Abstract
Parvalbumin (PV) is a Ca2+-binding protein found only in vertebrates. It is postulated to serve as a soluble relaxing factor in fast mammalian muscle. We have isolated a rat PV cDNA clone and used this as a probe to examine changes in PV mRNA during muscle and brain development. A cDNA library was constructed in PUC8/PUC9 plasmid vectors from adult poly(A)+ RNA isolated from rat gastrocnemius muscle. The library was screened with a 17-mer oligonucleotide encoding amino acids 28-33 of rat PV. One recombinant (9f) was confirmed as a PV clone by DNA sequencing and was shown to contain 73% of the protein coding sequence. Hybridization of clone 9f to RNA separated by electrophoresis revealed two species 700 and 1100 nucleotides long but genomic blotting indicates that PV may be a single copy gene. Highest levels of PV mRNA are found in the gastrocnemius, which is a fast contracting/relaxing muscle. Skin contains the next highest amount of PV mRNA followed, in order, by brain and the slow twitch soleus muscle. Rat muscle PV mRNA levels increase 15- to 20-fold between postnatal days 4 and 20 as measured by dot blot hybridization of total RNA, whereas only a slight increase was observed when young and adult brains were compared. The changes in PV mRNA during development appear to be selective, because mRNA coding for the structurally homologous Ca2+-binding protein calmodulin (CaM) was found to change only slightly in muscle. However, CaM mRNA levels decrease during the early days of brain ontogeny. Thus, the mRNAs that encode the homologous Ca2+-binding proteins PV and CaM appear to be developmentally regulated in a tissue-specific manner.
Full text
PDF


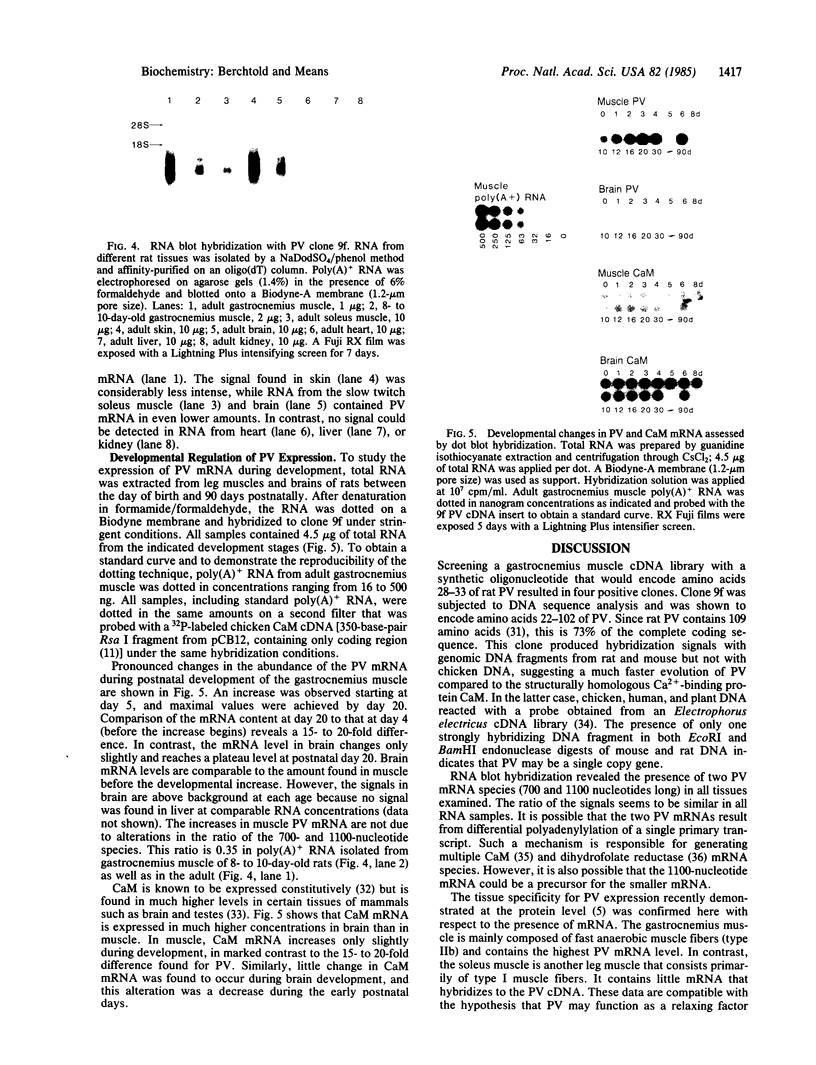

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold M. W., Celio M. R., Heizmann C. W. Parvalbumin in non-muscle tissues of the rat. Quantitation and immunohistochemical localization. J Biol Chem. 1984 Apr 25;259(8):5189–5196. [PubMed] [Google Scholar]
- Berchtold M. W., Heizmann C. W., Wilson K. J. Primary structure of parvalbumin from rat skeletal muscle. Eur J Biochem. 1982 Oct;127(2):381–389. doi: 10.1111/j.1432-1033.1982.tb06883.x. [DOI] [PubMed] [Google Scholar]
- Berchtold M. W., Wilson K. J., Heizmann C. W. Isolation of neuronal parvalbumin by high-performance liquid chromatography. Characterization and comparison with muscle parvalbumin. Biochemistry. 1982 Dec 7;21(25):6552–6557. doi: 10.1021/bi00268a035. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Carpenter C. D., Bruskin A. M., Hardin P. E., Keast M. J., Anstrom J., Tyner A. L., Brandhorst B. P., Klein W. H. Novel proteins belonging to the troponin C superfamily are encoded by a set of mRNAs in sea urchin embryos. Cell. 1984 Mar;36(3):663–671. doi: 10.1016/0092-8674(84)90346-5. [DOI] [PubMed] [Google Scholar]
- Celio M. R., Heizmann C. W. Calcium-binding protein parvalbumin as a neuronal marker. Nature. 1981 Sep 24;293(5830):300–302. doi: 10.1038/293300a0. [DOI] [PubMed] [Google Scholar]
- Celio M. R., Heizmann C. W. Calcium-binding protein parvalbumin is associated with fast contracting muscle fibres. Nature. 1982 Jun 10;297(5866):504–506. doi: 10.1038/297504a0. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Lagacé L., Bolton W. E., Boyd A. E., 3rd, Means A. R. Changes in calmodulin and its mRNA accompany reentry of quiescent (G0) cells into the cell cycle. Cell. 1984 Jan;36(1):73–81. doi: 10.1016/0092-8674(84)90075-8. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Dawid I. B. Isolation and characterization of calmodulin genes from Xenopus laevis. Mol Cell Biol. 1984 Mar;4(3):507–513. doi: 10.1128/mcb.4.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Elson N. A., Anderson W. F. Initiation of globin synthesis: assays. Methods Enzymol. 1974;30:101–127. doi: 10.1016/0076-6879(74)30014-6. [DOI] [PubMed] [Google Scholar]
