Abstract
An increased “dose” of endurance exercise training is associated with a greater maximal oxygen uptake (V̇o2max), a larger left ventricular (LV) mass, and improved heart rate and blood pressure control. However, the effect of lifelong exercise dose on metabolic and hemodynamic response during exercise has not been previously examined. We performed a cross-sectional study on 101 (69 men) seniors (60 yr and older) focusing on lifelong exercise frequency as an index of exercise dose. These included 27 who had performed ≤2 exercise sessions/wk (sedentary), 25 who performed 2–3 sessions/wk (casual), 24 who performed 4–5 sessions/wk (committed) and 25 who performed ≥6 sessions/wk plus regular competitions (Masters athletes) over at least the last 25 yr. Oxygen uptake and hemodynamics [cardiac output, stroke volume (SV)] were collected at rest, two levels of steady-state submaximal exercise, and maximal exercise. Doppler ultrasound measures of LV diastolic filling were assessed at rest and during LV loading (saline infusion) to simulate increased LV filling. Body composition, total blood volume, and heart rate recovery after maximal exercise were also examined. V̇o2max increased in a dose-dependent manner (P < 0.05). At maximal exercise, cardiac output and SV were largest in committed exercisers and Masters athletes (P < 0.05), while arteriovenous oxygen difference was greater in all trained groups (P < 0.05). At maximal exercise, effective arterial elastance, an index of ventricular-arterial coupling, was lower in committed exercisers and Masters athletes (P < 0.05). Doppler measures of LV filling were not enhanced at any condition, irrespective of lifelong exercise frequency. These data suggest that performing four or more weekly endurance exercise sessions over a lifetime results in significant gains in V̇o2max, SV, and heart rate regulation during exercise; however, improved SV regulation during exercise is not coupled with favorable effects on LV filling, even when the heart is fully loaded.
Keywords: aging, lifelong exercise, maximal exercise capacity, stroke volume, diastole
healthy aging is characterized by a progressive decline in maximal oxygen uptake (V̇o2max) (2, 20), which increases functional disability and risk of all-cause mortality (6). In longitudinal and cross-sectional studies, a smaller maximal cardiac output (Q̇c) and arteriovenous oxygen difference [(a-v)Do2] contribute to the decline in V̇o2max with senescence (4, 9, 28, 36).
The “dose” of exercise consists of a number of factors, including intensity (how hard), duration (how long), and frequency (how often). A greater frequency of exercise is associated with a greater V̇o2max (23), a larger left ventricular (LV) mass (23, 56), and improved heart rate and blood pressure regulation (37, 51). To date, no studies have examined the effect of lifelong exercise dose on physical fitness and exercise cardiovascular function. A greater depth of knowledge describing the metabolic and hemodynamic adaptations to different doses of lifelong exercise would be beneficial to determining the optimal exercise dose for minimizing the reduction in physical performance with aging.
The augmentation of stroke volume (SV) with endurance exercise training is primarily attributed to a larger end-diastolic volume (26, 46). In combination with LV compliance, rapid active relaxation and vigorous diastolic suction would enhance end-diastolic volume, while minimizing increases in filling pressure during exercise. However, compelling evidence of enhanced LV filling properties, including active relaxation and diastolic suction, are still lacking in endurance-trained individuals, even in those who perform near daily exercise (3, 8, 11, 31, 41). The apparent absence of a training effect may be related to methodological issues, including small sample size, heterogeneous exercise histories, or, importantly, an inability to comprehensively assess differences in LV loading conditions between trained and untrained participants.
Therefore, the purpose of this study was to examine the relationship between lifelong (>25 yr) exercise dose as tracked by exercise frequency and 1) metabolic and hemodynamic variables at rest and during exercise; 2) dynamic LV diastolic filling parameters at rest and during LV volume loading to simulate increased LV filling during exercise; and 3) postexercise heart rate recovery (HRR). We hypothesized that V̇o2max, exercise SV, dynamic LV diastolic filling properties, and HRR would be improved in a dose-dependent manner.
METHODS
Participant Recruitment
Participants were recruited primarily from the Cooper Center Longitudinal Study (58), a cohort of over 80,000 individuals in whom physical activity and cardiovascular risk factors have been quantified and followed for greater than 40 yr. The Masters athlete population was enriched through recruitment of top performers at regional and national endurance events. Participants were grouped based on lifelong exercise frequency [exercise session(s)/wk], with exercise being defined as periods of aerobic activity lasting at least 30 min. Sedentary participants exercised no more than once per week; “casual” exercisers engaged in 2–3 sessions per week; “committed” exercisers performed 4–5 sessions per week; and Masters athletes trained 6–7 times per week and participated in regular competitions. All participants were nonsmokers, were not taking any cardiovascular medications, had a 24-h ambulatory blood pressure <140/90 mmHg, body mass index ≤ 30 kg/m2, and a normal ECG and exercise stress echocardiogram. All participants were informed of the purpose and procedures used in the study and gave their written, informed consent to a protocol approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center in Dallas and Texas Health Presbyterian Hospital Dallas.
Measurement of Resting and Exercise Metabolic and Hemodynamic Variables
Oxygen uptake (V̇o2) (Douglas bag technique), hemodynamics, and blood pressures were determined at the following treadmill conditions: 1) quiet rest, 2) low-intensity (≈30–45% of V̇o2max) steady-state submaximal exercise, 3) moderate-intensity (≈60–75% of V̇o2max) steady-state submaximal exercise, and 4) maximal exercise. A small number of participants (n = 4) were tested on an upright cycle at the same conditions because of orthopedic concerns or participant request. Gas fractions were analyzed by mass spectrometry and ventilatory volumes by a Tissot spirometer, as previously reported (1). V̇o2max was defined as the highest V̇o2 measured from at least a 30-s Douglas bag. Q̇c was measured with the acetylene rebreathing method, which has been previously validated in this laboratory (29). Heart rate was measured via a 12-lead ECG, and SV was calculated as Q̇c/heart rate, while (a-v)Do2 was calculated from the Fick equation [(a-v)Do2 = V̇o2/Q̇c]. As differences in body size and composition may influence cardiovascular variables (9), SV was scaled relative to body surface area (BSA) (stroke index) and fat-free mass (FFM). Typical error of measurement expressed as a coefficient of variation (%) for test-retest reproducibility for V̇o2max and maximal Q̇c, SV, and heart rate for 86 participants in the current cohort is 4.4% (2.15 ± 0.64 vs. 2.19 ± 0.64 l/min), 11.1% (15.9 ± 4.6 vs. 15.2 ± 4.8 l/min), 11.9% (99 ± 30 vs. 93 ± 30 ml), and 3.5% (163 ± 12 vs. 161 ± 12 beats/min), respectively.
Resting and exercise blood pressures were measured on the left arm by ECG gated electrosphygmomanometry (Tango; SunTech Medical). Systemic vascular resistance (SVR) was calculated by the following formula: mean arterial pressure (MAP)/Q̇c × 80, where 80 is a conversion factor to dyn·s·cm−5. Effective arterial elastance (Ea), an index of ventricular-arterial coupling, was estimated as ESP/SV, in which ESP represents end-systolic blood pressure, by multiplying brachial systolic blood pressure by 0.9 (12). Ea was scaled relative to BSA, as previously reported (43).
Measurement of HRR after Maximal Exercise
Immediately after the cessation of maximal exercise, participants recovered in an upright seated position. Heart rate was continuously recorded, and HRR was analyzed at 1-min periods from 1–5 min of recovery. HRR is presented as beats per minute and R-R interval, which was calculated from the following formula: 60/heart rate × 1,000.
