Abstract
In the lung, acute reductions in oxygen lead to hypoxic pulmonary vasoconstriction, whereas prolonged exposures to hypoxia result in sustained vasoconstriction, pulmonary vascular remodeling, and the development of pulmonary hypertension. Data from both human subjects and animal models implicate a role for hypoxia-inducible factors (HIFs), oxygen-sensitive transcription factors, in pulmonary vascular responses to both acute and chronic hypoxia. In this review, we discuss work from our laboratory and others supporting a role for HIF in modulating hypoxic pulmonary vasoconstriction and mediating hypoxia-induced pulmonary hypertension, identify some of the downstream targets of HIF, and assess the potential to pharmacologically target the HIF system.
Keywords: hypoxia inducible factor, pulmonary hypertension, hypoxic pulmonary vasoconstriction, digoxin
reduced oxygen availability is a common occurrence in the lung, arising in both physiological (i.e., fetal development) and pathological (i.e., chronic lung disease) settings. As oxygen sensing and swift response to oxygen deprivation are essential for survival, regulation of oxygen homeostasis has evolved into a complex system. In higher organisms, adaptation to hypoxia involves the synchronized transcriptional regulation of a variety of genes and is coordinated, in large part, by hypoxia-inducible factors (HIFs), oxygen-sensitive transcription factors that have been described as “master regulators of oxygen homeostasis” (53). In this review, we will describe the HIF system, the regulation of HIFs in the lung, and the mounting evidence supporting a role for HIFs in pulmonary vascular responses to hypoxia.
HYPOXIA-INDUCIBLE FACTORS
The discovery of the first HIF, HIF-1, a little over 20 years ago (67), paved the way for an explosion of research implicating activation of HIF as a vital step in a variety of physiological processes associated with hypoxia, including embryogenesis (13, 28, 34, 43, 59), wound healing (40), control of respiration (52), red blood cell production (64, 65), and glucose and energy metabolism (63, 65). HIF has also been shown to play a critical role in pathological scenarios, such as pulmonary (9, 100) and systemic (65) hypertension, cancer (31, 61), and ischemic preconditioning (62, 66).
Identified as a hypoxia-sensitive transcription factor that controlled the expression of erythropoietin (67), HIF-1 is now regarded as a highly conserved transcription factor that is present in almost all cell types and regulates the oxygen-dependent expression of hundreds of genes (63). HIF-1 exists as a heterodimer, formed from HIF-1α and HIF-1β subunits. Concerted research efforts have demonstrated that the activity of HIF-1 is tightly regulated by oxygen availability (30, 53, 65). Under conditions in which oxygen is abundant, HIF-1β is ubiquitously expressed, whereas HIF-1α protein expression is typically quite low or altogether absent. As oxygen levels fall, HIF-1α protein levels and HIF-1 DNA binding activity increase (63). For nearly a decade after the discovery of HIF-1, the mechanism by which cells sensed changes in oxygen concentration and transduced the signal into an increase in HIF-1 activity and induction of hypoxia-inducible genes was a matter of intense speculation and investigation. In 2001, several laboratories simultaneously reported that HIF-1α ubiquitination and targeting for degradation required hydroxylation of the protein (27, 29, 44, 101). Under normoxic conditions, hydroxylation of HIF-1α occurs at two proline residues (Pro402 and Pro564 in human HIF-1α) located within the oxygen-dependent degradation domain in a reaction catalyzed by prolyl hydroxylase domain proteins (PHDs) using molecular oxygen as a substrate (17, 27, 29). Four PHD isoforms have been identified, of which PHD1, PHD2, and PHD3 hydroxylate HIF-1α, with PHD2 responsible for the majority of HIF-1α hydroxylation in vivo (3, 7, 46). The PHDs appear to be developmentally regulated, with higher levels observed in adult compared with fetal animals (56). Despite low levels in the fetus, however, PHD2 is clearly required for in utero development, since mice deficient for PHD2 die midgestation (85). In contrast, mice lacking PHD1 and PHD3 appear to develop normally and are viable (85).
Once hydroxylated, HIF-1α can interact with the tumor suppressor, von Hippel-Lindau (VHL) protein (Fig. 1), which recruits an E3 ubiquitin-protein ligase, allowing HIF-1α to be polyubiquitinated and targeted for 26S proteosomal degradation (41, 63). At reduced oxygen concentrations, PHD activity decreases, with almost complete inhibition of activity when oxygen levels fall below 2% (14). The consequent decline in HIF-1α hydroxylation results in protein stabilization and translocation into the nucleus, where it binds HIF-1β and recruits coactivator proteins to form a transcriptional complex at the HIF binding site (5′RCGTG3′) within the hypoxia response element, modulating the transcription of various target genes (26). Increased HIF-1α protein generally correlates with increased transcriptional activity, although HIF-1 transactivation is regulated by hydroxylation of HIF-1α at an asparagine residue (Asp803) within the COOH-terminal transactivation domain via factor inhibiting HIF-1 (FIH-1), which causes a conformational change in the protein and blocks the binding of the transcriptional coactivators CREB binding protein and p300 (18, 42).
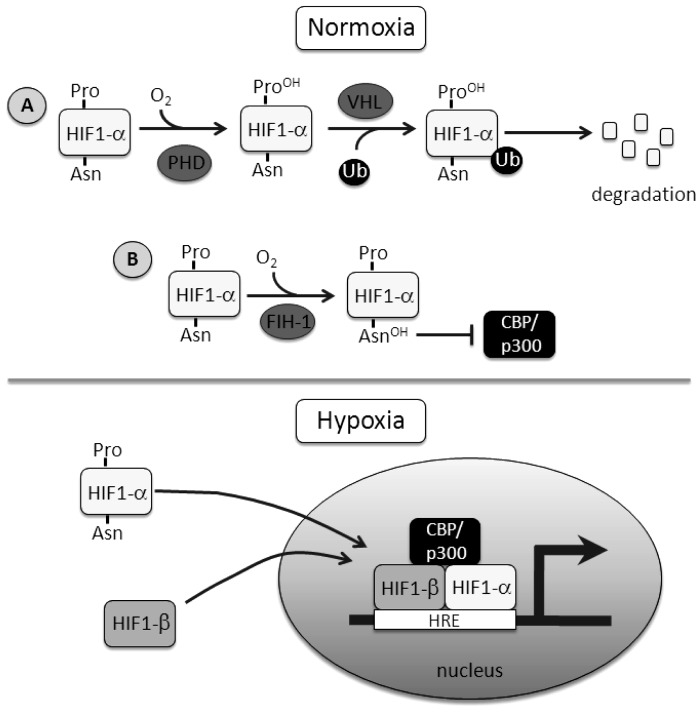
Fig. 1.
