Abstract
This study investigated mechanisms for the stressor-induced changes in muscle fatigability in men and women. Participants performed an isometric-fatiguing contraction at 20% maximal voluntary contraction (MVC) until failure with the elbow flexor muscles. Study one (n = 55; 29 women) involved two experimental sessions: 1) a high-stressor session that required a difficult mental-math task before and during a fatiguing contraction and 2) a control session with no mental math. For some participants (n = 28; 14 women), cortical stimulation was used to examine mechanisms that contributed to muscle fatigability during the high-stressor and control sessions. Study two (n = 23; nine women) determined the influence of a low stressor, i.e., a simple mental-math task, on muscle fatigability. In study one, the time-to-task failure was less for the high-stressor session than control (P < 0.05) for women (19.4%) and men (9.5%): the sex difference response disappeared when covaried for initial strength (MVC). MVC force, voluntary activation, and peak-twitch amplitude decreased similarly for the control and high-stressor sessions (P < 0.05). In study two, the time-to-task failure of men or women was not influenced by the low stressor (P > 0.05). The greater fatigability, when exposed to a high stressor during a low-force task, was not exclusive to women but involved a strength-related mechanism in both weaker men and women that accelerated declines in voluntary activation and slowing of contractile properties.
Keywords: voluntary activation, transcranial magnetic stimulation, sex differences, muscle fatigue, gender
psychosocial stress can impair motor performance during low-intensity isometric tasks. When young adults, for example, are exposed to an acute stressor (such as difficult mental math, unpredictable delivery of electrical stimulation, or a choice reaction time task), while sustaining isometric contractions with the upper limb, time-to-task failure decreases, and the amplitude of force fluctuations can increase (2, 4, 27, 36, 53). The influence of a stressor on steadiness and fatigability appears to be more prominent in women than men and in individuals with higher trait anxiety (4, 35, 53). These findings are important, because physiological responses to psychosocial stress are significant determinants of health (18) and therefore, predispose some populations to more health problems, such as work-related musculoskeletal disorders (38). Despite the detrimental short- and long-term repercussions of repeated exposure to an acute stressor on the musculoskeletal system, mechanisms underlying these interactions are not known.
Our initial findings in this area showed increased fatigability of a submaximal, sustained contraction (reduction in time to failure) for the elbow flexor muscles when performing a difficult mental-math task (high cognitive stressor) (53). The increased fatigability was greater in women than men and was also accompanied by an increase in indices of sympathetic activation [mean arterial pressure (MAP) and heart rate] (53). The reduction in time to failure with exposure to the stressor was associated with maximal strength, because weaker individuals exhibited a greater increase in fatigability when exposed to the difficult cognitive challenge. Because men were stronger than women, it was not clear in our initial study whether the stressor-induced reduction in time to failure was due to the strength alone (independent of sex) or specific to women (53). To address this issue, in the current study, we recruited and tested large numbers of men and women and of varying strength.
Strength is dependent on adequate voluntary command from supraspinal centers and activation of motor units that vary in contractile properties across motor-unit populations. Both voluntary activation and contractile properties are altered during fatiguing contractions (12, 19) and are potentially influenced by an acute stressor, but this has not been investigated. Increased sympathetic activation, which occurs during acute stress exposure, can, however, alter the intrinsic properties of muscle fibers by potentiating force of type II fast-twitch fibers and reduce the half-relaxation time of type I slow-twitch fibers in animal muscle (1) and human motor units with low recruitment thresholds (40). Men, who are typically stronger, usually possess a greater proportional area of type II fibers than women when analyzed from muscle biopsy samples [e.g., Porter et al. (39) and Simoneau and Bouchard (41)] and typically, more rapid contractile function (12, 33, 52). Therefore, the greater fatigability in weaker individuals when exposed to a stressor could be due to a selective influence of sympathetic activity on the different fibers and their contractile properties. We investigated the association between contractile properties and fatigability when a high and low cognitive stressor was imposed in both men and women.
Stressor-induced alterations in muscle fatigability could also involve adjustments from supraspinal sources, and this may differ among individuals of different strength and with varying activation of the sympathetic nervous system. Stressor-induced activation of the sympathetic nervous system causes release of neuromodulators (norepinephrine and serotonin) and hormones (epinephrine and cortisol) that impact cognitive (28), cardiovascular (8), and motor functions (22). Furthermore, descending command and synaptic input to the motoneuron pool may be altered during tasks that require more attention (17, 34). Hence, we assessed activation of the motor cortex during maximal voluntary contractions (MVCs). An increase in the force from cortical stimulation during a maximal effort contraction [superimposed twitch (SIT)] indicates suboptimal output from the motor cortex and voluntary activation is reduced (7, 44). Under control conditions, women are able to sustain fatiguing contractions in the elbow flexor muscles longer than men at the same relative intensity [for review, see Hunter (11)], but voluntary activation and its reduction were similar for both sexes (12, 19). It is unknown, however, whether the greater fatigability associated with an imposed stressor in individuals with weaker muscles can be attributed to the reduced activation from the motor cortex.
The first purpose of study one was to determine the associations between the stressor-induced modulation of fatigability and the strength (MVC force) and sex of the participants. The second purpose of study one was to determine the relative contributions of adjustments in voluntary activation and contractile function to the stressor-induced change in fatigability. We hypothesized that women would have greater decrements in time to failure of a submaximal isometric-fatiguing contraction than men when exposed to a high cognitive stressor, and this would be associated with 1) elbow flexor maximal strength, 2) slower rates of relaxation, and 3) greater reductions in voluntary activation in the elbow flexor muscles. In study two, men and women performed a simple mental-math test during the fatiguing contraction to determine the influence of a low cognitive stressor (simple mental math) on the time-to-task failure. We hypothesized that the low cognitive stressor would not influence fatigability in men and women.