Measurement of Body Composition
Body density and composition were determined by underwater weighing with correction for residual lung volume (59). Each subject performed at least three adequate measurements defined as a definite plateau in underwater weight, and the mean value was calculated.
Assessment of Dynamic LV Diastolic Filling
LV function was assessed using previously described techniques (1, 41). Briefly, a 6 Fr balloon-tipped fluid-filled catheter (Edwards Lifesciences) was placed using fluoroscopic guidance to measure pulmonary capillary wedge pressure (PCWP). PCWP was determined from three measurements obtained at end expiration, as previously described (1, 41). PCWP, Q̇c, and Doppler measures of dynamic LV diastolic filling measurements were obtained after at least 20 min of supine rest. LV filling was increased through a rapid infusion of warm (37°C) isotonic saline solution at 200 ml/min via an antecubital intravenous line to achieve total volume infusion of 10–15 ml/kg (NS15). In this cohort, there was a positive association between SV at NS15 and during moderate intensity submaximal exercise (R = 0.69, P < 0.05) and maximal exercise (R = 0.58, P < 0.05) using the acetylene rebreathing method.
Echocardiographic images were digitally acquired using an iE33 (Philips,) and ATL HDI5000 (Advanced Technology Laboratories) and were measured offline in Xcelera cardiovascular image management system (Philips). LV end-diastolic volume was determined using a modified Simpson's method, as previously described (1), and then scaled relative to BSA (left ventricular end-diastolic volume index).
Mitral inflow.
A 2-mm sample volume placed at the tips of the mitral valve leaflets to determine peak early and late mitral inflow velocities, and the corresponding early-to-late ratio was calculated.
Tissue Doppler imaging.
In the apical four-chamber view, a 2-mm sample volume was placed at the septal and lateral side of the mitral annulus. A mean value of septal and lateral values of the peak early (mean Em) mitral annular velocity was calculated (41).
Propagation velocity of early mitral inflow.
A color M-mode image of LV inflow was obtained with the sampling area positioned to extend from mid-atrium to the apex, directly through the mitral valve orifice. The scale was reduced to produce a clear aliasing within the early portion of the mitral inflow. The slope of first aliasing velocity from the mitral plane to 4 cm into the ventricle was used to measure propagation velocity of early mitral inflow. Using a five-chamber apical view with a 4-mm sample volume, the interval between aortic valve closing and mitral valve opening [isovolumic relaxation time (IVRT)] was determined.
Measurement of Total Blood Volume
Total blood volume (TBV) was measured using a carbon monoxide rebreathing method modified from that described by Burge and Skinner (7) and has been described in detail elsewhere (25). Typical error of measurement expressed as a coefficient of variation (%) for test-retest reproducibility for hemoglobin mass, which is used as a marker of carbon monoxide distribution, is ≈3% for repeated measures in our laboratory (25).
Statistical Analysis
One-factor ANOVA and repeated-measures ANOVAs were used to determine the effect of lifelong exercise frequency on subject characteristics, exercise metabolic and hemodynamics variables, Doppler measures of LV dynamic diastolic filling, and HRR. Tukey adjustments were used to control for multiple comparisons. Nonparametric data were analyzed via Kruskal-Wallis ANOVA on ranks. Pearson's correlations were used to establish the relationship between TBV and V̇o2max. All statistical analysis was performed using SigmaStat (Systat Software). P < 0.05 was considered statistically significant. Data are presented as means ± SD in Tables 1–4 and means ± SE in Figs. 1–4.
Table 1.
Participant characteristics
| Sedentary | Casual | Committed | Masters Athletes | ANOVA P Value | |
|---|---|---|---|---|---|
| n | 27 | 25 | 24 | 25 | |
| No. men (%) | 15 (56) | 18 (72) | 19 (79) | 17 (68) | |
| Age, yr | 69 ± 5 | 71 ± 6 | 69 ± 6 | 68 ± 3 | 0.201 |
| Weight, kg | 75 ± 11 | 76 ± 14 | 73 ± 11 | 66 ± 12*† | 0.015 |
| Height, cm | 169 ± 10 | 174 ± 10 | 173 ± 8 | 171 ± 10 | 0.336 |
| BSA, m2 | 1.87 ± 0.19 | 1.91 ± 0.23 | 1.88 ± 0.18 | 1.76 ± 0.21 | 0.058 |
| Body fat, % | 32 ± 8 | 30 ± 7 | 29 ± 5 | 22 ± 7*† | <0.001 |
| FFM, kg | 51 ± 9 | 53 ± 10 | 52 ± 9 | 51 ± 8 | 0.699 |
| HgB concentration, g/dl | 14 ± 1 | 13 ± 1 | 14 ± 1 | 14 ± 1 | 0.153 |
| TBV, ml/kg | 66 ± 11 | 69 ± 8 | 73 ± 8* | 83 ± 8*† | <0.001 |
| LVMI g/m2 | 50 ± 8 | 52 ± 8 | 62 ± 11*† | 67 ± 12*† | <0.001 |
Values are means ± SD; n. no. of subjects.
BSA, body surface area; FFM, fat-free mass; HgB, hemoglobin; TBV, total blood volume; LVMI, left ventricular mass index.
P < 0.05 vs. sedentary.
P < 0.05 vs. casual.
Table 4.
Left ventricular volumes, Doppler parameters, and PCWP at supine rest and saline volume infusion (NS15)
| Sedentary | Casual | Committed | Masters Athletes | |
|---|---|---|---|---|
| Supine rest | ||||
| Heart rate, beats/min | 72 ± 12 | 65 ± 8* | 63 ± 9* | 60 ± 7* |
| E/A ratio | 0.97 ± 0.24 | 1.02 ± 0.20 | 1.14 ± 0.32 | 1.10 ± 0.24 |
| Mean Em, cm/s | 9.2 ± 1.8 | 9.0 ± 1.4 | 9.4 ± 1.3 | 10.4 ± 2.2† |
| IVRT, ms | 122 ± 27 | 108 ± 17 | 117 ± 25 | 126 ± 27† |
| VP, cm/s | 43 ± 8 | 44 ± 10 | 45 ± 9 | 38 ± 6 |
| PCWP, mmHg | 8.7 ± 2.3 | 8.5 ± 1.6 | 8.4 ± 1.5 | 8.3 ± 1.6 |
| LVEDVI, ml/m2 | 55 ± 12 | 59 ± 12 | 59 ± 12 | 75 ± 14*†‡ |
| NS15 | ||||
| Heart rate, beats/min | 80 ± 11 | 74 ± 10 | 68 ± 10* | 64 ± 7*† |
| E/A ratio | 1.28 ± 0.31 | 1.17 ± 0.26 | 1.36 ± 0.38 | 1.27 ± 0.32 |
| Mean Em, cm/s | 10.1 ± 1.9 | 9.4 ± 1.6 | 10.2 ± 1.4 | 11.1 ± 2.2† |
| IVRT, ms | 97 ± 23 | 90 ± 16 | 95 ± 18 | 108 ± 25† |
| VP, cm/s | 55 ± 14 | 60 ± 15 | 55 ± 12 | 48 ± 11† |
| PCWP, mmHg | 15.2 ± 1.8 | 15.2 ± 2.8 | 14.7 ± 2.5 | 13.4 ± 2.3*† |
| LVEDVI, ml/m2 | 59 ± 12 | 62 ± 12 | 62 ± 12 | 80 ± 18*†‡ |
Values are means ±SD.
