Abstract
Brief, high-concentration (phasic) spikes in nucleus accumbens dopamine critically participate in aspects of food reward. Although physiological state (e.g., hunger, satiety) and associated hormones are known to affect dopamine tone in general, whether they modulate food-evoked, phasic dopamine specifically is unknown. Here, we used fast-scan cyclic voltammetry in awake, behaving rats to record dopamine spikes evoked by delivery of sugar pellets while pharmacologically manipulating central receptors for the gut “hunger” hormone ghrelin. Lateral ventricular (LV) ghrelin increased, while LV ghrelin receptor antagonism suppressed the magnitude of dopamine spikes evoked by food. Ghrelin was effective when infused directly into the lateral hypothalamus (LH), but not the ventral tegmental area (VTA). LH infusions were made in close proximity to orexin neurons, which are regulated by ghrelin and project to the VTA. Thus, we also investigated and found potentiation of food-evoked dopamine spikes by intra-VTA orexin-A. Importantly, intra-VTA blockade of orexin receptors attenuated food intake induced by LV ghrelin, thus establishing a behaviorally relevant connection between central ghrelin and VTA orexin. Further analysis revealed that food restriction increased the magnitude of dopamine spikes evoked by food independent of any pharmacological manipulations. The results support the regulation of food-evoked dopamine spikes by physiological state with endogenous fluctuations in ghrelin as a key contributor. Our data highlight a novel mechanism by which signals relating physiological state could influence food reinforcement and food-directed behavior.
Keywords: feeding, mesolimbic, nucleus accumbens, obesity, reward, voltammetry
Introduction
The prevalence of inexpensive, highly palatable foods is a major contributor to the soaring rate of obesity (Drewnowski and Specter, 2004). The positive reinforcing nature of these foods has led to a focus on mesolimbic circuitry. Feeding broadly increases dopamine release and turnover in the nucleus accumbens (NAc; Hernandez and Hoebel, 1988; Taber and Fibiger, 1997) and the magnitude of dopamine release is dependent on physiological state (i.e., hunger vs satiety; Wilson et al., 1995; Ahn and Phillips, 1999; Bassareo and Di Chiara, 1999). In addition to broad increases in dopamine concentration, food reward also evokes phasic spikes in striatal dopamine concentration (Day et al., 2007; Brown et al., 2011; Beeler et al., 2012), which are the result of burst firing of midbrain dopamine neurons (Sombers et al., 2009; Zweifel et al., 2009; Owesson-White et al., 2012). These brief dopamine spikes play a critical role in reinforcement learning (Day et al., 2007; Stuber et al., 2008; Steinberg et al., 2013) and are thought to drive food-seeking behaviors (Roitman et al., 2004; Wanat et al., 2010). Food restriction increases burst firing of dopamine neurons in anesthetized mice (Branch et al., 2013). However, the mechanisms by which physiological state change, induced by food restriction, increases dopamine system activity remain unknown.
Ghrelin, a peptide released by the stomach, is elevated by food restriction (Tschöp et al., 2000), and is the only known gut peptide associated with hunger (Cummings et al., 2004). Peripheral ghrelin crosses the blood–brain barrier (Banks et al., 2002) and ghrelin receptors are expressed throughout the brain (Zigman et al., 2006) including the ventral tegmental area (VTA)/substantia nigra–the site of striatal-projecting dopamine neurons. Ghrelin promotes food seeking through multiple brain circuits, including the arcuate nucleus of the hypothalamus (Nakazato et al., 2001), the lateral hypothalamus (LH; Olszewski et al., 2003), and ventral hippocampus (Kanoski et al., 2013) as well as the mesolimbic system (Abizaid et al., 2006). In addition to the VTA, many of these sites directly interact with the mesolimbic system. Indeed, both peripheral and central administration of ghrelin increases dopamine concentration in the NAc, as measured by microdialysis (Jerlhag et al., 2006; Jerlhag, 2008). Thus, ghrelin receptors pose a likely target for modulation of phasic dopamine signaling evoked by food.
We measured dopamine signaling in the NAc core using fast-scan cyclic voltammetry (FSCV) while rats (fed ad libitum or food restricted) retrieved sugar pellets delivered with a variable and randomly selected intertrial interval. Retrieval of each pellet was associated with a spike in dopamine concentration. We hypothesized that within-session central ghrelin manipulations would modulate these dopamine spikes and sought to determine site specificity for central ghrelin effects on phasic dopamine signaling.
Materials and Methods
Subjects.
Male Sprague Dawley rats (n = 47; Charles River) weighing 325–425 g at the time of testing were used. Rats were individually housed with lights on from 7:00 A.M.to 7:00 P.M. All training and experimental sessions took place during the light phase in standard operant chambers (Med Associates) with a food receptacle and magazine for the delivery of single 45 mg sugar pellets (3.58 kcal/g; BioServ). Rats were trained to retrieve sugar pellets that were delivered with a random intertrial interval (delivery interval range: 30–90 s; mean: 60 ± 8.2 s). Following 5 d of training, rats were surgically prepared for FSCV. After returning to presurgery body weight, rats were retrained for 2 d before the experimental session. Animal care and use was in accordance with the National Institutes for Health Guide for the Care and Use of Laboratory Animals, and approved by the Institutional Animal Care and Use Committee at the University of Illinois at Chicago.
Surgery.
Rats were anesthetized with ketamine hydrochloride (100 mg/kg, i.p.) and xylazine hydrochloride (10 mg/kg, i.p.). All implants were targeted relative to bregma using the rat brain atlas of Paxinos and Watson (2007). A guide cannula (Bioanalytical Systems) was implanted dorsal to the right NAc core (+1.3 mm AP, +1.5 mm ML, −2.5 mm DV). An infusion cannula (Plastics One) was also implanted [lateral ventricle (LV): 22 gauge, 11 mm cannula (GC313), −0.8 mm AP, −2.1 mm ML, −3.7 mm DV, angled 10° away from the midline; VTA: 26 gauge 11 mm cannula (C315), −5.8 mm AP, +2.9 mm ML, −6.5 mm DV, angled 15° away from the midline; LH: 22 gauge 11 mm cannula, −3.1 mm AP, +1.7 mm ML, −7.1 mm DV]. LH coordinates were selected to target orexin neurons (Fadel and Deutch, 2002), and VTA coordinates were chosen to maximize the likelihood of affecting VTA neurons that project to the NAc core (Ikemoto, 2010). A chlorinated silver reference electrode was placed in left forebrain. Stainless steel skull screws and dental cement secured implants to the skull.
Experimental protocol.
During an experimental session, rats were placed into operant chambers as above. FSCV in awake and behaving rats and analyte identification and quantification have been extensively described previously (Phillips et al., 2003; Cone et al., 2013). Briefly, a micromanipulator containing a glass-insulated carbon fiber (∼75 μm; Goodfellow) (recording) electrode was inserted into the NAc guide cannula. The recording electrode was then lowered into NAc and locked into place. A FSCV headstage (University of Washington EME Shop) was used to tether the rat, apply voltage changes, and measure resultant current changes. The electrode voltage was held at −0.4 V and ramped in a triangular fashion (−0.4 to +1.3 to −0.4 V; 400 V/s) at 10 Hz. In addition, an injector connected to a 10 μl Hamilton syringe was inserted into the infusion cannula. To verify that food reward reliably evoked phasic dopamine release, a single sugar pellet was delivered. If this failed to evoke dopamine release, the electrode was advanced 0.16 mm and the process was repeated.
