Abstract
In humans, β-adrenergic receptor activation causes a substantial portion of hypoxic vasodilation in skeletal muscle at rest and during forearm exercise. Recent evidence suggests that β-adrenergic receptors are either more sensitive or upregulated in young women vs. men. Therefore, we examined whether sex influences hypoxic vasodilation in 31 young subjects (15 women/16 men; 26 ± 1 yr). We also examined whether potential sex-related differences existed in a group of older adults (6 women/5 men; 61 ± 2 yr). All subjects performed forearm exercise at 10 and 20% of maximum under normoxic and hypoxic [80% arterial O2 saturation (So2)] conditions. Forearm vascular conductance (FVC; ml·min−1·100 mmHg−1) was calculated from blood flow (ml/min) and blood pressure (mmHg). At rest, young women demonstrated a greater vasodilator response to hypoxia compared with men (39 ± 12 vs. 13 ± 6%, P < 0.05). The absolute compensatory vasodilator response (hypoxic FVC-normoxic FVC) during exercise was similar between sexes, but the relative change was greater in young women at 10% (28 ± 5 vs. 17 ± 3%, P < 0.05) and 20% exercise (29 ± 4% vs. 15 ± 3%, P < 0.01). Additionally, the absolute changes in vasodilation after normalizing the response to forearm volume or workload were greater in young women during exercise (P < 0.05). Interestingly, the compensatory vasodilator responses between older women and men were similar at 10 and 20% exercise, regardless of whether the response is expressed as absolute, relative, or absolute change normalized for forearm volume or workload (P = 0.054–0.97). Our data suggest that the compensatory vasodilator response to hypoxic exercise is greater in young women compared with men. However, sex-specific differences appear to be lost with aging.
Keywords: vasodilation, hypoxia, sex, exercise
blood flow increases to exercising skeletal muscle, and this increase is driven primarily by vasodilation in the contracting muscles. Under conditions of systemic hypoxia (decreased oxygen availability) and during exercise (increased demand), there is a greater need for an increase in blood flow. The combination of submaximal dynamic exercise and hypoxia produces a compensatory vasodilation and augmented blood flow in contracting muscles relative to the same level of exercise under normoxic conditions and greater than that predicted by a simple summation of the individual responses (3, 7, 8, 23, 25, 27). The compensatory vasodilation during hypoxic exercise occurs to ensure an adequate oxygen delivery to the active muscles. Over the last several years, our group has conducted a series of studies in humans to evaluate various vasodilating substances as potential mediators of compensatory vasodilation during hypoxic exercise (5). During mild rhythmic handgripping, we found evidence that at least some of the compensatory vasodilation was mediated by vasodilating β-adrenergic receptors in the active limb (27). We later demonstrated that the β-adrenergic receptor component of compensatory vasodilation during mild rhythmic handgripping exercise was mediated through a nitric oxide pathway (4).
There is now substantial evidence suggesting that the control of vascular tone at rest and during exercise might be influenced by sex. Along these lines, women tend to demonstrate greater vasodilator responses to intra-arterial infusion of acetylcholine (when normalized to vasodilator capacity) relative to young men (10). Additionally, sex-related differences in the vasoconstrictor responsiveness to sympathetic stimulation and norepinephrine-induced vasoconstriction have been observed in young humans (13, 15, 17). Of specific interest to the current study, evidence also suggests that β-adrenergic receptors are either more sensitive or upregulated in young women vs. men (15, 17), and that the vasodilatory response to dynamic leg exercise under normoxic conditions is greater in young women compared with men (22). Taken together with our studies demonstrating that β-adrenergic receptor activation is responsible for a portion of hypoxic vasodilation, these findings may suggest that the compensatory vasodilator response could be greater in women compared with men.
With this information as background, the purpose of this study was to investigate whether hypoxic vasodilation is influenced by sex. We tested the hypothesis that young women would demonstrate a greater compensatory vasodilation during hypoxic exercise than their male counterparts. Interestingly, the compensatory vasodilator response to hypoxic exercise is attenuated in older adults (9). Moreover, the enhanced β-adrenergic-mediated vasodilation observed in young women appears to be reduced in older women (15). Therefore, to gain insight whether potential sex-related differences in hypoxic vasodilation exist with aging, we also examined the compensatory vasodilator responses in a group of older adults.
METHODS
All of the records used in this study were retrospectively analyzed from previous trials completed in our laboratory (7–9). The investigations from which the data were obtained had ethical approval from the institutional review board of the Mayo Clinic and were performed according to the Declaration of Helsinki. In total, 33 young (26 ± 1 yr) and 11 older (61 ± 2 yr) subjects gave their informed consent to participate in the specific studies. However, two of the young subjects participated in multiple protocols. The first protocol in which the subjects participated was used in the present analysis. Therefore, a total of 31 records from the young adults were used in the present study. Subjects were nonsmokers and were not taking any medications. Subjects reported normal daily activities, but no regular physical training. Studies were performed after an overnight fast and after the subjects refrained from exercise and caffeine for at least 24 h. Young female subjects were studied during the early follicular phase of the menstrual cycle or the placebo phase of oral contraceptives (1, 21).
Forearm Exercise
Subjects performed rhythmic forearm exercise with a handgrip device using the nondominant arm at 10 and 20% of each subject's maximal voluntary contraction (MVC). The weight was lifted 4–5 cm over a pulley at a duty cycle of 1 s contraction and 2 s relaxation (20 contractions/min) using a metronome to ensure correct timing.
Arterial and Venous Catheterization
A 20-gauge, 5-cm catheter was placed in the brachial artery of the exercising arm under aseptic conditions after local anesthesia (2% lidocaine). The catheter was connected to a three-port connector in series (11). One port was linked to a pressure transducer positioned at heart level (Model PX600F, Edwards Lifescience, Irvine, CA) to allow measurement of arterial pressure and was continuously flushed (3 ml/h) with heparinized saline with a stop-cock system to enable arterial blood sampling. Deep venous blood was sampled via an 18-gauge, 3-cm catheter inserted retrograde in an antecubital vein (16).
Heart Rate and Systemic Blood Pressure
Heart rate (HR) was recorded via continuous three-lead ECG. A pressure transducer connected to the arterial catheter measured beat-to-beat blood pressure (Cardiocap/5, Datex-Ohmeda, Louisville, CO).
Forearm Blood Flow
Brachial artery mean blood velocity and diameter were determined with a 12-MHz linear-array Doppler probe (model M12L, Vivid 7, General Electric, Milwaukee, WI). Brachial artery blood velocity was measured with a probe insonation angle previously calibrated to 60°. Brachial artery diameter measurements were obtained at end diastole between contractions during steady-state conditions. Forearm blood flow (FBF) was calculated as the product of mean blood velocity (cm/s) and brachial artery cross-sectional area (cm2) and expressed as milliliters per minute (ml/min). Mean blood velocity measurements included the velocity profiles across the entire duty cycle (both contraction and relaxation phases).
Systemic Hypoxia
A self-regulating partial rebreathe system that clamps end-tidal CO2 at baseline levels, despite large changes in minute ventilation, was used to generate hypoxic conditions (2, 26–28). The target arterial So2 (assessed via pulse oximetry) was ∼80%. End-tidal carbon dioxide concentrations were monitored (Cardiocap/5, Datex-Ohmeda, Louisville, CO), and ventilation was assessed via a turbine (model VMM-2a, Interface Associates, Laguna Nigel, CA).
Blood-Gas and Catecholamine Analysis
Brachial artery and deep venous blood samples were analyzed with a clinical blood-gas analyzer (Bayer 855 Automatic Blood Gas System, Boston, MA) for partial pressures of O2 and CO2 (Po2 and Pco2), pH, and So2. Arterial and venous O2 content were calculated using the measured Po2 and So2 values. Arterial and venous plasma catecholamine (epinephrine and norepinephrine) levels were determined by HPLC with electrochemical detection.