Schematic detailing the regulation of hypoxia-inducible factor 1 (HIF-1). A: under normoxic conditions, HIF-1α protein levels are regulated by prolyl hydroxylase domain (PHD) proteins and von Hipple-Lindau (VHL) protein, targeting the protein for degradation. B: transactivation of HIF is controlled by factor inhibiting HIF-1 (FIH-1), an asparaginyl hydroxylase. Hydroxylation of the asparagine residue prevents cofactor binding. During hypoxia, HIF-1α escapes both prolyl (Pro) and asparaginyl (Asn) hydroxylation, leading to protein accumulation, nuclear translocation, and formation and binding of the transcriptional complex to target genes. Ub, ubiquitin; CBP, CREB binding protein; HRE, hypoxia response element.
Both PHD and FIH-1 hydroxylation activity depend on oxygen availability (Fig. 1), with one atom of dioxygen incorporated in the prolyl or asparagyl residue, respectively, providing a molecular basis for the oxygen-sensing function of these enzymes (30, 53). Analysis of the kinetics of PHD and FIH-1 activity suggests that these enzymes are ideally suited as oxygen sensors, with in vitro derived Km values for oxygen binding of ∼100 μM and 70 μM for PHDs and FIH-1, respectively (33). This range of oxygen dependence has been confirmed by measurement of PHD activity in cellular extracts under different oxygen concentrations (6). Thus, based on their affinities for oxygen, PHDs and FIH-1 are well positioned to sense oxygen within the range of physiological tissue concentrations (30).
Later work revealed the existence of paralogs of the HIF-1α subunit. HIF-2α was identified based on its sequence similarity to HIF-1α (16, 87) and is regulated by the same PHD-dependent degradation pathways. HIF-2α also dimerizes with HIF-1β, but, in contrast to HIF-1α, exhibits a restricted, cell-specific expression pattern (53, 63). A third paralog, HIF-3α, has also been identified, but appears to function primarily as a negative regulator of HIF-1 (53).
Early studies revealed the oxygen dependence and kinetics of HIF-1 activation in the lung (95, 99), with in vivo hypoxic induction of HIF-1α mRNA occurring within 30 min (95). Using an isolated, ventilated, perfused ferret lung preparation, where rapid biopsies of hypoxic lung tissue could be readily obtained, significant HIF-1α protein accumulation was observed within 1 h of initiating hypoxic exposure, continued to increase for the duration of the hypoxic challenge, and decreased within 1 min on reoxygenation (99). With more reduced preparations, it became evident that HIF-1α protein levels and DNA binding increased in both endothelial and smooth muscle cells from pulmonary arteries (99). The rapid induction of HIF-1α observed in pulmonary arterial smooth muscle cells (PASMCs) exposed to 1% oxygen is most certainly a direct consequence of substrate-limited inhibition of PHD activity. Moderate levels of hypoxia (4% oxygen) also induce HIF-1α protein, but through a different mechanism, requiring longer duration of hypoxic exposure and involving production of endothelin-1 (ET-1) and downregulation of PHD expression (51).
HIF AND ACUTE PULMONARY RESPONSES TO HYPOXIA
In the systemic circulation, hypoxia causes vasodilation to increase oxygen delivery and meet the metabolic demands of the tissue. Conversely, the pulmonary circulation contracts as alveolar and arterial oxygen content decrease, as initially described in detail by von Euler and Lillestrand in 1946 (90). The exact reason for this phenomenon, termed hypoxic pulmonary vasoconstriction (HPV), remains in debate, although HPV is most commonly believed to be a residual feature from fetal development, where the hypoxic environment maintains a closed pulmonary circulation until birth, or a mechanism to match ventilation and perfusion by diverting blood flow from poorly oxygenated portions of the lung. In either case, decrements in alveolar oxygen tension give rise to an immediate, reversible contraction that varies in intensity by level of hypoxia, sex, and species (84). The exact mechanisms underlying oxygen sensing in the pulmonary vasculature are still under investigation, but are considered to require a coordinated response between the endothelial and smooth muscle cells (1, 84). In PASMCs, hypoxia leads to production of mitochondrial reactive oxygen species, calcium release from the sarcoplasmic reticulum, opening of calcium-permeable nonselective cation channels, inhibition of voltage-gated potassium channels, membrane depolarization, and subsequent activation of calcium influx through voltage-gated calcium channels (84). Increased production of vasoconstricting, and/or reduced production of vasodilating, factors by the neighboring endothelial cells (ECs) augment PASMC responses to hypoxia (84), indicating that, while the main contractile mechanics underpinning HPV reside in PASMCs, the endothelium certainly plays a modulatory role in the response.
The fact that the pulmonary circulation contracts almost immediately upon a reduction in oxygen tension would appear to rule out a role for regulation of the acute response at the level of transcription, and thus eliminate the possibility that HIF mediates HPV. However, the finding that individuals with Chuvash polycythemia, a rare genetic condition where germline hypomorphic mutations in VHL result in augmented HIF expression under nonhypoxic conditions, exhibited enhanced HPV (79) suggested a possible role for HIF in regulating acute pulmonary vascular responses to hypoxia. The proposal that HIF could regulate HPV finds further support in studies of humans with gain-of-function mutations in HIF-2α who also exhibit enhanced HPV (20). Determining whether the role of HIF lies in modulating targets believed to be directly involved in the contractile response (i.e., voltage-gated potassium or calcium channels) or in circulating factors known to be required for enhancement or priming of the smooth muscle during HPV (i.e., ET-1), remains to be determined.