- Desplan C., Thomasset M., Moukhtar M. Synthesis, molecular cloning, and restriction analysis of DNA complementary to vitamin D-dependent calcium-binding protein mRNA from rat duodenum. J Biol Chem. 1983 Mar 10;258(5):2762–2765. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garfinkel L. I., Periasamy M., Nadal-Ginard B. Cloning and characterization of cDNA sequences corresponding to myosin light chains 1, 2, and 3, troponin-C, troponin-T, alpha-tropomyosin, and alpha-actin. J Biol Chem. 1982 Sep 25;257(18):11078–11086. [PubMed] [Google Scholar]
- Gillis J. M., Thomason D., Lefèvre J., Kretsinger R. H. Parvalbumins and muscle relaxation: a computer simulation study. J Muscle Res Cell Motil. 1982 Dec;3(4):377–398. doi: 10.1007/BF00712090. [DOI] [PubMed] [Google Scholar]
- Goodman M., Pechère J. F., Haiech J., Demaille J. G. Evolutionary diversification of structure and function in the family of intracellular calcium-binding proteins. J Mol Evol. 1979 Nov;13(4):331–352. doi: 10.1007/BF01731373. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hastings K. E., Emerson C. P., Jr cDNA clone analysis of six co-regulated mRNAs encoding skeletal muscle contractile proteins. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1553–1557. doi: 10.1073/pnas.79.5.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heizmann C. W., Berchtold M. W., Rowlerson A. M. Correlation of parvalbumin concentration with relaxation speed in mammalian muscles. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7243–7247. doi: 10.1073/pnas.79.23.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfman D. M., Feramisco J. R., Fiddes J. C., Thomas G. P., Hughes S. H. Identification of clones that encode chicken tropomyosin by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1983 Jan;80(1):31–35. doi: 10.1073/pnas.80.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W., Siebert P. D., King M. W., Stucki P., Dugaiczyk A., Norman A. W. Molecular cloning of a vitamin D-dependent calcium-binding protein mRNA sequence from chick intestine. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4228–4232. doi: 10.1073/pnas.80.14.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karathanasis S. K., Norum R. A., Zannis V. I., Breslow J. L. An inherited polymorphism in the human apolipoprotein A-I gene locus related to the development of atherosclerosis. Nature. 1983 Feb 24;301(5902):718–720. doi: 10.1038/301718a0. [DOI] [PubMed] [Google Scholar]
- Katz L., Williams P. H., Sato S., Leavitt R. W., Helinski D. R. Purification and characterization of covalently closed replicative intermediates of ColEl DNA from Escherichia coli. Biochemistry. 1977 Apr 19;16(8):1677–1683. doi: 10.1021/bi00627a024. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Vanaman T. C. Calmodulin. Adv Protein Chem. 1982;35:213–321. doi: 10.1016/s0065-3233(08)60470-2. [DOI] [PubMed] [Google Scholar]
- Klug G., Reichmann H., Pette D. Rapid reduction in parvalbumin concentration during chronic stimulation of rabbit fast twitch muscle. FEBS Lett. 1983 Feb 21;152(2):180–182. doi: 10.1016/0014-5793(83)80374-3. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H. Structure and evolution of calcium-modulated proteins. CRC Crit Rev Biochem. 1980;8(2):119–174. doi: 10.3109/10409238009105467. [DOI] [PubMed] [Google Scholar]
