Abstract
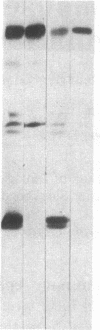
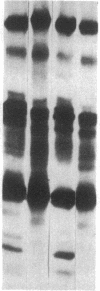
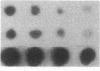
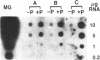
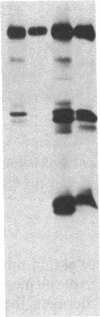
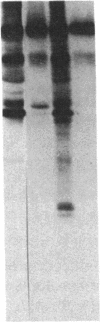
Mouse mammary epithelial cells (MMEC) secrete certain milk proteins only when cultured on floating collagen gels. We demonstrate here that modulation of milk proteins by substrata is manifested at several regulatory levels; (i) Cells cultured on floating collagen gels have 3- to 10-fold more casein mRNA than cells cultured on plastic or attached collagen gels. (ii) Cells on the latter two "flat" substrata, nevertheless, synthesize a significant amount of caseins, indicating that the remaining mRNA is functional. (iii) Cells on all substrata are inducible for casein mRNA and casein proteins by prolactin, but the extent of induction is greater on collagen than that on plastic--i.e., the substratum confers an altered degree of inducibility. (iv) Cells on all substrata synthesize casein proteins at rates proportional to the amount of casein mRNA, but the newly synthesized caseins in cells on plastic are degraded intracellularly, whereas those synthesized by cells on floating gels are secreted into the medium. (v) Cells on all substrata examined lose virtually all mRNA for whey acidic protein despite the fact that this mRNA is abundant in the mammary gland itself; we conclude that additional, as-yet-unknown, factors are necessary for synthesis and secretion of whey acidic protein in culture.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benya P. D., Shaffer J. D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982 Aug;30(1):215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Bissell M. J., Hall H. G., Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982 Nov 7;99(1):31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Chambard M., Verrier B., Gabrion J., Mauchamp J. Polarization of thyroid cells in culture: evidence for the basolateral localization of the iodide "pump" and of the thyroid-stimulating hormone receptor-adenyl cyclase complex. J Cell Biol. 1983 Apr;96(4):1172–1177. doi: 10.1083/jcb.96.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman J. T., Enami J., Pitelka D. R., Nandi S. Hormonal effects on intracellular and secreted casein in cultures of mouse mammary epithelial cells on floating collagen membranes. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4466–4470. doi: 10.1073/pnas.74.10.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman J. T., Pitelka D. R. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977 May;13(5):316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Green M. R., Pastewka J. V. Characterization of major milk proteins from BALB/c and C3H mice. J Dairy Sci. 1976 Feb;59(2):207–215. doi: 10.3168/jds.S0022-0302(76)84186-0. [DOI] [PubMed] [Google Scholar]
- Gupta P., Rosen J. M., D'Eustachio P., Ruddle F. H. Localization of the casein gene family to a single mouse chromosome. J Cell Biol. 1982 Apr;93(1):199–204. doi: 10.1083/jcb.93.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeuptle M. T., Suard Y. L., Bogenmann E., Reggio H., Racine L., Kraehenbuhl J. P. Effect of cell shape change on the function and differentiation of rabbit mammary cells in culture. J Cell Biol. 1983 May;96(5):1425–1434. doi: 10.1083/jcb.96.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L. G., Sippel A. E. Characterization and cloning of the mRNAs specific for the lactating mouse mammary gland. Eur J Biochem. 1982 Jun 15;125(1):131–141. doi: 10.1111/j.1432-1033.1982.tb06660.x. [DOI] [PubMed] [Google Scholar]
- Hobbs A. A., Richards D. A., Kessler D. J., Rosen J. M. Complex hormonal regulation of rat casein gene expression. J Biol Chem. 1982 Apr 10;257(7):3598–3605. [PubMed] [Google Scholar]
- Kaetzel C. S., Ray D. B. Immunochemical characterization with monoclonal antibodies of three major caseins and alpha-lactalbumin from rat milk. J Dairy Sci. 1984 Jan;67(1):64–75. doi: 10.3168/jds.S0022-0302(84)81267-9. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee E. Y., Parry G., Bissell M. J. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984 Jan;98(1):146–155. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. H., Murphree A. L., Benedict W. F. Expression and amplification of the N-myc gene in primary retinoblastoma. 1984 May 31-Jun 6Nature. 309(5967):458–460. doi: 10.1038/309458a0. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G., Pitot H. C. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations. Exp Cell Res. 1975 Aug;94(1):70–78. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- Piletz J. E., Heinlen M., Ganschow R. E. Biochemical characterization of a novel whey protein from murine milk. J Biol Chem. 1981 Nov 25;256(22):11509–11516. [PubMed] [Google Scholar]
- Ray D. B., Horst I. A., Jansen R. W., Kowal J. Normal mammary cells in long term culture. I. development of hormone-dependent functional monolayer cultures and assay of alpha-lactalbumin production. Endocrinology. 1981 Feb;108(2):573–583. doi: 10.1210/endo-108-2-573. [DOI] [PubMed] [Google Scholar]
- Ray D. B., Horst I. A., Jansen R. W., Mills N. C., Kowal J. Normal mammary cells in long term culture. II. prolactin, corticosterone, insulin, and triiodothyronine effects on alpha-lactalbumin production. Endocrinology. 1981 Feb;108(2):584–590. doi: 10.1210/endo-108-2-584. [DOI] [PubMed] [Google Scholar]
- Razooki Hasan H., White D. A., Mayer R. J. Extensive destruction of newly synthesized casein in mammary explants in organ culture. Biochem J. 1982 Jan 15;202(1):133–138. doi: 10.1042/bj2020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon J. M., Pitelka D. R. The influence of cell shape on the induction of functional differentiation in mouse mammary cells in vitro. In Vitro. 1981 Nov;17(11):1016–1028. doi: 10.1007/BF02618428. [DOI] [PubMed] [Google Scholar]
- Suard Y. M., Haeuptle M. T., Farinon E., Kraehenbuhl J. P. Cell proliferation and milk protein gene expression in rabbit mammary cell cultures. J Cell Biol. 1983 May;96(5):1435–1442. doi: 10.1083/jcb.96.5.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Wicha M. S., Lowrie G., Kohn E., Bagavandoss P., Mahn T. Extracellular matrix promotes mammary epithelial growth and differentiation in vitro. Proc Natl Acad Sci U S A. 1982 May;79(10):3213–3217. doi: 10.1073/pnas.79.10.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde C. J., Hasan H. R., Mayer R. J. Comparison of collagen gels and mammary extracellular matrix as substrata for study of terminal differentiation in rabbit mammary epithelial cells. Exp Cell Res. 1984 Apr;151(2):519–532. doi: 10.1016/0014-4827(84)90400-2. [DOI] [PubMed] [Google Scholar]