MATERIALS AND METHODS
Two studies were performed involving young, healthy men and women (18–35 yr). All participants were healthy with no known neurological or cardiovascular diseases and were naive to the protocol. Participants reported no history or current mental pathology, including anxiety disorders and/or depression. Each protocol was approved by the Institutional Review Board at Marquette University and conformed to the standards of the 1975 Helsinki Declaration. Each participant provided informed consent and attended a familiarization session, followed by several experimental sessions.
At the familiarization session, each participant practiced MVCs with the elbow flexor muscles. The physical-activity level for each participant was assessed with a questionnaire that estimated the relative kilocalorie expenditure/wk (23). The day of menstrual cycle was recorded for each experimental day for female participants. The 1st day of menstruation was considered day one of the cycle. Participants were also habituated to transcranial magnetic stimulation (TMS) and electrical stimulation of the brachial plexus.
Study One
Twenty-six men (20 ± 2 yr) and 29 women (20 ± 3 yr) participated in a familiarization and two experimental sessions (control and high-stressor sessions) that were counterbalanced among participants. The control and high-stressor sessions were ≥7 days apart and involved performing a fatiguing contraction with the elbow flexor muscles of the left arm. In the control session, each participant performed the contraction without performing the difficult cognitive task (i.e., mental math). During the stressor sessions, each participant was required to perform 4 min of mental math before and simultaneously while performing the fatiguing contraction at a target level that was equivalent to 20% of MVC force for as long as possible (see Experimental Protocol at Experimental Sessions for more detail). Hand dominance was estimated by the Edinburgh Handedness Inventory (37) (0.44 ± 0.5 vs. 0.70 ± 0.2 for men and women, respectively, with a ratio of one indicating complete right-handedness). To determine the time to failure across participants of varying levels of strength, we pooled data from the 55 participants tested in this study with data from our original study (53), where we used the same procedures and equipment as the present study. Hence, the data of 20 participants were added to increase the total to 75 participants for both studies combined for the strength-matched analysis.
A subset of participants from study one (14 women, 20 ± 2 yr; 14 men, 20 ± 3 yr) performed experimental sessions (control and high-stressor sessions) with TMS (elicited during MVCs and submaximal contractions) to quantify voluntary activation, motor-evoked potentials (MEPs), silent periods, and peak relaxation rates before and after the fatiguing contraction. Experimental sessions were counterbalanced among participants.
Study Two
To determine the influence of a simple cognitive task (low stressor) on time-to-task failure, 14 men (20 ± 2 yr) and nine women (20 ± 4 yr) attended an additional session to perform a simple mental-math task before and during a fatiguing contraction. Cortical stimulation was used to elicit force and electromyography (EMG) during MVCs and submaximal contractions. The procedures were the same as the high-stressor session except that the mental math was simple and a low-level stressor (see Cognitive Tasks).
Mechanical Recordings
Each participant was seated upright in an adjustable chair with the left arm slightly abducted and the elbow joint resting on a padded support. The elbow joint was flexed to 90° so that the forearm was parallel to the ground, and the force at the wrist was directed upward with activation of the elbow flexor muscles. The hand and forearm were placed in a modified wrist-hand-thumb orthosis (Orthomerica, Newport Beach, CA), and the forearm was placed midway between pronation and supination. Two nylon straps were placed over each shoulder to minimize shoulder movement. The force exerted by the wrist in the vertical direction was measured with a transducer (force-moment sensor; JR3, Woodland, CA) that was mounted on a custom-designed, adjustable support. The orthosis was attached to the force transducer. The force was recorded online at 500 samples/s, using a Power 1401 analog-to-digital (A-D) converter and Spike2 software [Cambridge Electronic Design (CED), Cambridge, UK], and displayed on a 19-in. monitor, 1.5 m in front of the subject. Each subject was asked to trace the horizontal cursor with the force signal for as long as possible during the fatiguing contraction. The force signal appeared on the screen from the right side of the monitor at 2.5 cm/s. The gain of the force signal was the same across sessions for each participant.
Electrical Recordings
EMG signals were recorded with bipolar surface electrodes (Ag-AgCl, 8-mm diameter; 16 mm between electrodes) that were placed over biceps brachii, brachioradialis, and triceps brachii muscles. The bipolar electrode configuration was placed longitudinally over the muscle belly, midway between the origin and insertion for each muscle, according to the European recommendations for surface EMG (9). Reference electrodes were placed on a bony prominence at the elbow. The EMG signal was amplified (100×) and band-pass filtered (13–1,000 Hz) with Coulbourn modules (Coulbourn Instruments, Allentown, PA) before being recorded directly to a computer with the Power 1401 A-D converter and Spike2 (CED). The EMG signals were digitized at 2,000 samples/s.
Motor cortex stimulation.
TMS was delivered via a round coil (13.5 cm outside diameter) over the vertex (Magstim 200; Magstim, Whitland, UK) to elicit MEPs in biceps brachii, brachioradialis, and triceps brachii muscles. The right cerebral hemisphere was stimulated by the direction of the current flow in the coil to activate the left limb preferentially. A single pulse was delivered over the motor cortex at an intensity that produced a large MEP in the agonist biceps muscle [minimum amplitude of 50% of maximal M wave (Mmax) during a brief MVC of the elbow flexor muscles] but only a small MEP in the antagonist triceps muscle amplitude (<20% of Mmax) when the M wave was maximal (49). TMS was delivered during voluntary contractions only, including during brief maximal and submaximal contractions before and after the fatiguing contraction (recovery), and also, during the fatiguing contraction (once at the start and once at task failure; Fig. 1).
