, and apoptotic signaling elements were investigated through western blot analysis. As shown in Figure 3f, Ad.IL3-MnSOD markedly induced cleavage of caspase-9 and -7, as well as poly (ADP-ribose) polymerase, a substrate for various caspases, indicating the activation of the caspase-9–caspase-7 pathway in HL60 cells by Ad.IL3-MnSOD. Ad.IL3-PPA infection increased the cleavage of caspase-9 and -7 in HL60 cells at a lower level than Ad.IL3-MnSOD. In addition, a significantly elevated level of Bax was shown in HL60 cells treated with Ad.IL3, which did not happen to either Ad.IL3-PPA or Ad.IL3-MnSOD. Furthermore, in KG-1 cells, both Ad.IL3 and Ad.IL3-MnSOD induced cleavage of caspase-7 and poly (ADP-ribose) polymerase, as compared with PBS and Ad.sp-E1A controls, and Ad.IL3-MnSOD showed much better effect than Ad.IL3. However, Ad.IL3-PPA did not significantly alter the cleavage of caspase-7 in KG-1 cells, suggesting a cell-type-dependent manner of the Ad.IL3-PPA-induced cytotoxicity. Our data also suggested that Ad.IL3-PPA possibly used some other intracellular factors to induce leukemia cell apoptosis.
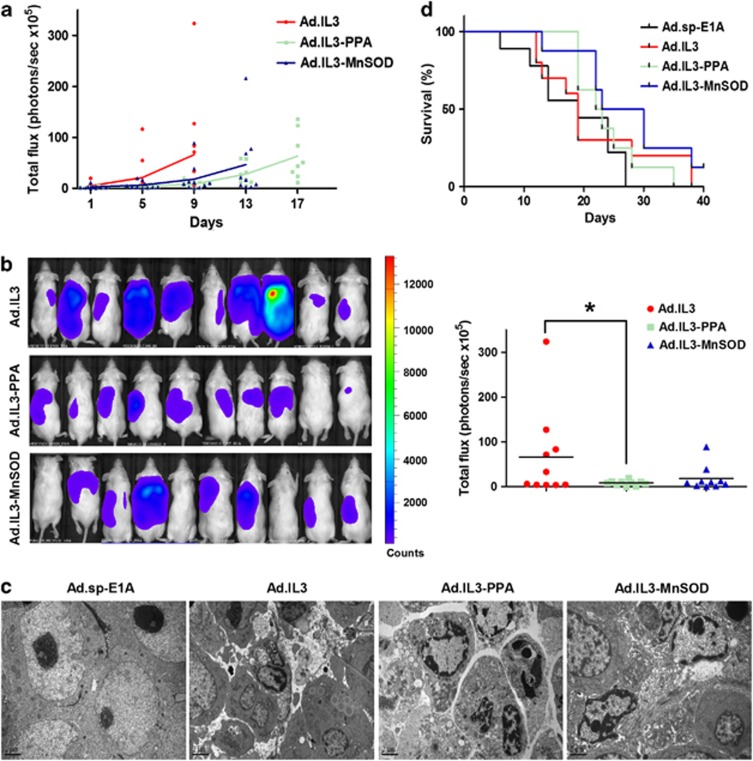
The in vivo antileukemia effect of Ad.IL3-PPA and Ad.IL3-MnSOD
We then evaluated the in vivo antileukemia effect of oncolytic adenoviruses Ad.IL3-PPA and Ad.IL3-MnSOD. HL60/Luc cells stably expressing Firefly luciferase were engrafted subcutaneously into nonobese diabetic/severe-combined immunodeficiency mice and monitored for bioluminescence. At day 27 posttransplantation, HL60/Luc tumors started to grow rapidly. Mice were then grouped randomly and treated with Ad.IL3, Ad.IL3-PPA, Ad.IL3-MnSOD or control virus Ad.sp-E1A. Leukemia cell burden was monitored for each group (n=10) until disease-related death started. Because mice in Ad.sp-E1A group started to die very early, the leukemia cell burdens were only compared among Ad.IL3, Ad.IL3-PPA and Ad.IL3-MnSOD groups. The growth of HL60/Luc cells in nonobese diabetic/severe-combined immunodeficiency mice evaluated by bioluminescence imaging in each group was presented in Figure 4a. Data showed that Ad.IL3-PPA and Ad.IL3-MnSOD treatments delayed the growth of HL60/Luc xenografts in mice, as compared with Ad.IL3. At day 9 after treatment, both Ad.IL3-PPA and Ad.IL3-MnSOD led to lower cancer cell burden than Ad.IL3, and a significant difference was achieved between Ad.IL3 and Ad.IL3-PPA groups (Figure 4b). Transmission electronic microscope analysis detected typical cell apoptosis in mice treated with Ad.IL3, Ad.IL3-PPA or Ad.IL3-MnSOD, but not Ad.sp-E1A control (Figure 4c). Furthermore, Ad.IL3, Ad.IL3-PPA and Ad.IL3-MnSOD increased 16.8, 29.3 and 48.4% of median survival, respectively, as compared with Ad.sp-E1A. A significant difference was achieved between Ad.sp-E1A and Ad.IL3-MnSOD groups (Figure 4d). Our data demonstrated the in vivo antileukemia effect of Ad.IL3-gene oncolytic adenoviruses.
