Abstract
Multiple myeloma is a malignancy of plasma cells predominantly located in the bone marrow. A number of bone morphogenetic proteins (BMPs) induce apoptosis in myeloma cells in vitro, and with this study we add BMP-9 to the list. BMP-9 has been found in human serum at concentrations that inhibit cancer cell growth in vitro. We here show that the level of BMP-9 in serum was elevated in myeloma patients (median 176 pg/ml, range 8–809) compared with healthy controls (median 110 pg/ml, range 8–359). BMP-9 was also present in the bone marrow and was able to induce apoptosis in 4 out of 11 primary myeloma cell samples by signaling through ALK2. BMP-9-induced apoptosis in myeloma cells was associated with c-MYC downregulation. The effects of BMP-9 were counteracted by membrane-bound (CD105) or soluble endoglin present in the bone marrow microenvironment, suggesting a mechanism for how myeloma cells can evade the tumor suppressing activity of BMP-9 in multiple myeloma.
Introduction
The hematological cancer multiple myeloma arises from terminally differentiated plasma cells and is primarily found in the bone marrow.1 Some cytokines in the bone marrow induce apoptosis in myeloma cells. We and others have earlier shown that bone morphogenetic proteins (BMP)-2, -4, -5, -6 and -7 induce growth arrest or apoptosis in myeloma cells in vitro.2, 3, 4, 5 Furthermore, high expression of BMP-6 in myeloma cells correlated positively with overall survival of multiple myeloma patients.6 BMPs may therefore act as tumor suppressors inhibiting myeloma development.
Multiple myeloma is usually associated with severe osteolytic lesions due to a disturbed balance between bone formation and resorption. In addition to the growth inhibitory effects on myeloma cells, BMPs mediate osteogenic differentiation of mesenchymal stem cells and promote bone formation.7 BMP-9 is one of the least characterized BMPs; nevertheless it may be one of the most potent BMPs in inducing osteogenic differentiation.8, 9 On the other hand, BMP-9 protected monocyte-derived osteoclasts from apoptosis.10 BMP-9 was present in biologically active concentrations (2–12 ng/ml) in serum of healthy individuals.11, 12 In humans, BMP-9 was found to be produced predominantly in the liver.13 BMP9 mRNA has also been found to be expressed in human bone cells.14
BMPs may signal through four type I receptors (ALK1, ALK2, ALK3 and ALK6) and three type II receptors (BMPRII, ActRIIA and ActRIIB).15 BMP-9 and -10 are the only known BMPs that bind the type I BMP-receptor ALK1, predominantly expressed by endothelial cells.16 However, in addition to signaling through ALK1, BMP-9 has also been shown to signal through ALK2.15, 17 Multiple myeloma cells do not express ALK1, but 85% of primary myeloma cells express ALK2 mRNA.6, 18 The role of BMPs in cancer is somewhat unclear, but BMP-9 and -10 have been reported to exert tumor suppressing activities in both breast and prostate cancer.19, 20, 21, 22 In contrast, autocrine BMP-9 signaling supported proliferation of ovarian as well as liver cancer cells.17, 23
Endoglin (CD105) is an accessory receptor for the TGF-β family of ligands. BMP-9 and -10 are the only known ligands that bind endoglin directly,24, 25 whereas endoglin can bind TGF-β, activin-A, BMP-2 and BMP-7 in complex with their respective receptors.26 Membrane-bound endoglin is necessary for TGF-β signaling through ALK1 and has been proposed to balance signaling through either ALK1, promoting proliferation, or through ALK5, which inhibits growth of endothelial cells.27 Endoglin is highly expressed by endothelial cells at sites of active angiogenesis28 and elevated levels were found in myeloma bone marrow,29 as well as in tumors such as breast, colon, brain, lung, prostate and cervical cancer (reviewed in Duff et al.30). Moreover, BMP-9 inhibited migration and proliferation of endothelial cells, as well as angiogenesis.11, 25
A soluble form of endoglin is shed from cells and its levels have been found to be elevated in various cancers and correlated with metastatic disease.31, 32, 33 It was reported that serum levels of endoglin were higher in multiple myeloma patients than in healthy controls and that high levels were associated with advanced disease, tumor growth and angiogenesis.34, 35
Here we show that BMP-9 induced apoptosis in multiple myeloma cells by signaling through ALK2, and that soluble as well as membrane-bound endoglin protected myeloma cells from BMP-9-induced apoptosis.
Materials and methods
Cell lines and reagents
The human multiple myeloma cell lines INA-6, ANBL-6, CAG and JJN-3 were kind gifts from Dr M Gramatzki (University of Erlangen-Nurnberg, Erlangen, Germany), Dr D Jelinek (Mayo Clinic, Rochester, MN, USA), Dr J Epstein (University of Arkansas for Medical Sciences, Little Rock, AR, USA) and Dr J Ball (University of Birmingham, Birmingham, UK), respectively. RPMI-8226 and U266 were from American Type Culture Collection (Rockville, MD, USA). IH-13 and KJON were established in our laboratory.
INA-6 and ANBL-6 cells were grown in 10% heat-inactivated fetal calf serum in RPMI-1640 (RPMI) supplemented with recombinant human interleukin (IL)-6 (1 ng/ml). IH-1 cells were maintained in 10% heat-inactivated human serum (Department of Immunology and Transfusion Medicine, St Olav's University Hospital, Trondheim, Norway) in RPMI and IL-6 (2 ng/ml). CAG, JJN-3, RPMI-8226 and U266 were grown in RPMI supplemented with 10%, 10%, 20% or 15% fetal calf serum, respectively. Cells were cultured at 37 °C in a humidified atmosphere containing 5% CO2. For experiments, 2% human serum in RPMI was used as medium, with IL-6 (1 ng/ml) added for IL-6-dependent cells. All recombinant human proteins were from R&D Systems (R&D Systems Europe Ltd., Abingdon, UK), except IL-6 (Biosource, Camarillo, CA, USA).
Primary cells
To obtain primary myeloma cells, CD138+ cells were isolated from bone marrow specimens obtained through the Norwegian Myeloma Biobank using RoboSep automated cell separator and the Human CD138 Positive Selection Kit (StemCell Technologies, Grenoble, France). The study was approved by the regional ethics committee (2011/2029) and all patients had given informed consent. Bone marrow stromal cells (BMSCs) were prepared as earlier described.36
Cell viability and proliferation
Cells were stained using the Apotest FITC kit (Nexins Research, Kattendijke, The Netherlands). Briefly, cells were incubated with annexin V fluorescein isothiocyanate (0.2 μg/ml) on ice for 1 h. Propidium iodide (PI) (1.4 μg/ml) was added 5 min before cells were analyzed using an LSRII flow cytometer (BD Biosciences, San Jose, CA, USA). Cells negative for both annexin V and PI staining were considered viable. CellTiter-Glo (Promega, Madison, WI, USA) measures ATP levels in cells by use of luciferase and was used to determine relative cell proliferation. Cells were seeded in 96-well optical plates and treated as indicated. CellTiter-Glo reagent was added according to the manufacturer's protocol and luminescence was determined using Victor 1420 multilabel counter (PerkinElmer Inc., Waltham, MA, USA).