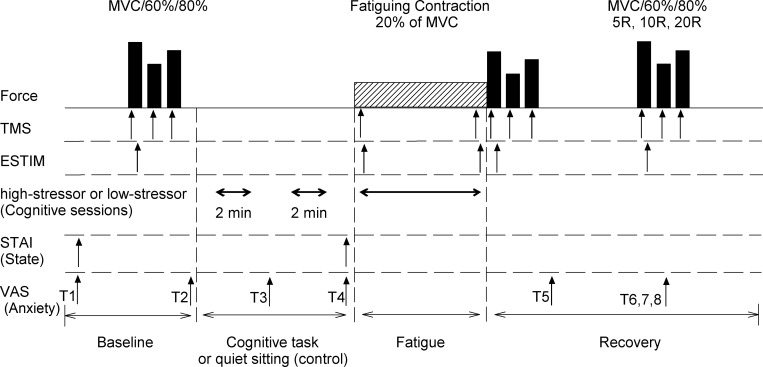
Fig. 1.
Experimental protocol. This is the general protocol. Not all participants were involved in the stimulation. Top row: order of force tasks performed by each subject with the elbow flexor muscles. Each participant performed 4 sets of maximal voluntary contractions (MVCs) and brief contractions at 60% and 80% of MVC. These were repeated during recovery [recovery at 5 min, 10 min, and 20 min (5R, 10R, and 20R)] from the fatiguing contraction. 2nd and 3rd rows: arrows denote the time points that transcranial magnetic stimulation (TMS) and electrical stimulation of the brachial plexus (ESTIM) were delivered in some participants (study 1, n = 28; study 2, n = 23). A difficult mental-math task (high stressor), a simple mental-math task (low-stressor task), or quiet rest (control session) was performed, 2 × 2 min (total of 4 min) before and then during the fatiguing contraction for each respective session. The State-Trait Anxiety Inventory (STAI) questionnaire was assessed twice throughout the protocol. Levels of anxiety using the Visual Analog Scale (VAS) were assessed throughout the protocol. T1–T2, 2 baseline assessments before intended arousal; T3, after the 1st 2-min bout of the cognitive task with no contraction (low- or high-stressor session) or quiet rest (control session); T4, after the 2nd 2-min bout of the cognitive task or quiet rest (control session); T5, immediately after the fatiguing contraction; T6–T8, 5, 10, and 20 min after the fatiguing contraction (recovery), respectively. Bottom row: order in which the events took place. Note that the schematic is not to scale for time or force.
Brachial plexus stimulation.
The brachial plexus was electrically stimulated to produce a maximal compound muscle action potential (Mmax) of the biceps brachii, brachioradialis, and triceps brachii muscles. Brachial plexus stimulation occurred during each MVC and after delivery of the cortical stimulation. Participants were allowed 1 s to recover maximal force after cortical stimulation before brachial stimulation was applied. A cathode was placed in the supraclavicular fossa and an anode on the acromion process. A constant-current stimulator (model DS7AH; Digitimer, Hertfordshire, UK) was used to deliver single stimuli (100 μs duration) to the brachial plexus.
Cognitive Tasks
During the high-stressor session (study one only), each participant performed serial subtraction by 13 from a four-digit number, with a response required every 3 s (36). Once participants made an error in the math or were not able to provide the correct answer within 3 s, they were asked to start the mental math again from a new number in the series. They performed the mental math before the fatiguing contraction (2 × 2-min bouts) and then continuously during the fatiguing contraction. Mental math is a well-established psychosocial technique to induce stress (18, 36) and was used in a previous study to increase levels of anxiety and stress (53).
During the low-stressor session (study two only), each participant performed a simple math task that was not designed to induce stress (53) but to involve similar cognitive processes of that during the high-stressor session. Participants subtracted by one from 50 continuously during the 4 min (2 × 2-min bouts) before the fatiguing contraction and during the fatiguing contraction in the low-stressor session.
Cognitive Assessment of Anxiety
Cognitive levels of anxiety were assessed throughout the protocol using the Visual Analog Scale (VAS) and the state portion of the State-Trait Anxiety Inventory (STAI) questionnaire, as we have detailed previously (19, 53). VAS, for anxiety, was recorded at eight time points during the protocol (Fig. 1): two baseline assessments before intended arousal (T1–T2); after the first 2-min bout of the cognitive task with no contraction (low- or high-stressor session) or quiet rest (control session; T3); after the second 2-min bout of the cognitive task or quiet rest (control session; T4); immediately after the fatiguing contraction (T5); and then, 5, 10, and 20 min after the fatiguing contraction (recovery; T6–T8, respectively).
The STAI-state questionnaire involved 20 statements that required a response on a four-point, Likert-type scale. Assessment of STAI was performed at baseline, after quiet sitting (control session) and after 4 min (2 × 2-min bouts) of the mental math (low- and high-stressor sessions; Fig. 1).
Cardiovascular Measurements
Heart rate and blood pressure were monitored at rest (baseline), during the cognitive tasks or quiet sitting (before the fatiguing contraction), and also, during each fatiguing contraction. Both heart rate and blood pressure were monitored with an automated beat-by-beat blood-pressure monitor (Finapres 2300; Datex-Ohmeda, Louisville, CO). The blood-pressure cuff was placed around the middle finger of the relaxed right hand with the arm placed on a table adjacent to the subject at heart level. The automated blood-pressure signal was calibrated for each participant to a manual blood pressure taken at rest. The blood-pressure signal was recorded online to a computer at 500 samples/s.
Experimental Protocol at Experimental Sessions
Each experimental session began with assessments of baseline levels of anxiety using the VAS and STAI (state levels). After the initial setup of the participant, stimulation of the motor cortex with TMS and brachial plexus with electrical stimulation was conducted to determine optimal levels of stimulation. These levels of stimulation remained constant throughout the rest of the protocol for that session. All procedures thereafter were performed in the following order for each experimental session (Fig. 1): 1) MVCs of the elbow flexor muscles; 2) VAS for anxiety before and after either quiet sitting (control session) or 4 min (2 × 2-min bouts) of mental tasks (high- and low-stressor sessions only); 3) STAI after the 4 min (2 × 2-min bouts) of either quiet sitting or mental math; 4) performance of a fatiguing contraction at 20% MVC force; and 5) recovery MVCs and assessment of cognitive and physiological arousal immediately after the fatiguing contraction and at 5-, 10-, and 20-min recovery.