Figure 4.
The in vivo antileukemia effect of Ad.IL3-gene. (a) Growth of HL60/Luc cells in mice treated with 2 × 109 plaque-forming units of Ad.IL3, Ad.IL3-MnSOD or Ad.IL3-PPA was evaluated by bioluminescence imaging. Ten mice per group were treated and analyzed until disease-related death started. Each data point represents a measurement from an individual mouse. The mean values in each group were connected by lines. (b) Bioluminescence images of HL60/Luc engrafted mice from treatment groups indicated at day 9 after treatment were shown on the left. Quantitative analysis of total leukemia cell burden in mice (photons/s × 105) was shown on the right (P<0.05). Each data point represents a measurement from an individual mouse. (c) Apoptotic cells in mice treated with Ad.IL3, Ad.IL3-MnSOD or Ad.IL3-PPA were observed under TEM. Bars show 2 μm. (d) Survival of nonobese diabetic/severe-combined immunodeficiency mice engrafted with HL60/Luc cells and treated with Ad.sp-E1A, Ad.IL3, Ad.IL3-MnSOD or Ad.IL3-PPA. Only disease-related death was counted.
Discussion
Viral infection is the first step for oncolytic adenoviruses to induce cancer cell death. However, because of low expression of CAR on leukemia cells, the entry of Ad5 into leukemia cells is difficult. We genetically inserted the sCAR-IL3 expression cassette into the viral genome of Ad.sp-E1A to form oncolytic adenovirus Ad.IL3. Our data suggested that sCAR-IL3 fusion protein could be expressed and presumably installed on viral surface during viral packaging, which led to successful infection of Ad.IL3 in leukemia cells though CD123. Previously, modifications of the oncolytic adenoviral fibers have focused on retargeting viruses through incorporating receptor-specific ligand peptides into the viral fibers,36, 37, 38 or altering viral tropism through the replacement of the fiber knob alone or together with the shaft domain to form chimeric fibers.14, 39, 40 A clinical study suggested that modification of adenoviral fiber knobs could circumvent the neutralizing antibody response in patients receiving multiple rounds of oncolytic adenoviruses.41 In our strategy, Ad.IL3 was retargeted to CD123 through expressing sCAR-IL3 and presumably incorporating it noncovalently to the fiber surface, resulting in sustainable infection capability of Ad.IL3. The successful infection of Ad.IL3 to leukemia cells suggested that engineering oncolytic adenoviruses to express sCAR-ligand fusion proteins may provide a universal strategy to redirect oncolytic adenoviruses to a variety of membrane receptors on cancer cells resisting to Ad5 infection.