Once a stable release site was confirmed, the experimental session began. Electrochemical data were synced with video and recorded during the entire session. After 10 pellets (mid-session), an infusion pump was activated to deliver an intracranial infusion. For LV experiments, n-octanoylated ghrelin (1 μg in 1 μl 0.9% saline; American Peptide), d-[Lys]-GHRP (1 μg in 1 μl 0.9% saline; Tocris Bioscience), or vehicle was infused at a rate of 2 μl/min through a 28 gauge injector (1 mm projection beyond the infusion cannula). Both LH and VTA rats received either n-octanoylated ghrelin [(0.4 μg in 0.3 μl artificial CSF (aCSF)] or vehicle at a rate of 0.15 μl/min. LH injectors were 28 gauge (1 mm projection) and VTA injectors were 33 gauge (2 mm projection). After the recording session, electrodes were removed; rats were disconnected from the headstage and returned to their home cage. Ad libitum chow intake was then monitored for the next 30 min. Following experiments, all recording electrodes were calibrated in a flow cell as described previously (Sinkala et al., 2012) to convert detected current to concentration. The average calibration factor for all electrodes used in these experiments was 43.47 ± 1.9 nm/nA.
Data analysis.
All rats retrieved and consumed all pellets before and after pharmacological manipulations. Individual trials were background subtracted and dopamine concentration during the 10 s before to 10 s after pellet retrieval was extracted from voltammetric data using principal component analysis (Heien et al., 2004; Day et al., 2007). We calculated peak dopamine concentration evoked by pellet retrieval by finding the maximum dopamine concentration during the 1 s before to 1 s after retrieval of each individual pellet. These values were averaged and compared before and after LV, LH, or VTA infusions.
Verification of cannula placements and recording sites.
After experiments, LV cannula placements were verified by injecting angiotensin II into the LV (50 ng in 2 μl saline; Sigma-Aldrich) and measuring home cage water intake. Angiotensin II caused drinking (at least 7 ml in 30 min) in all rats. Following completion of experiments, rats were deeply anesthetized with sodium pentobarbital (100 mg/kg) and a small electrolytic lesion was made at the voltammetry recording site using a polyimide-insulated stainless steel electrode (A-M Systems). Rats were then transcardially perfused with cold 0.01 m PBS followed by 10% buffered formalin solution (Sigma). Brains from rats with LV cannula were removed and stored in formalin. Brains from LH and VTA cannulated rats were removed and stored in formalin for 24 h and transferred to 30% sucrose in 0.1 m phosphate buffer (PB). All brains were sectioned at 40 μm on a cryostat. NAc sections were mounted and lesion locations were verified using light microscopy in conjunction with the rat brain atlas of Paxinos and Watson (2007). All recordings were confirmed to be from the NAc core (Fig. 1A–D). Sections containing LH or VTA were stored in 5% sucrose/0.01% NaN3 in 0.1 m PB until immunohistochemical staining. Of the rats that received LH infusions, a subset was immunohistochemically stained for orexin-A, and in all six rats (aCSF: n = 3; ghrelin: n = 3), cannula tips were confirmed to be in close proximity (∼100 μm) to orexin-A-positive cell bodies. Of the rats that received VTA infusions, a subset was immunohistochemically stained for tyrosine hydroxylase (TH), and in all 10 rats (aCSF: n = 3; ghrelin: n = 3; orexin-A: n = 4), cannula tips were confirmed to be in TH-positive regions of the VTA. Cannula tips from all remaining animals were confirmed to be in their targeted locations (LH or VTA) using light microscopy.
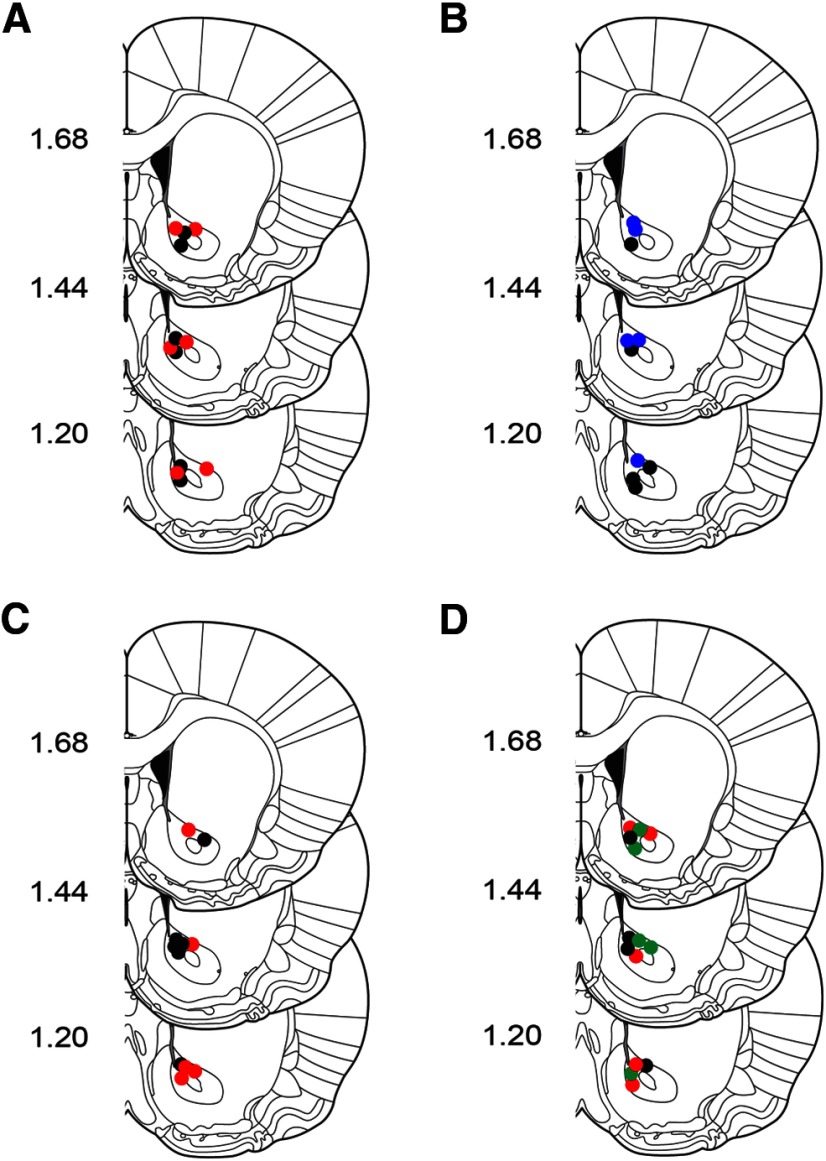
Figure 1.
Summary of NAc recording sites. Coronal brain sections modified from Paxinos and Watson (2007). Colored circles indicate approximate recording locations. Numbers at left indicate approximate distance from bregma. A, Recording locations from ad libitum fed rats that received LV saline (n = 6) or ghrelin (n = 6). B, Recording locations from food-restricted rats that received LV saline (n = 5) or d-[Lys]-GHRP (n = 5). C, Recording locations from ad libitum fed rats that received LH aCSF (n = 6) or ghrelin (n = 5). D, Recording locations from ad libitum fed rats that received intra-VTA aCSF (n = 4), ghrelin (n = 5), or orexin-A (n = 5). Black, vehicle (saline or aCSF); red, ghrelin; blue, d-[Lys]-GHRP; green, orexin-A.