Cell cycle analysis
JJN-3 cells were seeded at 200 000 cells/ml and cultured for 18 h with or without BMP-4, BMP-6 or BMP-9. A modified version of the Vindeløv method was used to prepare nuclei for flow cytometric DNA analysis, and is described elsewhere.37, 38 Data was acquired with an LSRII flow cytometer (BD) and analyzed in FlowJo (Tree Star, Inc., Ashland, OR, USA) using the Watson pragmatic model.
Immunoblotting
Cells were treated as indicated, washed with ice cold phosphate-buffered saline (PBS) and lysed for 15 min on ice. The lysis buffer contained 1% Nonidet P40 (NP-40) (Sigma-Aldrich, St Louis, MO, USA), 150 mM NaCl, 50 mM Tris-HCl (pH 7.5), protease inhibitor cocktail (Roche, Basel, Switzerland), 1 mM Na3VO4 and 50 mM NaF. Samples were electrophorated on NuPAGE Bis-Tris gels with MOPS running buffer (Invitrogen, Carlsbad, CA, USA). Gels were blotted onto nitrocellulose membranes, blocked with 5% nonfat dry milk in Tris-buffered saline with 0.01% Tween 20 (TBS-T) and incubated overnight with primary antibodies as indicated. Primary antibodies used were: c-MYC (BD Norge AS, Trondheim, Norway), phospho-SMAD1/5/8 (Cell Signaling Technology, Medprobe, Oslo, Norway), endoglin (Santa Cruz Biotechnology, Inc., Heidelberg, Germany) and GAPDH (Abcam, Cambridge, UK). Blots were washed in TBS-T before incubation for 1 h with horseradish peroxidase-conjugated secondary antibodies (Dako Cytomation, Glostrup, Denmark). The blots were washed thoroughly with TBS-T before bands were detected using SuperSignal West Femto (Thermo Fisher Scientific, Waltham, MA, USA) as luminescence substrate and Kodak Image Station 2000R (Kodak, Rochester, NY, USA).
Quantitative reverse transcription-PCR
Total RNA was isolated from cells using the RNeasy Mini Kit (Qiagen, Crawley, UK) and complementary DNA was synthesized from total RNA using the High Capacity RNA-to-cDNA kit (Applied Biosystems, Carlsbad, CA, USA). PCR was performed using StepOne Real-Time PCR System and Taqman Gene Expression Assays (Applied Biosystems). The Taqman assays used were: ALK1/ACVRL1 (Hs00163543_m1), ALK2/ACVR1 (Hs00153836_m1), ALK3/BMPR1A (Hs01034909_g1), ALK6/BMPR1B (Hs00176144_m1), ActRIIA/ACVR2A (Hs00155658_m1), ActRIIB/ACVR2B (Hs00609603_m1), BMPRII/BMPR2 (Hs00176148_m1), ENG (Hs00923997_g1), GAPDH (Hs99999905_m1) and ID1 (Hs00357821_g1). The comparative Ct method was used to estimate relative changes in gene expression using GAPDH as housekeeping gene.
Transfections
Myeloma cell lines were transfected using the Nucleofector device (Amaxa biosystems, Cologne, Germany) and the Amaxa Nucleofector Kit R for INA-6 or T for CAG (Lonza, Basel, Switzerland), as previously described.39 For each transfection, cells were treated with 1 μM ON-TARGETplus Non-Targeting pool, SMARTpool ACVR1 or ENG siRNAs (Dharmacon RNAi technologies by Thermo Scientific, Lafayette, CO, USA).
Cytokine measurements
Patient serum samples were collected for the Nordic Myeloma Study Group during a randomized phase 3 clinical trial on pamidronate dosage in the years 2001–2005.40 Only samples that were taken before start of treatment were included (n=138). For comparison, serum was collected from 58 age- and gender-matched healthy volunteers. Bone marrow plasma samples from 16 patients were also obtained as described.41 The presence of BMP-9 and soluble endoglin in bone marrow plasma and cell culture supernatants was assayed using human BMP-9 and endoglin/CD105 DuoSet ELISAs (R&D Systems). Serum samples were analyzed using a multiplex assay (MILLIPLEX MAP Human Angiogenesis/Growth Factor Magnetic Bead Panel, HAGP1MAG-12K, Millipore Corporation, Billerica, MA, USA) for determination of BMP-9. For measurements of soluble endoglin in serum, the DuoSet ELISA was used. All samples were run in duplicates and values above or below the standard curves were set to the highest or lowest standard value, respectively.
Endoglin cell membrane expression
Myeloma cells and BMSCs were washed with PBS containing 0.1% bovine serum albumin (BSA) (PBS/BSA). Cells were then labeled with phycoerythrin-conjugated antibodies: isotype control or endoglin (CD105) (BD Norge AS). After 20 min of incubation, cells were washed with PBS/BSA and resuspended in PBS. Data were acquired using an LSRII flow cytometer and analyzed using FlowJo software.
Coculture experiments
For cocoulture experiments a method applying an automated fluorescence microscope platform was used.36 Briefly, BMSCs were allowed to adhere to 96-well plates before INA-6 cells and BMPs were added. Cell membrane integrity was evaluated as a measure of viability using YO-PRO-1 DNA stain (Invitrogen) that labels apoptotic cells. Myeloma cells and BMSCs were distinguished by size, shape and intensity of DRAQ5 nuclear staining (eBioscience, San Diego, CA, USA). The ScanR microscope-based screening platform was used for automated image acquisition and images were analyzed using the Olympus ScanR Analysis software (Olympus, Hamburg, Germany).
Statistics
Statistical calculations were done using IBM SPSS Statistics 20 (IBM Corp., Armonk, NY, USA). For serum samples, all patients were included, except for analysis of progression-free survival where 18 patients were omitted due to missing data. Comparisons between groups were done using Mann–Whitney U-test. Survival analysis was done by the Kaplan–Meier method. Correlations were analyzed using Spearman's rank correlation test. The level of statistical significance was P<0.05 (two-tailed).