MnSOD is a nuclear DNA-encoded mitochondrial matrix protein catalyzing the conversion of superoxide radicals to hydrogen peroxide, thus eliminating the superoxide produced in the mitochondria.42, 43, 44 Previous studies have demonstrated abnormal activity of MnSOD in tumor cells.45, 46 However, the role of MnSOD in the progression of different tumors is still controversial. MnSOD was shown promoting breast cancer metastasis and anoikis resistance,47 as well as enhancing lung cancer cell invasion through upregulating FoxM1 and MMP2 expression.48 On the other hand, overexpression of MnSOD was shown to inhibit cell proliferation or suppress malignant phenotype of pancreatic cancer cells,49, 50 breast cancer cells51 and prostate cancer cells.51, 52 Furthermore, oncolytic adenovirus carrying gene encoding MnSOD was shown to significantly suppress colon cancer cell growth both in vitro and in vivo through activating the Bax–cytochrome c–caspase-9–caspase-3 apoptotic signaling pathway.34 However, there are few studies addressing the therapeutic effect of MnSOD on leukemia. In our study, oncolytic adenovirus Ad.IL3-MnSOD successfully forced the expression of MnSOD in CD123+ leukemia cells, and induced cell apoptosis through caspase-9–caspase-7 pathway. Notably, the Ad.IL3-MnSOD infection resulted in an almost complete cleavage of full-length caspase-9 in HL60 cells, reflecting a high level of caspase-9 activation. In addition, Ad.IL3-MnSOD significantly induced apoptosis, and prolonged survival of mice engrafted with HL60 leukemia cells. These data indicate that MnSOD could be used as a leukemia suppressor gene, and oncolytic adenovirus Ad.IL3-MnSOD could be valuable for AML therapy pending clinical investigations.
Lectins have provided useful tools for analyzing glycofiles and biomarkers for a variety of cancer types, including ovarian cancer,53 aggressive breast cancer,54 liver cancer,55 and pancreatic cancer.56 Furthermore, some purified lectins such as Maackia amurensis seeds lectin,57 concanavalin A58 and Polygonatum cyrtonema lectin59 have been shown to elicit anticancer effect through inducing apoptosis or autophagy. PPA is a monocot mannose-binding lectin accumulated in the tuber of P. pedatisecta, an Araceae species. Previously, we demonstrated that a recombinant PPA preferentially recognized drug-resistant leukemic K562/ADR cells and lung cancer H460/5Fu cells, as compared with their parental cell lines. Pretreatment with PPA significantly enhanced phagocytosis of K562/ADR cells by macrophages in vivo, and the cell membrane target of PPA on K562/ADR was determined to be sarcolemmal membrane-associated protein.60 Meanwhile, we determined that PPA could be exogenously expressed in a variety of cancer cells through gene delivery, and subsequently induced cell death. Further investigations revealed that PPA translocated into the nucleus and colocalized with DNA. The methylosome that contains methylosome protein 50 and protein arginine methyltransferase 5 was demonstrated to mediate the PPA-induced cell death.35 In this report, oncolytic adenovirus Ad.IL3-PPA significantly induced apoptosis in HL60 leukemia cells, and suppressed HL60 proliferation both in vitro and in vivo. The caspase-9–caspase-7 pathway was suggested to be responsible for the Ad.IL3-PPA-induced apoptosis in HL60 cells. Our study demonstrated for the first time that PPA could act as an antileukemia gene. Further preclinical and clinical studies may provide insights into using Ad.IL3-PPA as a novel agent in antileukemia therapies.
In general, we provided a novel strategy to redirect oncolytic adenoviruses to CD123+ AML cells though carrying a sCAR-IL3 expression cassette in the viral genome. Adenoviruses expressing sCAR-ligand may serve as a universal retargeting strategy to a variety of membrane receptors on cancer cells resisting to Ad5 infection. Furthermore, Ad.IL3 further armed with genes including MnSOD and PPA achieved significant antileukemia effects both in vitro and in vivo, although still not promising, pending further modification. Our data suggest that clinical investigation into the antileukemia effect of Ad.IL3-MnSOD and Ad.IL3-PPA may provide novel antileukemia agents for future AML therapies.