Immunohistochemistry.
Standard methods were adapted for orexin-A and TH immunohistochemistry (Mahler and Aston-Jones, 2012; McCutcheon et al., 2012). Briefly, sections containing either LH or VTA were placed in 0.3% H2O2 for 10 min and then blocking solution containing either 3% normal donkey serum (orexin-A) or 3% normal goat serum (TH) and 0.3% Triton-X for 2 h. Sections were incubated overnight at room temperature in blocking solution with primary antibody (goat anti-orexin-A, 1:2000, SC-8070; Santa Cruz Biotechnology; rabbit anti-TH, 1:1000, AB-152; Millipore). Next, sections were incubated in biotinylated secondary antibody (orexin-A: 2 h, 1:500, Santa Cruz Biotechnology; TH: 1.5 h, 1:500, Vector Laboratories). Sections were then transferred to an avidin–biotin complex (1:500; Vector Laboratories) for 1.5 h (orexin-A) or 45 min (TH) before being reacted with DAB (0.02%; Vector Laboratories) for 3–7 min. Finally, sections were mounted, dehydrated, and coverslipped.
Assessing the role of VTA orexin receptors in food intake induced by LV ghrelin.
Rats were fed ad libitum during all phases of this experiment. Naive rats (n = 6) were anesthetized and prepared for stereotaxic surgery as described above. A single cannula was targeted to the left LV and bilateral cannulae were directed at the VTA. We used the same cannulae, coordinates, and procedures as described above. Following recovery, rats had two nights of pre-exposure to 20 of the same sugar pellets that were used in the voltammetry sessions. Next, rats were habituated to the testing procedure. The test chamber was a standard clear plastic rodent cage with a wire top, which was identical to the home cage except without bedding, to help account for spillage. A small receptacle was filled with 10 g of sugar pellets, placed in the corner of the cage, and secured to the floor. Rats were allowed to freely consume sugar pellets for 1 h before they were removed from the testing chamber.
On test days, we infused either the orexin receptor antagonist SB-334867 (SB; 1 μg in 0.3 μl 50% DMSO in sterile water) or vehicle bilaterally into the VTA, followed by ghrelin (1 μg in 1 μl 0.9% saline) or vehicle into the left LV. Infuser gauge and infusion speeds were identical to those used above. Following infusions, animals were placed in the test chamber and allowed to freely consume sugar pellets for 1 h. After the session, rats were returned to their home cage and total consumption was recorded. Thus, the four treatments were as follows: LV-vehicle, VTA-vehicle; LV-vehicle, VTA-SB; LV-ghrelin, VTA-SB; LV-ghrelin, VTA-vehicle. Each rat received all treatments, which were counterbalanced across days. Testing sessions occurred once every other day. Following completion, LV cannula locations were confirmed with angiotensin II. Rats were then transcardially perfused, brains removed, sliced on a cryostat, and tissue was prepared for light microscopy as described above for verification of VTA cannula locations.
Statistical analysis.
Peak dopamine concentration evoked during pellet retrieval was compared using a two-way [epoch (pre, post-infusion) × treatment (vehicle, ghrelin)] ANOVA, with Tukey's HSD post hoc tests where appropriate. In the voltammetry experiments, food intake was compared using an unpaired t test (two groups) or one-way ANOVA (>2 groups). Food intake following concurrent LV and VTA manipulations was compared using a two-way repeated-measures ANOVA[LV treatment (vehicle, ghrelin) × VTA treatment (vehicle, SB)] ANOVA. Statistical analyses were performed using GraphPad 5.0 (Prism), Statistica 10 (StatSoft), or SPSS Version 20.0 (IBM).
Results
LV ghrelin potentiates food-evoked dopamine spikes in ad libitum fed rats
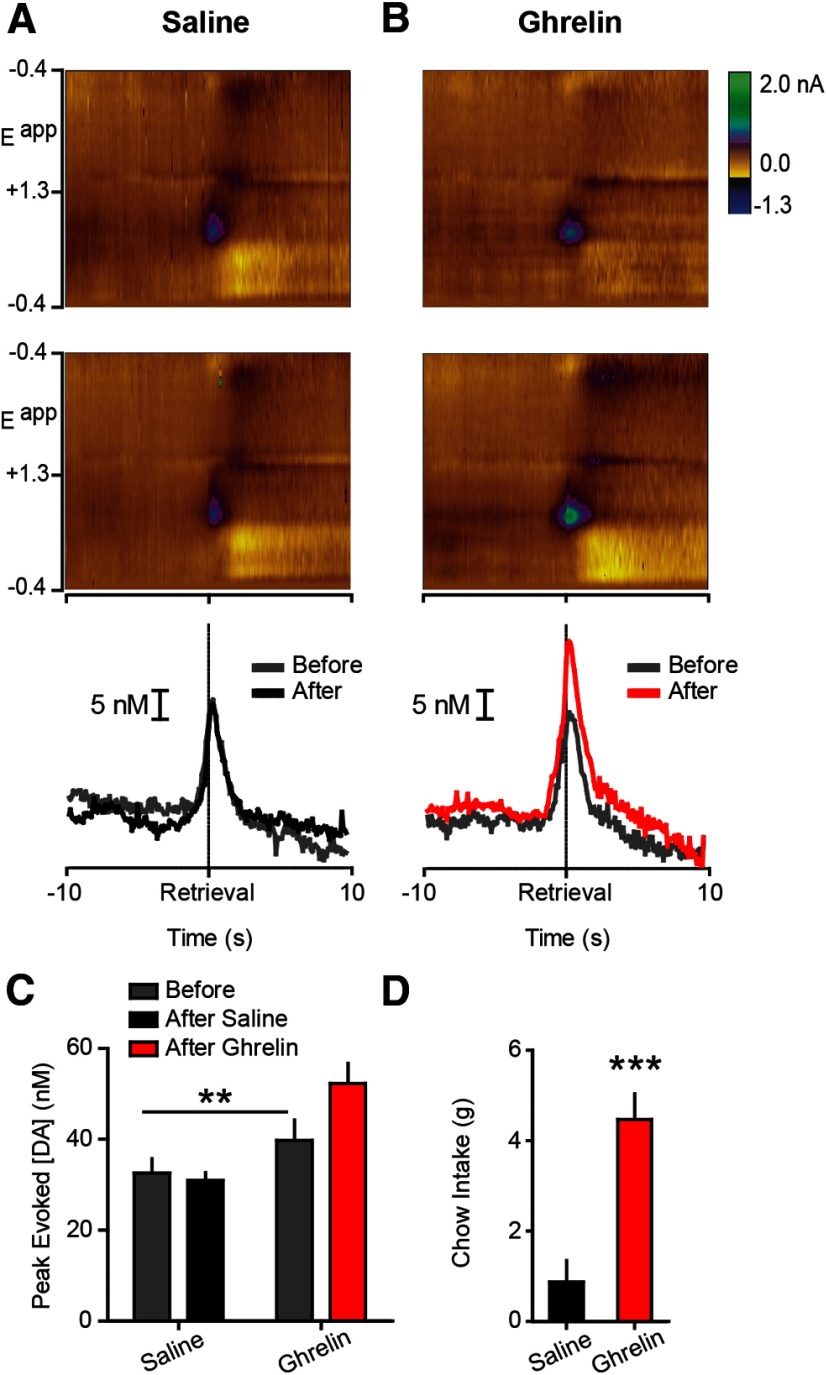
To determine whether ghrelin centrally regulates phasic dopamine signaling evoked by food, we used ad libitum fed rats with cannula in the LV and infused vehicle (1 μl saline; n = 6) or ghrelin (1 μg; n = 6) into the LV mid-session. LV ghrelin increased the magnitude of dopamine spikes evoked during pellet retrieval (epoch × treatment interaction (F(1,10) = 15.96, p < 0.01); post hoc: post-ghrelin p < 0.01 vs all comparisons; Fig. 2A–C). Ghrelin significantly augmented postrecording session home cage chow intake relative to vehicle (t(10) = 4.816, p < 0.001; Fig. 2D).