Results
BMP-9 induced apoptosis and growth arrest in myeloma cell lines and primary cells
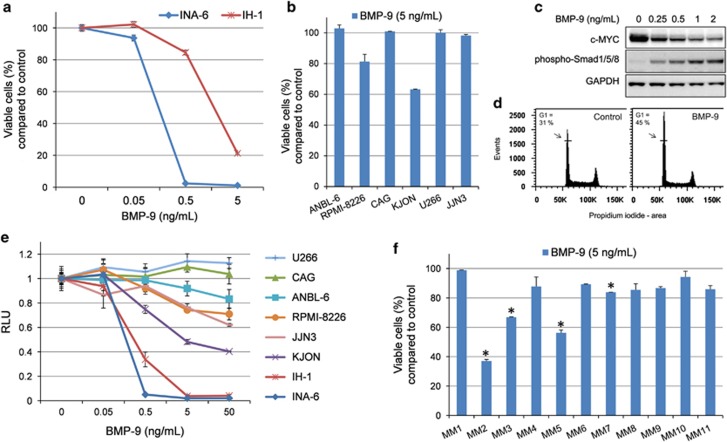
To determine whether BMP-9 could induce apoptosis in myeloma cells, the eight myeloma cell lines INA-6, ANBL-6, CAG, KJON, IH-1, RPMI-8226, U266 and JJN-3 were treated with BMP-9 before cell viability was measured using annexin V labeling. The INA-6 and IH-1 cell lines were highly sensitive toward BMP-9-induced apoptosis (Figure 1a). BMP-9 also induced apoptosis in the myeloma cell lines RPMI-8226 and KJON, whereas in the remaining four cell lines no effect of BMP-9 on apoptosis was observed (Figure 1b). We have earlier shown that BMP-induced apoptosis in myeloma cells was dependent on downregulation of c-MYC.5, 42 Likewise, BMP-9 also induced c-MYC downregulation in a dose-dependent manner in INA-6 cells (Figure 1c).
Figure 1.
BMP-9-induced apoptosis and growth arrest in myeloma cells. (a, b) Human myeloma cell lines were treated for 3 days with the indicated concentrations of BMP-9. Cell viability was assessed by flow cytometry following annexin V fluorescein isothiocyanate and propidium iodide (PI) labeling. Cells that were negative for annexin V and PI were considered viable. (c) INA-6 cells were treated for 20 h with the indicated concentrations of BMP-9, subjected to cell lysis, electrophoresis and immunoblotting with antibodies detecting c-MYC, phosphorylated SMAD1/5/8 or GAPDH. (d) JJN-3 cells were treated with BMP-9 for 24 h and subjected to cell cycle analysis. (e) Human myeloma cell lines were treated for 3 days with the indicated concentrations of BMP-9. Cell growth was evaluated using CellTiter-Glo. Relative luciferase units (RLU) reflected the amount of ATP in each well. (f) CD138+ cells from patients were treated with BMP-9 (5 ng/ml) for 3 days before cell viability was measured as described in (a). The data in a–d are representative of three independent experiments, whereas experiments using primary cells (f) were performed once. Data are presented as means±1 s.d. Asterisks (b, f) indicate a significant lower cell viability of at least 15% compared with control (P<0.05, one-tailed t-test).
Earlier we observed that the JJN-3 cell line was growth arrested by BMP-4 or BMP-6 treatment, whereas there was no effect on apoptosis (Supplementary Figure 1). Similarly, after 24 h of BMP-9 treatment a significant proportion of the JJN-3 cells were arrested in the G1-phase of the cell cycle as compared with control cells (Figure 1d).
We also chose to study growth inhibition in myeloma cells by quantifying intracellular ATP levels as a measure of relative cell proliferation (Figure 1e). The INA-6 and IH-1 cell lines were growth inhibited by BMP-9 at concentrations <0.5 ng/ml, whereas CAG and U266 were not affected even at a concentration of 50 ng/ml BMP-9. The other cell lines showed varying degrees of sensitivity to BMP-9. Taken together, we concluded that of the cell lines tested here, two were resistant (CAG and U266) and two were only growth arrested (JJN-3 and ANBL-6), whereas the remaining four became apoptotic and/or growth arrested following treatment with BMP-9.
BMP-9 and -10 are highly homologous and both have been shown to suppress breast and prostate cancer cells.19, 20, 21, 22 We therefore tested whether also BMP-10 had an effect on myeloma cells. However, even at a concentration of 400 ng/ml no effect of BMP-10 on apoptosis or growth arrest in INA-6 and IH-1 cells was seen (data not shown).
Finally, we treated isolated CD138+ cells from 11 myeloma patients with BMP-9 (5 ng/ml). A significant reduction in cell viability of at least 15% compared with untreated cells was seen in 4 out of 11 samples (Figure 1f), indicating that BMP-9 induces apoptosis also in some primary myeloma cell samples.
BMP-9 signaled through ALK2 in myeloma cells
The observation that BMP-9 had a strong effect on myeloma cells, whereas BMP-10 had no effect, suggested that BMP-9 signaled through another BMP type I receptor than the common BMP-9/10 receptor ALK1 in these cells.