Acknowledgments
This work was supported by National Natural Science Foundation of China Grant 30801379. We thank Shibing Wang and Ruibo Zhao for technical assistance.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- Liu XY. Targeting gene—virotherapy of cancer and its prosperity. Cell Res. 2006;16:879–886. doi: 10.1038/sj.cr.7310108. [DOI] [PubMed] [Google Scholar]
- Curiel DT. The development of conditionally replicative adenoviruses for cancer therapy. Clin Cancer Res. 2000;6:3395–3399. [PubMed] [Google Scholar]
- Hawkins LK, Lemoine NR, Kirn D. Oncolytic biotherapy: a novel therapeutic plafform. Lancet Oncol. 2002;3:17–26. doi: 10.1016/s1470-2045(01)00618-0. [DOI] [PubMed] [Google Scholar]
- Kirn D, Martuza RL, Zwiebel J. Replication-selective virotherapy for cancer: biological principles, risk management and future directions. Nat Med. 2001;7:781–787. doi: 10.1038/89901. [DOI] [PubMed] [Google Scholar]
- Cafferata EG, Maccio DR, Lopez MV, Viale DL, Carbone C, Mazzolini G, et al. A novel A33 promoter-based conditionally replicative adenovirus suppresses tumor growth and eradicates hepatic metastases in human colon cancer models. Clin Cancer Res. 2009;15:3037–3049. doi: 10.1158/1078-0432.CCR-08-1161. [DOI] [PubMed] [Google Scholar]
- Yu de B, Zhong SY, Yang M, Wang YG, Qian QJ, Zheng S, et al. Potent antitumor activity of double-regulated oncolytic adenovirus-mediated ST13 for colorectal cancer. Cancer Sci. 2009;100:678–683. doi: 10.1111/j.1349-7006.2009.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang KJ, Wang YG, Cao X, Zhong SY, Wei RC, Wu YM, et al. Potent antitumor effect of interleukin-24 gene in the survivin promoter and retinoblastoma double-regulated oncolytic adenovirus. Hum Gene Ther. 2009;20:818–830. doi: 10.1089/hum.2008.205. [DOI] [PubMed] [Google Scholar]
- O'Shea CC, Johnson L, Bagus B, Choi S, Nicholas C, Shen A, et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6:611–623. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim PH, Yoo JY, Yoon AR, Choi HJ, Seong J, et al. Double E1B 19 kDa- and E1B 55 kDa-deleted oncolytic adenovirus in combination with radiotherapy elicits an enhanced anti-tumor effect. Gene Therapy. 2009;16:1111–1121. doi: 10.1038/gt.2009.72. [DOI] [PubMed] [Google Scholar]
- Bewley MC, Springer K, Zhang YB, Freimuth P, Flanagan JM. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science. 1999;286:1579–1583. doi: 10.1126/science.286.5444.1579. [DOI] [PubMed] [Google Scholar]
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, et al. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Qian W, Liu J, Tong Y, Yan S, Yang C, Yang M, et al. Enhanced antitumor activity by a selective conditionally replicating adenovirus combining with MDA-7/interleukin-24 for B-lymphoblastic leukemia via induction of apoptosis. Leukemia. 2008;22:361–369. doi: 10.1038/sj.leu.2405034. [DOI] [PubMed] [Google Scholar]
- Jin J, Liu H, Yang C, Li G, Liu X, Qian Q, et al. Effective gene-viral therapy of leukemia by a new fiber chimeric oncolytic adenovirus expressing TRAIL: in vitro and in vivo evaluation. Mol Cancer Ther. 2009;8:1387–1397. doi: 10.1158/1535-7163.MCT-08-0962. [DOI] [PubMed] [Google Scholar]
- Wang G, Li G, Liu H, Yang C, Yang X, Jin J, et al. E1B 55-kDa deleted, Ad5/F35 fiber chimeric adenovirus, a potential oncolytic agent for B-lymphocytic malignancies. J Gene Med. 2009;11:477–485. doi: 10.1002/jgm.1326. [DOI] [PubMed] [Google Scholar]