Figure 2.
Ghrelin increases phasic dopamine evoked by food reward in ad libitum fed rats. A, Top, Average colorplot (10 trials/rat; n = 6) depicting current changes (color) as a function of electrode potential (y-axis) in the 10 s before and after (x-axis) pellet retrieval. Dopamine [identified by its oxidation (approximately +0.6 V; green) and reduction (approximately −0.2 V; light yellow) features] was transiently evoked during pellet retrieval, before vehicle infusion. Middle, Average colorplot after LV vehicle infusion. Bottom, Average dopamine concentration aligned to retrieval before and after LV saline extracted from individual colorplots using chemometric analysis (Heien et al., 2004). B, Average colorplots before (top) and after LV ghrelin (middle; 10 trials/rat; n = 6). Average dopamine concentration aligned to pellet retrieval before and after LV ghrelin (bottom). C, Peak dopamine evoked during pellet retrieval, before and after LV vehicle or ghrelin. D, Postsession chow intake following LV vehicle or ghrelin. Error bars indicate mean ± SEM; **p < 0.01, ***p < 0.001.
Central ghrelin receptor antagonism suppresses food-evoked dopamine spikes in food restricted rats
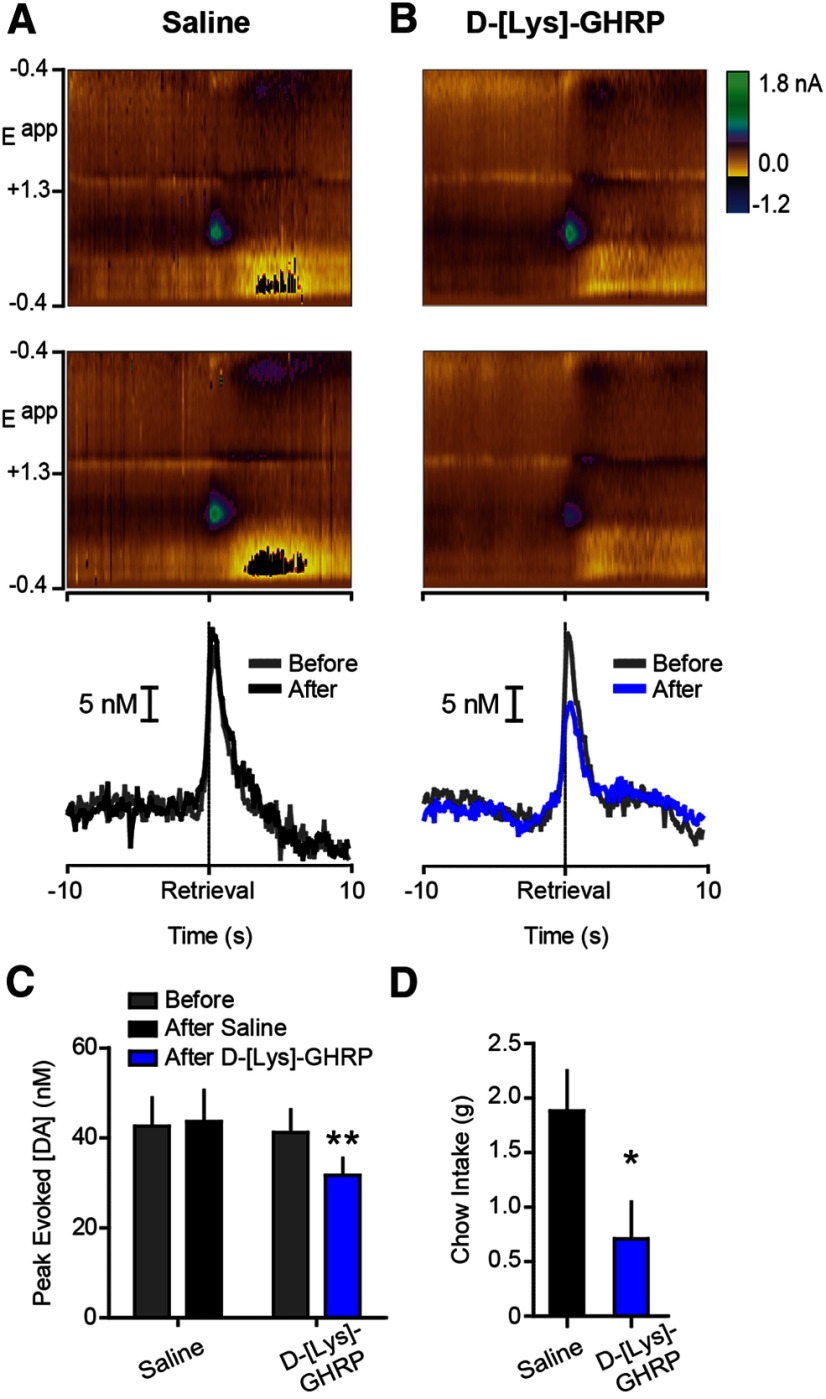
We next sought to determine whether endogenous ghrelin modulates dopamine spikes evoked by food. Rats were food restricted and either vehicle (1 μl saline; n = 5) or the ghrelin receptor antagonist d-[Lys]-GHRP (1 μg; n = 5) was infused into the LV mid-session. Ghrelin receptor antagonism suppressed dopamine spikes evoked during pellet retrieval (epoch × treatment interaction (F(1,8) = 13.17, p < 0.01); post hoc: d-[Lys]-GHRP p < 0.01 vs pre-infusion; Fig. 3A–C). LV d-[Lys]-GHRP also suppressed postrecording session home cage chow intake (t(8) = 2.346, p < 0.05; Fig. 3D).
Figure 3.
Ghrelin receptor antagonism attenuates phasic dopamine evoked by food reward in food-restricted rats. A, B, Top, middle, Conventions are identical to those in Figure 2 except before and after vehicle (10 trials/rat; n = 5) or d-[Lys]-GHRP (10 trials/rat; n = 5). Bottom, Average dopamine concentration aligned to pellet retrieval before and after LV saline or d-[Lys]-GHRP. C, Peak dopamine evoked during pellet retrieval, before and after LV saline or d-[Lys]-GHRP. D, Postsession chow intake following LV saline or d-[Lys]-GHRP. Error bars indicate mean ± SEM; *p < 0.05, **p < 0.01.