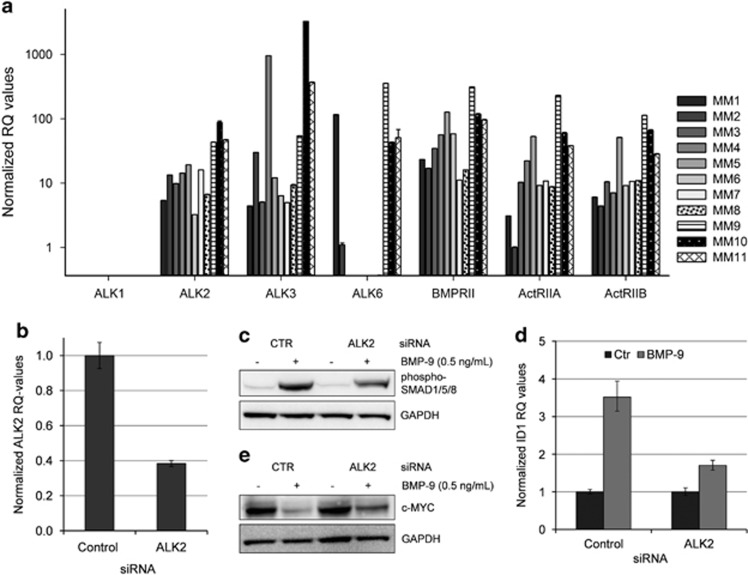
Indeed, primary myeloma cells did not express ALK1 mRNA (Figure 2a). In contrast, they all contained mRNA encoding the BMP type I receptors ALK2, ALK3 and in some cases also ALK6. The three type II receptors BMPRII, ActRIIA and ActRIIB were expressed in all primary myeloma cell samples. Interestingly, ALK2, as well as all BMP type II receptors, has been reported to bind BMP-9.15 Using quantitative reverse transcription-PCR we found that INA-6 cells express ALK2, barely detectable levels of ALK3 and no ALK6 (data not shown). To show that BMP-9 actually signaled through ALK2 in myeloma cells, we first incubated cells with dorsomorphin homolog 1, an inhibitor of BMP-receptor-activated SMADs.43 The inhibitor counteracted BMP-9-induced apoptosis in INA-6 as well as in IH-1 cells (Supplementary Figure 2), indicating that, as with other BMPs, also BMP-9-induced apoptosis was dependent on activation of BMP-receptor-activated SMADs. Knockdown experiments were then performed in the INA-6 cell line using ALK2 small interfering RNA (siRNA). INA-6 cells transfected with ALK2 siRNA showed a reduction of ALK2 mRNA levels to ∼40% of control levels (Figure 2b). Two days post transfection, INA-6 cells were incubated with or without BMP-9 for 1 h. Pre-treatment with ALK2 siRNA reduced the level of BMP-9-induced SMAD1/5/8 phosphorylation as determined by immunoblotting (Figure 2c). Furthermore, to determine whether the function of activated SMAD1/5/8 was affected, we looked at the well-known SMAD target gene ID1.44 The relative amounts of ID1 mRNA were determined by quantitative reverse transcription-PCR in cells treated with BMP-9. The BMP-9-induced increase of ID1 mRNA was significantly reduced in cells treated with ALK2 siRNA as compared with non-targeting control siRNA (Figure 2d). BMP-6 also used ALK2 for activation of SMAD1/5/8 in INA-6 cells (Supplementary Figure 3). We have earlier shown that BMP-induced apoptosis in myeloma cells occurs through SMAD-dependent downregulation of c-MYC.5 BMP-9-induced downregulation of c-MYC was indeed counteracted by the use of ALK2 siRNA (Figure 2e). In summary, our results clearly indicate that ALK2 is the BMP type I receptor used for BMP-9 signaling in myeloma cells.
Figure 2.
BMP-9 signaled through ALK2 in myeloma cells. (a) Comparison of the expression of different BMP receptors in 11 patient samples. The relative levels of mRNA were determined by quantitative reverse transcription (QRT)-PCR and compared with the expression of ActRIIA in patient MM2, which was set to 1. (b) QRT-PCR showing knockdown of ALK2 mRNA in siRNA-treated INA-6 cells. The cells were transfected with either Non-Targeting control or ALK2 siRNA and cells were collected at day 2 post transfection. (c) Transfected INA-6 cells were treated 2 days post transfection with BMP-9 (0.5 ng/ml) for 1 h and subjected to immunoblotting with antibodies detecting phosphorylated SMAD1/5/8 or GAPDH. (d) Cells treated as in (c) and analyzed for expression of ID1 mRNA by QRT-PCR. (e) INA-6 cells transfected as in (b). Cells were treated with or without BMP-9 for 20 h and subjected to immunoblotting with antibodies detecting c-MYC or GAPDH. Error bars represent 1 s.d. RQ, relative quantitation.
Soluble and membrane-bound endoglin sequestered BMP-9 and antagonized the growth inhibitory effects of BMP-9
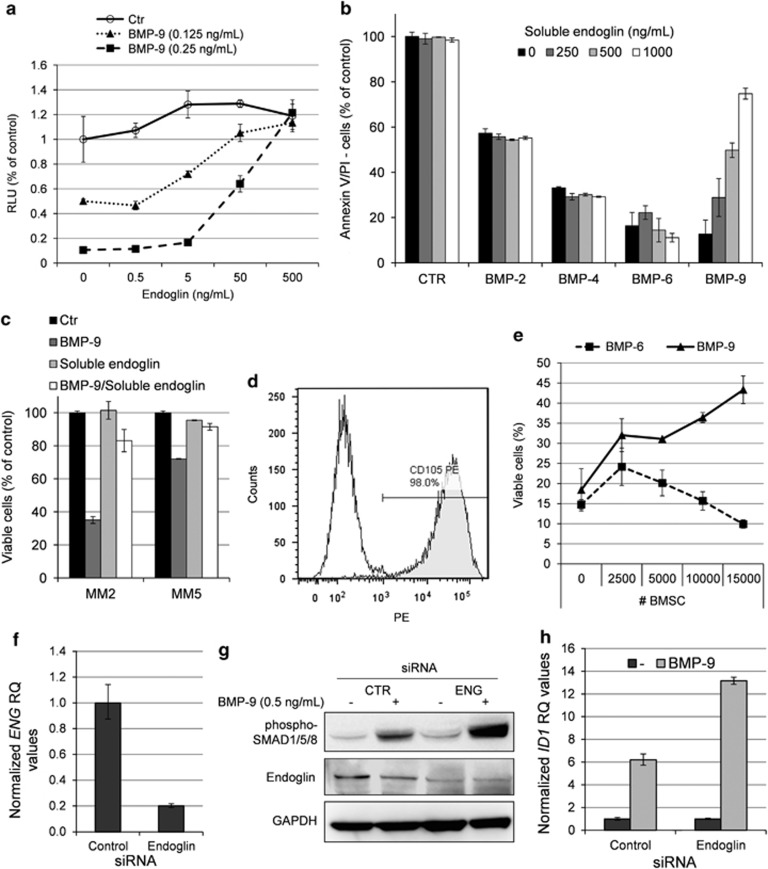
Betaglycan and endoglin are auxiliary, type III receptors of the TGF-beta superfamily, and BMP-9 has previously been shown to bind endoglin even in the absence of BMP receptor.24, 25 To clarify whether type III receptors were able to influence BMP-9 signaling in myeloma cells, we incubated cells with BMP-9 in the absence or presence of soluble recombinant human betaglycan or endoglin. Betaglycan had no effect on BMP-9 signaling under the given conditions (data not shown). Endoglin, on the other hand, antagonized BMP-9 signaling in a dose-dependent manner (Figure 3a). INA-6 cells express the type I receptor ALK2 and minute amounts of ALK3, and are not responsive to BMP-2, -4 or -5 that signal through ALK3 (Ro et al.4 and data not shown). Thus, only BMP-6, -7 and, as shown here, BMP-9 have reported effects on INA-6 cells. To determine the effects of endoglin on other BMPs, we therefore chose to use IH-1 cells that express all known type I receptors, except for ALK1, and which is generally sensitive to BMPs.4 Addition of soluble endoglin did not affect BMP-2-, -4- or -6-induced apoptosis in IH-1 cells (Figure 3b), indicating that endoglin inhibits BMP-9, but not BMP-2, BMP-4 or BMP-6. The inhibitory effect of soluble endoglin on BMP-9-induced apoptosis was also seen in primary myeloma cells from patient MM2 and MM5 (Figure 3c).