E/A ratio, the ratio of peak early-to-late mitral inflow velocities; Mean Em, mean peak early mitral annular velocity; IVRT, isovolumic relaxation time; Vp, propagation velocity; PCWP, pulmonary capillary wedge pressure; LVEDVI, left ventricular end-diastolic volume index.
P < 0.05 vs. sedentary.
P < 0.05 vs. casual.
P < 0.05 vs. committed.
Fig. 1.
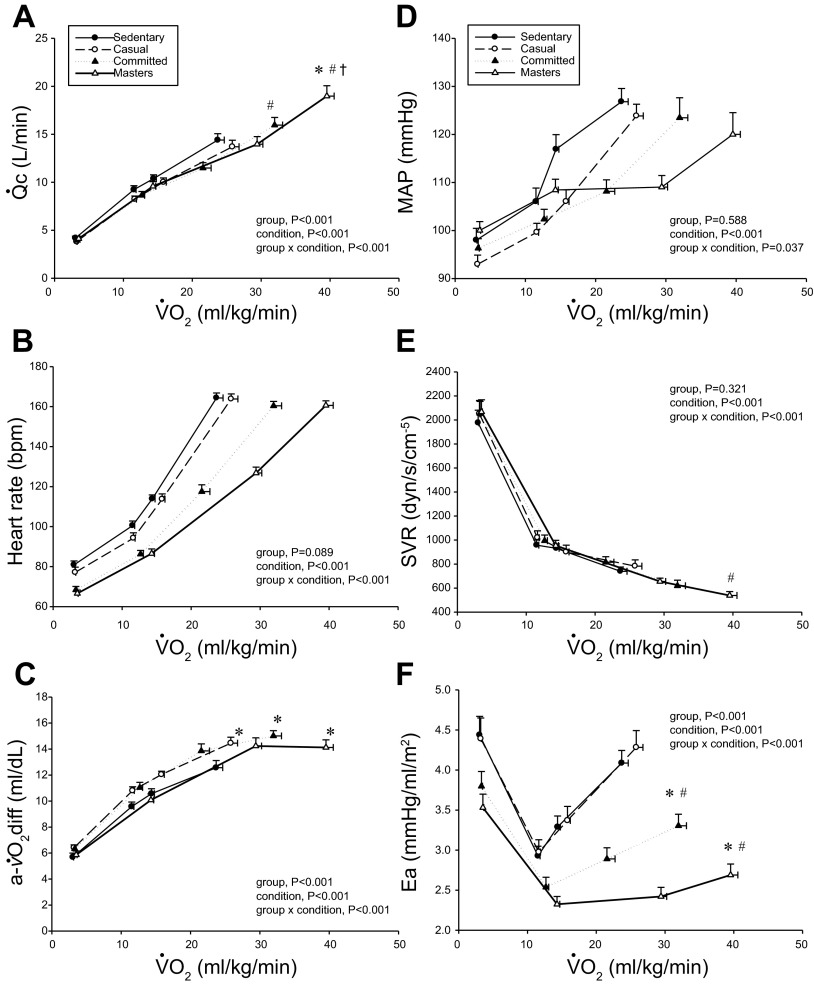
Cardiac output (Q̇c; A), heart rate (B), arteriovenous oxygen difference [a-v̇O2diff; (a-v)Do2; C], mean arterial pressure (MAP; D), systemic vascular resistance (SVR; E), and effective arterial elastance (Ea; F) as a function of oxygen uptake (V̇o2) scaled relative to total body mass. Values are means ± SE. *P < 0.05 vs. sedentary. #P < 0.05 vs. casual. †P < 0.05 vs. committed. Only statistically significant results at maximal exercise are indicated. Please refer to text for further details.
Fig. 4.
Heart rate recovery expressed in bpm (A) and R-R interval (B) after maximal exercise. Values are means ± SE. *P < 0.05, committed exercisers vs. sedentary. †P < 0.05, Masters athletes vs. sedentary.
RESULTS
Participants Characteristics
The LV pressure-volume curves and MRI measurements of cardiac morphology in this cohort have been reported (4a). The Masters athletes were lighter, had a smaller BSA, and were leaner, but were similar in terms of FFM (Table 1). TBV scaled relative to total body mass (ml/kg) and LV mass index were larger in Masters athletes and committed exercisers compared with sedentary exercisers (P < 0.05) (Table 1).
Resting Metabolic and Hemodynamics Variables
Resting metabolic and hemodynamic variables are presented in Table 2. Stroke index was larger, while heart rate and Ea were lower in the Masters athletes compared with sedentary and casual exercisers (P < 0.05). MAP, SVR, and (a-v)Do2 were not different among groups.
Table 2.
Resting parameters
| Sedentary | Casual | Committed | Masters Athletes | ANOVA P Value | |
|---|---|---|---|---|---|
| V̇o2 | |||||
| ml/min | 229 ± 56 | 214 ± 48 | 244 ± 63 | 230 ± 48 | 0.726 |
| ml·kg−1·min−1 | 3.0 ± 0.5 | 3.2 ± 0.4 | 3.3 ± 0.7 | 3.5 ± 0.7* | 0.037 |
| ml·kg FFM−1·min−1 | 4.5 ± 0.8 | 4.6 ± 0.6 | 4.7 ± 1.0 | 4.6 ± 0.8 | 0.920 |
| Q̇c, l/min | 4.2 ± 1.0 | 3.8 ± 0.8 | 3.9 ± 0.9 | 4.1 ± 1.2 | 0.619 |
| QI, l·m−2·min−1 | 2.2 ± 0.5 | 2.0 ± 0.3 | 2.1 ± 0.4 | 2.3 ± 0.5 | 0.130 |
| Q̇c, ml·kg FFM−1·min−1 | 82.6 ± 15.0 | 72.8 ± 14.5 | 75.3 ± 14.6 | 81.4 ± 17.1 | 0.070 |
| Heart rate, beats/min | 81 ± 10 | 77 ± 13 | 68 ± 9*† | 67 ± 9*† | <0.001 |
| SV, ml | 53 ± 13 | 51 ± 16 | 59 ± 18 | 63 ± 19 | 0.056 |
| SI, ml/m2 | 28 ± 7 | 27 ± 7 | 31 ± 8 | 35 ± 9*† | 0.002 |
| SV, ml/kg FFM | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.1 ± 0.3 | 1.2 ± 0.3† | 0.007 |
| MAP, mmHg | 98 ± 13 | 93 ± 10 | 96 ± 10 | 100 ± 9 | 0.115 |
| Ea, mmHg·ml−1·m−2 | 4.4 ± 1.2 | 4.4 ± 1.3 | 3.8 ± 0.9 | 3.5 ± 0.8*† | 0.009 |
| SVR, dyn·s·cm−5 | 1,976 ± 547 | 2,051 ± 557 | 2,053 ± 505 | 2,067 ± 504 | 0.586 |
| (a-v)Do2, ml/dl | 6 ± 2 | 6 ± 1 | 6 ± 2 | 6 ± 1 | 0.206 |
Values are means ± SD.
V̇o2, oxygen uptake; Q̇c, cardiac output; QI, cardiac index; SV, stroke volume; SI, stroke index; MAP, mean arterial pressure; Ea, effective arterial elastance index; SVR, systemic vascular resistance; (a-v)Do2, arteriovenous oxygen difference.
P < 0.05 vs. sedentary.
P < 0.05 vs. casual.
Steady-State Submaximal and Maximal Exercise
Maximal exercise metabolic variables are presented in Table 3. Irrespective of unit, V̇o2max was greater in committed exercisers and Master athletes compared with sedentary and casual exercisers (P < 0.05).