- Saito Y, Kitamura H, Hijikata A, Tomizawa-Murasawa M, Tanaka S, Takagi S, et al. Identification of therapeutic targets for quiescent, chemotherapy-resistant human leukemia stem cells. Sci Transl Med. 2010;2:17–19. doi: 10.1126/scitranslmed.3000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quere R, Andradottir S, Brun AC, Zubarev RA, Karlsson G, Olsson K, et al. High levels of the adhesion molecule CD44 on leukemic cells generate acute myeloid leukemia relapse after withdrawal of the initial transforming event. Leukemia. 2011;25:515–526. doi: 10.1038/leu.2010.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosen N, Park CY, Tatsumi N, Oji Y, Sugiyama H, Gramatzki M, et al. CD96 is a leukemic stem cell-specific marker in human acute myeloid leukemia. Proc Natl Acad Sci USA. 2007;104:11008–11013. doi: 10.1073/pnas.0704271104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CT, Upchurch D, Szilvassy SJ, Guzman ML, Howard DS, Pettigrew AL, et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14:1777–1784. doi: 10.1038/sj.leu.2401903. [DOI] [PubMed] [Google Scholar]
- Munoz L, Nomdedeu JF, Lopez O, Carnicer MJ, Bellido M, Aventin A, et al. Interleukin-3 receptor alpha chain (CD123) is widely expressed in hematologic malignancies. Haematologica. 2001;86:1261–1269. [PubMed] [Google Scholar]
- Florian S, Sonneck K, Hauswirth AW, Krauth MT, Schernthaner GH, Sperr WR, et al. Detection of molecular targets on the surface of CD34+/CD38− stem cells in various myeloid malignancies. Leuk Lymphoma. 2006;47:207–222. doi: 10.1080/10428190500272507. [DOI] [PubMed] [Google Scholar]
- van Rhenen A, van Dongen GA, Kelder A, Rombouts EJ, Feller N, Moshaver B, et al. The novel AML stem cell associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood. 2007;110:2659–2666. doi: 10.1182/blood-2007-03-083048. [DOI] [PubMed] [Google Scholar]
- Bakker AB, van den Oudenrijn S, Bakker AQ, Feller N, van Meijer M, Bia JA, et al. C-type lectin-like molecule-1: a novel myeloid cell surface marker associated with acute myeloid leukemia. Cancer Res. 2004;64:8443–8450. doi: 10.1158/0008-5472.CAN-04-1659. [DOI] [PubMed] [Google Scholar]
- Stomski FC, Sun Q, Bagley CJ, Woodcock J, Goodall G, Andrews RK, et al. Human interleukin-3 (IL-3) induces disulfide-linked IL-3 receptor alpha- and beta-chain heterodimerization, which is required for receptor activation but not high-affinity binding. Mol Cell Biol. 1996;16:3035–3046. doi: 10.1128/mcb.16.6.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth AW, Florian S, Printz D, Sotlar K, Krauth MT, Fritsch G, et al. Expression of the target receptor CD33 in CD34+/CD38−/CD123+ AML stem cells. Eur J Clin Invest. 2007;37:73–82. doi: 10.1111/j.1365-2362.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- Graf M, Hecht K, Reif S, Pelka-Fleischer R, Pfister K, Schmetzer H. Expression and prognostic value of hemopoietic cytokine receptors in acute myeloid leukemia (AML): implications for future therapeutical strategies. Eur J Haematol. 2004;72:89–106. doi: 10.1046/j.0902-4441.2003.00184.x. [DOI] [PubMed] [Google Scholar]
- Feuring-Buske M, Frankel AE, Alexander RL, Gerhard B, Hogge DE. A diphtheria toxin-interleukin 3 fusion protein is cytotoxic to primitive acute myeloid leukemia progenitors but spares normal progenitors. Cancer Res. 2002;62:1730–1736. [PubMed] [Google Scholar]
- Black JH, McCubrey JA, Willingham MC, Ramage J, Hogge DE, Frankel AE. Diphtheria toxin-interleukin-3 fusion protein (DT(388)IL3) prolongs disease-free survival of leukemic immunocompromised mice. Leukemia. 2003;17:155–159. doi: 10.1038/sj.leu.2402744. [DOI] [PubMed] [Google Scholar]
- Jin L, Lee EM, Ramshaw HS, Busfield SJ, Peoppl AG, Wilkinson L, et al. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor α chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell. 2009;5:31–42. doi: 10.1016/j.stem.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Li GC, Li N, Zhang YH, Li X, Wang YG, Liu XY, et al. Mannose-exposing myeloid leukemia cells detected by the sCAR-PPA fusion protein. Int J Hematol. 2009;89:611–617. doi: 10.1007/s12185-009-0308-3. [DOI] [PubMed] [Google Scholar]