Figure 3.
Endoglin inhibited BMP-9, but not BMP-2, BMP-4 or BMP-6. (a) INA-6 cells were treated for 3 days with indicated concentrations of BMP-9 in the presence of increasing amounts of recombinant human soluble endoglin. Relative cell growth was measured using the CellTiter-Glo assay. (b) IH-1 cells were treated for 3 days with BMP-2 (200 ng/ml), BMP-4 (20 ng/ml), BMP-6 (300 ng/ml) or BMP-9 (5 ng/ml) in the presence of increasing amounts of soluble endoglin. Cell viability was measured by annexin V and PI staining. (c) Myeloma cells from patient MM2 and MM5 were treated with BMP-9 (5 ng/ml), endoglin (1 μg/ml) or a combination of both for 3 days before cell viability was measured using annexin V and PI. Untreated cells were used as control. (d) BMSCs were incubated with PE-labeled endoglin or isotype control antibodies and analyzed by flow cytometry. (e) INA-6 cells were grown in coculture with increasing numbers of BMSCs in the presence of BMP-9 (0.25 ng/ml) or BMP-6 (50 ng/ml). Cell membrane integrity was evaluated as a measure of viability using the YO-PRO-1 DNA stain as described in the Materials and Methods section. (f) QRT-PCR showing knockdown of endoglin (ENG) mRNA in siRNA-treated CAG cells. The cells were transfected with either Non-targeting Control or ENG siRNA and cells were collected 2 days post transfection. (g) Transfected CAG cells were treated 2 days post transfection with BMP-9 (0.5 ng/ml) for 1 h and subjected to immunoblotting with antibodies detecting phosphorylated SMAD1/5/8, endoglin or GAPDH. (h) CAG cells treated as in (f) and analyzed for expression of ID1 mRNA by QRT-PCR. Error bars represent 1 s.d. RQ, relative quantitation.
We also tested the effects of the recombinant human antagonists such as chordin-like 1, crossveinless-2, follistatin, gremlin and twisted gastrulation on BMP-9 signaling. Chordin-like 1 and twisted gastrulation counteracted the effect of BMP-9 on INA-6 proliferation, but only when used at 10 μg/ml (Supplementary Figure 4). The other antagonists had no significant effects, suggesting that there are few naturally occurring antagonists of BMP-9.
We further hypothesized that presence of endoglin in the bone marrow microenvironment could be one way for myeloma cells to evade BMP-9-induced apoptosis. Measurements of soluble endoglin and BMP-9 in bone marrow plasma from 16 myeloma patients showed presence of both soluble endoglin (median 14.1 ng/ml, range 9–22) and BMP-9 (median 33.9 pg/ml, range 5–160). These data indicate that soluble endoglin is present in the bone marrow at a level that inhibits BMP-9-induced apoptosis in myeloma cells, as shown in Figure 3a.
A potentially important source of endoglin in the bone marrow is membrane-bound endoglin. Membrane-bound endoglin was present on >95% of expanded BMSCs from myeloma patients (Figure 3d). Interestingly, BMSCs protected INA-6 cells from BMP-9-induced apoptosis, but not BMP-6-induced apoptosis (Figure 3e), consistent with the finding that endoglin binds BMP-9 but not BMP-6.
Some myeloma cell lines were unresponsive to BMP-9-induced growth inhibition (Figure 1). Having also shown that endoglin is capable of inhibiting BMP-9, we hypothesized that one way in which myeloma cells could be protected from BMP-9-induced growth inhibition was by expressing endoglin on their cell surface. In contrast to INA-6 cells, CAG cells express endoglin (Supplementary Figure 5). To further explore the role of endoglin in BMP-9 signaling, we knocked down endoglin in the CAG cell line using siRNA transfection. Knockdown of endoglin (Figures 3f and g) led to increased BMP-9 signaling, as shown directly by immunoblot showing increased phosphorylation of SMAD1/5/8 (Figure 3g) and indirectly by quantitative reverse transcription-PCR showing increased BMP-9 mediated induction of SMAD-responsive ID1 (Figure 3h).
To summarize, these results suggest that myeloma cells in the bone marrow may be protected from BMP-9-induced growth suppression or apoptosis by endoglin expressed on myeloma or BMSC surfaces, or endoglin in a soluble form.