Table 3.
Maximal exercise parameters
| Sedentary | Casual | Committed | Masters Athletes | ANOVA P Value | |
|---|---|---|---|---|---|
| V̇o2max | |||||
| l/min | 1.8 ± 0.5 | 2.0 ± 0.6 | 2.4 ± 0.6* | 2.6 ± 0.5*† | <0.001 |
| ml·kg−1·min−1 | 24 ± 5 | 26 ± 5 | 32 ± 6*† | 40 ± 5*†‡ | <0.001 |
| ml·kg FFM−1·min−1 | 35 ± 7 | 37 ± 6 | 46 ± 6*† | 51 ± 7*† | <0.001 |
| Maximal heart rate, beats/min | 164 ± 13 | 164 ± 12 | 160 ± 11 | 161 ± 11 | 0.522 |
| RER | 1.21 ± 0.11 | 1.19 ± 0.06 | 1.17 ± 0.06 | 1.19 ± 0.08 | 0.564 |
Values are means ± SD.
V̇o2max, maximal oxygen uptake; RER, respiratory exchange ratio.
P < 0.05 vs. sedentary.
P < 0.05 vs. casual.
P < 0.05 vs. committed.
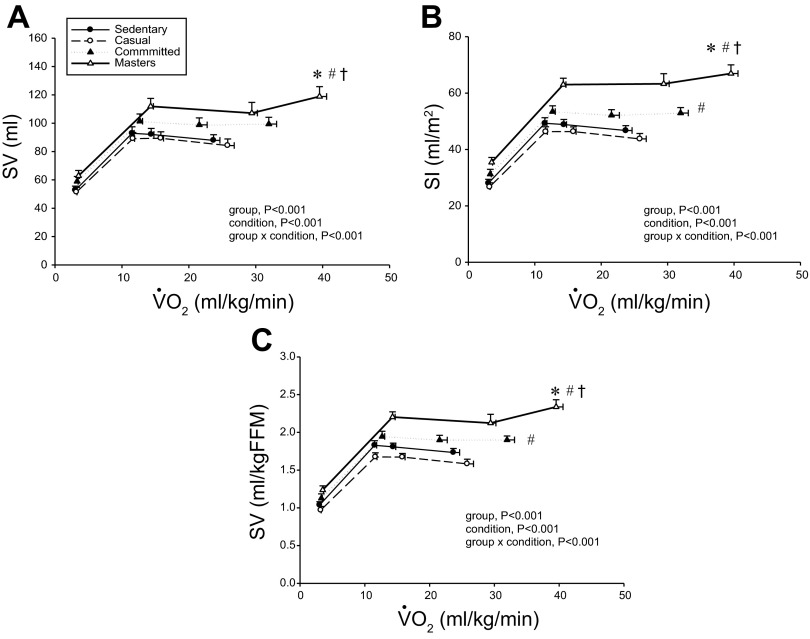
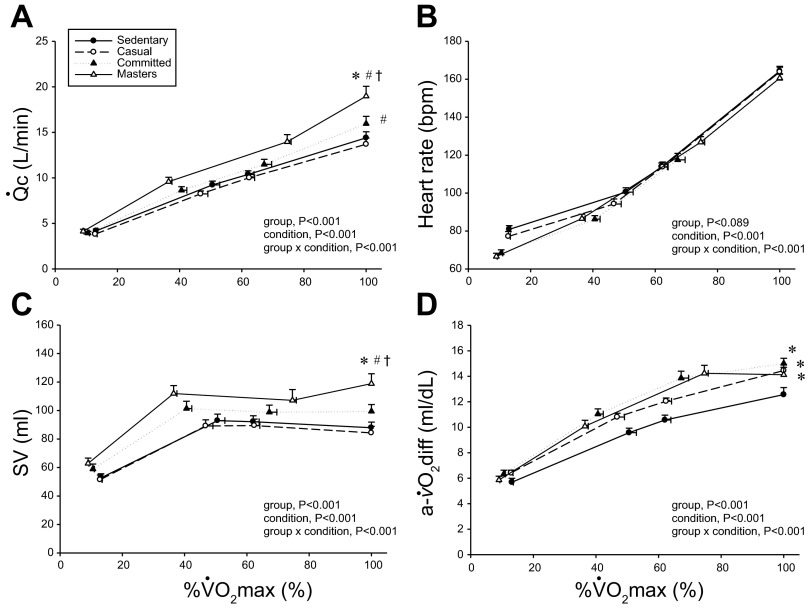
Figures 1 and 2 depict the relationship between V̇o2 expressed relative to total body mass (ml·kg−1·min−1) and as a percentage of V̇o2max (%V̇o2max) (Fig. 3) vs. Q̇c, SV, and (a-v)Do2 at rest, both levels of submaximal exercise, and maximal exercise. Plots of Q̇c vs. V̇o2 were basically superimposable among the groups, with committed exercisers and Masters athletes achieving a greater maximal Q̇c compared with sedentary and casual exercise groups (Figs. 1A and 3A). Heart rate response during submaximal exercise was lower in committed exercisers and Masters athletes relative to any V̇o2 expressed relative to total body mass (rightward shift) (Fig. 1B), but the response was not different among groups when depicted as a percentage of V̇o2max (Fig. 3B). Absolute and scaled SV were larger (upward shift) in committed exercisers and Masters athletes during submaximal and maximal exercise, while the casual exercisers mirrored the sedentary group (Figs. 2, A–C). While the response of MAP during exercise varied among the groups, MAP was not different at any condition (Fig. 1D).
Fig. 2.
Absolute stroke volume (SV; A), stroke index (SI; B), and SV scaled relative to fat-free mass (FFM; C) as a function of V̇o2 scaled relative to total body mass. Values are means ± SE. *P < 0.05 vs. sedentary. #P < 0.05 vs. casual. †P < 0.05 vs. committed. Only statistically significant results at maximal exercise are indicated. Please refer to text for further details.
Fig. 3.
Q̇c (A), heart rate (B), absolute SV (C), and (a-v)Do2 (D) as a function of V̇o2 as a percentage of maximum (%max; V̇o2max). Values are means ± SE. bpm, Beats/min. *P < 0.05 vs. sedentary. #P < 0.05 vs. casual. †P < 0.05 vs. committed. Only statistically significant results at maximal exercise are indicated. Please refer to text for further details.
Plots of (a-v)Do2 vs. V̇o2 scaled to total body mass and as a percentage of V̇o2max show that systemic oxygen extraction was greater (upward shift) in all trained groups compared with the sedentary group during moderate-intensity submaximal and maximal exercise (P < 0.05) (Figs. 1C and 3D). Masters athletes had a lower SVR during moderate-intensity submaximal exercise (P < 0.05 vs. sedentary and casual) and at maximal exercise (P < 0.05 vs. casual exercisers) (Fig. 1E). Ea was lower in Masters athletes compared with the sedentary and casual groups during submaximal exercise (downward shift) and was lower in committed exercisers and Masters athletes compared with the other two groups at maximal exercise (P < 0.05) (Fig. 1F).
HRR after Maximal Exercise
As shown in Fig. 4, A and B, heart rate was lower, while R-R interval was longer at 3–5 min of recovery in the committed exercisers and Masters athletes compared with sedentary subjects. The relative recovery of heart rate from maximal heart rate was only longer after 2 min of recovery in committed exercisers and Masters athletes when HRR was expressed as an R-R interval (P < 0.05 vs. sedentary).