Experimental Design
Each subject completed a resting baseline condition (5 min) followed by rhythmic forearm exercise at 10% MVC, which was immediately increased to 20% MVC during normoxia and normocapnic hypoxia. Forearm exercise was performed at each intensity for 5 min. Exposure to normoxia or hypoxia was alternated and randomized. All hypoxia trails included a 5-min normoxia (prehypoxia) period immediately before the level of inspired O2 was titrated to achieve an arterial So2 of ∼80%. The prehypoxia period served as a normoxic baseline to calculate the hypoxia-mediated changes in blood flow at rest and during exercise. The titration of inspired O2 to achieve the target arterial So2 level occurred over a 5- to 10-min period. A rest period of at least 20 min was allowed between each exercise trial. During each condition (normoxia and hypoxia), arterial and venous blood was sampled at rest and at steady-state exercise for blood-gas analysis and plasma catecholamine determination.
Data Analysis and Statistics
Data were collected at 200 Hz and analyzed off-line with signal processing software (WinDaq, DATAQ Instruments, Akron, OH). Mean arterial pressure (MAP) was determined from the brachial artery pressure waveform, and HR was determined from the electrocardiogram. Ventilation, end-tidal CO2, and So2 (pulse oximetry) were determined by averaging minutes 4 and 5 at rest and during each exercise intensity. FBF and arterial pressure were determined by averaging values from the 4th min at rest and each exercise bout. Forearm vascular conductance (FVC) was calculated as (FBF/arterial pressure) × 100 and expressed as ml·min−1·100 mmHg−1. Compensatory vasodilation was calculated as the absolute and relative difference in FVC between hypoxic and normoxic conditions at both rest and during exercise. Blood-gas and catecholamine values were determined from blood samples obtained during normoxia and hypoxia. Arterial (a)-venous (v) oxygen difference during forearm exercise was calculated by the blood samples. The v-a norepinephrine difference was calculated by the difference between venous and arterial norepinephrine concentrations.
All values are expressed as means ± SE. To examine the effects of sex on the compensatory vasodilator response to hypoxic exercise, we compared via ANOVA the magnitude of change in FVC (steady-state FVC during hypoxic exercise − steady state FVC during normoxic exercise) between the young men and women. To examine if sex-related differences exist with aging, we also compared the compensatory vasodilator response to hypoxic exercise via ANOVA in a group of older adults. Hemodynamic, respiratory, blood-gas, and catecholamine responses during each gas condition (normoxia and hypoxia) at rest and during exercise were compared via repeated-measures ANOVA to examine potential differences between sexes within each age group. Tukey post hoc analysis determined where statistical differences occurred. Statistical difference was set a priori at P < 0.05.
RESULTS
Subject demographics are presented in Table 1. There were no differences in age between men and women in each respective age group. However, height, weight, body mass index, forearm volume, and MVC were all lower in women compared with men in each respective age group (P < 0.01 for all).
Table 1.
Subject characteristics
| Young |
Older |
|||
|---|---|---|---|---|
| Variable | Men | Women | Men | Women |
| Age, yr | 26 ± 1 | 26 ± 1 | 59 ± 3 | 62 ± 3 |
| Height, cm | 178 ± 2 | 167 ± 2* | 179 ± 11 | 65 ± 3* |
| Weight, kg | 81 ± 2 | 63 ± 2* | 85 ± 3 | 63 ± 2* |
| BMI, kg/m2 | 25.4 ± 0.5 | 22.4 ± 0.6* | 26.8 ± 0.9 | 23.5 ± 1.1* |
| FAV, ml | 1,107 ± 32 | 759 ± 29* | 1,022 ± 59 | 692 ± 35* |
| MVC, kg | 47 ± 2 | 29 ± 1* | 43 ± 3 | 26 ± 3* |
Values are means ± SE.
BMI, body mass index; FAV, forearm volume; MVC, maximal voluntary contraction.
P < 0.05 vs. men.
Young Adults
Systemic hemodynamic and respiratory responses.
Under resting conditions MAP, HR and minute ventilation were similar between men and women for both the normoxia and hypoxia trials (Table 2). However, women demonstrated a lower end-tidal CO2 at rest compared with men. By design, end-tidal CO2 was maintained during all conditions and, therefore, remained lower in the women during exercise. As expected, HR and minute ventilation increased as a consequence of systemic hypoxia and incremental forearm exercise in both men and women. MAP increased with exercise only in the men and was significantly greater compared with women during exercise at 20% MVC under normoxic and hypoxic conditions.
Table 2.
Systemic hemodynamic and respiratory responses at rest and with incremental exercise during normoxia and hypoxia in men and women
| Normoxia |
Hypoxia |
|||||
|---|---|---|---|---|---|---|
| Rest | 10% | 20% | Rest | 10% | 20% | |
| Young adults | ||||||
| Men (n = 16) | ||||||
| Mean arterial pressure, mmHg | 90 ± 1 | 92 ± 1† | 95 ± 1† | 90 ± 1 | 92 ± 1† | 94 ± 2† |
| Heart rate, beats/min | 60 ± 2 | 64 ± 2† | 68 ± 3† | 73 ± 3* | 77 ± 3*† | 80 ± 3*† |
| Minute ventilation, l/min (btps) | 7.5 ± 0.6 | 8.8 ± 0.7† | 10.4 ± 1.0† | 13.3 ± 1.5* | 16.6 ± 2.2*† | 18.6 ± 2.2*† |
| End-tidal CO2, % | 5.3 ± 0.1 | 5.4 ± 0.1 | 5.4 ± 0.1 | 5.3 ± 0.1 | 5.3 ± 0.1 | 5.4 ± 0.1 |
| Women (n = 15) | ||||||
| Mean arterial pressure, mmHg | 86 ± 2 | 87 ± 2 | 87 ± 2‡ | 88 ± 2 | 88 ± 2 | 87 ± 2‡ |
| Heart rate, beats/min | 62 ± 3 | 67 ± 3† | 68 ± 3† | 76 ± 3* | 79 ± 4* | 82 ± 4*† |
| Minute ventilation, l/min (btps) | 6.0 ± 0.5 | 7.9 ± 0.7† | 9.3 ± 0.8† | 10.8 ± 1.9* | 13.0 ± 1.8* | 15.1 ± 2.0*† |
| End-tidal CO2, % | 4.8 ± 0.1 | 4.8 ± 0.1 | 4.9 ± 0.1 | 4.8 ± 0.1 | 4.8 ± 0.1 | 4.9 ± 0.1 |
| Older adults | ||||||
| Men (n = 5) | ||||||
| Mean arterial pressure, mmHg | 106 ± 4 | 111 ± 6† | 114 ± 6† | 106 ± 5 | 113 ± 6† | 113 ± 6† |
| Heart rate, beats/min | 68 ± 4 | 70 ± 5† | 71 ± 5 | 81 ± 7* | 82 ± 7*† | 81 ± 8* |
| Minute ventilation l/min (btps) | 8.8 ± 0.8 | 8.9 ± 0.8 | 10.3 ± 1.6 | 12.5 ± 2.4* | 14.6 ± 1.7* | 17.2 ± 2.4*† |
| End-tidal CO2, % | 5.0 ± 0.2 | 5.1 ± 0.1 | 5.1 ± 0.1 | 4.9 ± 0.2 | 5.1 ± 0.2 | 5.0 ± 0.2 |
| Women (n = 6) | ||||||
| Mean arterial pressure, mmHg | 98 ± 7 | 102 ± 7† | 106 ± 6† | 100 ± 6 | 100 ± 7 | 102 ± 5 |
| Heart rate, beats/min | 62 ± 2 | 66 ± 3† | 70 ± 3† | 76 ± 2* | 77 ± 3*† | 80 ± 5*† |
| Minute ventilation, 1/min (btps) | 5.9 ± 0.3 | 6.5 ± 0.5 | 9.0 ± 1.7† | 11.0 ± 1.1* | 14.5 ± 2.4* | 16.7 ± 4.0*† |
| End-tidal CO2, mmHg | 5.3 ± 0.2 | 5.3 ± 0.2 | 5.3 ± 0.1 | 5.3 ± 0.1 | 5.3 ± 0.2 | 5.3 ± 0.1 |
Values are means ± SE; n, no. of subjects.
P < 0.05 vs. normoxia.
P < 0.05 vs. rest.
P < 0.05 vs. men.
Forearm exercise.