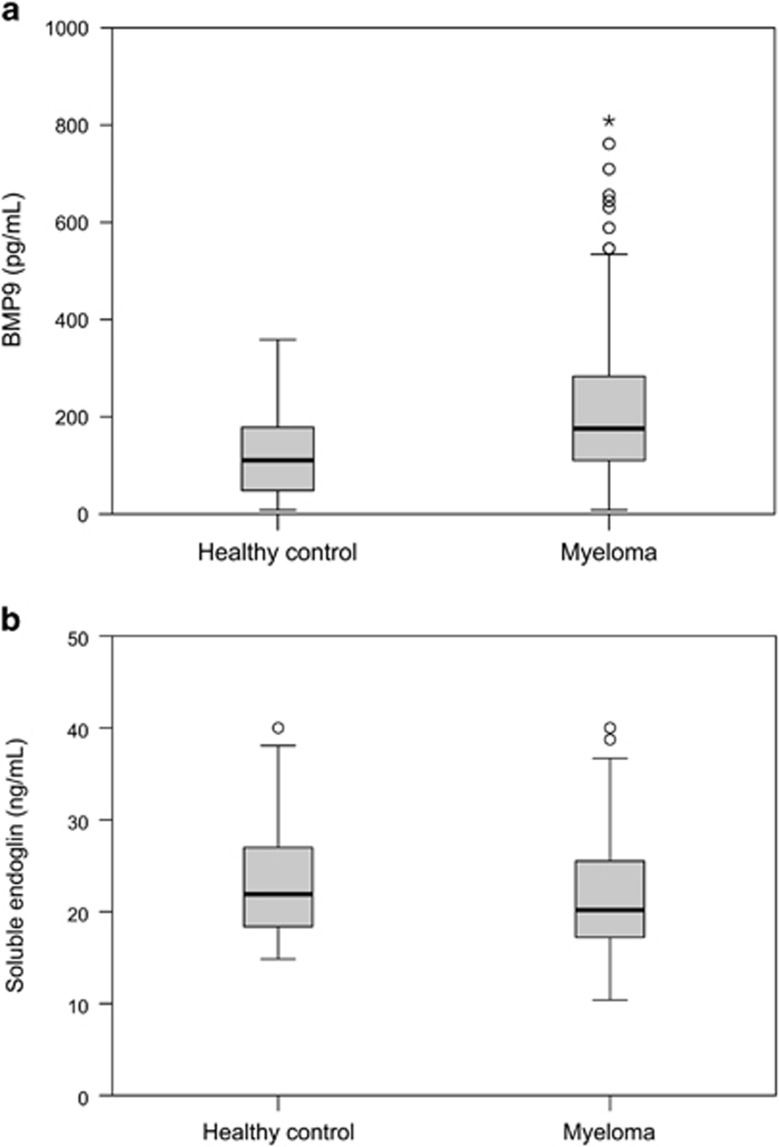
Soluble endoglin and BMP-9 were also measured in serum from a larger patient group (n=138) and compared with levels in serum from healthy individuals (n=58). Median serum BMP-9 concentration in myeloma patients (176 pg/ml, range 8–809) was significantly higher (P=0.002) than in healthy controls (110 pg/ml, range 8–359) (Figure 4a). We found no significant increase in the levels of soluble endoglin in myeloma patients (20.2 ng/ml, range 10.4–40.0) compared with healthy controls (21.9 ng/ml, range 14.8–40.0) (Figure 4b). Neither BMP-9 nor soluble endoglin levels had any influence on overall survival or progression-free survival (data not shown). We also analyzed the data to see whether there were any correlations between BMP-9 or endoglin and the percentage of plasma cells in bone marrow, serum levels of M-protein, β2-microglobulin or albumin, disease stage (International Staging System) or bone disease. Only minor correlations were found between serum levels of BMP-9 and M-protein and albumin (data not shown).
Figure 4.
Serum concentrations of BMP-9 and soluble endoglin. The concentrations of BMP-9 (a) and soluble endoglin (b) were measured in serum samples from 138 patients and 58 healthy controls. The dark lines indicate median concentrations. Outliers and extreme outliers are indicated by points and asterisks, respectively. The top of the box represents the 75th percentile, whereas the bottom represents the 25th percentile.
Discussion
Many BMPs are potent inhibitors of myeloma cell growth in vitro, causing growth arrest or apoptosis in a number of cell lines and primary cells. However, some cells evade the growth inhibitory effects of BMPs, as was also the case here with BMP-9. There are several possible reasons for this, and one obvious reason would be lack of appropriate receptors. For BMPs to exert their growth inhibitory functions on myeloma cells, they must bind specific BMP receptors to induce phosphorylation of receptor-activated SMADs; SMAD1/5/8.5 Myeloma cells in general express all known BMP receptors, except ALK1. There are some variations, particularly in expression of ALK6, where detectable levels were found in 5 out of 11 patient samples tested here. Nevertheless, even when BMPs bind correctly and induce activation of SMADs, the cells may evade growth inhibition. In line with this, we found no correlation between expression of ALK2 and BMP-9 sensitivity in primary myeloma cells. Myeloma cells could also possibly evade growth inhibitory effects of BMP by defect SMAD1/5/8 function after phosphorylation by cyclin-dependent kinases, as described for TGF-β activated SMAD2.45 Other ways that may render cells resistant to BMP-induced growth inhibition are lack of downstream components of the SMAD-signaling pathway, as shown in lymphoma cells.46 Owing to scarcity of material, we chose to use one concentration of BMP-9 (5 ng/ml) when analyzing primary cells. Using this concentration, 4 out of 11 samples showed a significant downregulation of cell viability by at least 15% as compared with control. The effects of BMP-9 are dose dependent, thus from these analyses it is not possible to tell if more patient samples would be sensitive if higher concentrations of BMP-9 had been used.
We have earlier shown that BMP induced apoptosis in myeloma cells by downregulation of c-MYC and that myeloma cells depend on c-MYC for survival.5, 42 Also in the case of BMP-9, cell death correlated with downregulation of c-MYC protein, underscoring the importance of c-MYC expression for myeloma cell survival. Moreover, translocations placing MYC in the proximity of immunoglobulin enhancers may be one of the ways myeloma cells become resistant to BMP-induced apoptosis.5
The BMP ligand–receptor interaction is highly promiscuous; one receptor can bind different ligands, whereas one ligand can activate different sets of receptors. Previous studies have shown that BMP-9 signals by binding to the type I receptors ALK1 and ALK2. The binding to ALK1 is stronger than the binding to ALK2, and binding of BMP-9 to ALK2 is strongly enhanced in the presence of type II receptors.25 Indeed, using a panel of recombinant human BMP receptors, we found that ALK1 completely blunted BMP-9-induced growth inhibition on INA-6 cells, whereas ALK2 had no detectable effect (data not shown).
We show for the first time that serum levels of BMP-9 are increased in myeloma patients compared with healthy controls. Soluble endoglin was previously found to be increased in myeloma sera and correlated with advanced stages of the disease.34 In our material we could not find a significant increase in serum levels of soluble endoglin in myeloma patients compared with healthy controls. Nevertheless, we show that soluble endoglin is also present in the bone marrow of myeloma patients at levels that inhibit BMP-9-induced apoptosis in myeloma cells. We found that myeloma-derived BMSCs express membrane-bound endoglin, but there is also an increased amount of endoglin-expressing endothelial cells in the bone marrow of myeloma patients compared with healthy controls.29 These, and other sources of endoglin, may scavenge BMP-9 from the surroundings of myeloma cells, thereby protecting them from the growth inhibitory effects of BMP-9. Interestingly, endoglin expressed on the surface of myeloma cells also inhibited BMP-9 signaling, as shown by knockdown experiments in CAG cells. Thus, targeting endoglin using neutralizing antibodies could represent a promising approach for myeloma treatment.
In conclusion we here show that myeloma cells can be growth arrested or killed by low doses of BMP-9, and that the effects of BMP-9 is mediated by ALK2 in these cells. BMP-9, together with the previously described BMP-2, -4, -5, -6 and -7, thus has the potential to counteract myelomagenesis in the bone marrow, and for the first time we show that BMP-9 is increased in myeloma patient serum compared with healthy controls. The cellular source for BMP-9 remains to be investigated. We also present data indicating that BMP-9, in contrast to BMP-2, -4 or -6, may be scavenged by soluble as well as membrane-bound endoglin. Thus, endoglin may regulate the availability of this cytokine for myeloma cells in the bone marrow microenvironment and could be a promising target for therapy.