LV Volumes, Filling Pressure, and Doppler Measures of LV Filling and Active Relaxation at Rest and During Volume Infusion
Doppler indexes of LV diastolic filling at supine rest and during LV loading are shown in Table 4. Left ventricular end-diastolic volume index was larger in Master athletes at supine rest and during saline infusion (P < 0.05). Except for a faster mean Em and a slower IVRT in the Masters athletes compared with casual exercisers (P < 0.05), there were no differences in Doppler measures at rest among the groups. These differences in mean Em and IVRT between casual exercisers and Masters athletes persisted during LV volume loading. Propagation velocity of early mitral inflow was slower in Masters athletes compared with the sedentary group (P < 0.05), which in part likely reflects a lower PCWP (P < 0.05 vs. sedentary) in this group at the same condition.
Blood Volume and V̇o2max
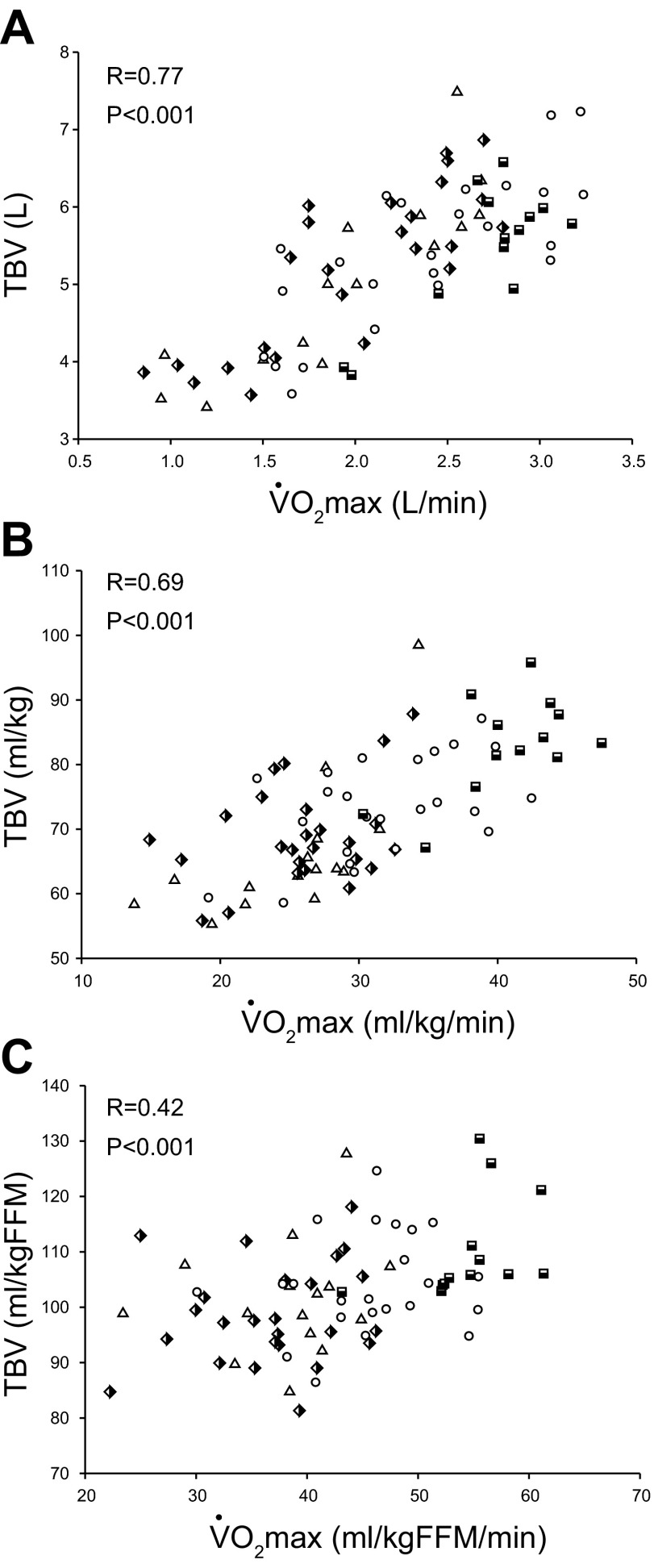
As depicted in Fig. 5A, there was a positive relationship between absolute levels of TBV and V̇o2max in all participants (R = 0.77, P < 0.001). Plots (Fig. 5, B and C) show that the direction of this relationship remained intact when TBV and V̇o2max were scaled relative to total body mass (R = 0.69, P < 0.001) or FFM (R = 0.42, P < 0.001).
Fig. 5.
Absolute (A) and total blood volume (TBV) scaled relative to total body mass (B) and FFM (C) as a function of absolute and scaled V̇o2max. Triangles [sedentary, 15 subjects (9 men)]; semi-filled diamonds [casual exercisers, 25 subjects (18 men)]; circles [committed exercisers, 24 subjects (19 men)]; semi-filled squares [Masters athletes, 13 subjects (11 men)].
DISCUSSION
The primary novel finding from this study is that four or more weekly endurance exercise sessions for at least 25 yr resulted in enhanced O2 transport and utilization during maximal exercise compared with lower frequencies of lifelong exercise. Higher frequencies of lifelong endurance exercise also resulted in favorable effects on SV regulation (ventricular-arterial coupling) and heart rate control during and after exercise. By collecting measures of LV mass, TBV, and Doppler measures of LV filling at rest and during saline infusion, the findings of this present study indicate that a larger SV during exercise is coupled with cardiac growth and TBV expansion, but not necessarily with beneficial effects on LV filling properties, even when the heart is fully loaded. Thus the current findings suggest that four or more weekly exercise sessions over a lifetime appears to be an effective strategy to preserving cardiovascular structure and function during exercise in seniors.
The Effect of Lifelong Exercise Frequency on V̇o2max, Exercise Hemodynamics, and (a-v)Do2
An important observation of this study was a strong relationship between V̇o2max and lifelong exercise frequency, which is consistent with previous findings in men only (23). We extend these finding by showing that: 1) a low frequency of lifelong exercise (2–3 sessions/wk) exerts a modest effect on V̇o2max expressed relative to total body mass (≈8%), and 2) the addition of one to two exercise sessions above this level (4–5 sessions/wk) is sufficient stimulus to limit the reduction in V̇o2max with aging. Our present findings complement a recent study in elite octogenarian athletes, who had performed at least 4 sessions/wk over 50 yr, resulting in an 80% greater V̇o2max (38 ± 1 vs. 21 ± 1 ml·kg−1·min−1) compared with age- and sex-matched controls (57) and are comparable to values observed in healthy untrained adults 40–50 yr younger (10).
Consistent with previous cross-sectional studies in men and women, the present study demonstrated that a high level of lifelong endurance exercise training is associated with a larger Q̇c and SV during submaximal and maximal exercise (26, 33, 36, 46). However, the effect of endurance training on maximal SV when begun later in life (>60 yr) is less clear, with several (17, 22, 52, 53), but not all (4, 18), studies reporting improvements. The reason for the discrepancy in study findings is unclear, but may be due to methodological differences, including measurement techniques, the mode of training, the length and intensity of the training stimulus, and the overall health of study participants. Given this uncertainty, further research is needed to establish the role of exercise dose, including intensity, frequency, and duration on maximal SV when exercise training is started later in life.
Irrespective of age or sex, increases in exercise SV with endurance-type training are primarily attributed to a larger end-diastolic volume, resulting from a combination of LV remodeling with increased compliance, hypervolemia, and potentially faster diastolic filling rates (1, 24, 26). In this present study, a greater lifelong exercise frequency was associated with a larger TBV and LV mass. A similar observation for LV mass was reported in men aged 18–77 yr (23), suggesting that LV growth and hypervolemia are influenced by frequency of endurance exercise training.