Initial measures.
One initial elbow flexor MVC was performed to determine if the participant was within 5% of previous sessions and to calculate force for the submaximal fatiguing contraction at 20% MVC. To quantify voluntary activation, four sets of brief control contractions (2–3 s) with the elbow flexor muscles were separated by 2 min of rest to minimize fatigue. Each set involved performance of a MVC, followed by brief contractions of 60% and 80% MVC. Within each set of contractions, the start of each contraction was separated by 3–4 s. Peak forces in the MVC from two of the four trials needed to be within 5% of each other; if this did not occur, additional trials were performed until this was accomplished. TMS was delivered during each contraction, and brachial plexus stimulation was only delivered during the MVCs.
Fatiguing contraction.
A fatiguing contraction was performed with the elbow flexor muscles at 20% MVC force during each experimental session. The participant matched the target force, as displayed on the monitor, and was encouraged before the fatiguing contraction to sustain the force for as long as possible. Verbal encouragement was not provided during the fatiguing contraction to avoid interruption of cognitive tasks and to maintain consistency throughout sessions. The fatiguing contraction was terminated when participants were not able to maintain the target force (i.e., their force declined by 10%). Task failure was detected automatically using a custom-designed program (Spike2; CED) that monitored the force signal, and this time was recorded as the time-to-task failure. To minimize the influence of transient fluctuations in motor output on the criteria for task failure, the task was terminated only after force fell below the predetermined threshold for 2 s out of a 4-s interval.
Recovery measures.
Measures of MVC force, voluntary activation, and VAS for anxiety were assessed at the following times: immediately upon task failure and 5, 10, and 20 min after termination of the fatiguing contraction (T5–T8; see Fig. 1).
Data Analysis
The MVC force was quantified as the average value over a 0.5-s interval that was centered about the peak of the MVC. The torque for the MVC and submaximal contractions was calculated as the product of force and the distance between the elbow joint and the point at which the wrist was attached to the force transducer (moment arm). Rates of change for several variables were calculated by subtracting the value at or immediately after task failure from the baseline value and normalizing the absolute change to the average time to failure.
Heart rate and MAP were calculated for the start of the first 2-min bout and at 30 s and 90 s after the start of each 2-min bout of either quiet rest (control) or mental math (high stressor) before the fatiguing contraction. For the low-stressor task, heart rate and MAP (before the fatiguing contraction) were unable to be analyzed for six participants (three men), due to artefact in the signal. These data are therefore not reported. Heart rate and MAP were also recorded during each fatiguing contraction and analyzed by comparing ∼15-s averages at 25% intervals. For each interval, the blood-pressure signal was analyzed for the mean peaks [systolic blood pressure (SBP)], mean troughs [diastolic blood pressure (DBP)], and number of pulses/s (multiplied by 60 to determine heart rate). MAP was calculated for each epoch with the following equation: MAP = DBP + 1/3(SBP − DBP). Rate-pressure product was calculated as the heart rate multiplied by the MAP (heart rate × MAP). Rate-pressure product is an indicator of cardiac workload and the oxygen requirement of the heart (51).
The amplitude of the SIT elicited by TMS is quantified as a percentage of the voluntary force measured immediately before TMS (7). The SIT amplitude was used to calculate voluntary activation, which was quantified by expressing the amplitude of the SIT (elicited by TMS) as a fraction of the estimated amplitude of the response evoked by the same stimulus at rest [estimated resting twitch (eRT)] (48). Only voluntary activation data are reported, because the results were similar to the SIT (percent MVC). Because motor cortical and spinal-cord excitability increase with activity (10), a control resting twitch was not able to be achieved at rest. Therefore, the amplitude of the resting twitch was estimated rather than measured directly (48). During the sets of brief maximal and submaximal contractions (MVC followed by 60% and 80% MVC contractions), TMS was delivered, and the resting twitch was estimated by extrapolation of the linear relation between the amplitude of the SIT and voluntary force. One regression analysis was performed for each set of brief contractions. The y-intercept was taken as the estimated amplitude of the resting twitch evoked by TMS. The amplitude of the eRT can be accurately determined from three data points in fresh or fatigued muscle when the contractions are >50% MVC (48). Voluntary activation (percent) was calculated as a percentage measured by cortical stimulation [(1 − SIT/eRT) × 100] (48). Data points were excluded (8.6%) at different time points when the regression of the estimated twitch was r < 0.9.
Contractile properties of the elbow flexor muscle fibers were also assessed. The amplitude of the eRT was used as an index of the force-generating capacity of the elbow flexor muscles, and the decline of the force after cortical stimulus was used to determine the peak relaxation rate of the whole muscle (47). Peak relaxation rates were determined during each MVC by calculating the steepest falling of the force during the EMG silence immediately following TMS (47). This was determined as the highest negative derivative of the force for an interval of 10 ms between two cursors placed on either side of the decline in force during the silent period. The steepest rate of force decline was normalized to the total force (MVC + SIT) before the silent period (47).
The amplitude and area of MEPs and M wave were measured between two cursors placed at the start and end of the waveform for the bicep brachii, brachioradialis, and triceps brachii muscles. Because the M-wave amplitude and area showed similar changes throughout, only M-wave area is reported. Additionally, the MEP areas for the biceps and brachioradialis muscles were similar in their changes between the stressor and control sessions, so only biceps MEP areas are reported. M waves were elicited after each MEP, but because there were no changes in the M wave, the MEP is represented as a percentage change from their baseline values. When TMS is delivered during a voluntary contraction, the MEP is followed by a period of near silence in the EMG (15). The silent period was measured as the interval from the stimulus to the resumption of continuous EMG. Voluntary torque was quantified by calculation of the mean torque over a 500-ms period immediately before TMS at the start and end of each sustained fatiguing contraction, during control and recovery of MVCs, and during the submaximal contractions at 60% and 80% MVC.