Central ghrelin potentiates food-evoked dopamine spikes in a site-specific manner
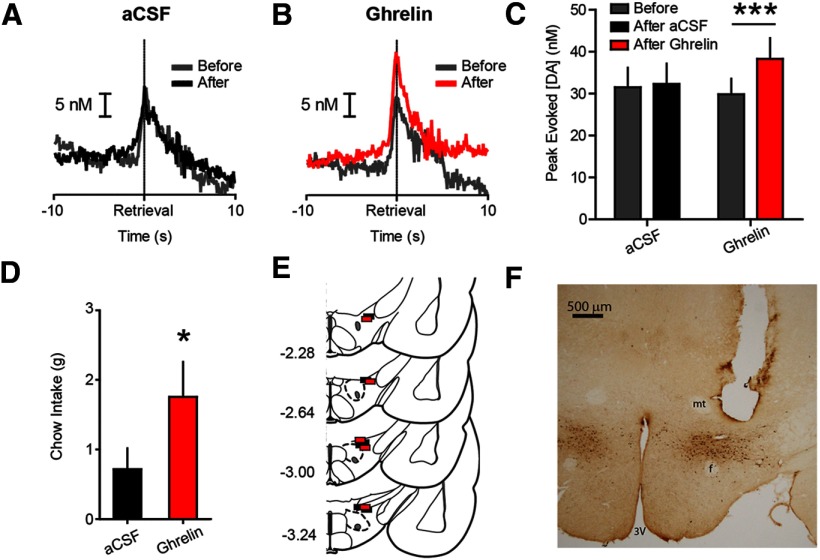
To determine ghrelin's site of action on phasic dopamine signaling, we targeted the LH due to its sensitivity to ghrelin (Olszewski et al., 2003) and strong link with the mesolimbic system (Peyron et al., 1998; Fadel and Deutch, 2002; Watabe-Uchida et al., 2012). The LH of ad libitum fed rats was infused, mid-session, with either vehicle (0.3 μl aCSF; n = 6) or ghrelin (0.4 μg; n = 5). LH ghrelin potentiated dopamine spikes evoked during pellet retrieval (epoch × treatment interaction (F(1,9) = 22.30, p < 0.01); post hoc: LH ghrelin p < 0.001 vs pre-infusion; Fig. 4A–C)– recapitulating the effects of intraventricular ghrelin. LH ghrelin also increased postrecording session home cage chow intake compared with vehicle (t(9) = 2.519, p < 0.05; Fig. 4D). All cannula tips were confirmed to be in the LH (Fig. 4E) and immunohistochemistry indicated infusions were near orexin-A-positive cell bodies (Fig. 4F).
Figure 4.
Phasic dopamine evoked during pellet retrieval is potentiated by intra-LH ghrelin. A, Average dopamine concentration evoked during pellet retrieval before (gray) and after (black) intra-LH aCSF infusion (n = 6). B, Average dopamine concentration evoked during pellet retrieval before (gray) and after (red) intra-LH ghrelin infusion (n = 5). C, Peak dopamine evoked during pellet retrieval before and after intra-LH infusion of aCSF or ghrelin. D, Intra-LH ghrelin increased postsession chow intake, relative to vehicle. E, Coronal brain sections modified from Paxinos and Watson (2007). Colored squares indicate approximate locations of cannula tips from all rats used in LH infusion experiments (black, aCSF; red, ghrelin). Numbers at left indicate approximate distance from bregma in millimeters. F, Representative coronal brain slice with LH cannula stained for orexin-A. 3V, third ventricle; f, fornix; mt, mammillothalamic tract. Error bars indicate mean ± SEM; *p < 0.05, ***p < 0.001.
As ghrelin acts in the VTA (Abizaid et al., 2006) to influence feeding and food-directed motivation (Skibicka et al., 2013), we sought to determine whether intra-VTA ghrelin modulates phasic dopamine signaling. In addition, our data suggested, and considerable evidence supports, a link between ghrelin and LH orexin neurons (Olszewski et al., 2003; Toshinai et al., 2003; Perello et al., 2010). Given that orexin regulates the excitability of VTA dopamine neurons (Korotkova et al., 2003; Borgland et al., 2006), we also investigated whether the effects of LH ghrelin could be recapitulated by intra-VTA orexin-A. In ad libitum fed rats, we infused vehicle (0.3 μl aCSF; n = 4), ghrelin (0.4 μg; n = 5), or orexin-A (1 μg; n = 5) into the VTA mid-session. Intra-VTA orexin-A, but not ghrelin, increased the magnitude of dopamine spikes evoked during pellet retrieval (epoch × treatment interaction (F(2,11) = 8.69, p < 0.01); post hoc: orexin-A p < 0.01 compared with before infusion; Fig. 5A–D). Both intra-VTA ghrelin and orexin-A potentiated postrecording session home cage chow intake compared with vehicle (F(2,11) = 5.18, both p < 0.05); post hoc: ghrelin, orexin-A p < 0.05 vs vehicle; Fig. 5E). All cannula tips were confirmed to be in the VTA by light microscopy (Fig. 5F) and immunohistochemistry for TH (Fig. 5G).
Figure 5.
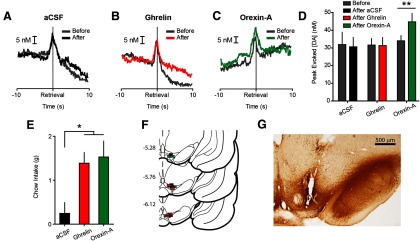
Intra-VTA orexin-A, but not ghrelin, potentiates phasic NAc dopamine evoked during pellet retrieval. A, Average dopamine concentration evoked during pellet retrieval before (gray) and after (black) intra-VTA aCSF infusion (n = 4). B, Average dopamine concentration evoked during pellet retrieval before (gray) and after (red) intra-VTA ghrelin infusion (n = 5). C, Average dopamine concentration evoked during pellet retrieval before (gray) and after (green) intra-VTA orexin-A infusion (n = 5). D, Peak dopamine evoked during pellet retrieval before and after intra-VTA infusion of aCSF, ghrelin, or orexin-A. E, Intra-VTA ghrelin and orexin-A significantly increased postsession chow intake, relative to vehicle. F, Coronal brain sections modified from Paxinos and Watson (2007). Colored squares indicate approximate locations of cannula tips from all rats used in VTA infusion experiments (black, aCSF; red, ghrelin; green, orexin-A). Numbers at left indicate approximate distance from bregma in millimeters. G, Representative coronal brain slice with VTA cannula stained for TH. Error bars indicate mean ± SEM; *p < 0.05, **p < 0.01.
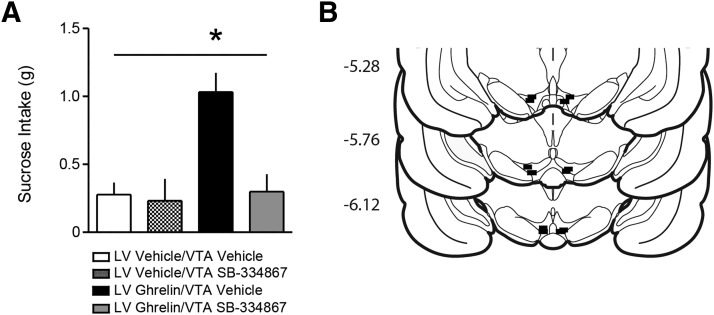
LV ghrelin-induced feeding is suppressed by VTA orexin receptor antagonism
The results of our voltammetry experiments suggest that orexin action in the VTA is a possible mechanism by which LV and LH ghrelin could regulate dopamine signaling. Thus, we sought to provide evidence for a functional relationship between central ghrelin and VTA orexin. We tested whether VTA orexin signaling was critical for feeding induced by central ghrelin infusions in ad libitum fed rats (n = 6). We found that bilateral blockade of VTA orexin receptors with SB significantly attenuated the ability of LV ghrelin to promote intake of rewarding food (LV treatment × VTA treatment interaction (F(1,5) = 8.59, p < 0.05); Fig. 6A). As before, cannula tips were confirmed to be in the LV by angiotensin-induced drinking and in the VTA by light microscopy (Fig. 6B).