Coupled with enhanced LV compliance (1, 4a, 30), rapid LV active relaxation and vigorous diastolic suction would maximize LV end-diastolic volume, while attenuating increases in filling pressure in highly trained individuals (55). Previous work, including from our laboratory, has examined the effect of lifelong exercise training on early diastolic filling properties, including active relaxation and diastolic suction at rest and during exercise in seniors, with the majority reporting only modest improvements (3, 8, 31, 40, 41). Similar finding have been reported in young athletes compared with age-matched controls (3, 8), suggesting that any potential training-related improvements in echocardiographic parameters are not diminished with age. The apparent lack of a training effect on Doppler measures of LV filling at any age may reflect small sample sizes, large variations in subject's exercise histories, and notably an inability to comprehensively assess differences in LV loading conditions between trained and untrained individuals. The latter factor is particularly relevant issue given the load-dependency of Doppler measures of dynamic LV diastolic filling (19).
One previous study from our laboratory (41) collected invasive measures of LV filling conditions and reported minimal improvements in dynamic LV filling parameters in Masters athletes compared with sedentary controls. This present study in a much larger cohort shows that resting noninvasive measures of LV relaxation and suction are not improved at any frequency of lifelong training. Collectively, these findings seem to suggest that training-related improvements in SV during exercise are not coupled with significant enhancements in LV relaxation and diastolic suction at rest or during LV volume loading, a model analogous to increased preload during exercise. However, a key limitation to our findings includes that Doppler measures were collected while the subjects were supine, which may not reflect 1) hemodynamics and LV filling response that occur during upright exercise (39), or 2) changes in LV contractility during exercise, which would facilitate the ventricle contracting below its equilibrium volume engaging diastolic suction (35). Supporting this contention is previous work in our laboratory that showed that early diastolic intraventricular pressure gradients, an index of diastolic suction, increased progressively with incremental exercise workloads in Masters athletes, but only initially increased with low-intensity exercise in healthy, age-matched controls (40). Therefore, future studies should assess intraventricular pressure gradients and ventricular twist mechanics during exercise in a large cohort of senior athletes and controls to clearly determine the effect of lifelong exercise training on LV diastolic filling.
Early studies in small cohorts (21, 45) reported that measures of systolic function (ejection fraction, end-systolic volume) are improved during exercise in trained seniors, although this is not a consistent finding (46). A reduction in aortic impedance via a reduction in aortic stiffness (51) and SVR, improved ventricular-arterial coupling (22, 50), and potentially enhanced cardiac β-adrenergic sensitivity (54) would effectively augment LV emptying during exercise in trained seniors. Unfortunately, systolic volumes were not collected in this present study; thus we can only speculate on the effect of systolic function on SV regulation in our trained participants. However, Ea, an index that characterizes the total arterial load imposed on the LV, was lower during submaximal and maximal exercise in committed exercisers and Masters athletes, pointing toward improved arterial compliance and ventricular-arterial coupling in trained seniors, which is consistent with a previous observation in young trained men (38) and after 1 yr of training in previously untrained seniors (22). Therefore, these results highlight that improved ventricular-arterial coupling is a notable training-related adaptation for enhancing SV regulation during exercise in seniors.
We also found that maximal (a-v)Do2 was significantly greater in trained seniors, which has been reported in a prior study (36). Interestingly, we observed that even a low frequency of lifelong exercise (2–3 sessions/wk) improved oxygen extraction by metabolically active tissue during maximal exercise. These findings are important, as maximal (a-v)Do2 is reported to decline with aging (4, 9, 36), which probably in part reflects physical deconditioning in addition to the aging process itself, as maximal (a-v)Do2 is normalized in seniors with several months of exercise training (4, 53). Several mechanisms appear to underlie this improved oxygen extraction in trained seniors, including the preservation of FFM, increased number of mitochondria and enzymes activity (14), increased vasodilator response (44), and enhanced blood flow redistribution to exercising muscle mass (4).
The Effect of Lifelong Exercise Frequency on Heart Rate Control During and Recovery From Exercise
An important observation in this present study is that the relationship between V̇o2 relative to maximum effort (i.e., at the same relative workload) and heart rate was superimposable between our groups during exercise (Fig. 3B). Our results suggest that heart rate is precisely regulated in response to changes in SV during exercise to maintain the linear relationship between Q̇c and metabolic demand, as depicted in Fig. 1A. The current findings and previous data (36, 42) demonstrate that the slope of the relationship between blood flow and metabolic demand during exercise is not influenced by age, sex, or fitness in healthy individuals. In some conditions, such as postural orthostatic tachycardia syndrome, a compensatory heart rate response is demonstrated due to a smaller SV to “normalize” the slope relationship between Q̇c and O2 delivery during exercise (49); however, this compensation may not be sufficient to “normalize” this slope in populations with metabolic disease or heart failure, with or without preserved ejection fraction (5, 13, 32).
In contrast to the regulation of HR during exercise, HRR immediately after the cessation of exercise is exclusively due to restoration of vagal tone, while longer recovery durations reflect the balance between vagal tone restoration and sympathetic tone withdrawal (16). Because of its clear association with vagal activity, HRR may be used as a measure of “global cardiovascular health” in healthy individuals and patients with cardiovascular disease (15, 48). We observed that ≈97% of our participants achieved a HRR of >12 beats at 1 min (15) and >22 beats at 2 min (48), indicators of an appropriate HRR after maximal exercise.
Heart rate was slower, while R-R interval was longer at 3–5 min of recovery in highly trained seniors (Fig. 5, A and B). Cross-sectional (15, 48) and longitudinal training studies (49) have reported that a greater fitness level is associated with either a slower heart rate or a faster relative decline in heart rate at recovery periods ranging from 30 s to 5 min. Studies (37, 51) from our laboratory have reported that weekly exercise frequency influences heart rate and blood pressure regulation in younger adults and seniors over a 1-yr training period. Of particular note, improvements were noted after 3–6 mo of training, suggesting that exercise training-related improvements in autonomic function may be observed after a relatively short period of training. We were, therefore, surprised that we did not observe a more marked decay in the early phase (1 min) of HRR, a period highly influenced by vagal reactivation, in our trained seniors. It is possible that the beneficial effects of lifelong exercise frequency on heart rate “recovery” are more evident before our earliest postexercise assessment, or, more likely, that a “normal” HRR before 2 min is more difficult to improve at any dose of lifelong exercise training.
Study Limitations
First, factors other than those measured in this study (genetics, lifelong physical activity levels) may influence V̇o2max and central hemodynamics; therefore, we cannot exclude the possibility that the improved metabolic and hemodynamic responses in our trained subjects may be related in part to factors other than exercise training. Second, this present study used a simple approach to allocate participants into lifelong exercise groups. The usage of such an approach limits any conclusions based on other components of exercise training program, including intensity and duration or mode, all of which may have an important impact on the metabolic and hemodynamic response during exercise. Third, our trained cohort was predominantly men, because we could only find a limited number of women whose lifelong exercise volume met our criteria. Further studies in a larger cohort of women are required to confirm these present results, or alternatively to elucidate whether sex may influence exercise function in response to different frequencies of lifelong exercise. Finally, our senior subjects were nonobese and normotensive and were screened for occult cardiovascular disease; therefore, it is unclear whether the present findings are applicable to the larger population.