Statistical Analysis
Data are reported as means ± SD within the text and tables and displayed as means ± SE in the figures. Separate repeated-measures ANOVAs for time and session with sex as a between-subject factor (men vs. women) were used to compare various dependent variables. For study one, an analysis of covariance also compared time-to-task failure across sessions with repeated measures on session (control vs. high stressor), sex as a between-subject factor, and covarying for maximal strength. Repeated-measures factors included session (control vs. high stressor or control vs. low stressor), fatigue (baseline, after fatiguing task, and recovery), and time [before vs. after the cognitive stressor tasks (before the fatiguing contraction) and during the fatiguing contraction (0%, 25%, 50%, 75%, and 100% of time to failure)]. Specifically, the statistical designs were as follows for the dependent variables: 1) session × sex for time-to-task failure; 2) session × fatigue × sex for comparison of MVC, voluntary activation, eRT, MEP area, silent-period duration, and peak rates of relaxation; 3) session × time × sex for anxiety, heart rate, and MAP before and after the mental math (before the fatiguing contraction); and 4) session × time × sex for MEP area, MAP, and heart rate during the fatiguing contraction. A separate repeated-measures ANOVA was performed for study two to compare control with low-stressor session.
Independent t-tests were used to compare men and women for various physical characteristics, such as age, weight, height, handedness, STAI (trait) anxiety levels, and physical activity levels. The strength of an association is reported as the squared Pearson product-moment correlation coefficient (r2). A multiple linear regression was performed to determine predictors for the stressor-induced reduction in time to failure. A significance level of P ≤ 0.05 was used to identify statistical significance.
RESULTS
Men and women were similar in age, physical activity, and trait anxiety levels (P > 0.01) but differed in height and weight (P < 0.01; see Table 1).
Table 1.
Subject characteristics for men and women for all subjects in study 1 (n = 55) and those that participated in cortical stimulation (n = 28)
| Variable | Men | Women | P (Sex Effect) |
|---|---|---|---|
| (n = 55) | |||
| Age, yr | 20 ± 2 | 20 ± 3 | P = 0.67 |
| Height, cm | 181.8 ± 14.2 | 171.3 ± 10.7 | P = 0.002 |
| Body mass, kg | 76.0 ± 9.0 | 67.6 ± 8.4 | P < 0.001 |
| STAI, trait | 32.3 ± 7.0 | 33.3 ± 6.5 | P = 0.45 |
| PA, metabolic equivalents · h−1 · wk−1 | 56.0 ± 37.3 | 58.2 ± 39.0 | P = 0.81 |
| (n = 28) | |||
| Age, yr | 20 ± 2 | 20 ± 3 | P = 0.67 |
| Height, cm | 179.7 ± 4.0 | 168.6 ± 8.0 | P < 0.001 |
| Body mass, kg | 78.7 ± 9.2 | 68.5 ± 8.1 | P = 0.006 |
| STAI, trait | 31.4 ± 7.5 | 33.3 ± 6.7 | P = 0.48 |
| PA, metabolic equivalents · h−1 · wk−1 | 55.5 ± 39.5 | 56.8 ± 46.7 | P = 0.93 |
The last column indicates P values for the comparison of men with women.
STAI, State-Trait Anxiety Inventory; PA, physical activity.
Study One: High Stressor vs. Control
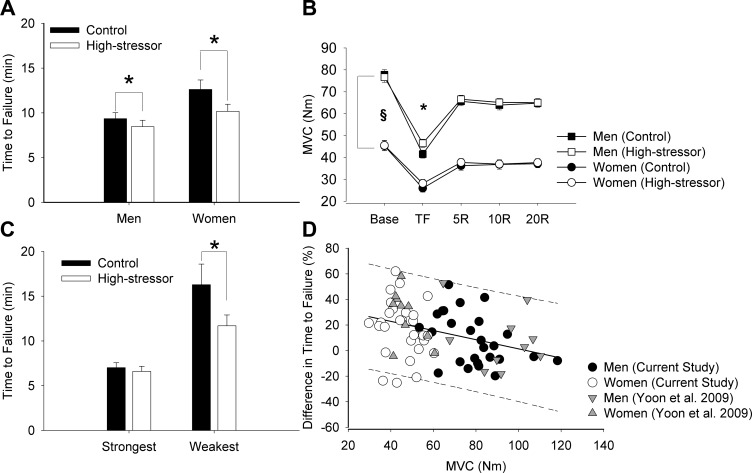
Time-to-task failure was briefer for the high-stressor session compared with the control session (session effect, P < 0.001). The reduction in time to failure from the control session to the high-stressor session was 19.4% for women and 9.5% for men (session × sex, P = 0.05; Fig. 2A). Men had a briefer time to failure than women for both sessions (8.9 ± 3.5 min vs. 11.4 ± 5.1 min, respectively; sex effect, P = 0.03). When the time to failure was covaried for maximal strength of each participant, there was still a session effect (P = 0.006), but the sex difference in time-to-task failure disappeared (sex effect, P = 0.87) with no interaction (session × sex, P = 0.60). Neither time-to-task failure nor the difference in time to failure between control and high-stressor sessions was associated with the day of the menstrual cycle in the women (P > 0.05).
Fig. 2.