HIF AND PULMONARY HYPERTENSION
Pulmonary hypertension is defined as a resting mean pulmonary artery pressure ≥ 25 mmHg and results from various etiologies, including chronic hypoxia (CH) associated with several lung diseases (74). Pathophysiology results from sustained active vasoconstriction and pulmonary vascular remodeling, the latter of which is characterized by medial thickening, enhanced muscularity, and migration and proliferation of PASMCs (70, 82). Ultimately, right ventricular hypertrophy becomes a serious complication with the potential for ensuing right ventricular failure. The majority of work addressing the effects of CH on the pulmonary circulation has been performed in adult animals, even though increased pulmonary arterial pressure, remodeling, and contraction also occur in infants exposed to perinatal hypoxia. It is widely recognized that regulation of pulmonary arterial pressure is influenced by postnatal age, and it is unclear which mechanisms identified in the adult translate to the neonate. The focus of this section will be on mechanisms identified in adult animals.
Rodents exposed to CH develop vasoconstriction and vascular remodeling reminiscent of humans with hypoxia-associated pulmonary hypertension and are, therefore, useful models to identify cellular modifications contributing to disease pathogenesis (70, 82). PASMCs isolated from chronically hypoxic rats exhibit elevated intracellular calcium concentration ([Ca2+]i) (68), which in turn leads to enhanced cell contraction (73), migration (36), and proliferation (47). Initial reports of downregulated voltage-gated potassium channels (77, 91) and membrane depolarization (83) suggested that voltage-gated Ca2+ channels might be responsible for the increased [Ca2+]i; however, it is now well established that sustained calcium influx is mediated primarily by upregulation of nonselective cation channels (68), which are composed of canonical transient receptor potential (TRPC) proteins (39, 92). Instead, reduced voltage-gated potassium channel expression, which is HIF dependent (8, 71, 94), may contribute to remodeling by reducing apoptosis (55).
PASMC proliferation is also regulated by Na+/H+ exchanger (NHE) activity, which extrudes H+ ions for Na+ ions and controls intracellular pH (pHi) (54). Moreover, NHE isoform 1 (NHE1) expression and activity, and thus pHi, are elevated in PASMCs isolated from chronically hypoxic animals (58, 69), and NHE inhibitors prevented CH-induced pulmonary vascular remodeling and development of pulmonary hypertension (54). The role of NHE1 in hypoxia-induced pulmonary hypertension was confirmed using NHE1 null mice, which were protected from CH-induced elevations in right ventricular pressure (used as an estimate of pulmonary arterial pressure) and pulmonary vascular remodeling (103), and in vitro silencing of NHE1 by short interfering RNA, which inhibited PASMC proliferation and migration in response to hypoxia (102).
The role of HIF-1 in regulating the development and maintenance of hypoxia-induced pulmonary hypertension has been investigated using transgenic mice. Indistinguishable from their wild-type littermates under normoxia, mice with a loss-of-function mutation in one allele encoding the HIF-1α protein (Hif1a+/−) (28) are partially protected from developing CH-induced PH, exhibiting reduced vascular remodeling and attenuated increases in right ventricular pressure and hypertrophy (100). In Hif1a+/− mice, CH also fails to upregulate expression of TRPCs or NHE1, elevate [Ca2+]i, alkalinize pHi, or reduce voltage-gated potassium channel expression or activity (69, 71, 93, 94). Consistent with these findings, gain-of-function studies demonstrated that expressing HIF-1α under nonhypoxic conditions mimicked the effects of hypoxia, resulting in downregulated voltage-gated potassium channel subunits, increased TRPC and NHE1 expression, enhanced NHE activity, and elevated pHi (69, 93, 94).
Other HIF target genes may also participate in the development of hypoxic PH. First isolated from cultured porcine aortic ECs in 1988 (97), ET-1 is a HIF target, potent vasoconstrictor, and stimulator of vascular smooth muscle cell proliferation (23). CH increases lung, pulmonary artery, and plasma ET-1 levels, as well as the expression of ET-1 receptors (37, 72). In animal models, ET-1 receptor antagonists partially prevent and/or reverse established CH-induced pulmonary hypertension (23, 72). In addition to being a HIF target, as described earlier in this review, ET-1 also upregulates HIF-1α in PASMCs (38, 51), creating a feedforward mechanism to enhance HIF-1 expression and potentiate the development of pulmonary hypertension (Fig. 2). Consistent with this hypothesis, ET-1 receptor antagonism prevented upregulation of HIF-1α in PASMCs and rats exposed to hypoxia (51). In contrast, ET-1 failed to augment HIF-1α in aortic smooth muscle cells, suggesting a feature not shared by all vascular smooth muscle.
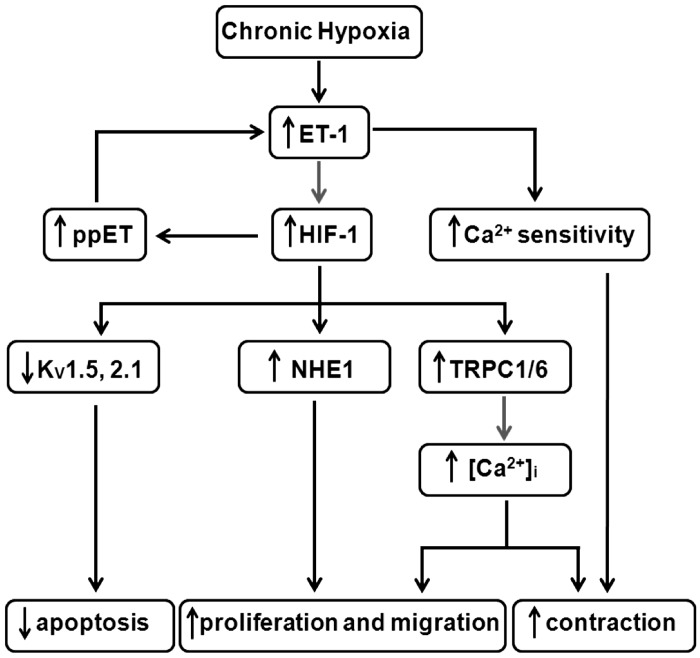
Fig. 2.