Women demonstrated a substantially lower absolute FBF and FVC at 10 and 20% MVC compared with men (Tables 3 and 4). Exposure to hypoxia at rest resulted in an increase in the absolute FBF and FVC in women (P < 0.01), but not men (P = 0.17–0.20) (Table 3). Consequently, the relative change in FBF and FVC (compared with normoxia) at rest was substantially greater in young women compared with men (40 ± 12 vs. 17 ± 6% for FBF and 39 ± 12 vs. 13 ± 6% for FVC; P < 0.05 for both). Both men and women demonstrated higher absolute FBF and FVC during exercise under hypoxic compared with normoxic conditions (main effect of hypoxia; P < 0.05; Table 3). The absolute change in FBF and FVC between the normoxic and hypoxic exercise conditions were similar between young women and men (Table 3 and Fig. 1A). However, the relative compensatory vasodilator responses to hypoxic exercise at 10 and 20% MVC (%increase in FVC during steady-state hypoxic exercise compared with steady-state FVC during normoxic exercise) were substantially greater in young women compared with men (Fig. 1B).
Table 3.
Forearm hemodynamics at rest and with incremental exercise during normoxia and hypoxia in men and women
| Rest | 10% | 20% | |
|---|---|---|---|
| Young adults | |||
| FBF, ml/min | |||
| Men (n = 16) | |||
| Normoxia | 80 ± 9 | 250 ± 17† | 426 ± 28† |
| Hypoxia | 86 ± 8 | 289 ± 17*† | 486 ± 29*† |
| Women (n = 15) | |||
| Normoxia | 41 ± 4 | 137 ± 11†‡ | 224 ± 20†‡ |
| Hypoxia | 52 ± 6* | 172 ± 18*†‡ | 273 ± 24*†‡ |
| FVC, ml×min−1×100 mmHg−1 | |||
| Men (n = 16) | |||
| Normoxia | 89 ± 9 | 270 ± 16† | 446 ± 28† |
| Hypoxia | 96 ± 9 | 312 ± 16*† | 510 ± 29*† |
| Women (n = 15) | |||
| Normoxia | 47 ± 5 | 157 ± 12†‡ | 254 ± 22†‡ |
| Hypoxia | 60 ± 6* | 201 ± 19*†‡ | 323 ± 26*†‡ |
| Older adults | |||
| FBF, ml/min | |||
| Men (n = 5) | |||
| Normoxia | 78 ± 6 | 263 ± 35† | 430 ± 68† |
| Hypoxia | 87 ± 9 | 320 ± 42*† | 471 ± 74*† |
| Women (n = 6) | |||
| Normoxia | 56 ± 10 | 170 ± 18†‡ | 262 ± 40†‡ |
| Hypoxia | 66 ± 15 | 242 ± 26*†‡ | 285 ± 41†‡ |
| FVC, ml×min−1×100 mmHg−1 | |||
| Men (n = 5) | |||
| Normoxia | 73 ± 5 | 237 ± 30† | 379 ± 56† |
| Hypoxia | 82 ± 7 | 286 ± 38*† | 424 ± 73*† |
| Women (n = 6) | |||
| Normoxia | 58 ± 11 | 169 ± 21†‡ | 251 ± 40†‡ |
| Hypoxia | 65 ± 14 | 202 ± 28*†‡ | 280 ± 39*†‡ |
Values are means ± SE; n, no. of subjects. FBF, forearm blood flow; FVC, forearm vascular conductance.
P < 0.05 vs. normoxia.
P < 0.01 vs. previous exercise intensity.
P < 0.05 vs. men.
Table 4.
Forearm hemodynamics normalized for forearm volume and workload during normoxia and hypoxia in men and women
| Rest | 10% MVC | 20% MVC | 10% MVC | 20% MVC | |
|---|---|---|---|---|---|
| FBF normalized to FAV, ml×min−1×100 ml FAV−1 |
FBF normalized to workload, ml×min−1×100 g weight lifted−1 |
||||
| Young | |||||
| Men (n = 16) | |||||
| Normoxia | 7.3 ± 0.8 | 22.4 ± 1.2† | 38.4 ± 2.2† | 5.3 ± 0.3 | 4.6 ± 0.2 |
| Hypoxia | 7.7 ± 0.6 | 26.0 ± 1.1*† | 43.9 ± 2.3*† | 6.2 ± 0.3* | 5.2 ± 0.2* |
| Women (n = 15) | |||||
| Normoxia | 5.4 ± 0.7‡ | 18.3 ± 1.6†‡ | 29.9 ± 3.0†‡ | 5.1 ± 0.4 | 3.9 ± 0.3 |
| Hypoxia | 7.0 ± 0.8* | 22.7 ± 2.4†* | 36.4 ± 3.4†* | 6.3 ± 2.4* | 4.7 ± 0.3* |
| FVC normalized to FAV, ml×min−1×100 mmHg−1×100 ml FAV−1 |
FVC normalized to workload, ml×min−1×100 mmHg−1×100 g weight lifted−1 |
||||
| Men (n = 16) | |||||
| Normoxia | 8.1 ± 0.8 | 24.3 ± 1.1† | 40.3 ± 2.1† | 5.8 ± 0.3 | 4.8 ± 0.2 |
| Hypoxia | 8.6 ± 0.7 | 28.1 ± 1.0*† | 46.2 ± 2.3*† | 6.7 ± 0.2* | 5.5 ± 0.2* |
| Women (n = 15) | |||||
| Normoxia | 6.3 ± 0.8 | 21.0 ± 1.9† | 34.1 ± 3.4† | 5.9 ± 0.5 | 4.4 ± 0.3 |
| Hypoxia | 8.1 ± 0.9* | 26.9 ± 2.6*† | 43.2 ± 3.9*† | 7.5 ± 0.7* | 5.6 ± 0.3* |
| FBF normalized to FAV, ml×min−1×100 ml FAV−1 |
FBF normalized to workload, ml×min−1×100 g weight lifted−1 |
||||
| Older | |||||
| Men (n = 5) | |||||
| Normoxia | 7.7 ± 0.5 | 26.3 ± 4.1† | 42.9 ± 7.3† | 6.1 ± 0.05 | 4.8 ± 0.5 |
| Hypoxia | 8.5 ± 0.7 | 32.2 ± 5.7*† | 46.9 ± 8.1† | 7.5 ± 0.8* | 5.3 ± 0.5 |
| Women (n = 6) | |||||
| Normoxia | 8.1 ± 1.5 | 24.7 ± 2.7† | 37.6 ± 5.6† | 6.3 ± 0.4 | 4.9 ± 0.6 |
| Hypoxia | 9.3 ± 2.0 | 28.9 ± 3.3*† | 40.5 ± 4.7*† | 7.3 ± 0.6* | 5.3 ± 0.5* |
| FVC normalized to FAV, ml×min−1×100 mmHg−1×100 ml FAV−1 |
FVC normalized to workload, ml×min−1×100 mmHg−1×100 g weight lifted−1 |
||||
| Men (n = 5) | |||||
| Normoxia | 7.2 ± 0.5 | 23.7 ± 3.7† | 37.7 ± 5.9† | 5.5 ± 0.4 | 4.3 ± 0.4 |
| Hypoxia | 8.0 ± 0.4 | 28.7 ± 4.8*† | 42.1 ± 7.1*† | 6.6 ± 0.6* | 4.7 ± 0.5 |
| Women (n = 6) | |||||
| Normoxia | 8.3 ± 1.6 | 24.7 ± 3.2† | 36.0 ± 5.6† | 6.2 ± 0.6 | 4.7 ± 0.7 |
| Hypoxia | 9.2 ± 2.0 | 29.3 ± 4.2*† | 40.3 ± 5.3*† | 7.3 ± 0.8* | 5.3 ± 0.7* |
Values are means ± SE; n, no. of subjects.
P < 0.05 vs. normoxia.
P < 0.01 vs. previous exercise intensity.
P < 0.05 vs. men.
Fig. 1.
Compensatory vasodilator response expressed as the absolute (A) and relative (B) difference in steady-state forearm vascular conductance (FVC) between hypoxic and normoxic exercise in young adults. A: the absolute (Δ) increase in FVC during hypoxic exercise was similar between young men (n = 16) and women (n = 15). B: young women demonstrated a greater relative (%) increase in FVC during hypoxic exercise at 10 and 20% maximal voluntary contraction (MVC) compared with young men (n = 16). Values are means ± SE. *P < 0.05 vs. men.