Time-to-task failure (A), MVCs (B), time-to-task failure when separating weakest and strongest (C), association between the difference in time to failure between sessions (%) and MVC torque (D). A: study 1 (n = 55). Men had briefer time to failure than women for both the control and high-stressor sessions (P < 0.01). Both men and women were more fatigable during the high-stressor session compared with the control session (*P < 0.01). B: MVC torque of the elbow flexor muscles for men and women is shown at baseline (Base), at task failure (TF), and at 5, 10, and 20 min throughout recovery. MVC torque was greater for men than women before, immediately after, and throughout recovery for both sessions (§P < 0.05). Reduction in strength decreased after the fatiguing contraction (*P < 0.05), similarly for men and women across sessions (P > 0.05). C: 10 strongest and 10 weakest participants were chosen from the combined data of the current study and the Yoon et al. study (53). Weaker individuals (all women, n = 10) had the highest reduction in time-to-task failure when the high stressor was imposed (average = 24%, *P = 0.01) during the fatiguing contraction. In contrast, for the stronger individuals (all men, n = 10), the difference was 6% (P = 0.90). D: initial MVC torque was negatively correlated with the relative difference in time-to-task failure between the control and stressor session (P < 0.001, r = −0.37, r2 = 0.13, n = 75). The regression line is solid with the 95% predicted interval indicated (dotted lines).
Men were stronger than women for both sessions (sex effect, P < 0.001). MVC torque was not different between the control and high-stressor sessions (session effect, P = 0.37) for men or women (session × sex, P > 0.05; Fig. 2B). We pooled data from our initial study (53) (n = 20) with the current data set (n = 55) and chose the 10 strongest and 10 weakest participants from the combined pool of 75 participants. Average MVC for the weakest group was 38.1 ± 4.0 Nm (all women) and for the strongest group was 103.5 ± 8.0 Nm (all men). Time to failure was briefer in the high-stressor session in the weak group compared with the strong group (session × strength, P = 0.03; Fig. 2C). The weakest participants had the greatest decrement in time to failure between the control and high-stressor sessions (23.9 ± 23.3%), whereas the strongest group had the least difference (6.1 ± 14.6%).
Whereas the relative reductions in MVC torque after the fatiguing contractions did not differ between sessions and sexes (P > 0.05), the initial MVC torque was negatively correlated with the relative difference in time-to-task failure between the control and high-stressor sessions (r = −0.37, r2 = 0.13, P < 0.001; Fig. 2D). In addition, linear regression analysis indicated that the initial MVC predicted the change in time to failure (r = −0.34, P = 0.01). When performing the correlation separately for men and women, the relationship was significant for men (r = −0.42, P = 0.03) but not women (r = −0.22, P = 0.18). Time to failure, maximal strength, and reduction in strength for the men and women in study one, who participated in cortical stimulation (n = 28), are described in Table 2.
Table 2.
Time to failure, maximal voluntary contraction (MVC), and reduction in strength (between start and end of fatiguing contraction) for control and high-stressor sessions (n = 28) and for control and low-stressor sessions (n = 23)
| Variable | Control | High Stressor | P (Session Effect) |
|---|---|---|---|
| (n = 28) | |||
| Time to failure, min | 11.0 ± 5.4 | 9.0 ± 3.4 | P = 0.002 |
| Maximal strength (MVC, Nm) | 61.8 ± 23.7 | 61.5 ± 23.5 | P = 0.37 |
| Reduction in strength, % | 42.8 ± 9.8 | 40.4 ± 11.7 | P = 0.33 |
| Control | Low-Stressor | ||
| (n = 23) | |||
| Time to failure, min | 10.7 ± 5.9 | 10.1 ± 6.0 | P = 0.32 |
| Maximal strength (MVC, Nm) | 66.5 ± 23.6 | 66.7 ± 22.2 | P = 0.83 |
| Reduction in strength, % | 41.8 ± 10.4 | 41.4 ± 10.9 | P = 0.82 |
Voluntary Activation
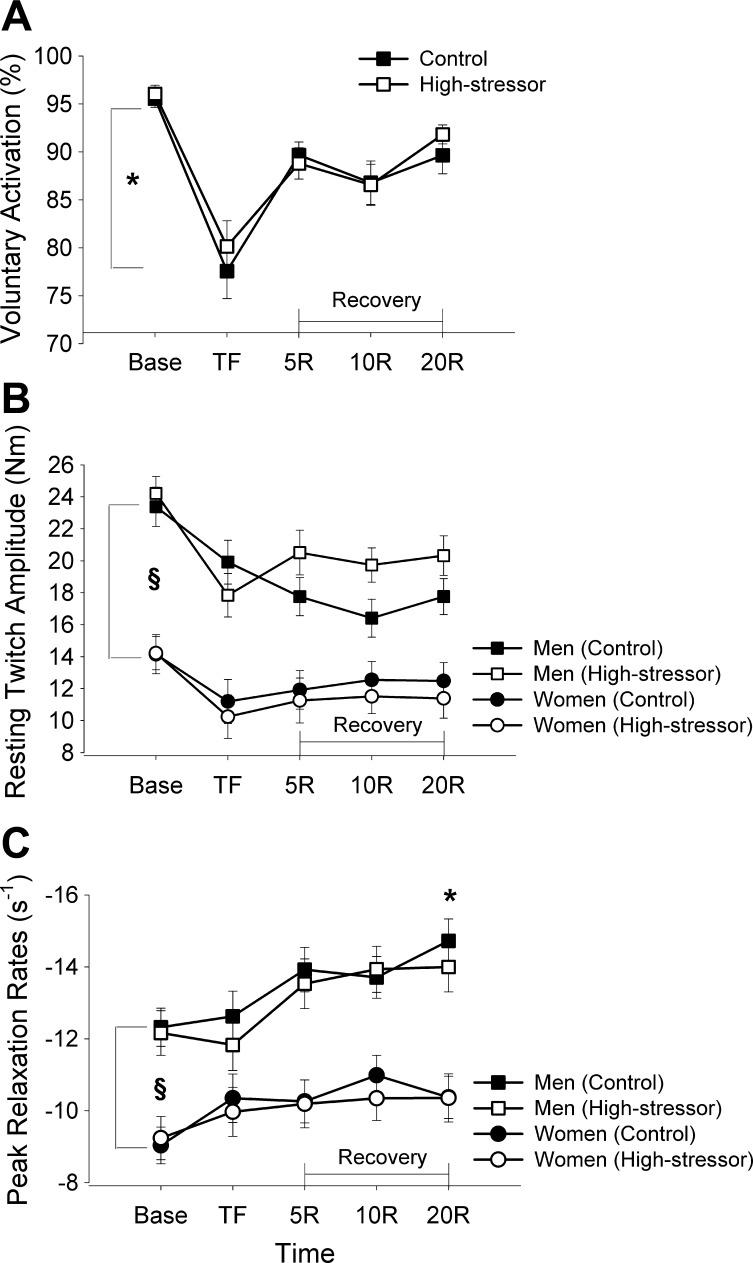
Voluntary activation was similar at baseline for the high-stressor and the control sessions (session effect at baseline, P = 0.48). The reduction in voluntary activation was similar for both sessions (session × fatigue, P = 0.54; Fig. 3A) for men and women after the fatiguing contraction (session × fatigue × sex, P = 0.46). The difference between the control and high-stressor session in time to failure was not associated with the difference in voluntary activation between sessions (P = 0.71). Voluntary activation increased within 5 min of recovery but was not fully recovered to baseline values within 20 min (fatigue effect during recovery, P < 0.001). Recovery was similar for the control and high-stressor sessions for men and women (sex effect, P = 0.42), and there were no interactions of time, session, and sex (P > 0.05).