- Liu TF, Urieto JO, Moore JE, Miller MS, Lowe AC, Thorburn A, et al. Diphtheria toxin fused to variant interleukin-3 provides enhanced binding to the interleukin-3 receptor and more potent leukemia cell cytotoxicity. Exp Hematol. 2004;32:277–281. doi: 10.1016/j.exphem.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gu J, Zhao L, He L, Qian W, Wang J, et al. Complete elimination of colorectal tumor xenograft by combined manganese superoxide dismutase with tumor necrosis factor-related apoptosis-inducing ligand gene virotherapy. Cancer Res. 2006;66:4291–4298. doi: 10.1158/0008-5472.CAN-05-1834. [DOI] [PubMed] [Google Scholar]
- Lu Q, Li N, Luo J, Yu M, Huang Y, Wu X, et al. Pinellia pedatisecta agglutinin interacts with the methylosome and induces cancer cell death. Oncogenesis. 2012;1:e29. doi: 10.1038/oncsis.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leja J, Yu D, Nilsson B, Gedda L, Zieba A, Hakkarainen T, et al. Oncolytic adenovirus modified with somatostatin motifs for selective infection of neuroendocrine tumor cells. Gene Therapy. 2011;18:1052–1062. doi: 10.1038/gt.2011.54. [DOI] [PubMed] [Google Scholar]
- Nokisalmi P, Pesonen S, Escutenaire S, Sarkioja M, Raki M, Cerullo V, et al. Oncolytic adenovirus ICOVIR-7 in patients with advanced and refractory solid tumors. Clin Cancer Res. 2010;16:3035–3043. doi: 10.1158/1078-0432.CCR-09-3167. [DOI] [PubMed] [Google Scholar]
- Wakayama M, Abei M, Kawashima R, Seo E, Fukuda K, Ugai H, et al. E1A, E1B double-restricted adenovirus with RGD-fiber modification exhibits enhanced oncolysis for CAR-deficient biliary cancers. Clin Cancer Res. 2007;13:3043–3050. doi: 10.1158/1078-0432.CCR-06-2103. [DOI] [PubMed] [Google Scholar]
- He X, Liu J, Yang C, Su C, Zhou C, Zhang Q, et al. 5/35 Fiber-modified conditionally replicative adenovirus armed with p53 shows increased tumor-suppressing capacity to breast cancer cells. Hum Gene Ther. 2011;22:283–292. doi: 10.1089/hum.2010.058. [DOI] [PubMed] [Google Scholar]
- Bauerschmitz GJ, Ranki T, Kangasniemi L, Ribacka C, Eriksson M, Porten M, et al. Tissue-specific promoters active in CD44+CD24−/low breast cancer cells. Cancer Res. 2008;68:5533–5539. doi: 10.1158/0008-5472.CAN-07-5288. [DOI] [PubMed] [Google Scholar]
- Raki M, Sarkioja M, Escutenaire S, Kangasniemi L, Haavisto E, Kanerva A, et al. Switching the fiber knob of oncolytic adenoviruses to avoid neutralizing antibodies in human cancer patients. J Gene Med. 2011;13:253–261. doi: 10.1002/jgm.1565. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Hoult JR, Blake DR. Oxidants, inflammation, and anti-inflammatory drugs. FASEB J. 1988;2:2867–2873. doi: 10.1096/fasebj.2.13.2844616. [DOI] [PubMed] [Google Scholar]
- Richter C, Park JW, Ames BN. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci USA. 1988;85:6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck Y, Oren R, Amit B, Levanon A, Gorecki M, Hartman JR. Human Mn superoxide dismutase cDNA sequence. Nucleic Acids Res. 1987;15:9076. doi: 10.1093/nar/15.21.9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberley LW, Buettner GR. Role of superoxide dismutase in cancer: a review. Cancer Res. 1979;39:1141–1149. [PubMed] [Google Scholar]
- Hu Y, Rosen DG, Zhou Y, Feng L, Yang G, Liu J, et al. Mitochondrial manganese-superoxide dismutase expression in ovarian cancer: role in cell proliferation and response to oxidative stress. J Biol Chem. 2005;280:39485–39492. doi: 10.1074/jbc.M503296200. [DOI] [PubMed] [Google Scholar]