Perspectives
One of the debilitating effects of aging is a progressive reduction in functional capacity as assessed by V̇o2max. While it has been clearly established that vigorous lifelong exercise training minimizes the reduction in physical function and exercise function in seniors, there is very limited information on the optimal “dose” of lifetime exercise to obtain such benefits. This current study examined one component of the overall exercise “dose” and demonstrated that performing four or more endurance exercise sessions weekly over a lifetime is associated with a greater V̇o2max, oxygen delivery and extraction, and improved ventricular-arterial coupling and heart rate control during exercise. It is important to note that, while the largest values of V̇o2max and exercise function were observed in Master athletes, their level of training and competition are likely beyond the feasibility of most individuals. Instead, seniors who had consistently performed 4–5 exercise sessions/wk, and therefore likely reflect the current recommendations for weekly physical activity (≈150 min/wk) (34), still exhibited significant improvements in V̇o2max, exercise function, and cardiovascular control variables compared with healthy controls and, therefore, should be viewed as the minimum frequency of lifelong exercise to attenuate the loss of functional capacity and exercise function with aging. Our results showed that aging effects on resting Doppler measures of LV relaxation and diastolic suction are not prevented even when subjected to many decades of stimulus.
Conclusion
In summary, these present findings emphasize that lifelong exercise training frequency favorably influences V̇o2max, SV, systemic oxygen extraction, and ventricular-arterial coupling during exercise in healthy seniors, with the most prominent effect observed when four or more weekly endurance exercise sessions are performed throughout a lifetime.
GRANTS
This project was supported by the National Institute on Aging (Grant RO1 AG-17479).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.C.C.-R., J.L.H., P.S.B., N.F., S.S., M.D.P., K.B., S.L., E.D., and B.D.L. performed experiments; G.C.C.-R. and B.D.L. analyzed data; G.C.C.-R., J.L.H., P.S.B., N.F., S.S., M.D.P., K.B., S.L., E.D., and B.D.L. interpreted results of experiments; G.C.C.-R. prepared figures; G.C.C.-R. drafted manuscript; G.C.C.-R., J.L.H., P.S.B., N.F., S.S., and B.D.L. edited and revised manuscript; J.L.H., P.S.B., and B.D.L. conception and design of research; B.D.L. approved final version of manuscript.
REFERENCES
- 1.Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation 110: 1799–1805, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Astrand I, Astrand PO, Hallback I, Kilbom A. Reduction in maximal oxygen uptake with age. J Appl Physiol 35: 649–654, 1973 [DOI] [PubMed] [Google Scholar]
- 3.Baldi JC, McFarlane K, Oxenham H, Whalley GA, Walsh H, Doughty RN. Left ventricular diastolic filling and systolic function of young and older trained and untrained men. J Appl Physiol 95: 2570–2575, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation 100: 1085–1094, 1999 [DOI] [PubMed] [Google Scholar]
- 4a.Bhella P, Hastings J, Fujimoto N, Shibata S, Carrick-Ranson G, Adams-Huet B, Palmer M, Levine BD. The impact of lifelong exercise “dose” on left ventricular compliance and distensibility. JACC, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR, Levine BD. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail 13: 1296–1304, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blair S, Kohl H, Barlow C, Paffenbarger R, Gibbons L, Macera C. Physical fitness and all-cause mortality: a prospective study of men and women. JAMA 262: 2395–2401, 1989 [DOI] [PubMed] [Google Scholar]
- 7.Burge CM, Skinner SL. Determination of hemoglobin mass and blood volume with CO: evaluation and application of a method. J Appl Physiol 79: 623–631, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Carrick-Ranson G, Doughty RN, Whalley GA, Walsh HJ, Gamble GD, Baldi JC. The larger exercise stroke volume in endurance-trained men does not result from increased left ventricular early or late inflow or tissue velocities. Acta Physiol (Oxf) 205: 520–531, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Carrick-Ranson G, Hastings JL, Bhella PS, Shibata S, Fujimoto N, Palmer D, Boyd K, Levine BD. The effect of age-related differences in body size and composition on cardiovascular determinants of V̇o2max. J Gerentol A Biol Sci Med Sci 68: 608–616, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrick-Ranson G, Hastings JL, Bhella PS, Shibata S, Levine BD. The effect of exercise training on left ventricular relaxation and diastolic suction at rest and during orthostatic stress after bed rest. Exp Physiol 98: 501–513, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Carrick-Ranson G, Walsh H, Whalley GA, Lalande S, Gusso S, Doughty RN, Baldi JC. Mitral and tissue Doppler evaluation of diastolic filling response during rest and submaximal exercise in trained and untrained subjects. In: American College of Sports Medicine Annual Meeting Denver, CO: American College of Sports Medicine, 2006 [Google Scholar]
- 12.Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol 38: 2028–2034, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Chomsky DB, Lang CC, Rayos GH, Shyr Y, Yeoh TK, Pierson RN, 3rd, Davis SF, Wilson JR. Hemodynamic exercise testing. A valuable tool in the selection of cardiac transplantation candidates. Circulation 94: 3176–3183, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol 72: 1780–1786, 1992 [DOI] [PubMed] [Google Scholar]
- 15.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 341: 1351–1357, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Coote JH. Recovery of heart rate following intense dynamic exercise. Exp Physiol 95: 431–440, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Ehsani AA, Ogawa T, Miller TR, Spina RJ, Jilka SM. Exercise training improves left ventricular systolic function in older men. Circulation 83: 96–103, 1991 [DOI] [PubMed] [Google Scholar]
- 18.Ehsani AA, Spina RJ, Peterson LR, Rinder MR, Glover KL, Villareal DT, Binder EF, Holloszy JO. Attenuation of cardiovascular adaptations to exercise in frail octogenarians. J Appl Physiol 95: 1781–1788, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Firstenberg MS, Levine BD, Garcia MJ, Greenberg NL, Cardon L, Morehead AJ, Zuckerman J, Thomas JD. Relationship of echocardiographic indices to pulmonary capillary wedge pressures in healthy volunteers. J Am Coll Cardiol 36: 1664–1669, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Fleg J, Morrell C, Bos A, Brant L, Talbot L, Wright J, Lakatta E. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation 112: 674–682, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Fleg JL, Schulman SP, O'Connor FC, Gerstenblith G, Becker LC, Fortney S, Goldberg AP, Lakatta EG. Cardiovascular responses to exhaustive upright cycle exercise in highly trained older men. J Appl Physiol 77: 1500–1506, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Fujimoto N, Prasad A, Hastings JL, Arbab-Zadeh A, Bhella PS, Shibata S, Palmer D, Levine BD. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation 122: 1797–1805, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gates PE, Tanaka H, Graves J, Seals DR. Left ventricular structure and diastolic function with human ageing. Relation to habitual exercise and arterial stiffness. Eur Heart J 24: 2213–2220, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Gledhill N, Cox D, Jamnik R. Endurance athletes' stroke volume does not plateau: major advantage is diastolic function. Med Sci Sports Exerc 26: 1116–1121, 1994 [PubMed] [Google Scholar]