Fig. 3.
Voluntary activation (A), estimated resting twitch (eRT; B), and peak relaxation rates (C) for the biceps brachii of the control and high-stressor sessions. Values are represented as the mean ± SE. Base indicates baseline measures before the fatiguing contraction; TF indicates measures recorded immediately upon task failure. Voluntary activation was similar for the control and high-stressor sessions before, immediately after the fatiguing contraction, and throughout recovery (P > 0.05). Voluntary activation was significantly different from baseline (*P < 0.05). eRT and the peak relaxation rates were greater and faster (respectively) for men than women (§P < 0.05) but were not affected by the high-stressor task (P > 0.05).
Contractile Properties
Estimated resting twitch.
eRT was similar at baseline for the control and high-stressor sessions (P > 0.05). Men had greater resting-twitch amplitudes than women at baseline, after the fatiguing contraction, and throughout recovery for all three sessions (sex effect, P < 0.001). The amplitude of the twitch decreased after the fatiguing contraction, similarly for the control and high-stressor sessions (session × fatigue, P = 0.12) and for men and women (fatigue × sex, P = 0.18; Fig. 3B). The eRT did not return to baseline levels at 20 min of recovery (fatigue effect at 20 min recovery, P < 0.01).
Peak relaxation rates.
The peak rate of relaxation was similar at baseline between the control and high-stressor sessions (session effect at baseline, P = 0.92). Men had faster peak relaxation rates than women before and after the fatiguing contraction (sex effect, P = 0.004). Peak rates of relaxation were not different after the fatiguing contraction (fatigue effect, P = 0.33) for either session (session × fatigue, P = 0.13) for men and women (session × fatigue × sex, P = 0.88). Peak rates of relaxation increased after the fatiguing contraction throughout recovery, up to 20 min (fatigue effect during recovery, P = 0.005; Fig. 3C), for both men and women (fatigue × sex, P = 0.09).
Baseline peak relaxation rates were correlated with the time to failure for the control session (r = 0.46, r2 = 0.21, P = 0.02) so that individuals with a faster peak rate of relaxation had a briefer time-to-task failure. The association was not significant for the high-stressor session (r = 0.27, r2 = 0.07, P = 0.16). Furthermore, the change in time to failure when exposed to the high stressor was not correlated with baseline peak rates of relaxation (P = 0.25) nor with the change in peak relaxation rate from the control session to the high-stressor session (P = 0.72).
Motor-Evoked Potentials
M-wave area of the biceps brachii elicited during MVCs did not change from baseline, throughout the fatiguing contraction, or after the fatiguing contraction (fatigue effect, P > 0.05) or during recovery (fatigue effect during recovery, P = 0.44). M-wave area was similar for the control and high-stressor sessions (P > 0.05). Hence, any change in MEP size was not due to changes in the size of the M wave. There was no difference in M-wave area between men and women (P > 0.05).
MEP area of biceps brachii elicited during MVCs and the 20% contraction increased (fatigue effect, P < 0.001) similarly in the high-stressor and control sessions from baseline to after the fatiguing contraction (126 ± 44% for the control session and 131 ± 44% for the high-stressor session during the MVC, session × fatigue, P = 0.68) for both men and women (session × fatigue × sex, P = 0.15). There was no main effect of sex (P = 0.19). The MEP area had returned to levels similar to baseline within 5 min after the fatiguing contraction (time effect from baseline to 5-min recovery, P = 0.67) for both sessions with no interactions (P > 0.05).
Silent Period
Silent period during MVCs.
The silent-period duration elicited during the MVCs at baseline was longer in the high-stressor session compared with the control session (P < 0.05). The silent-period duration increased from baseline to task failure (time effect after fatigue, P = 0.001) more so during the control session (194 ± 69 ms to 241 ± 110 ms) compared with the high-stressor session (228 ± 106 ms to 234 ± 102 ms, session × fatigue, P = 0.02), because of the longer-duration silent period at baseline during the high-stressor task. During recovery, the silent period remained elevated for the high-stressor session relative to control (quadratic interaction of time × session, P = 0.04).
Silent period during 20% fatiguing contraction.