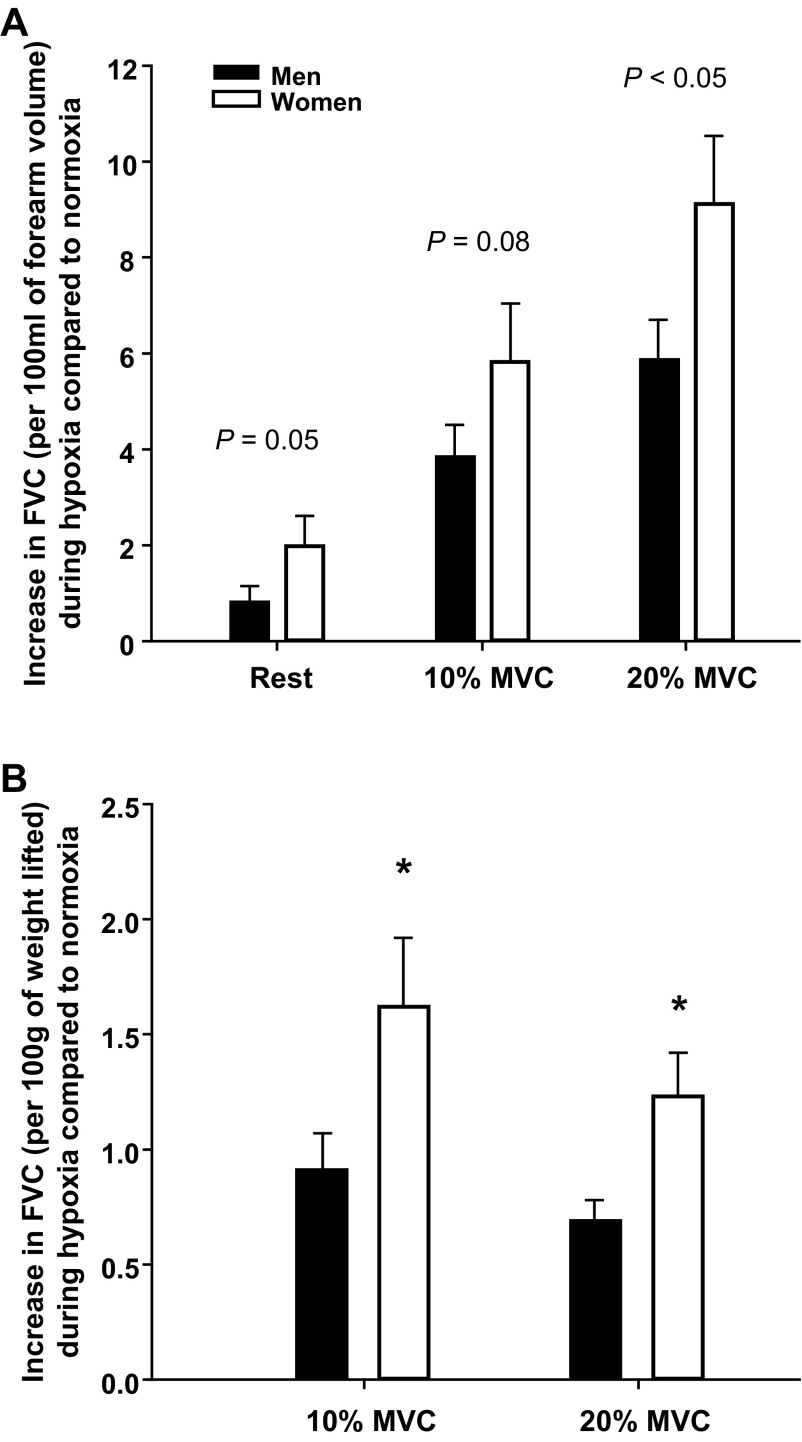
To address the discrepant findings reported above (absolute vs. relative changes) and account for differences in forearm volume and the absolute workload used for each relative exercise intensity between men and women, we analyzed the data two additional ways. First, we examined whether sex-related differences existed in the absolute change in vasodilation (FVC) after normalizing the response to forearm volume (by dividing FVC by forearm volume; ml·min−1·100 mmHg−1·100 ml forearm volume−1). In both young men and women, the FVC normalized to forearm volume was greater during hypoxic compared with normoxic exercise at 10 and 20% MVC (P < 0.01; Table 4). However, the magnitude of the increases in FVC normalized to forearm volume between normoxic and hypoxic conditions either tended to be or were greater in young women compared with young men (Fig. 2A).
Fig. 2.
Absolute change in vasodilation (FVC) during hypoxia compared with normoxia normalized to forearm volume or workload in young adults. A: the young women (n = 15) tended to have a greater increase in FVC normalized for forearm volume (per 100 ml) between normoxic and hypoxic conditions compared with young men (n = 16). B: young women demonstrated a greater increase in FVC normalized for workload (per 100 g of weight lifted). Values are means ± SE. *P < 0.05 vs. men.
Since there were significant differences in the absolute weight used for each relative intensity (10 and 20% MVC) between groups, our second approach examined whether sex-related differences existed in the absolute change in vasodilation (FVC) after normalizing the response for workload (by dividing FVC by the weight lifted; ml·min−1·100 mmHg−1·100 g weight lifted−1). In both young men and women, the FVC normalized to workload was greater during hypoxic compared with normoxic exercise at 10 and 20% MVC (P < 0.01; Table 4). This analytic approach clearly demonstrates that the magnitude of change in the vasodilator response to hypoxic exercise after correcting for workload is enhanced in young women (Fig. 2B). Moreover, this approach also suggests that the lack of difference between sexes in the absolute change in FVC during hypoxic exercise (Fig. 1A) might be due to the significantly different absolute workloads used between young men and women.
Blood gases.
Women demonstrated lower arterial and venous oxygen content than men at rest and with incremental forearm exercise under both normoxia and hypoxia conditions (P < 0.05 for all) (Table 5). Hypoxia reduced arterial Po2 and arterial and venous oxygen content at rest and with incremental forearm exercise in both men and women (P < 0.05 for all). Hypoxia also resulted in a reduced a-v oxygen difference compared with normoxia during incremental exercise in both men and women. Forearm oxygen consumption increased similarly with normoxic and hypoxic exercise at 10 and 20% MVC in men. However, oxygen consumption in women was slightly yet significantly (P < 0.05) greater during hypoxic exercise at 20% MVC compared with the same intensity of normoxic exercise (Table 5). These differences persisted after normalizing oxygen consumption to forearm volume (per 100 ml).
Table 5.