Figure 6.
Bilateral intra-VTA orexin antagonism attenuates feeding induced by LV ghrelin. A, Sucrose pellet consumption following LV ghrelin is suppressed by bilateral VTA infusions of the orexin receptor antagonist SB-334867 but not vehicle (n = 6). B, Coronal brain sections modified from Paxinos and Watson (2007). Black rectangles indicate approximate VTA infusion locations. Numbers at left indicate approximate distance from bregma. Error bars indicate mean ± SEM; *LV × VTA interaction p < 0.05.
Physiological state modulates food-evoked dopamine spikes
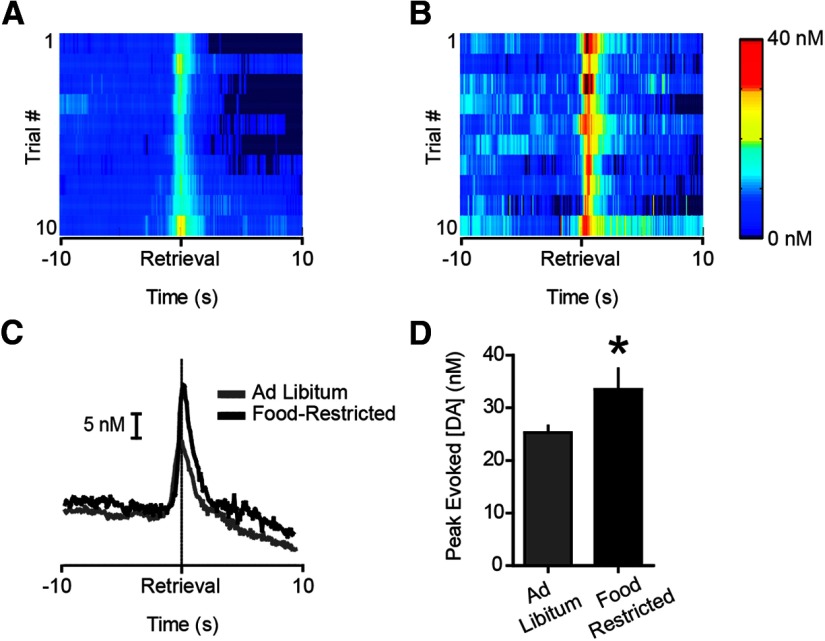
Our pharmacological manipulations suggested that endogenous signaling related to physiological state influences phasic dopamine signaling. Thus, it was critical to determine whether caloric restriction results in measurable differences in the phasic dopamine response to food. To investigate this possibility, we combined data from all ad libitum fed (n = 37) and food-restricted rats (n = 10) used in this study and compared the trials that occurred before any pharmacological manipulations were made. Consistent with the notion that the physiological state endogenously regulates phasic dopamine signaling, food restriction significantly augmented dopamine spikes evoked during pellet retrieval (t(45) = 2.61, p < 0.05; Fig. 7A–D).
Figure 7.
Phasic dopamine evoked during pellet retrieval is augmented by food restriction. A, Heatplot depicting average dopamine concentration per trial for all ad libitum fed rats from the experiments presented in this manuscript (n = 37). Each row represents the average dopamine concentration for each of the 10 pellet retrievals before any infusions were made. B, Same conventions as in (A) but for all food-restricted rats used in the experiments presented in this manuscript (n = 10). C, Average dopamine concentration evoked during pellet retrieval in ad libitum (gray) versus food-restricted (black) rats. D, Peak dopamine concentration evoked during pellet retrieval is augmented by food-restriction. Error bars indicate mean ± SEM; *p < 0.05.
Discussion
We found that the gut hormone ghrelin acts centrally to regulate phasic dopamine spikes evoked by food reward via action in the LH, but not VTA. The effect of LH ghrelin was recapitulated by intra-VTA orexin-A, and food intake induced by LV ghrelin was attenuated following blockade of VTA orexin receptors, suggesting a potential mechanism of action for LV and LH ghrelin. Importantly, food restriction augmented dopamine spikes evoked by food independent of any pharmacological manipulations, implying that endogenous signaling related to physiological state can influence the phasic dopamine response to food reward. Indeed, food restriction increases burst firing of dopamine neurons in anesthetized mice (Branch et al., 2013) and potentiates dopamine release evoked during feeding (Wilson et al., 1995). Food restriction could broadly affect multiple signals related to physiological state, including blood glucose levels, leptin secretion, and vagal tone. However, given that ghrelin is elevated by food restriction (Tschöp et al., 2000; Cummings et al., 2004), these data suggest that one way physiological state dynamically tunes the phasic dopamine response to food reward is through central ghrelin signaling.
The LH has long been known to link energy status and food-motivated behavior (Margules and Olds, 1962; Berthoud and Münzberg, 2011). The results of our LH manipulations suggest that metabolic hormones can act in the LH to modulate the phasic dopamine response to food reward. In addition, ghrelin affects food intake and food-related reinforcement through actions on LH orexin neurons (Olszewski et al., 2003; Toshinai et al., 2003; Perello et al., 2010). Thus, the effect of intra-VTA orexin-A on phasic dopamine release and the ability of intra-VTA orexin receptor antagonism to blunt feeding in response to LV ghrelin suggests a potential mechanism by which LV and LH ghrelin could enhance food-evoked dopamine spikes. Indeed, intra-VTA orexin-A increases, while intra-VTA orexin receptor antagonism decreases the magnitude of phasic dopamine evoked by electrical stimulation of the VTA (España et al., 2010, 2011). We extend these findings by showing that orexin-A in the VTA increases the magnitude of phasic dopamine evoked by behaviorally relevant natural rewards. Additionally, food intake induced by LV ghrelin is blunted in orexin knock-out mice (Toshinai et al., 2003). The results of our concurrent LV and VTA manipulations highlight a critical role for VTA orexin in feeding induced by central ghrelin. Together, these data suggest that ghrelin acts via the LH as an interface between physiological state and motivational circuitry and highlights a mechanism by which peripheral feeding hormones influence food-specific signaling and food-directed behavior, particularly in areas of the brain linked to reward seeking.