- 25.Gore CJ, Rodriguez FA, Truijens MJ, Townsend NE, Stray-Gundersen J, Levine BD. Increased serum erythropoietin but not red cell production after 4 wk of intermittent hypobaric hypoxia (4,000–5,500 m). J Appl Physiol 101: 1386–1393, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Hagberg J, Goldberg A, Lakatta L, O'Connor F, Becker L, Lakatta E, Fleg J. Expanded blood volumes contribute to the increased cardiovascular performance of endurance-trained older men. J Appl Physiol 85: 484–489, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Higginbotham M, Kenneth M, Williams R, Coleman R, Cobb F. Physiologic basis for the age-related decline in aerobic work capacity. Am J Cardiol 57: 1374–1379, 1986 [DOI] [PubMed] [Google Scholar]
- 29.Jarvis SS, Levine BD, Prisk GK, Shykoff BE, Elliott AR, Rosow E, Blomqvist CG, Pawelczyk JA. Simultaneous determination of the accuracy and precision of closed-circuit cardiac output rebreathing techniques. J Appl Physiol 103: 867–874, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Levine BD, Lane LD, Buckey JC, Friedman DB, Blomqvist CG. Left ventricular pressure-volume and Frank-Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation 84: 1016–1023, 1991 [DOI] [PubMed] [Google Scholar]
- 31.Levy WC, Cerqueira MD, Abrass IB, Schwartz RS, Stratton JR. Endurance exercise training augments diastolic filling at rest and during exercise in healthy young and older men. Circulation 88: 116–126, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Lewis SF, Haller RG, Cook JD, Blomqvist CG. Metabolic control of cardiac output response to exercise in McArdle's disease. J Appl Physiol 57: 1749–1753, 1984 [DOI] [PubMed] [Google Scholar]
- 33.McCole SD, Brown MD, Moore GE, Zmuda JM, Cwynar JD, Hagberg JM. Enhanced cardiovascular hemodynamics in endurance-trained postmenopausal women athletes. Med Sci Sports Exerc 32: 1073–1079, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA, Castaneda-Sceppa C. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 116: 1094–1105, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Nikolic S, Yellin EL, Tamura K, Vetter H, Tamura T, Meisner JS, Frater RW. Passive properties of canine left ventricle: diastolic stiffness and restoring forces. Circ Res 62: 1210–1222, 1988 [DOI] [PubMed] [Google Scholar]
- 36.Ogawa T, Spina R, Martin W, Kohrt W, Schechtman K, Holloszy J, Ehsani A. Effects of aging, sex and physical training on cardiovascular responses to exercise. Circulation 86: 494–503, 1992 [DOI] [PubMed] [Google Scholar]
- 37.Okazaki K, Iwasaki K, Prasad A, Palmer MD, Martini ER, Fu Q, Arbab-Zadeh A, Zhang R, Levine BD. Dose-response relationship of endurance training for autonomic circulatory control in healthy seniors. J Appl Physiol 99: 1041–1049, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Otsuki T, Maeda S, Iemitsu M, Saito Y, Tanimura Y, Ajisaka R, Miyauchi T. Systemic arterial compliance, systemic vascular resistance, and effective arterial elastance during exercise in endurance-trained men. Am J Physiol Regul Integr Comp Physiol 295: R228–R235, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Poliner LR, Dehmer GJ, Lewis SE, Parkey RW, Blomqvist CG, Willerson JT. Left ventricular performance in normal subjects: a comparison of the responses to exercise in the upright and supine positions. Circulation 62: 528–534, 1980 [DOI] [PubMed] [Google Scholar]
- 40.Popovic ZB, Prasad A, Garcia MJ, Arbab-Zadeh A, Borowski A, Dijk E, Greenberg NL, Levine BD, Thomas JD. Relationship among diastolic intraventricular pressure gradients, relaxation, and preload: impact of age and fitness. Am J Physiol Heart Circ Physiol 290: H1454–H1459, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Prasad A, Popovic ZB, Arbab-Zadeh A, Fu Q, Palmer D, Dijk E, Greenberg NL, Garcia MJ, Thomas JD, Levine BD. The effects of aging and physical activity on Doppler measures of diastolic function. Am J Cardiol 99: 1629–1636, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Proctor D, Beck K, Shen P, Eickhoff T, Halliwill J, Joyner M. Influence of age and gender on cardiac output-V̇o2 relationships during submaximal cycle ergometer. J Appl Physiol 84: 599–605, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation 112: 2254–2262, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Rinder MR, Spina RJ, Ehsani AA. Enhanced endothelium-dependent vasodilation in older endurance-trained men. J Appl Physiol 88: 761–766, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Schulman SP, Fleg JL, Goldberg AP, Busby-Whitehead J, Hagberg JM, O'Connor FC, Gerstenblith G, Becker LC, Katzel LI, Lakatta LE, Lakatta EG. Continuum of cardiovascular performance across a broad range of fitness levels in healthy older men. Circulation 94: 359–367, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Seals D, Hagberg J, Spina R, Rogers M, Schechtman K, Ehsani A. Enhanced left ventricular performance in endurance trained older men. Circulation 89: 198–205, 1994 [DOI] [PubMed] [Google Scholar]
- 47.Seals DR, Hagberg JM, Hurley BF, Ehsani AA, Holloszy JO. Endurance training in older men and women. I. Cardiovascular responses to exercise. J Appl Physiol 57: 1024–1029, 1984 [DOI] [PubMed] [Google Scholar]
- 48.Shetler K, Marcus R, Froelicher VF, Vora S, Kalisetti D, Prakash M, Do D, Myers J. Heart rate recovery: validation and methodologic issues. J Am Coll Cardiol 38: 1980–1987, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Shibata S, Fu Q, Bivens TB, Hastings JL, Wang W, Levine BD. Short-term exercise training improves the cardiovascular response to exercise in the postural orthostatic tachycardia syndrome. J Physiol 590: 3495–3505, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shibata S, Hastings JL, Prasad A, Fu Q, Okazaki K, Palmer MD, Zhang R, Levine BD. “Dynamic” Starling mechanism: effects of ageing and physical fitness on ventricular-arterial coupling. J Physiol 586: 1951–1962, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shibata S, Levine BD. Biological aortic age derived from the arterial pressure waveform. J Appl Physiol 110: 981–987, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spina R, Ogawa T, Coggan A, Holloszy J, Ehsani A. Exercise training improves left ventricular contractile response to beta-adrenergic agonist. J Appl Physiol 72: 307–311, 1992 [DOI] [PubMed] [Google Scholar]
- 53.Spina R, Ogawa T, Kohrt W, Holloszy J, Ehsani A. Differences in cardiovascular adaptations to endurance exercise training between older men and women. J Appl Physiol 75: 849–855, 1993 [DOI] [PubMed] [Google Scholar]
- 54.Spina R, Turner M, Ehsani A. Beta-adrenergic-mediated improvement in left ventricular function by exercise training in older men. Am J Physiol Heart Circ Physiol 274: H397–H404, 1998 [DOI] [PubMed] [Google Scholar]
- 55.Stickland MK, Welsh RC, Petersen SR, Tyberg JV, Anderson WD, Jones RL, Taylor DA, Bouffard M, Haykowsky MJ. Does fitness level modulate the cardiovascular hemodynamic response to exercise? J Appl Physiol 100: 1895–1901, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000 [DOI] [PubMed] [Google Scholar]
- 57.Trappe S, Hayes E, Galpin A, Kaminsky L, Jemiolo B, Fink W, Trappe T, Jansson A, Gustafsson T, Tesch P. New records in aerobic power among octogenarian lifelong endurance athletes. J Appl Physiol 114: 3–10, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei M, Kampert JB, Barlow CE, Nichaman MZ, Gibbons LW, Paffenbarger RS, Jr, Blair SN. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA 282: 1547–1553, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Wilmore JH, Behnke AR. An anthropometric estimation of body density and lean body weight in young men. J Appl Physiol 27: 25–31, 1969 [DOI] [PubMed] [Google Scholar]