Arterial and venous blood-gas responses at rest and with incremental exercise during normoxia and hypoxia in men and women
| Normoxia |
Hypoxia |
|||||
|---|---|---|---|---|---|---|
| Rest | 10% | 20% | Rest | 10% | 20% | |
| Young adults | ||||||
| Men (n = 16) | ||||||
| SaO2, % | 97 ± 0 | 97 ± 0 | 97 ± 0 | 81 ± 1* | 82 ± 1* | 82 ± 0* |
| PaO2, Torr | 103 ± 2 | 104 ± 2 | 107 ± 2 | 46 ± 1* | 47 ± 1* | 48 ± 1* |
| PvO2, Torr | 40 ± 3 | 28 ± 2† | 28 ± 1† | 29 ± 1* | 24 ± 1*† | 25 ± 1* |
| Arterial O2 content, ml/1 | 189 ± 4 | 189 ± 5 | 190 ± 4 | 157 ± 4* | 161 ± 4* | 165 ± 5* |
| Venous O2 content, ml/1 | 129 ± 8 | 85 ± 6† | 88 ± 5† | 95 ± 6* | 74 ± 6*† | 76 ± 7*† |
| a-v O2, ml/1 | 61 ± 6 | 104 ± 6 | 102 ± 6 | 63 ± 5 | 87 ± 7* | 88 ± 6* |
| O2 consumption, ml/min | 4.6 ± 0.7 | 26.2 ± 2.4† | 42.9 ± 3.3† | 5.1 ± 0.5 | 25.2 ± 2.4† | 42.5 ± 3.7† |
| Normalized for FAV, ml×min−1×100 ml−1 | 0.37 ± 0.06 | 2.34 ± 0.19† | 3.86 ± 0.27† | 0.43 ± 0.05 | 2.26 ± 0.20† | 3.84 ± 0.33† |
| Women (n = 15) | ||||||
| SaO2, % | 97 ± 0 | 97 ± 0 | 97 ± 0 | 83 ± 1* | 83 ± 1* | 83 ± 1* |
| PaO2, Torr | 109 ± 2 | 112 ± 2 | 110 ± 2 | 49 ± 1*‡ | 49 ± 1* | 49 ± 1* |
| PvO2, Torr | 39 ± 2 | 25 ± 1† | 27 ± 1 | 30 ± 1* | 23 ± 1*†‡ | 24 ± 1*† |
| Arterial O2 content, ml/1 | 160 ± 3‡ | 161 ± 3‡ | 161 ± 3‡ | 135 ± 3*‡ | 138 ± 2*‡ | 140 ± 2*‡ |
| Venous O2 content, ml/1 | 108 ± 6‡ | 61 ± 3†‡ | 67 ± 3†‡ | 82 ± 3* | 55 ± 3*†‡ | 54 ± 2*†‡ |
| a-v O2, ml/1 | 52 ± 6 | 100 ± 3† | 95 ± 3† | 53 ± 4 | 83 ± 4*† | 86 ± 3*† |
| O2 consumption, ml/min | 2.0 ± 0.3‡ | 13.9 ± 1.3†‡ | 21.1 ± 2.1†‡ | 2.4 ± 0.3‡ | 14.0 ± 1.4†‡ | 23.4 ± 2.2*†‡ |
| Normalized for FAV, ml×min−1×100 ml−1 | 0.27 ± 0.04 | 1.85 ± 0.17† | 2.82 ± 0.30†‡ | 0.32 ± 0.04 | 1.87 ± 0.20† | 3.13 ± 0.32*† |
| Older adults | ||||||
| Men (n = 5) | ||||||
| SaO2, % | 97 ± 0 | 96 ± 0 | 96 ± 0 | 82 ± 1* | 80 ± 1* | 81 ± 1* |
| PaO2, Torr | 101 ± 2 | 96 ± 1 | 97 ± 3 | 45 ± 1* | 43 ± 1* | 46 ± 1* |
| PvO2, Torr | 38 ± 2 | 26 ± 2† | 25 ± 1† | 31 ± 2* | 23 ± 1*† | 22 ± 1*† |
| Arterial O2 content, ml/1 | 182 ± 5 | 191 ± 6 | 191 ± 5 | 153 ± 5* | 154 ± 6* | 161 ± 4* |
| Venous O2 content, ml/1 | 140 ± 13 | 89 ± 10† | 80 ± 8† | 115 ± 5* | 73 ± 8*† | 70 ± 5† |
| a-v O2, ml/1 | 51 ± 4 | 102 ± 9† | 110 ± 7† | 38 ± 6* | 81 ± 5*† | 91 ± 3*† |
| O2 consumption, ml/min | 4.0 ± 0.4 | 26.4 ± 3.6† | 46.5 ± 6.3† | 3.2 ± 0.4 | 25.5 ± 3.3† | 42.1 ± 5.7† |
| Normalized for FAV, ml×min−1×100 ml−1 | 0.40 ± 0.05 | 2.69 ± 0.53† | 4.69 ± 0.83† | 0.32 ± 0.19 | 2.59 ± 0.51† | 4.21 ± 0.67† |
| Women (n = 6) | ||||||
| SaO2, % | 97 ± 0 | 96 ± 0 | 96 ± 0 | 81 ± 1* | 80 ± 1* | 80 ± 1* |
| PaO2, Torr | 99 ± 3 | 98 ± 3 | 96 ± 3 | 47 ± 1* | 46 ± 1*‡ | 46 ± 2* |
| PvO2, Torr | 36 ± 3 | 24 ± 2† | 28 ± 4 | 31 ± 2*‡ | 22 ± 1*† | 24 ± 1*† |
| Arterial O2 content, ml/1 | 161 ± 7‡ | 164 ± 8‡ | 168 ± 8‡ | 136 ± 7*‡ | 138 ± 5*‡ | 140 ± 7*‡ |
| Venous O2 content, ml/1 | 108 ± 10 | 65 ± 7† | 77 ± 18† | 95 ± 14*‡ | 59 ± 1† | 59 ± 7*† |
| a-v O2, ml/1 | 53 ± 10 | 99 ± 6† | 91 ± 11† | 41 ± 9*‡ | 80 ± 6*† | 81 ± 5† |
| O2 consumption, ml/min | 2.9 ± 0.6 | 16.5 ± 1.5†‡ | 23.1 ± 4.0†‡ | 2.3 ± 0.5 | 15.4 ± 1.5†‡ | 22.0 ± 2.0†‡ |
| Normalized for FAV, ml×min−1×100 ml−1 | 0.43 ± 0.10 | 2.42 ± 0.25† | 3.37 ± 0.58† | 0.33 ± 0.19 | 2.24 ± 0.22† | 3.18 ± 0.28† |
Values are means ± SE; n, no. of subjects. SaO2, arterial O2 saturation; PaO2, arterial Po2; PvO2, venous Po2; a-v, arterial-venous.
P < 0.05 vs. normoxia.
P < 0.05 vs. rest.
P < 0.05 vs. men.
Catecholamines.
Hypoxia increased arterial epinephrine in men and women (P < 0.05) (Table 6). There was a further increase in arterial epinephrine with incremental exercise under hypoxic conditions for both sexes (P < 0.05). Venous epinephrine during exercise (10 and 20% MVC) was also greater during hypoxia compared with normoxia in men and women (P < 0.05). Additionally, systemic hypoxia increased venous norepinephrine concentrations at rest and during exercise at 10% MVC in men and women. The v-a norepinephrine difference at rest was also greater during hypoxia in men and women compared with normoxic conditions (P < 0.05).
Table 6.
Epinephrine and norepinephrine at rest and incremental exercise during normoxia and hypoxia in men and women
| Normoxia |
Hypoxia |
|||||
|---|---|---|---|---|---|---|
| Rest | 10% | 20% | Rest | 10% | 20% | |
| Young adults | ||||||
| Men (n = 16) | ||||||
| Arterial norepinephrine | 133 ± 9 | 139 ± 10 | 159 ± 9† | 147 ± 10 | 160 ± 10† | 172 ± 10† |
| Venous norepinephrine | 191 ± 10 | 181 ± 8 | 186 ± 8 | 226 ± 17* | 210 ± 13*† | 208 ± 11† |
| v-a norepinephrine difference | 58 ± 10 | 42 ± 6† | 28 ± 8† | 79 ± 12* | 50 ± 8† | 36 ± 7† |
| Arterial epinephrine | 41 ± 6 | 46 ± 5 | 52 ± 5† | 66 ± 7* | 86 ± 9*† | 91 ± 8*† |
| Venous epinephrine | 17 ± 3 | 34 ± 4† | 42 ± 4† | 22 ± 3 | 56 ± 5* | 73 ± 7*† |
| Women (n = 15) | ||||||
| Arterial norepinephrine | 120 ± 22 | 131 ± 25 | 137 ± 25† | 139 ± 28 | 136 ± 23 | 144 ± 25† |
| Venous norepinephrine | 180 ± 26 | 165 ± 25 | 172 ± 25 | 218 ± 24* | 185 ± 22*† | 179 ± 21† |
| v-a norepinephrine difference | 59 ± 8 | 34 ± 6† | 35 ± 6† | 79 ± 9* | 49 ± 6† | 35 ± 7† |
| Arterial epinephrine | 30 ± 2 | 28 ± 3‡ | 36 ± 4‡ | 48 ± 5* | 56 ± 6*‡ | 72 ± 11*† |
| Venous epinephrine | 14 ± 1 | 25 ± 3†‡ | 30 ± 3†‡ | 20 ± 3 | 48 ± 4*† | 70 ± 8*† |
| Older adults | ||||||
| Men (n = 5) | ||||||
| Arterial norepinephrine | 204 ± 7 | 219 ± 13 | 240 ± 14† | 231 ± 10* | 256 ± 20 | 264 ± 25 |
| Venous norepinephrine | 298 ± 27 | 275 ± 21 | 286 ± 19 | 351 ± 37* | 330 ± 29* | 318 ± 20* |
| v-a norepinephrine difference | 93 ± 22 | 55 ± 10† | 47 ± 8† | 121 ± 27 | 74 ± 11 | 55 ± 7† |
| Arterial epinephrine | 69 ± 6 | 68 ± 8 | 69 ± 11 | 93 ± 9* | 106 ± 10* | 148 ± 21*† |
| Venous epinephrine | 41 ± 8 | 48 ± 7 | 66 ± 9† | 49 ± 7 | 84 ± 15* | 106 ± 18*† |
| Women (n = 6) | ||||||
| Arterial norepinephrine | 234 ± 47 | 270 ± 54† | 282 ± 44† | 272 ± 43 | 308 ± 29 | 356 ± 47*† |
| Venous norepinephrine | 410 ± 49 | 369 ± 61† | 363 ± 50† | 508 ± 74 | 431 ± 35 | 438 ± 49* |
| v-a norepinephrine difference | 175 ± 14‡ | 99 ± 18† | 82 ± 12† | 237 ± 52‡ | 123 ± 15† | 88 ± 19† |
| Arterial epinephrine | 59 ± 11 | 66 ± 15 | 61 ± 13 | 99 ± 13* | 125 ± 23* | 184 ± 58*† |
| Venous epinephrine | 49 ± 21 | 54 ± 15 | 75 ± 22† | 70 ± 27 | 104 ± 27*† | 134 ± 32*† |
Values are means ± SE in pg/ml; n, no. of subjects.