Acknowledgments
The authors are grateful for valuable technical help from Hanne Hella, Lill Anny Gunnes Grøseth, Glenn Buene and Solveig Kvam. This work was supported by grants from the Norwegian Cancer Society, the Kristian Gerhard Jebsen Foundation for Medical Research, the Research Council of Norway, the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology, and Guldahl's legacy.
Author Contributions
OEO and TH planned and performed the experiments and wrote the paper, KFW, KM, TKV, TBR, BFS and SHM performed the experiments, TS planned the experiments, AKM, IT and AW provided the patient material and AS planned the experiments and wrote the paper.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Mahindra A, Laubach J, Raje N, Munshi N, Richardson PG, Anderson K. Latest advances and current challenges in the treatment of multiple myeloma. Nat Rev Clin Oncol. 2012;9:135–143. doi: 10.1038/nrclinonc.2012.15. [DOI] [PubMed] [Google Scholar]
- Kawamura C, Kizaki M, Yamato K, Uchida H, Fukuchi Y, Hattori Y, et al. Bone morphogenetic protein-2 induces apoptosis in human myeloma cells with modulation of STAT3. Blood. 2000;96:2005–2011. [PubMed] [Google Scholar]
- Hjertner O, Hjorth-Hansen H, Borset M, Seidel C, Waage A, Sundan A. Bone morphogenetic protein-4 inhibits proliferation and induces apoptosis of multiple myeloma cells. Blood. 2001;97:516–522. doi: 10.1182/blood.v97.2.516. [DOI] [PubMed] [Google Scholar]
- Ro TB, Holt RU, Brenne AT, Hjorth-Hansen H, Waage A, Hjertner O, et al. Bone morphogenetic protein-5, -6 and -7 inhibit growth and induce apoptosis in human myeloma cells. Oncogene. 2004;23:3024–3032. doi: 10.1038/sj.onc.1207386. [DOI] [PubMed] [Google Scholar]
- Holien T, Vatsveen TK, Hella H, Rampa C, Brede G, Groseth LA, et al. Bone morphogenetic proteins induce apoptosis in multiple myeloma cells by Smad-dependent repression of MYC. Leukemia. 2012;26:1073–1080. doi: 10.1038/leu.2011.263. [DOI] [PubMed] [Google Scholar]
- Seckinger A, Meissner T, Moreaux J, Goldschmidt H, Fuhler GM, Benner A, et al. Bone morphogenic protein 6: a member of a novel class of prognostic factors expressed by normal and malignant plasma cells inhibiting proliferation and angiogenesis. Oncogene. 2009;28:3866–3879. doi: 10.1038/onc.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukicevic S, Grgurevic L. BMP-6 and mesenchymal stem cell differentiation. Cytokine Growth Factor Rev. 2009;20:441–448. doi: 10.1016/j.cytogfr.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85-A:1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT, et al. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;11:1312–1320. doi: 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- Fong D, Bisson M, Laberge G, McManus S, Grenier G, Faucheux N, et al. Bone morphogenetic protein-9 activates Smad and ERK pathways and supports human osteoclast function and survival in vitro. Cell Signal. 2013;25:717–728. doi: 10.1016/j.cellsig.2012.12.003. [DOI] [PubMed] [Google Scholar]
- David L, Mallet C, Keramidas M, Lamande N, Gasc JM, Dupuis-Girod S, et al. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res. 2008;102:914–922. doi: 10.1161/CIRCRESAHA.107.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera B, Inman GJ. A rapid and sensitive bioassay for the simultaneous measurement of multiple bone morphogenetic proteins. Identification and quantification of BMP4, BMP6 and BMP9 in bovine and human serum. BMC Cell Biol. 2009;10:20. doi: 10.1186/1471-2121-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidart M, Ricard N, Levet S, Samson M, Mallet C, David L, et al. BMP9 is produced by hepatocytes and circulates mainly in an active mature form complexed to its prodomain. Cell Mol Life Sci. 2012;69:313–324. doi: 10.1007/s00018-011-0751-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttapreyasri S, Koontongkaew S, Phongdara A, Leggat U. Expression of bone morphogenetic proteins in normal human intramembranous and endochondral bones. Int J Oral Maxillofac Surg. 2006;35:444–452. doi: 10.1016/j.ijom.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A. Bone morphogenetic proteins: a critical review. Cell Signal. 2011;23:609–620. doi: 10.1016/j.cellsig.2010.10.003. [DOI] [PubMed] [Google Scholar]
- David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood. 2007;109:1953–1961. doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- Herrera B, van Dinther M, Ten Dijke P, Inman GJ. Autocrine bone morphogenetic protein-9 signals through activin receptor-like kinase-2/Smad1/Smad4 to promote ovarian cancer cell proliferation. Cancer Res. 2009;69:9254–9262. doi: 10.1158/0008-5472.CAN-09-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hose D, Moreaux J, Meissner T, Seckinger A, Goldschmidt H, Benner A, et al. Induction of angiogenesis by normal and malignant plasma cells. Blood. 2009;114:128–143. doi: 10.1182/blood-2008-10-184226. [DOI] [PubMed] [Google Scholar]
- Ye L, Kynaston H, Jiang WG. Bone morphogenetic protein-9 induces apoptosis in prostate cancer cells, the role of prostate apoptosis response-4. Mol Cancer Res. 2008;6:1594–1606. doi: 10.1158/1541-7786.MCR-08-0171. [DOI] [PubMed] [Google Scholar]
- Ye L, Kynaston H, Jiang WG. Bone morphogenetic protein-10 suppresses the growth and aggressiveness of prostate cancer cells through a Smad independent pathway. J Urol. 2009;181:2749–2759. doi: 10.1016/j.juro.2009.01.098. [DOI] [PubMed] [Google Scholar]
- Wang K, Feng H, Ren W, Sun X, Luo J, Tang M, et al. BMP9 inhibits the proliferation and invasiveness of breast cancer cells MDA-MB-231. J Cancer Res Clin Oncol. 2011;137:1687–1696. doi: 10.1007/s00432-011-1047-4. [DOI] [PubMed] [Google Scholar]