- Kuwano R., Usui H., Maeda T., Fukui T., Yamanari N., Ohtsuka E., Ikehara M., Takahashi Y. Molecular cloning and the complete nucleotide sequence of cDNA to mRNA for S-100 protein of rat brain. Nucleic Acids Res. 1984 Oct 11;12(19):7455–7465. doi: 10.1093/nar/12.19.7455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagacé L., Chandra T., Woo S. L., Means A. R. Identification of multiple species of calmodulin messenger RNA using a full length complementary DNA. J Biol Chem. 1983 Feb 10;258(3):1684–1688. [PubMed] [Google Scholar]
- Mariash C. N., Seelig S., Oppenheimer J. H. A rapid, inexpensive, quantitative technique for the analysis of two-dimensional electrophoretograms. Anal Biochem. 1982 Apr;121(2):388–394. doi: 10.1016/0003-2697(82)90498-5. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means A. R. Calmodulin: properties, intracellular localization, and multiple roles in cell regulation. Recent Prog Horm Res. 1981;37:333–367. doi: 10.1016/b978-0-12-571137-1.50011-6. [DOI] [PubMed] [Google Scholar]
- Means A. R., Tash J. S., Chafouleas J. G. Physiological implications of the presence, distribution, and regulation of calmodulin in eukaryotic cells. Physiol Rev. 1982 Jan;62(1):1–39. doi: 10.1152/physrev.1982.62.1.1. [DOI] [PubMed] [Google Scholar]
- Monahan J. J., Harris S. E., Woo S. L., Robberson D. L., O'Malley B. W. The synthesis and properties of the complete complementary DNA transcript of ovalbumin mRNA. Biochemistry. 1976 Jan 13;15(1):223–233. doi: 10.1021/bi00646a034. [DOI] [PubMed] [Google Scholar]
- Munjaal R. P., Chandra T., Woo S. L., Dedman J. R., Means A. R. A cloned calmodulin structural gene probe is complementary to DNA sequences from diverse species. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2330–2334. doi: 10.1073/pnas.78.4.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima Y., Fujii-Kuriyama Y., Muramatsu M., Ogata K. Alternative transcription and two modes of splicing results in two myosin light chains from one gene. Nature. 1984 Mar 22;308(5957):333–338. doi: 10.1038/308333a0. [DOI] [PubMed] [Google Scholar]
- Pechère J. F., Derancourt J., Haiech J. The participation of parvalbumins in the activation-relaxation cycle of vertebrate fast skeletal-muscle. FEBS Lett. 1977 Mar 15;75(1):111–114. doi: 10.1016/0014-5793(77)80064-1. [DOI] [PubMed] [Google Scholar]
- Putkey J. A., Ts'ui K. F., Tanaka T., Lagacé L., Stein J. P., Lai E. C., Means A. R. Chicken calmodulin genes. A species comparison of cDNA sequences and isolation of a genomic clone. J Biol Chem. 1983 Oct 10;258(19):11864–11870. [PubMed] [Google Scholar]
- Rosen J. M., Woo S. L., Holder J. W., Means A. R., O'Malley B. W. Preparation and preliminary characterization of purified ovalbumin messenger RNA from the hen oviduct. Biochemistry. 1975 Jan 14;14(1):69–78. doi: 10.1021/bi00672a012. [DOI] [PubMed] [Google Scholar]
- Schloss J. A., Silflow C. D., Rosenbaum J. L. mRNA abundance changes during flagellar regeneration in Chlamydomonas reinhardtii. Mol Cell Biol. 1984 Mar;4(3):424–434. doi: 10.1128/mcb.4.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Nunberg J. H., Schimke R. T. Size heterogeneity in the 3' end of dihydrofolate reductase messenger RNAs in mouse cells. Cell. 1980 Nov;22(2 Pt 2):361–370. doi: 10.1016/0092-8674(80)90346-3. [DOI] [PubMed] [Google Scholar]
- Smoake J. A., Song S. Y., Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Distribution and developmental changes of the enzyme and its protein activator in mammalian tissues and cells. Biochim Biophys Acta. 1974 Apr 25;341(2):402–411. doi: 10.1016/0005-2744(74)90233-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]