P < 0.05 vs. normoxia.
P < 0.05 vs. rest.
P < 0.05 vs. men.
Older Adults
Systemic hemodynamic and respiratory responses.
Under resting conditions MAP, HR, minute ventilation, and end-tidal CO2 did not differ between older men and women for both the normoxia and hypoxia trials (Table 2). HR and minute ventilation were higher during hypoxia at rest and during incremental exercise compared with normoxic conditions in both men and women (P < 0.05 for both). MAP increased with normoxic and hypoxic exercise in the older men, whereas it was only increased during normoxic exercise in the older women.
Forearm exercise.
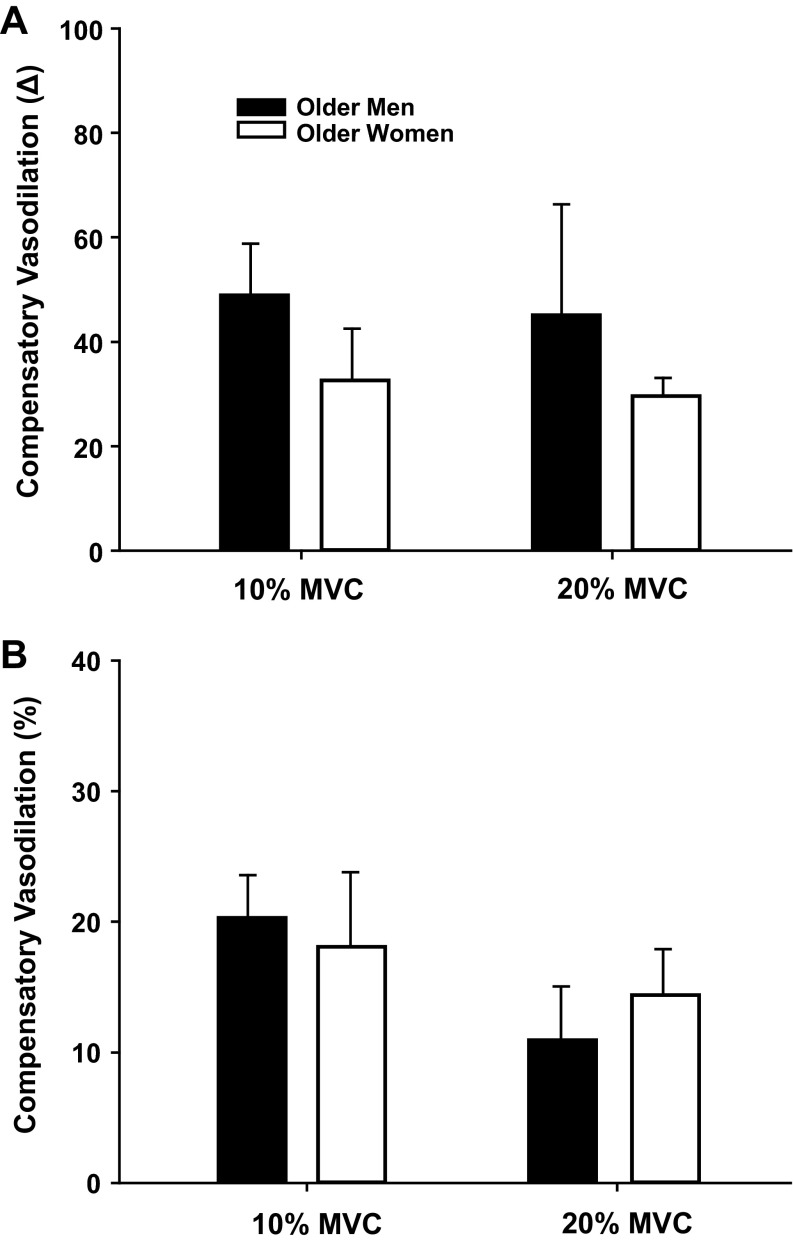
Similar to the group of young adults, older women demonstrated a substantially lower absolute FBF and FVC at 10 and 20% MVC compared with men (Tables 3 and 4). Under resting conditions FBF and FVC were unaffected by exposure to hypoxia in both men and women. However, the absolute FBF and FVC during exercise under hypoxic compared with normoxic conditions were augmented in both older men and women (main effect of hypoxia; P < 0.05; Table 3). Of particular interest to the present study, the compensatory vasodilator responses to hypoxic exercise at 10 and 20% MVC were similar between the older men and women (Fig. 3). As expected from our laboratory's previously published work (9), the compensatory vasodilator responses at 20% MVC were substantially less in older compared with young adults.
Fig. 3.
Compensatory vasodilator response expressed as the absolute (A) and relative (B) difference in steady-state FVC between hypoxic and normoxic exercise in older adults. The absolute (A) and relative (B) compensatory vasodilator response at 10 and 20% MVC did not differ between older men (n = 5) and women (n = 6). Values are means ± SE.
Similar to the group of young adults, older women had substantially smaller forearm volumes and performed the forearm exercise at lower absolute weights for each relative intensity (10 and 20% MVC) compared with the older men (P < 0.01). Therefore, we also analyzed the absolute changes in FVC after normalizing the response for either forearm volume (per 100 ml) or workload (per 100 g of weight lifted) in the older adults. Utilizing these approaches revealed similar results as those reported above (Table 4). That is, the compensatory responses to hypoxia at rest and during exercise were similar between older men and women (Fig. 4).
Fig. 4.
Absolute change in vasodilation (FVC) during hypoxia compared with normoxia normalized to forearm volume (A) or workload (B) in older adults. The increase in FVC normalized for forearm volume (per 100 ml; A) or workload (per 100 g of weight lifted; B) during hypoxia was similar between older men (n = 5) and women (n = 6). Values are means ± SE.
Blood gases.
Older women demonstrated lower arterial oxygen content than men at rest and with incremental forearm exercise under both normoxia and hypoxia conditions (P < 0.05 for all) (Table 5). Hypoxia reduced arterial Po2 and arterial and venous oxygen content at rest and with incremental forearm exercise in both men and women (P < 0.05 for all). Hypoxia also resulted in a reduced a-v oxygen difference compared with normoxia at rest and during incremental exercise in both men and women. Forearm oxygen consumption was lower in older women compared with men during both exercise intensities. However, these differences were absent after normalizing oxygen consumption to forearm volume (per 100 ml). The increase in forearm oxygen consumption with exercise was similar between normoxia and hypoxia for both older men and women (Table 5).
Catecholamines.