It is noteworthy that we did not observe an effect of intra-VTA ghrelin on phasic dopamine signaling. Ghrelin receptors are present on VTA dopamine neurons (Abizaid et al., 2006) and previous evidence suggests that administration of ghrelin into the VTA increases dopamine in the NAc shell (Jerlhag et al., 2007). However, the present study measured dopamine in the NAc core, suggesting that there could be regional differences in ghrelin's effects on dopamine signaling. It is possible that ghrelin receptors are expressed on distinct pools of VTA dopamine neurons that selectively project to subterritories other than the core. Indeed, VTA dopamine neurons respond differently to pharmacological challenges based on their projection targets (Lammel et al., 2008). Another possibility is that intra-VTA ghrelin could generally increase the activity of VTA dopamine neurons without altering phasic, event-specific dopamine signals, a phenomenon that would not be well captured using FSCV. Future studies that involve tracing the projections of ghrelin receptor expressing VTA dopamine neurons in addition to comparing the effects of VTA ghrelin on dopamine fluctuations in distinct striatal subregions will speak to these discrepancies.
We have identified one region where ghrelin regulates (LH) and another region where ghrelin does not regulate (VTA) phasic dopamine signaling. However, ghrelin receptors are expressed throughout the brain (Zigman et al., 2006) and central ghrelin diffuses to a variety of brain nuclei, including the LH and VTA but also the arcuate nucleus of the hypothalamus and nucleus of the solitary tract (NTS; Cabral et al., 2013). Given that recent work has identified previously unknown projections from the NTS to the VTA (Alhadeff et al., 2012), additional sites abundant in ghrelin receptors require further study. Many ghrelin receptor-expressing brain regions could either directly or indirectly regulate dopamine neurons. When given to humans, ghrelin increases the BOLD response to images of food across multiple brain regions linked to appetitive behavior (striatum, amygdala, and orbitofrontal cortex; Malik et al., 2008). Thus, the LH is unlikely to be the only brain site where ghrelin influences phasic dopamine signaling.
While VTA ghrelin did not affect phasic dopamine signaling, it did increase home cage food intake suggesting that phasic dopamine signaling may not be a reliable predictor of consummatory behavior (for review, see Salamone and Correa, 2012). This result is consistent with previous research indicating that manipulations of NAc dopamine do not alter free-feeding intake (Koob et al., 1978; Taber and Fibiger, 1997; Baldo et al., 2002). Dopamine may play a more critical role in motivating behavior toward high-effort, high-value options. Blocking NAc dopamine shifts choices away from operant responses in favor of freely available chow, but leaves total caloric intake unchanged (Salamone et al., 1991; Koch et al., 2000). The converse is true of hyperdopaminergic mice, as these animals will exert more effort to obtain food, but do not consume more food overall (Beeler et al., 2010). Furthermore, a recent report indicates that feeding elicited by intra-VTA ghrelin does not require NAc dopamine receptor activation (Skibicka et al., 2013). Thus, that VTA ghrelin promotes food intake in the absence of changes in phasic dopamine signaling is consistent with the notion that NAc dopamine is not required for normal food intake and VTA ghrelin can regulate feeding in a dopamine independent manner. Moreover, it speaks to VTA cell groups other than NAc core-projecting dopamine neurons as playing a critical role in feeding.
Even though changes in phasic dopamine release may not directly contribute to food consumption, our data have broad implications for food-related behaviors. Recent optogenetic experiments have revealed that phasic dopamine signaling is sufficient for aspects of behavioral reinforcement (Tsai et al., 2009; Witten et al., 2011) and reinforcement learning (Steinberg et al., 2013). Cues that signal the availability of food or the opportunity to respond for food evoke phasic dopamine release in the NAc core (Roitman et al., 2004; Day et al., 2007; Stuber et al., 2008), where dopamine is critical for pavlovian and instrumental learning (Smith-Roe and Kelley, 2000; Di Ciano et al., 2001) as well as performance (Gore and Zweifel, 2013). Genetic manipulations that attenuate phasic dopamine release impair cue-mediated learning motivated by food reward (Zweifel et al., 2009). As mentioned above, NAc dopamine may direct food-seeking behavior according to cost-benefit relationships among available food sources (Salamone et al., 1991; Koch et al., 2000; Beeler et al., 2010). Phasic dopamine signaling may also play a role in these processes, as it has been shown to encode effort and cost variables related to food seeking (Day et al., 2010; Gan et al., 2010; Wanat et al., 2010; Sugam et al., 2012). Physiological state may also factor into such decisions, as ghrelin shifts food preferences toward higher calorie options (Perello et al., 2010).Together, our data suggest that modulation of phasic dopamine signaling by ghrelin and physiological state may play a role in a wide range of food-directed behaviors.
Energy-related signals, such as leptin and ghrelin, have been shown to alter the activity of dopamine neurons in vitro (Abizaid et al., 2006) and in anesthetized animals (Hommel et al., 2006). Additionally, ghrelin broadly increases dopamine levels in the NAc (Jerlhag et al., 2006; Jerlhag, 2008). Thus, in addition to the previously reported changes in general excitatory drive, our data indicate that the hunger signal ghrelin influences the dopamine response to events temporally linked to food approach and consumption. Regulation of phasic dopamine signaling by ghrelin is thus a means by which physiological state could influence a wide range of behavioral phenomena, including learning, reinforcement, and incentive motivation. Given that obesity is fueled, in part, by a failure to limit approach and consumption in response to food reward, our data suggest that LH ghrelin and VTA orexin receptors represent targets for therapeutic interventions.
Footnotes
This work was supported by National Institutes of Health grants R01-DA025634 (M.F.R.) and K01-DA033380 (J.E.M.). J.J.C. was supported by the National Center for Advancing Translational Sciences GrantTL1TR000049. Additional support was provided by the University of Illinois at Chicago Campus Research Board Pilot Research program (J.E.M.), the Chicago Biomedical Consortium (J.J.C.), and the University of Illinois at Chicago Dean's Scholar Fellowship (J.J.C.). We thank Dr. Steve Mahler for sharing orexin-A immunohistochemistry protocols and Dr. Joshua Jones for sharing VTA cannula coordinates.
The authors declare no competing financial interests.