Proposed role of HIF-1 in hypoxic pulmonary hypertension. Initially, hypoxia induces the synthesis and release of endothelin-1 (ET-1) from pulmonary endothelial and arterial smooth muscle cells (PASMCs). ET-1 binds to receptors on the PASMCs, increasing Ca2+ sensitivity of the contractile apparatus and upregulating HIF-1α, which induces ET-1 transcription and results in a feedforward mechanism to augment HIF activation. Downstream targets of HIF-1 include voltage-gated K+ channels (including Kv1.5 and Kv2.1), Na+/H+ exchanger isoform 1 (NHE1), and canonical transient receptor potential (TRPC) proteins (TRPC1 and TRPC6), which coordinate to regulate PASMC contraction, growth/survival, and migration. ppET, prepro-endothelin; [Ca2+]i, intracellular Ca2+ concentration.
HIF-2α, with predominant lung expression in ECs and epithelial cells, also appears to play a role in the pathogenesis of CH-induced pulmonary hypertension, as mice with heterozygous deficiency for HIF-2α exhibited blunted right ventricular pressures and vascular remodeling (9). Evidence for a role for HIF-2α can also be found in Tibetan natives, an altitude-tolerant population, in which a loss-of-function mutation in Hif2a was found to correlate with reduced pulmonary artery pressures (88). Conversely, a genetic mutation leading to HIF-2α overexpression was associated with development of pulmonary hypertension in humans (20, 22) and in mice (86). Further implication for HIF-1 and HIF-2 in the development of pulmonary hypertension comes from individuals with Chuvash polycythemia and animal models with deletion of VHL, both of which exhibit elevated HIF levels and increased susceptibility to developing pulmonary hypertension (11, 25). Consistent with aforementioned results, when HIF-1α was selectively deleted in smooth muscle in adult animals using a tamoxifen-inducible smooth muscle heavy chain myosin driven Cre recombinase, hypoxic pulmonary hypertension and vascular remodeling were markedly attenuated (5). Surprisingly, mice with noninducible homozygous HIF-1α (32) and HIF-2α (75) deletion targeted to vascular smooth muscle cells and ECs, respectively, exhibited enhanced pulmonary hypertension. The explanation for the dichotomous results between targeted homozygous and inducible targeted homozygous or global heterozygous genetic modifications in HIF-1α and HIF-2α has yet to be resolved and clearly requires further investigation. Nonetheless, taken as a whole, the data suggest a potential feedforward model in the pathogenesis of CH-induced pulmonary hypertension whereby enhanced HIF-2α expression in ECs during CH might lead to increased ET-1 production, augmented HIF-1α in PASMCs, and upregulation of HIF target genes.
Finally, inflammation due to CH is another component likely contributing to the pathogenesis of pulmonary hypertension. In human patients and animal models, inflammation is an early consequence of hypoxic exposure (10, 21, 89), and studies in chronically hypoxic rats revealed that mast cell accumulation, increased inflammatory cell infiltrates, and recruitment of circulating monocytic and progenitor cells preceded and/or contributed to vascular remodeling (10, 50). Following recruitment, these cells can exert paracrine effects on the vasculature via release of vasoactive, pro-proliferative, and chemotactic metabolites (21, 49) and/or stimulate production of collagen and facilitate endothelial-mesenchymal transdifferentiation (81). The proinflammatory environment created in the pulmonary vasculature during CH involves several known HIF target genes, including nuclear factor (NF)-κB (89), stromal cell-derived factor-1 (10), and vascular endothelial growth factor (89). Taken together, these data suggest that, in addition to direct effects in ECs and PASMCs, HIF may also contribute to the development of hypoxia-induced pulmonary hypertension by promoting an inflammatory milieu.
In addition to inducing production of inflammatory cytokines, NF-κB can act to further increase HIF levels (15, 21). Moreover, activation of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (mTOR) signaling pathway is a frequent downstream target of inflammation and leads to upregulation of both HIF and NF-κB (21), setting up a potential feedforward mechanism between hypoxia, HIF, and inflammation. It is conceivable that the phenotypic switching in cell metabolism and subsequent hyper-proliferation/enhanced cell survival that occurs during pulmonary hypertension could be a consequence of this considerable cross talk, since mTOR is critical for the hypoxia-induced switch to glycolysis in PASMCs (4, 24), while upregulation of HIF leads to decreased oxygen consumption and increased glycolysis in ECs from pulmonary hypertensive patients (19). The exact role of inflammation in driving the activation of mTOR, NF-κB, and HIF and hypoxia-induced abnormalities in metabolic phenotype in the pulmonary circulation remains unclear and warrants further investigation.
POTENTIAL FOR THERAPEUTIC TARGETING OF HIF
Until the last 5 years, pharmacological targeting of HIF was quite difficult. However, in a study published in 2008, Zhang et al. (104) reported the use of a high-throughput screening assay for HIF activity to identify a number of clinically used compounds in the Johns Hopkins Drug Library that inhibited HIF activity. Of the identified compounds, several have now been used in preclinical studies to evaluate the ability to target HIF pharmacologically (35, 60, 96, 98, 104) and to determine the effect of such targeting on hypoxic responses in the lung (2). The first, digoxin, a cardiac glycoside that has been used for decades to treat heart failure based on its inotropic potential, was shown to inhibit HIF-1α protein accumulation and block HIF-1 activity in vivo (98, 104). Daily injection with digoxin also prevented the upregulation of known HIF-1 target genes in lung tissue from chronically hypoxic mice and the development of hypoxic pulmonary hypertension, as evidenced by normalization of right ventricular pressure, lack of vascular remodeling, and normal PASMC intracellular Ca2+ and pHi values (2). Interestingly, digoxin reduced, but did not normalize, right ventricular hypertrophy, perhaps suggesting a direct effect of hypoxia on the right ventricle independent of HIF-1 or the pulmonary vascular effects of hypoxia.