Hypoxia increased arterial epinephrine in older men and women (P < 0.05) (Table 6). There was a further increase in arterial epinephrine at 20% MVC exercise under hypoxic conditions for both sexes (P < 0.05). Venous epinephrine during exercise (10 and 20% MVC) was also greater during hypoxia compared with normoxia in men and women (P < 0.05). Systemic hypoxia increased venous norepinephrine concentrations at rest and during incremental exercise in older men, whereas it was only elevated at 20% MVC in older women.
DISCUSSION
To our knowledge, this is the first study to examine whether sex influences hypoxic vasodilation at rest and during exercise. When examining the compensatory vasodilator response as an absolute increase in FVC during hypoxic exercise compared with normoxic exercise (hypoxic exercise FVC − normoxic exercise FVC), there was no significant difference between young men and women (Fig. 1A). However, expressing the compensatory vasodilator response as a relative (%) change revealed a substantially greater response in young women compared with men (Fig. 1B). These discrepancies highlight the interpretative issues associated with quantifying and comparing exercise hyperemic and vasodilator responses between conditions and groups. On one hand, using the absolute change to compare the responsiveness to hypoxic exercise would suggest that there is no difference between young men and women. However, on the other hand, comparing the relative change suggests young women have an enhanced responsiveness. As mentioned earlier, the changes in absolute values may be skewed by differences in forearm volume and absolute workloads used (for each relative exercise intensity) between the young men and women. Theoretically, the young men should have a greater vasodilator capacity (greater volume) and demonstrate greater absolute FBF and FVC (simply due to higher workloads). Interestingly, normalizing the absolute responses for either forearm volume or workload would suggest young women demonstrate a greater compensatory vasodilation (Fig. 2). Based on this analysis, our preferred interpretation is that compensatory vasodilation is greater in young women than young men for a given combination of metabolic stimuli and hypoxia. Our present data also suggest that the sex-related differences in compensatory vasodilation during hypoxic exercise might be lost with aging, regardless of how the data are expressed (Figs. 3 and 4).
Sex-related differences in vascular control and peripheral blood flow at rest and during exercise have been previously shown to exist in humans. When normalized for vasodilator capacity, young women tend to show a greater FBF responsiveness to the endothelial-dependent vasodilator acetylcholine (10). Additionally, Parker and colleagues (22) found that the absolute femoral artery vasodilation and hyperemic response to graded single-leg exercise under normoxic conditions, when expressed at absolute and relative workloads, as well as normalized to the estimated quadriceps muscle mass, was greater in young women compared with men. Conversely, the absolute FBF and vasodilator responses to mild and moderate (10 and 20% MVC) intensity normoxic exercise in the present study were substantially lower in the young women compared with men (Table 3). When comparing the FBF and FVC responses between similar absolute workloads (20% MVC for women and 10% MVC for men; 12.6 ± 0.5 vs. 10.4 ± 0.4 kg), sex-related differences were no longer apparent (Table 3). The discrepancies between the two studies in regards to blood flow and vasodilator responses during normoxic exercise might be related to the significant between-limb (arm vs. leg) heterogeneity in vascular responsiveness that has been reported in humans (29). Despite the differences between our present data and those previously reported during normoxic exercise, the present study clearly demonstrates for the first time that the vasodilator responsiveness to hypoxia at rest and during incremental forearm exercise is enhanced in young women compared with men.
Potential Mechanisms for Greater Compensatory Vasodilation in Young Women
In several previous studies, our laboratory has observed a graded release of epinephrine with incremental hypoxic forearm exercise (4, 7–9, 27, 28), thus suggesting that β-adrenergic receptor-mediated vasodilation might contribute to the compensatory vasodilator response. Indeed, local inhibition of β-adrenergic receptors (via intra-arterial infusion of propranolol) blunts the vasodilator response to hypoxia at rest and during mild forearm exercise (26, 27). Interestingly, it appears that the β-adrenergic receptors in the peripheral vasculature are either more sensitive or upregulated in young women vs. men (17). Moreover, the β-adrenergic receptors offset peripheral α-adrenergic vasoconstriction in young women, but not young men (15). Therefore an enhanced β-adrenergic receptor sensitivity and activation could contribute in part to the augmented vasodilation in young women during hypoxic exercise. Importantly, if β-adrenergic receptor activation does contribute to the sex-related differences in compensatory vasodilation, it is not likely explained by the magnitude of change in circulating levels of epinephrine during hypoxic exercise, as this was similar in young men and women (Table 5). Of particular interest to the second aim of the present study, the ability of the β-adrenergic receptors to offset vasoconstrictor tone appears to be lost in older postmenopausal women (15) and may explain the lack of sex-related differences in hypoxic compensatory vasodilation with aging observed in the present study (Figs. 3 and 4).
Hypoxia often results in an enhanced sympathetic vasoconstrictor activity directed toward skeletal muscle during exercise compared with normoxic conditions (14). At rest, young women often demonstrate lower sympathetic vasoconstrictor responsiveness to pharmacological and nonpharmacological stimuli than men (13, 15, 17). Therefore, it is possible that young women have less sympathetic vasoconstrictor restraint during hypoxic exercise than men, which may explain the greater compensatory vasodilation observed in the present study. It could also be argued that the greater compensatory vasodilation in young women might reflect an enhanced functional sympatholysis during hypoxic exercise. However, recent data demonstrate that the degree of functional sympatholysis of α1- and α2-adrenergic-mediated vasoconstriction is similar in young men and women during normoxic exercise (20). Moreover, the level of oxygen availability does not appear to influence the degree of functional sympatholysis during forearm exercise (6, 28).
Last, it is also possible that circulating female sex hormones play a role in the greater vasodilator response to hypoxic exercise in young women. Along these lines, estrogen modulates vascular tone during dynamic exercise (24) and in response to hypoxia (19). In humans, serum levels of estradiol are correlated with FBF responses to intra-arterial acetylcholine infusions (10), and the greatest FBF responses to reactive hyperemia in young women occur during the late follicular phase of the menstrual cycle, which corresponds to the highest level of circulating levels of estradiol (1). Taken together, these findings might suggest estrogen potentially plays a role in the enhanced vasodilator response to hypoxic exercise in young women. However, it should be noted that all of the young women in the present study participated during the early follicular phase of the menstrual cycle or the placebo phase of oral contraceptives, when circulating levels of estrogen were likely low. Therefore, any contribution of estrogen to the enhanced vasodilator responsiveness to hypoxic exercise observed in the present study would have likely been a result of the chronic, rather than acute, effects of estrogen.
Experimental Considerations
In the present study, young women demonstrated a greater vasodilator response to hypoxia at rest and during exercise than young men. The finding that sex-related differences exist in the vasodilator responses to hypoxic exercise is based on the calculation of compensatory vasodilation (%change in FVC during steady-state hypoxic exercise compared with steady-state FVC during normoxic exercise). Conversely, examination of the absolute changes in FVC between normoxic and hypoxic exercise would suggest that the vasodilator responses to hypoxic exercise are similar between young men and women (Table 3). Controversy exists over the appropriate way to express vasodilator responsiveness (absolute vs. relative), especially when blood flow varies between conditions before the vasodilator stimulus. In the present study, FBF and FVC were substantially lower in young women compared with men during normoxic exercise. The calculation for compensatory vasodilation during hypoxic exercise takes into account any differences in blood flow and vascular conductance during the respective normoxic trial and serves as an indicator of the amount of vasodilation needed to keep oxygen delivery to the contracting muscles constant. Additionally, it could be argued that the lack of sex-related differences in the compensatory vasodilator response to hypoxic exercise when expressed as an absolute change in young adults is a product of the men having larger forearm volumes and/or muscle mass and performing exercise at greater absolute workloads. Indeed, when we normalized the vasodilator responses to hypoxic exercise for either forearm volume or workload in the young adults, women demonstrated an enhanced responsiveness.
It is possible that the lack of change in hypoxic-mediated vasodilation at rest in the young men is a result of significantly higher baseline FBF and FVC values compared with young women (Table 3). That is, the higher baseline blood flow and vasodilation might limit the ability to detect significant vasodilation to hypoxia alone. However, it can be argued that the lack of a significant hypoxia-induced vasodilation at rest in young men might be related to the 1) highly variable individual vasodilator responses to hypoxia at rest; or 2) sex-related differences in the vasoconstrictor responsiveness to sympathetic stimulation (13, 15, 17). The latter would suggest that the sympathoexcitatory nature of systemic hypoxia (14) might mask peripheral vasodilation at rest in young men. Last, exposure to acute systemic hypoxia at rest causes cutaneous vasoconstriction in the hand and fingers of men (12, 18). If sex-related differences exist in the regulation of the hand and finger circulations, specifically in response to a sympathoexcitatory stimulus, it is possible that the attenuated hypoxic-mediated vasodilation at rest observed in young men compared with women could be a result of an enhanced cutaneous vasoconstriction in the hand and fingers.