References
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschöp MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Phillips AG. Dopaminergic correlates of sensory-specific satiety in the medial prefrontal cortex and nucleus accumbens of the rat. J Neurosci. 1999;19:RC29. doi: 10.1523/JNEUROSCI.19-19-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153:647–658. doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res. 2002;137:165–177. doi: 10.1016/S0166-4328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- Banks WA, Tschöp M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302:822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89:637–641. doi: 10.1016/S0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- Beeler JA, Daw N, Frazier CR, Zhuang X. Tonic dopamine modulates exploitation of reward learning. Front Behav Neurosci. 2010;4:170. doi: 10.3389/fnbeh.2010.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler JA, McCutcheon JE, Cao ZF, Murakami M, Alexander E, Roitman MF, Zhuang X. Taste uncoupled from nutrition fails to sustain the reinforcing properties of food. Eur J Neurosci. 2012;36:2533–2546. doi: 10.1111/j.1460-9568.2012.08167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Münzberg H. The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol Behav. 2011;104:29–39. doi: 10.1016/j.physbeh.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Branch SY, Goertz RB, Sharpe AL, Pierce J, Roy S, Ko D, Paladini CA, Beckstead MJ. Food restriction increases glutamate receptor-mediated burst firing of dopamine neurons. J Neurosci. 2013;33:13861–13872. doi: 10.1523/JNEUROSCI.5099-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HD, McCutcheon JE, Cone JJ, Ragozzino ME, Roitman MF. Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. Eur J Neurosci. 2011;34:1997–2006. doi: 10.1111/j.1460-9568.2011.07914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral A, Fernandez G, Perello M. Analysis of brain nuclei accessible to ghrelin present in the cerebrospinal fluid. Neuroscience. 2013;253:406–415. doi: 10.1016/j.neuroscience.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone JJ, Chartoff EH, Potter DN, Ebner SR, Roitman MF. Prolonged high fat diet reduces dopamine reuptake without altering DAT gene expression. PLoS One. 2013;8:e58251. doi: 10.1371/journal.pone.0058251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am J Physiol Endocrinol Metab. 2004;287:E297–304. doi: 10.1152/ajpendo.00582.2003. [DOI] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Day JJ, Jones JL, Wightman RM, Carelli RM. Phasic nucleus accumbens dopamine release encodes effort- and delay-related costs. Biol Psychiatry. 2010;68:306–309. doi: 10.1016/j.biopsych.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. J Neurosci. 2001;21:9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewnowski A, Specter SE. Poverty and obesity: the role of energy density and energy costs. Am J Clin Nutr. 2004;79:6–16. doi: 10.1093/ajcn/79.1.6. [DOI] [PubMed] [Google Scholar]
- España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Melchior JR, Roberts DC, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology. 2011;214:415–426. doi: 10.1007/s00213-010-2048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/S0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Gan JO, Walton ME, Phillips PE. Dissociable cost and benefit encoding of future rewards by mesolimbic dopamine. Nat Neurosci. 2010;13:25–27. doi: 10.1038/nn.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore BB, Zweifel LS. Genetic reconstruction of dopamine d1 receptor signaling in the nucleus accumbens facilitates natural and drug reward responses. J Neurosci. 2013;33:8640–8649. doi: 10.1523/JNEUROSCI.5532-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heien ML, Johnson MA, Wightman RM. Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Anal Chem. 2004;76:5697–5704. doi: 10.1021/ac0491509. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiol Behav. 1988;44:599–606. doi: 10.1016/0031-9384(88)90324-1. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. Neurosci Biobehav Rev. 2010;35:129–150. doi: 10.1016/j.neubiorev.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E. Systemic administration of ghrelin induces conditioned place preference and stimulates accumbal dopamine. Addict Biol. 2008;13:358–363. doi: 10.1111/j.1369-1600.2008.00125.x. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addict Biol. 2006a;11:45–54. doi: 10.1111/j.1369-1600.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, Engel JA. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol. 2007;12:6–16. doi: 10.1111/j.1369-1600.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Fortin SM, Ricks KM, Grill HJ. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol Psychiatry. 2013;73:915–923. doi: 10.1016/j.biopsych.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Schmid A, Schnitzler HU. Role of nucleus accumbens dopamine D 1 and D 2 receptors in instrumental and Pavlovian paradigms of conditioned reward. Psychopharmacology. 2000;152:67–73. doi: 10.1007/s002130000505. [DOI] [PubMed] [Google Scholar]
- Koob GF, Riley SJ, Smith SC, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi and olfactory tubercle on feeding, locomotor activity, and amphetamine anorexia in the rat. J Comp Physiol Psychol. 1978;92:917–927. doi: 10.1037/h0077542. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Häckel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Aston-Jones GS. Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 2012;32:13309–13326. doi: 10.1523/JNEUROSCI.2277-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7:400–409. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Margules DL, Olds J. Identical “feeding” and “rewarding” systems in the lateral hypothalamus of rats. Science. 1962;135:374–375. doi: 10.1126/science.135.3501.374. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Conrad KL, Carr SB, Ford KA, McGehee DS, Marinelli M. Dopamine neurons in the ventral tegmental area fire faster in adolescent rats than in adults. J Neurophysiol. 2012;108:1620–1630. doi: 10.1152/jn.00077.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Li D, Grace MK, Billington CJ, Kotz CM, Levine AS. Neural basis of orexigenic effects of ghrelin acting within lateral hypothalamus. Peptides. 2003;24:597–602. doi: 10.1016/S0196-9781(03)00105-0. [DOI] [PubMed] [Google Scholar]
- Owesson-White CA, Roitman MF, Sombers LA, Belle AM, Keithley RB, Peele JL, Carelli RM, Wightman RM. Sources contributing to the average extracellular concentration of dopamine in the nucleus accumbens. J Neurochem. 2012;121:252–262. doi: 10.1111/j.1471-4159.2012.07677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th edition. London: Elsevier Academic; 2007. [Google Scholar]
- Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, Rovinsky SA, Woloszyn J, Yanagisawa M, Lutter M, Zigman JM. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol Psychiatry. 2010;67:880–886. doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Robinson DL, Stuber GD, Carelli RM, Wightman RM. Real-time measurements of phasic changes in extracellular dopamine concentration in freely moving rats by fast-scan cyclic voltammetry. Methods Mol Med. 2003;79:443–464. doi: 10.1385/1-59259-358-5:443. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology. 1991;104:515–521. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- Sinkala E, McCutcheon JE, Schuck MJ, Schmidt E, Roitman MF, Eddington DT. Electrode calibration with a microfluidic flow cell for fast-scan cyclic voltammetry. Lab Chip. 2012;12:2403–2408. doi: 10.1039/c2lc40168a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibicka KP, Shirazi RH, Rabasa-Papio C, Alvarez-Crespo M, Neuber C, Vogel H, Dickson SL. Divergent circuitry underlying food reward and intake effects of ghrelin: dopaminergic VTA-accumbens projection mediates ghrelin's effect on food reward but not food intake. Neuropharmacology. 2013;73:274–283. doi: 10.1016/j.neuropharm.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Smith-Roe SL, Kelley AE. Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci. 2000;20:7737–7742. doi: 10.1523/JNEUROSCI.20-20-07737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sombers LA, Beyene M, Carelli RM, Wightman RM. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci. 2009;29:1735–1742. doi: 10.1523/JNEUROSCI.5562-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH. A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci. 2013;16:966–973. doi: 10.1038/nn.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, Bonci A. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science. 2008;321:1690–1692. doi: 10.1126/science.1160873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugam JA, Day JJ, Wightman RM, Carelli RM. Phasic nucleus accumbens dopamine encodes risk-based decision-making behavior. Biol Psychiatry. 2012;71:199–205. doi: 10.1016/j.biopsych.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber MT, Fibiger HC. Feeding-evoked dopamine release in the nucleus, accumbens: regulation by glutamatergic mechanisms. Neuroscience. 1997;76:1105–1112. doi: 10.1016/S0306-4522(96)00450-2. [DOI] [PubMed] [Google Scholar]
- Toshinai K, Date Y, Murakami N, Shimada M, Mondal MS, Shimbara T, Guan JL, Wang QP, Funahashi H, Sakurai T, Shioda S, Matsukura S, Kangawa K, Nakazato M. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology. 2003;144:1506–1512. doi: 10.1210/en.2002-220788. [DOI] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Kuhnen CM, Phillips PE. Delays conferred by escalating costs modulate dopamine release to rewards but not their predictors. J Neurosci. 2010;30:12020–12027. doi: 10.1523/JNEUROSCI.2691-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Wilson C, Nomikos GG, Collu M, Fibiger HC. Dopaminergic correlates of motivated behavior: importance of drive. J Neurosci. 1995;15:5169–5178. doi: 10.1523/JNEUROSCI.15-07-05169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, Stuber GD, Tye KM, Janak PH, Deisseroth K. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJ, Paladini CA, Phillips PE, Palmiter RD. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci U S A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]