In mice beginning digoxin treatment after hypoxia-induced pulmonary hypertension had already developed, pHi and [Ca2+]i were still normalized; however, vascular remodeling was unaltered (2). In rats, the smooth muscle that appears in the small intra-acinar pulmonary vessels during exposure to CH can be reversed with return to normoxic conditions for 6 wk (76), suggesting that de-remodeling is possible if the inciting conditions are removed. Whether the lack of de-remodeling observed with digoxin reflects insufficient time to reverse the changes in muscularity of the small vessels, or, once established, vascular remodeling is maintained via HIF-independent mechanisms, remains to be determined. Right ventricular systolic pressures were also reduced, indicating a slowing of the progression of pulmonary hypertension (2). The ability of digoxin to attenuate further increases in right ventricular pressure in the face of maintained vascular remodeling suggests that vasoconstriction likely plays a more prominent role in maintaining increased pulmonary arterial pressure with longer exposures to CH. This notion is supported by reports showing that perfusion with Rho kinase inhibitors, which alleviates enhanced myofilament Ca2+ sensitization, acutely normalized or markedly reduced pulmonary arterial pressure in chronically hypoxic rats (48) and mice (12), respectively.
Another compound that was found to inhibit HIF activity, albeit via different mechanisms, was the topical antiseptic, acriflavine (35). This compound does not alter HIF-1α protein levels; rather, acriflavine inhibits HIF activity by preventing subunit dimerization (35). In experiments performed with chronically hypoxic rats, which develop more robust pulmonary hypertension than mice exposed to CH, daily administration of acriflavine significantly reduced right ventricular pressure and right ventricular hypertrophy (2). Vascular responses to hypoxia were also reduced, with acriflavine decreasing remodeling of the small pulmonary vessels and normalizing PASMC resting [Ca2+]i in rats subjected to CH (2).
It is important to note that, while both digoxin and acriflavine have beneficial effects in the rodent models of hypoxic pulmonary hypertension, it is impossible to completely rule out that these drugs could have exerted their effects independent of HIF inhibition. That HIF target genes were reduced in the lungs of digoxin-treated animals, and acriflavine inhibits HIF activity by a distinct molecular mechanism (35) and does not have other known mechanisms of action that overlap with digoxin, suggests the effects of these drugs were likely due to pharmacological inhibition of HIF; nevertheless, caution should be used when attributing the effects solely to HIF inhibition.
Another method to potentially target the HIF system is via iron supplementation, since PHD activity is iron sensitive (29). Indeed, infusion of iron reduced, whereas iron chelation increased, the elevation in pulmonary arterial pressures in response to both acute (78) and sustained hypoxia (80). As with the drugs discussed above, it is not possible to rule out nonspecific effects of iron supplementation; however, these data are consistent with the proposal that HIF inhibition could be protective during hypoxic pulmonary hypertension.
CONCLUSIONS
The unique responses of the pulmonary vasculature to hypoxia have been widely studied, yet the exact mechanism(s) underlying these responses remains to be fully elucidated. Data from humans and animal models implicate HIF as both a modulator of acute HPV and a mediator of the development of hypoxia-associated pulmonary hypertension. In patients, digoxin has been used in the treatment of right ventricular failure during pulmonary hypertension (35, 45, 57); however, without data supporting a positive effect of digoxin on survival and pulmonary arterial pressure, the use of this drug in patients with pulmonary hypertension remains controversial. Future studies using HIF inhibitors, including digoxin and acriflavine, will be needed to verify the role of HIF in HPV and to determine whether these agents will prove useful in treating pulmonary hypertension.
GRANTS
The work from the author's laboratory described in this review was supported by grants from the National Heart, Lung, and Blood Institute (HL-67191 and HL-73859).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.A.S. and S.S.L. drafted manuscript; L.A.S. and S.S.L. edited and revised manuscript; L.A.S. and S.S.L. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was made possible by contributions from several talented and dedicated lab members, including Tish Weigand, Clark Undem, Sarah Pisarcik, Jian Wang, Michele Fallon, Eon Rios, E. Miles Whitman, Julie Maylor, and Wenju Lu, and through ongoing collaborations with J. T. Sylvester and Gregg L. Semenza.
REFERENCES
- 1.Aaronson PI, Robertson TP, Knock GA, Becker S, Lewis TH, Snetkov V, Ward JP. Hypoxic pulmonary vasoconstriction: mechanisms and controversies. J Physiol 570: 53–58, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abud EM, Maylor J, Undem C, Punjabi A, Zaiman AL, Myers AC, Sylvester JT, Semenza GL, Shimoda LA. Digoxin inhibits development of hypoxic pulmonary hypertension in mice. Proc Natl Acad Sci U S A 109: 1239–1244, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem 279: 38458–38465, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1α-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol 294: H570–H578, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Ball MK, Waypa GB, Mungai PT, Nielsen JM, Czech L, Dudley VJ, Beussink L, Dettman RW, Berkelhamer SK, Steinhorn RH, Shah SJ, Schumacker PT. Regulation of hypoxia-induced pulmonary hypertension by vascular smooth muscle HIF-1α. Am J Respir Crit Care Med. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berchner-Pfannschmidt U, Tug S, Trinidad B, Oehme F, Yamac H, Wotzlaw C, Flamme I, Fandrey J. Nuclear oxygen sensing: induction of endogenous prolyl-hydroxylase 2 activity by hypoxia and nitric oxide. J Biol Chem 283: 31745–31753, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1α in normoxia. EMBO J 22: 4082–4090, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thébaud B, Bonnet S, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, Archer SL. An abnormal mitochondrial-hypoxia inducible factor-1α-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation 113: 2630–2641, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Brusselmans K, Compernolle V, Tjwa M, Wiesener MS, Maxwell PH, Collen D, Carmeliet P. Heterozygous deficiency of hypoxia-inducible factor-2α protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J Clin Invest 111: 1519–1527, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke DL, Frid MG, Kunrath CL, Karoor V, Anwar A, Wagner BD, Strassheim D, Stenmark KR. Sustained hypoxia promotes the development of a pulmonary artery-specific chronic inflammatory microenvironment. Am J Physiol Lung Cell Mol Physiol 297: L238–L250, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bushuev VI, Miasnikova GY, Sergueeva AI, Polyakova LA, Okhotin D, Gaskin PR, Debebe Z, Nekhai S, Castro OL, Prchal JT, Gordeuk VR. Endothelin-1, vascular endothelial growth factor and systolic pulmonary artery pressure in patients with Chuvash polycythemia. Haematologica 91: 744–749, 2006 [PubMed] [Google Scholar]