- Ye L, Bokobza S, Li J, Moazzam M, Chen J, Mansel RE, et al. Bone morphogenetic protein-10 (BMP-10) inhibits aggressiveness of breast cancer cells and correlates with poor prognosis in breast cancer. Cancer Sci. 2010;101:2137–2144. doi: 10.1111/j.1349-7006.2010.01648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera B, Garcia-Alvaro M, Cruz S, Walsh P, Fernandez M, Roncero C, et al. BMP9 is a proliferative and survival factor for human hepatocellular carcinoma cells. PLoS One. 2013;8:e69535. doi: 10.1371/journal.pone.0069535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castonguay R, Werner ED, Matthews RG, Presman E, Mulivor AW, Solban N, et al. Soluble endoglin specifically binds bone morphogenetic proteins 9 and 10 via its orphan domain, inhibits blood vessel formation, and suppresses tumor growth. J Biol Chem. 2011;286:30034–30046. doi: 10.1074/jbc.M111.260133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharpfenecker M, van Dinther M, Liu Z, van Bezooijen RL, Zhao Q, Pukac L, et al. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci. 2007;120:964–972. doi: 10.1242/jcs.002949. [DOI] [PubMed] [Google Scholar]
- Barbara NP, Wrana JL, Letarte M. Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamily. J Biol Chem. 1999;274:584–594. doi: 10.1074/jbc.274.2.584. [DOI] [PubMed] [Google Scholar]
- Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, et al. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J. 2004;23:4018–4028. doi: 10.1038/sj.emboj.7600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker L, Arthur HM. Endoglin expression in early development is associated with vasculogenesis and angiogenesis. Mech Dev. 2002;110:193–196. doi: 10.1016/s0925-4773(01)00562-7. [DOI] [PubMed] [Google Scholar]
- Pruneri G, Ponzoni M, Ferreri AJ, Decarli N, Tresoldi M, Raggi F, et al. Microvessel density, a surrogate marker of angiogenesis, is significantly related to survival in multiple myeloma patients. Br J Haematol. 2002;118:817–820. doi: 10.1046/j.1365-2141.2002.03654.x. [DOI] [PubMed] [Google Scholar]
- Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: evidence and potential applications. FASEB J. 2003;17:984–992. doi: 10.1096/fj.02-0634rev. [DOI] [PubMed] [Google Scholar]
- Wang JM, Wilson PB, Kumar S, Pye D, Hunter RD. Quantitation of endothelial cell specific protein E-9 employing a single monoclonal antibody in an indirect sandwich ELISA. J Immunol Methods. 1994;171:55–64. doi: 10.1016/0022-1759(94)90228-3. [DOI] [PubMed] [Google Scholar]
- Li C, Guo B, Wilson PB, Stewart A, Byrne G, Bundred N, et al. Plasma levels of soluble CD105 correlate with metastasis in patients with breast cancer. Int J Cancer. 2000;89:122–126. doi: 10.1002/(sici)1097-0215(20000320)89:2<122::aid-ijc4>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Calabro L, Fonsatti E, Bellomo G, Alonci A, Colizzi F, Sigalotti L, et al. Differential levels of soluble endoglin (CD105) in myeloid malignancies. J Cell Physiol. 2003;194:171–175. doi: 10.1002/jcp.10200. [DOI] [PubMed] [Google Scholar]
- Tsirakis G, Pappa CA, Spanoudakis M, Chochlakis D, Alegakis A, Psarakis FE, et al. Clinical significance of sCD105 in angiogenesis and disease activity in multiple myeloma. Eur J Intern Med. 2012;23:368–373. doi: 10.1016/j.ejim.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Pappa C, Alexandrakis M, Boula A, Psarakis F, Kolovou A, Bantouna V, et al. Emerging roles of endoglin/CD105 and angiogenic cytokines for disease development and progression in multiple myeloma patients. Hematol Oncol. 2013;34:201–205. doi: 10.1002/hon.2044. [DOI] [PubMed] [Google Scholar]
- Misund K, Baranowska KA, Holien T, Rampa C, Klein DC, Borset M, et al. A method for measurement of drug sensitivity of myeloma cells co-cultured with bone marrow stromal cells. J Biomol Screen. 2013;18:637–646. doi: 10.1177/1087057113478168. [DOI] [PubMed] [Google Scholar]
- Vindelov LL, Christensen IJ, Nissen NI. A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry. 1983;3:323–327. doi: 10.1002/cyto.990030503. [DOI] [PubMed] [Google Scholar]
- Holien T, Olsen OE, Misund K, Hella H, Waage A, Ro TB, et al. Lymphoma and myeloma cells are highly sensitive to growth arrest and apoptosis induced by artesunate. Eur J Haematol. 2013;91:339–346. doi: 10.1111/ejh.12176. [DOI] [PubMed] [Google Scholar]
- Fagerli UM, Holt RU, Holien T, Vaatsveen TK, Zhan F, Egeberg KW, et al. Overexpression and involvement in migration by the metastasis-associated phosphatase PRL-3 in human myeloma cells. Blood. 2008;111:806–815. doi: 10.1182/blood-2007-07-101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimsing P, Carlson K, Turesson I, Fayers P, Waage A, Vangsted A, et al. Effect of pamidronate 30 mg versus 90 mg on physical function in patients with newly diagnosed multiple myeloma (Nordic Myeloma Study Group): a double-blind, randomised controlled trial. Lancet Oncol. 2010;11:973–982. doi: 10.1016/S1470-2045(10)70198-4. [DOI] [PubMed] [Google Scholar]
- Wader KF, Fagerli UM, Holt RU, Stordal B, Borset M, Sundan A, et al. Elevated serum concentrations of activated hepatocyte growth factor activator in patients with multiple myeloma. Eur J Haematol. 2008;81:380–383. doi: 10.1111/j.1600-0609.2008.01130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holien T, Vatsveen TK, Hella H, Waage A, Sundan A. Addiction to c-MYC in multiple myeloma. Blood. 2012;120:2450–2453. doi: 10.1182/blood-2011-08-371567. [DOI] [PubMed] [Google Scholar]
- Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR, et al. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol. 2010;5:245–253. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchynskyi O, ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem. 2002;277:4883–4891. doi: 10.1074/jbc.M111023200. [DOI] [PubMed] [Google Scholar]
- Baughn LB, Di Liberto M, Niesvizky R, Cho HJ, Jayabalan D, Lane J, et al. CDK2 phosphorylation of Smad2 disrupts TGF-beta transcriptional regulation in resistant primary bone marrow myeloma cells. J Immunol. 2009;182:1810–1817. doi: 10.4049/jimmunol.0713726. [DOI] [PubMed] [Google Scholar]
- Huse K, Bakkebo M, Walchli S, Oksvold MP, Hilden VI, Forfang L, et al. Role of Smad proteins in resistance to BMP-induced growth inhibition in B-cell lymphoma. PLoS One. 2012;7:e46117. doi: 10.1371/journal.pone.0046117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.