- Kamarajugadda S, Cai Q, Chen H, Nayak S, Zhu J, He M, et al. Manganese superoxide dismutase promotes anoikis resistance and tumor metastasis. Cell Death Dis. 2013;4:e504. doi: 10.1038/cddis.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PM, Wu TC, Shieh SH, Wu YH, Li MC, Sheu GT, et al. MnSOD promotes tumor invasion via upregulation of FoxM1–MMP2 axis and related with poor survival and relapse in lung adenocarcinomas. Mol Cancer Res. 2013;11:261–271. doi: 10.1158/1541-7786.MCR-12-0527. [DOI] [PubMed] [Google Scholar]
- Weydert C, Roling B, Liu J, Hinkhouse MM, Ritchie JM, Oberley LW, et al. Suppression of the malignant phenotype in human pancreatic cancer cells by the overexpression of manganese superoxide dismutase. Mol Cancer Ther. 2003;2:361–369. [PubMed] [Google Scholar]
- Cullen JJ, Weydert C, Hinkhouse MM, Ritchie J, Domann FE, Spitz D, et al. The role of manganese superoxide dismutase in the growth of pancreatic adenocarcinoma. Cancer Res. 2003;63:1297–1303. [PubMed] [Google Scholar]
- Duan H, Zhang HJ, Yang JQ, Oberley LW, Futscher BW, Domann FE. MnSOD up-regulates maspin tumor suppressor gene expression in human breast and prostate cancer cells. Antioxid Redox Signal. 2003;5:677–688. doi: 10.1089/152308603770310356. [DOI] [PubMed] [Google Scholar]
- Venkataraman S, Jiang X, Weydert C, Zhang Y, Zhang HJ, Goswami PC, et al. Manganese superoxide dismutase overexpression inhibits the growth of androgen-independent prostate cancer cells. Oncogene. 2005;24:77–89. doi: 10.1038/sj.onc.1208145. [DOI] [PubMed] [Google Scholar]
- Wu J, Xie X, Liu Y, He J, Benitez R, Buckanovich RJ, et al. Identification and confirmation of differentially expressed fucosylated glycoproteins in the serum of ovarian cancer patients using a lectin array and LC-MS/MS. J Proteome Res. 2012;11:4541–4552. doi: 10.1021/pr300330z. [DOI] [PubMed] [Google Scholar]
- Drake PM, Schilling B, Niles RK, Prakobphol A, Li B, Jung K, et al. Lectin chromatography/mass spectrometry discovery workflow identifies putative biomarkers of aggressive breast cancers. J Proteome Res. 2012;11:2508–2520. doi: 10.1021/pr201206w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn YH, Shin PM, Oh NR, Park GW, Kim H, Yoo JS. A lectin-coupled, targeted proteomic mass spectrometry (MRM MS) platform for identification of multiple liver cancer biomarkers in human plasma. J Proteomics. 2012;75:5507–5515. doi: 10.1016/j.jprot.2012.06.027. [DOI] [PubMed] [Google Scholar]
- Li C, Simeone DM, Brenner DE, Anderson MA, Shedden KA, Ruffin MT, et al. Pancreatic cancer serum detection using a lectin/glyco-antibody array method. J Proteome Res. 2009;8:483–492. doi: 10.1021/pr8007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Alvarez JA, Krishnan H, Shen Y, Acharya NK, Han M, McNulty DE, et al. Plant lectin can target receptors containing sialic acid, exemplified by podoplanin, to inhibit transformed cell growth and migration. PLoS One. 2012;7:e41845. doi: 10.1371/journal.pone.0041845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Min MW, Bao JK. Induction of apoptosis by concanavalin A and its molecular mechanisms in cancer cells. Autophagy. 2009;5:432–433. doi: 10.4161/auto.5.3.7924. [DOI] [PubMed] [Google Scholar]
- Liu B, Cheng Y, Bian HJ, Bao JK. Molecular mechanisms of Polygonatum cyrtonema lectin-induced apoptosis and autophagy in cancer cells. Autophagy. 2009;5:253–255. doi: 10.4161/auto.5.2.7561. [DOI] [PubMed] [Google Scholar]
- Chen K, Yang X, Wu L, Yu M, Li X, Li N, et al. Pinellia pedatisecta agglutinin targets drug resistant K562/ADR leukemia cells through binding with sarcolemmal membrane associated protein and enhancing macrophage phagocytosis. PLoS One. 2013;8:e74363. doi: 10.1371/journal.pone.0074363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.