- 12.Cahill E, Rowan SC, Sands M, Banahan M, Ryan D, Howell K, McLoughlin P. The pathophysiological basis of chronic hypoxic pulmonary hypertension in the mouse: vasoconstrictor and structural mechanisms contribute equally. Exp Physiol 97: 796–806, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Compernolle V, Brusselmans K, Franco D, Moorman A, Dewerchin M, Collen D, Carmeliet P. Cardia bifida, defective heart development and abnormal neural crest migration in embryos lacking hypoxia-inducible factor-1α. Cardiovasc Res 60: 569–579, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Ehrismann D, Flashman E, Genn DN, Mathioudakis N, Hewitson KS, Ratcliffe PJ, Schofield CJ. Studies on the activity of the hypoxia-inducible-factor hydroxylases using an oxygen consumption assay. Biochem J 401: 227–234, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med 364: 656–665, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1α regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci U S A 94: 4273–4278, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43–54, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Fandrey J, Gorr TA, Gassmann M. Regulating cellular oxygen sensing by hydroxylation. Cardiovasc Res 71: 642–651, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Fijalkowska I, Xu W, Comhair SA, Janocha AJ, Mavrakis LA, Krishnamachary B, Zhen L, Mao T, Richter A, Erzurum SC, Tuder RM. Hypoxia inducible-factor1α regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am J Pathol 176: 1130–1138, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Formenti F, Beer PA, Croft QP, Dorrington KL, Gale DP, Lappin TR, Lucas GS, Maher ER, Maxwell PH, McMullin MF, O'Connor DF, Percy MJ, Pugh CW, Ratcliffe PJ, Smith TG, Talbot NP, Robbins PA. Cardiopulmonary function in two human disorders of the hypoxia-inducible factor (HIF) pathway: von Hippel-Lindau disease and HIF-2α gain-of-function mutation. FASEB J 25: 2001–2011, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frohlich S, Boylan J, McLoughlin P. Hypoxia-induced inflammation in the lung: a potential therapeutic target in acute lung injury? Am J Respir Cell Mol Biol 48: 271–279, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Gale DP, Harten SK, Reid CDL, Tuddenham EGD, Maxwell PH. Autosomal dominant erythrocytosis and pulmonary arterial hypertension associated with an activating HIF2 alpha mutation. Blood 112: 919–921, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Galie N, Manes A, Branzi A. The endothelin system in pulmonary arterial hypertension. Cardiovasc Res 61: 227–237, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Goncharova EA. mTOR and vascular remodeling in lung diseases: current challenges and therapeutic prospects. FASEB J 27: 1796–1807, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hickey MM, Richardson T, Wang T, Mosqueira M, Arguiri E, Yu H, Yu QC, Solomides CC, Morrisey EE, Khurana TS, Christofidou-Solomidou M, Simon MC. The von Hippel-Lindau Chuvash mutation promotes pulmonary hypertension and fibrosis in mice. J Clin Invest 120: 827–839, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirota K, Semenza GL. Regulation of hypoxia-inducible factor 1 by prolyl and asparaginyl hydroxylases. Biochem Biophys Res Commun 338: 610–616, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292: 464–468, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev 12: 149–162, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 30: 393–402, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Kim WY, Perera S, Zhou B, Carretero J, Yeh JJ, Heathcote SA, Jackson AL, Nikolinakos P, Ospina B, Naumov G, Brandstetter KA, Weigman VJ, Zaghlul S, Hayes DN, Padera RF, Heymach JV, Kung AL, Sharpless NE, Kaelin WG, Jr, Wong KK. HIF2α cooperates with RAS to promote lung tumorigenesis in mice. J Clin Invest 119: 2160–2170, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YM, Barnes EA, Alvira CM, Ying L, Reddy S, Cornfield DN. Hypoxia inducible factor-1α in pulmonary artery smooth muscle cells lowers vascular tone by decreasing myosin light chain phosphorylation. Circ Res 112: 1230–1233, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koivunen P, Hirsila M, Kivirikko KI, Myllyharju J. The length of peptide substrates has a marked effect on hydroxylation by the hypoxia-inducible factor prolyl 4-hydroxylases. J Biol Chem 281: 28712–28720, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Kotch LE, Iyer NV, Laughner E, Semenza GL. Defective vascularization of HIF-1α-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Dev Biol 209: 254–267, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Lee K, Zhang H, Qian DZ, Rey S, Liu JO, Semenza GL. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc Natl Acad Sci U S A 106: 17910–17915, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Leggett K, Maylor J, Undem C, Lai N, Lu W, Schweitzer KS, King LS, Myers AC, Sylvester JT, Sidhaye VK, Shimoda LA. Hypoxia-induced migration in pulmonary arterial smooth muscle cells requires calcium-dependent upregulation of aquaporin 1. Am J Physiol Lung Cell Mol Physiol 303: L343–L353, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H, Chen SJ, Chen YF, Meng QC, Durand J, Oparil S, Elton TS. Enhanced endothelin-1 and endothelin receptor gene expression in chronic hypoxia. J Appl Physiol 77: 1451–1459, 1994 [DOI] [PubMed] [Google Scholar]