Our secondary aim in the present study was to gain insight whether potential sex-related differences in hypoxic vasodilation exist with aging. To address this aim, we retrospectively examined the compensatory vasodilator responses in a group of 11 older adults. This approach only allowed for comparisons in small groups of older men (n = 5) and women (n = 6) and may explain the lack of difference in compensatory vasodilation during hypoxic exercise between older adults in the present study. However, it should be noted that the compensatory responses between older men and women were very similar at 10 and 20% MVC, regardless of how the data are expressed (Figs. 3 and 4). Moreover, several other variables reported in the present study (Tables 3–5) for older adults reached significance, despite the smaller group sizes. Therefore, it is unlikely that the inclusion of additional older subjects would reveal large sex-related differences in the compensatory vasodilator response to hypoxic exercise, as was observed in the young adults. Nonetheless, the data related to potential sex-related differences with aging reported in the present study should be treated with some caution due to the small sample sizes of each group.
Conclusions
The present study provides novel evidence that sex-related differences might exist (depending on analytic approach) in the compensatory vasodilator response to hypoxic exercise between young women and men. The mechanisms responsible for the discrepancies between sexes are currently unclear. However, when considered in the context that 1) at least some of the compensatory vasodilation during hypoxia is mediated by vasodilating β-adrenergic receptors (26, 27); and 2) β-receptors are either more sensitive or upregulated in young (but not older) women (15, 17), our data may suggest that the augmented compensatory vasodilator responses to hypoxia in young women are a result of greater β-adrenergic receptor activation.
GRANTS
This study was supported by National Institutes of Health research Grants HL-46493 (to M.J. Joyner), AR-55819 (to D.P. Casey), and by Clinical and Translational Science Award RR-024150. The Caywood Professorship via the Mayo Foundation also supported this research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.P.C. and M.J.J. conception and design of research; D.P.C., J.R.S., and M.J.J. performed experiments; D.P.C. and J.R.S. analyzed data; D.P.C., J.R.S., and M.J.J. interpreted results of experiments; D.P.C. and J.R.S. prepared figures; D.P.C. drafted manuscript; D.P.C., J.R.S., and M.J.J. edited and revised manuscript; D.P.C., J.R.S., and M.J.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Branton Walker, Shelly Roberts, Jean Knutson, Karen Krucker, Chistopher Johnson, Brandon Madery, Rachel Elvebak, Lakshmi (Madhuri) Somaraju, Heather Tonyan, and Pam Engrav for technical assistance. We also thank the volunteers for time.
REFERENCES
- 1.Adkisson EJ, Casey DP, Beck DT, Gurovich AN, Martin JS, Braith RW. Central, peripheral and resistance arterial reactivity: fluctuates during the phases of the menstrual cycle. Exp Biol Med (Maywood) 235: 111–118, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banzett RB, Garcia RT, Moosavi SH. Simple contrivance “clamps” end-tidal Pco2 and Po2 despite rapid changes in ventilation. J Appl Physiol 88: 1597–1600, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD, Saltin B. Determinants of maximal oxygen uptake in severe acute hypoxia. Am J Physiol Regul Integr Comp Physiol 284: R291–R303, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Casey DP, Curry TB, Wilkins BW, Joyner MJ. Nitric oxide-mediated vasodilation becomes independent of beta-adrenergic receptor activation with increased intensity of hypoxic exercise. J Appl Physiol 110: 687–694, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casey DP, Joyner MJ. Local control of skeletal muscle blood flow during exercise: influence of available oxygen. J Appl Physiol 111: 1527–1538, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casey DP, Joyner MJ, Claus PL, Curry TB. Vasoconstrictor responsiveness during hyperbaric hyperoxia in contracting human muscle. J Appl Physiol 114: 217–224, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey DP, Madery BD, Curry TB, Eisenach JH, Wilkins BW, Joyner MJ. Nitric oxide contributes to the augmented vasodilatation during hypoxic exercise. J Physiol 588: 373–385, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casey DP, Madery BD, Pike TL, Eisenach JH, Dietz NM, Joyner MJ, Wilkins BW. Adenosine receptor antagonist and augmented vasodilation during hypoxic exercise. J Appl Physiol 107: 1128–1137, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casey DP, Walker BG, Curry TB, Joyner MJ. Ageing reduces the compensatory vasodilatation during hypoxic exercise: the role of nitric oxide. J Physiol 589: 1477–1488, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietz NM. Gender and nitric oxide-mediated vasodilation in humans. Lupus 8: 402–408, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol 480: 361–368, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fahim M. Effect of hypoxic breathing on cutaneous temperature recovery in man. Int J Biometeorol 36: 5–9, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Hachiya T, Hashimoto I, Saito M, Blaber AP. Peripheral vascular responses of men and women to LBNP. Aviat Space Environ Med 83: 118–124, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Hanada A, Sander M, Gonzalez-Alonso J. Human skeletal muscle sympathetic nerve activity, heart rate and limb haemodynamics with reduced blood oxygenation and exercise. J Physiol 551: 635–647, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the beta-adrenergic receptors. J Physiol 589: 5285–5297, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joyner MJ, Nauss LA, Warner MA, Warner DO. Sympathetic modulation of blood flow and O2 uptake in rhythmically contracting human forearm muscles. Am J Physiol Heart Circ Physiol 263: H1078–H1083, 1992 [DOI] [PubMed] [Google Scholar]
- 17.Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol 36: 1233–1238, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Kollai M. Responses in cutaneous vascular tone to transient hypoxia in man. J Auton Nerv Syst 9: 497–512, 1983 [DOI] [PubMed] [Google Scholar]
- 19.Lahm T, Patel KM, Crisostomo PR, Markel TA, Wang M, Herring C, Meldrum DR. Endogenous estrogen attenuates pulmonary artery vasoreactivity and acute hypoxic pulmonary vasoconstriction: the effects of sex and menstrual cycle. Am J Physiol Endocrinol Metab 293: E865–E871, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Limberg JK, Eldridge MW, Proctor LT, Sebranek JJ, Schrage WG. Alpha-adrenergic control of blood flow during exercise: effect of sex and menstrual phase. J Appl Physiol 109: 1360–1368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Herr MD, Proctor DN. Sex differences in leg vasodilation during graded knee extensor exercise in young adults. J Appl Physiol 103: 1583–1591, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Roach RC, Koskolou MD, Calbet JA, Saltin B. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol Heart Circ Physiol 276: H438–H445, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Rogers J, Sheriff DD. Role of estrogen in nitric oxide- and prostaglandin-dependent modulation of vascular conductance during treadmill locomotion in rats. J Appl Physiol 97: 756–763, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Rowell LB, Saltin B, Kiens B, Christensen NJ. Is peak quadriceps blood flow in humans even higher during exercise with hypoxemia? Am J Physiol Heart Circ Physiol 251: H1038–H1044, 1986 [DOI] [PubMed] [Google Scholar]
- 26.Weisbrod CJ, Minson CT, Joyner MJ, Halliwill JR. Effects of regional phentolamine on hypoxic vasodilatation in healthy humans. J Physiol 537: 613–621, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilkins BW, Pike TL, Martin EA, Curry TB, Ceridon ML, Joyner MJ. Exercise intensity-dependent contribution of beta-adrenergic receptor-mediated vasodilatation in hypoxic humans. J Physiol 586: 1195–1205, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilkins BW, Schrage WG, Liu Z, Hancock KC, Joyner MJ. Systemic hypoxia and vasoconstrictor responsiveness in exercising human muscle. J Appl Physiol 101: 1343–1350, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wray DW, Richardson RS. Aging, exercise, and limb vascular heterogeneity in humans. Med Sci Sports Exerc 38: 1804–1810, 2006 [DOI] [PubMed] [Google Scholar]