- 38.Li M, Liu Y, Jin F, Sun X, Li Z, Fang P, Shi H, Jiang X. Endothelin-1 induces hypoxia inducible factor 1α expression in pulmonary artery smooth muscle cells. FEBS Lett 586: 3888–3893, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 95: 496–505, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Liu L, Marti GP, Wei X, Zhang X, Zhang H, Liu YV, Nastai M, Semenza GL, Harmon JW. Age-dependent impairment of HIF-1α expression in diabetic mice: Correction with electroporation-facilitated gene therapy increases wound healing, angiogenesis, and circulating angiogenic cells. J Cell Physiol 217: 319–327, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu YV, Baek JH, Zhang H, Diez R, Cole RN, Semenza GL. RACK1 competes with HSP90 for binding to HIF-1α and is required for O(2)-independent and HSP90 inhibitor-induced degradation of HIF-1α. Mol Cell 25: 207–217, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev 15: 2675–2686, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature 386: 403–407, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. Independent function of two destruction domains in hypoxia-inducible factor-α chains activated by prolyl hydroxylation. EMBO J 20: 5197–5206, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McLaughlin VV, Rich S. Pulmonary hypertension. Curr Probl Cardiol 29: 575–634, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Metzen E, Berchner-Pfannschmidt U, Stengel P, Marxsen JH, Stolze I, Klinger M, Huang WQ, Wotzlaw C, Hellwig-Burgel T, Jelkmann W, Acker H, Fandrey J. Intracellular localisation of human HIF-1α hydroxylases: implications for oxygen sensing. J Cell Sci 116: 1319–1326, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Mogami H, Kojima I. Stimulation of calcium entry is prerequisite for DNA synthesis induced by platelet-derived growth factor in vascular smooth muscle cells. Biochem Biophys Res Commun 196: 650–658, 1993 [DOI] [PubMed] [Google Scholar]
- 48.Nagaoka T, Morio Y, Casanova N, Bauer N, Gebb S, McMurtry I, Oka M. Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 287: L665–L672, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Nicolls MR, Voelkel NF. Hypoxia and the lung: beyond hypoxic vasoconstriction. Antioxid Redox Signal 9: 741–743, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ono S, Westcott JY, Voelkel NF. PAF antagonists inhibit pulmonary vascular remodeling induced by hypobaric hypoxia in rats. J Appl Physiol 73: 1084–1092, 1992 [DOI] [PubMed] [Google Scholar]
- 51.Pisarcik S, Maylor J, Lu W, Yun X, Undem C, Sylvester JT, Semenza GL, Shimoda LA. Activation of hypoxia-inducible factor-1 in pulmonary arterial smooth muscle cells by endothelin-1. Am J Physiol Lung Cell Mol Physiol 304: L549–L561, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prabhakar NR, Kumar GK, Nanduri J. Intermittent hypoxia-mediated plasticity of acute O2 sensing requires altered red-ox regulation by HIF-1 and HIF-2. Ann N Y Acad Sci 1177: 162–168, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev 92: 967–1003, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quinn DA, Dahlberg CG, Bonventre JP, Scheid CR, Honeyman T, Joseph PM, Thompson BT, Hales CA. The role of Na+/H+ exchange and growth factors in pulmonary artery smooth muscle cell proliferation. Am J Respir Cell Mol Biol 14: 139–145, 1996 [DOI] [PubMed] [Google Scholar]
- 55.Remillard CV, Yuan JX. Activation of K+ channels: an essential pathway in programmed cell death. Am J Physiol Lung Cell Mol Physiol 286: L49–L67, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Resnik ER, Herron JM, Lyu SC, Cornfield DN. Developmental regulation of hypoxia-inducible factor 1 and prolyl-hydroxylases in pulmonary vascular smooth muscle cells. Proc Natl Acad Sci U S A 104: 18789–18794, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rich S, Seidlitz M, Dodin E, Osimani D, Judd D, Genthner D, McLaughlin V, Francis G. The short-term effects of digoxin in patients with right ventricular dysfunction from pulmonary hypertension. Chest 114: 787–792, 1998 [DOI] [PubMed] [Google Scholar]
- 58.Rios EJ, Fallon M, Wang J, Shimoda LA. Chronic hypoxia elevates intracellular pH and activates Na+/H+ exchange in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 289: L867–L874, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Ryan HE, Lo J, Johnson RS. HIF-1α is required for solid tumor formation and embryonic vascularization. EMBO J 17: 3005–3015, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sarkar K, Cai Z, Gupta R, Parajuli N, Fox-Talbot K, Darshan MS, Gonzalez FJ, Semenza GL. Hypoxia-inducible factor 1 transcriptional activity in endothelial cells is required for acute phase cardioprotection induced by ischemic preconditioning. Proc Natl Acad Sci U S A 109: 10504–10509, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 29: 625–634, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Semenza GL. Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta 1813: 1263–1268, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell 148: 399–408, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Semenza GL. Involvement of oxygen-sensing pathways in physiologic and pathologic erythropoiesis. Blood 114: 2015–2019, 2009 [DOI] [PubMed] [Google Scholar]
- 65.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 24: 97–106, 2009 [DOI] [PubMed] [Google Scholar]
- 66.Semenza GL. Surviving ischemia: adaptive responses mediated by hypoxia-inducible factor 1. J Clin Invest 106: 809–812, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12: 5447–5454, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shimoda LA. 55th Bowditch Lecture: Effects of chronic hypoxia on the pulmonary circulation: role of HIF-1. J Appl Physiol 113: 1343–1352, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shimoda LA, Fallon M, Pisarcik S, Wang J, Semenza GL. HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 291: L941–L949, 2006 [DOI] [PubMed] [Google Scholar]
- 70.Shimoda LA, Laurie SS. Vascular remodeling in pulmonary hypertension. J Mol Med 91: 297–309, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shimoda LA, Manalo DJ, Sham JS, Semenza GL, Sylvester JT. Partial HIF-1α deficiency impairs pulmonary arterial myocyte electrophysiological responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 281: L202–L208, 2001 [DOI] [PubMed] [Google Scholar]
- 72.Shimoda LA, Sham JS, Liu Q, Sylvester JT. Acute and chronic hypoxic pulmonary vasoconstriction: a central role for endothelin-1? Respir Physiol Neurobiol 132: 93–106, 2002 [DOI] [PubMed] [Google Scholar]
- 73.Shimoda LA, Wang J, Sylvester JT. Ca2+ channels and chronic hypoxia. Microcirculation 13: 657–670, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 54, Suppl 1: S43–S54, 2009 [DOI] [PubMed] [Google Scholar]
- 75.Skuli N, Liu L, Runge A, Wang T, Yuan L, Patel S, Iruela-Arispe L, Simon MC, Keith B. Endothelial deletion of hypoxia-inducible factor-2α alters vascular function and tumor angiogenesis. Blood 114: 469–477, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sluiter I, van Heijst A, Haasdijk R, Kempen MB, Boerema-de Munck A, Reiss I, Tibboel D, Rottier RJ. Reversal of pulmonary vascular remodeling in pulmonary hypertensive rats. Exp Mol Pathol 93: 66–73, 2012 [DOI] [PubMed] [Google Scholar]
- 77.Smirnov SV, Robertson TP, Ward JP, Aaronson PI. Chronic hypoxia is associated with reduced delayed rectifier K+ current in rat pulmonary artery muscle cells. Am J Physiol Heart Circ Physiol 266: H365–H370, 1994 [DOI] [PubMed] [Google Scholar]
- 78.Smith TG, Balanos GM, Croft QP, Talbot NP, Dorrington KL, Ratcliffe PJ, Robbins PA. The increase in pulmonary arterial pressure caused by hypoxia depends on iron status. J Physiol 586: 5999–6005, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith TG, Brooks JT, Balanos GM, Lappin TR, Layton DM, Leedham DL, Liu C, Maxwell PH, McMullin MF, McNamara CJ, Percy MJ, Pugh CW, Ratcliffe PJ, Talbot NP, Treacy M, Robbins PA. Mutation of von Hippel-Lindau tumour suppressor and human cardiopulmonary physiology. PLoS Med 3: e290, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith TG, Talbot NP, Privat C, Rivera-Ch M, Nickol AH, Ratcliffe PJ, Dorrington KL, Leon-Velarde F, Robbins PA. Effects of iron supplementation and depletion on hypoxic pulmonary hypertension: two randomized controlled trials. JAMA 302: 1444–1450, 2009 [DOI] [PubMed] [Google Scholar]
- 81.Stenmark KR, Davie NJ, Reeves JT, Frid MG. Hypoxia, leukocytes, and the pulmonary circulation. J Appl Physiol 98: 715–721, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297: L1013–L1032, 2009 [DOI] [PubMed] [Google Scholar]
- 83.Suzuki H, Twarog BM. Membrane properties of smooth muscle cells in pulmonary hypertensive rats. Am J Physiol Heart Circ Physiol 242: H907–H915, 1982 [DOI] [PubMed] [Google Scholar]
- 84.Sylvester JT, Shimoda LA, Aaronson PI, Ward JP. Hypoxic pulmonary vasoconstriction. Physiol Rev 92: 367–520, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takeda K, Ho VC, Takeda H, Duan LJ, Nagy A, Fong GH. Placental but not heart defects are associated with elevated hypoxia-inducible factor α levels in mice lacking prolyl hydroxylase domain protein 2. Mol Cell Biol 26: 8336–8346, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tan Q, Kerestes H, Percy MJ, Pietrofesa R, Chen L, Khurana TS, Christofidou-Solomidou M, Lappin TR, Lee FS. Erythrocytosis and pulmonary hypertension in a mouse model of human HIF2A gain of function mutation. J Biol Chem 288: 17134–17144, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev 11: 72–82, 1997 [DOI] [PubMed] [Google Scholar]
- 88.Tissot van Patot MC, Gassmann M. Hypoxia: adapting to high altitude by mutating EPAS-1, the gene encoding HIF-2α. High Alt Med Biol 12: 157–167, 2011 [DOI] [PubMed] [Google Scholar]
- 89.Voelkel NF, Mizuno S, Bogaard HJ. The role of hypoxia in pulmonary vascular diseases: a perspective. Am J Physiol Lung Cell Mol Physiol 304: L457–L465, 2013 [DOI] [PubMed] [Google Scholar]
- 90.von Euler U, Liljestrand G. Observations on the pulmonary arterial blood pressure in the cat. Acta Physiol Scand 12: 301–320, 1946 [Google Scholar]
- 91.Wang J, Juhaszova M, Rubin LJ, Yuan XJ. Hypoxia inhibits gene expression of voltage-gated K+ channel α subunits in pulmonary artery smooth muscle cells. J Clin Invest 100: 2347–2353, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang J, Shimoda LA, Sylvester JT. Capacitative calcium entry and TRPC channel proteins are expressed in rat distal pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L848–L858, 2004 [DOI] [PubMed] [Google Scholar]
- 93.Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res 98: 1528–1537, 2006 [DOI] [PubMed] [Google Scholar]
- 94.Whitman EM, Pisarcik S, Luke T, Fallon M, Wang J, Sylvester JT, Semenza GL, Shimoda LA. Endothelin-1 mediates hypoxia-induced inhibition of voltage-gated K+ channel expression in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 294: L309–L318, 2008 [DOI] [PubMed] [Google Scholar]
- 95.Wiener CM, Booth G, Semenza GL. In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochem Biophys Res Commun 225: 485–488, 1996 [DOI] [PubMed] [Google Scholar]
- 96.Wong CC, Zhang H, Gilkes DM, Chen J, Wei H, Chaturvedi P, Hubbi ME, Semenza GL. Inhibitors of hypoxia-inducible factor 1 block breast cancer metastatic niche formation and lung metastasis. J Mol Med 90: 803–815, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yanagisawa M, Kurihara H, Kimura S, Goto K, Masaki T. A novel peptide vasoconstrictor, endothelin, is produced by vascular endothelium and modulates smooth muscle Ca2+ channels. J Hypertens Suppl 6: S188–S191, 1988 [DOI] [PubMed] [Google Scholar]
- 98.Yoshida T, Zhang H, Iwase T, Shen J, Semenza GL, Campochiaro PA. Digoxin inhibits retinal ischemia-induced HIF-1α expression and ocular neovascularization. FASEB J 24: 1759–1767, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu AY, Frid MG, Shimoda LA, Wiener CM, Stenmark K, Semenza GL. Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor-1 in the lung. Am J Physiol Lung Cell Mol Physiol 275: L818–L826, 1998 [DOI] [PubMed] [Google Scholar]
- 100.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. J Clin Invest 103: 691–696, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu F, White SB, Zhao Q, Lee FS. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci U S A 98: 9630–9635, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu L, Hales CA. Silencing of NHE1 attenuates PASMC proliferation, hypertrophy and migration via E2F1. Am J Respir Cell Mol Biol 45: 923–930, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yu L, Quinn DA, Garg HG, Hales CA. Deficiency of the NHE1 gene prevents hypoxia-induced pulmonary hypertension and vascular remodeling. Am J Respir Crit Care Med 177: 1276–1284, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang H, Qian DZ, Tan YS, Lee K, Gao P, Ren YR, Rey S, Hammers H, Chang D, Pili R, Dang CV, Liu JO, Semenza GL. Digoxin and other cardiac glycosides inhibit HIF-1α synthesis and block tumor growth. Proc Natl Acad Sci U S A 105: 19579–19586, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]