The duration of the silent period was similar for the control and high-stressor sessions at the start of the fatiguing contraction (session effect, P = 0.37) and did not increase during the 20% fatiguing contraction for the control (160 ± 39 ms to 172 ± 59 ms) or the high-stressor session (168 ± 51 ms to 173 ± 59 ms, session × fatigue, P = 0.56) with no overall session effect (P = 0.59) for either men or women (session × sex interaction, P = 0.67).
Cardiovascular Responses
Cardiovascular responses to high stressor before the fatiguing contraction.
Heart rates were similar at baseline for both the control and high-stressor sessions (session effect, P = 0.17) with no effect of sex (P = 0.99). Heart rate was greater during the 4 min of mental math (high-stressor session) compared with the quiet sitting (control session; 81.9 ± 2.7 vs. 76.7 ± 2.5 beats/min, respectively; session effect, P = 0.04). There was no effect of sex (P = 0.59) and no interactions (P > 0.05). MAP was similar at baseline across sessions (P = 0.17) for both men and women (session × sex, P = 0.79) and increased within 30 s into the mental-math task (high-stressor session) compared with quiet sitting (control session; session × time, P = 0.002). MAP remained elevated during the 4 min of mental math compared with the quiet sitting (101.3 ± 4.8 vs. 83.9 ± 0.8 mmHg, respectively; session effect, P < 0.001). MAP response was greater for the men compared with the women during the high-stressor session (session × sex, P = 0.02).
Cardiovascular response during the fatiguing contraction.
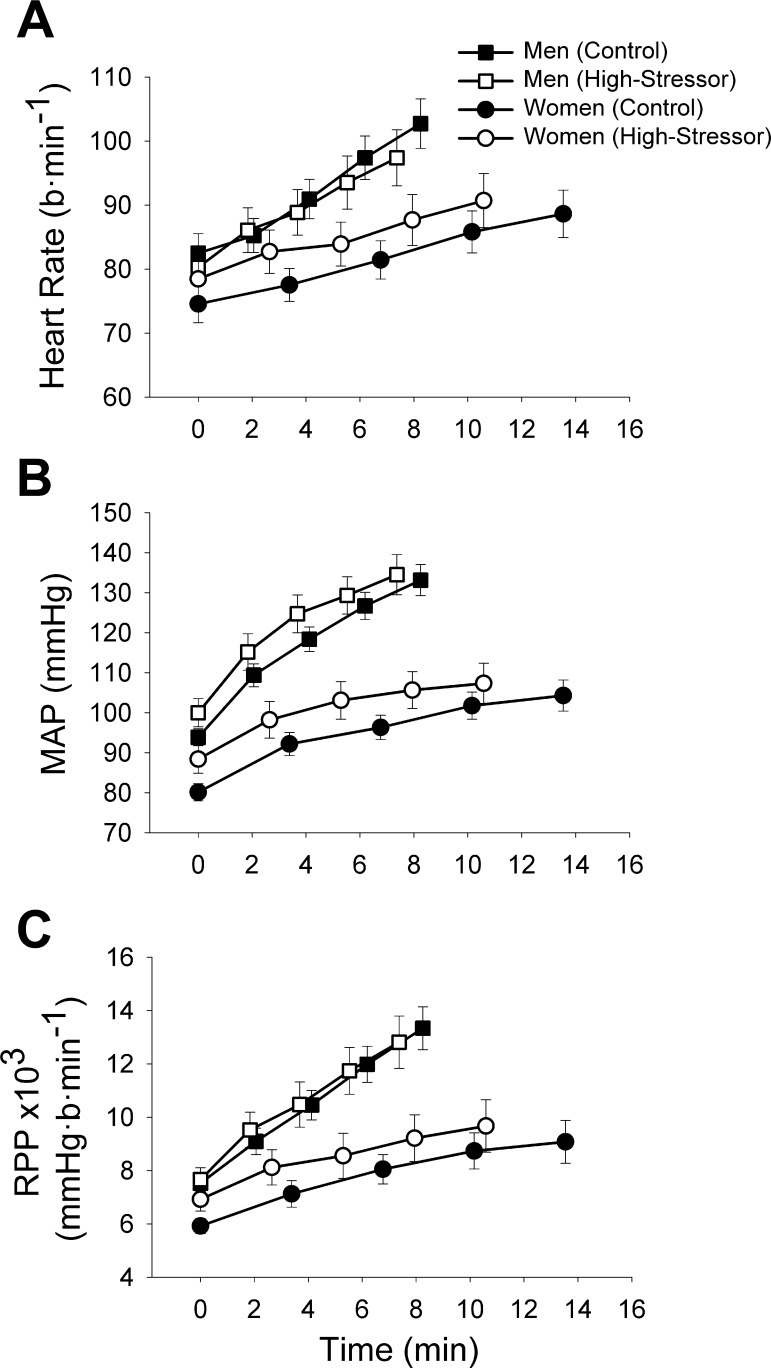
Heart rate increased from the start of the fatiguing contraction to task failure (time effect, P < 0.001), similarly for the control and high-stressor sessions (session × time, P = 0.09; Fig. 4A) for men and women (session × sex × time, P = 0.91). The change in heart rate from the start of the contraction to task failure was not associated with the stressor-induced reduction in time to failure (P > 0.05).
Fig. 4.
The heart rate (A), mean arterial pressure (MAP; B), and rate-pressure product (RPP; C) during the fatiguing contraction for the control and high-stressor sessions. The values are presented as mean ± SE at 25% increments of the time-to-task failure for men (squares) and women (circles). Averages of 15-s intervals were used for the MAP and heart rate. Heart rate, MAP, and the RPP increased throughout the fatiguing contraction (P < 0.05) for the high-stressor and the control sessions similarly (P > 0.05).
MAP increased throughout the fatiguing contraction from the start of the fatiguing contraction to task failure (time effect, P < 0.001), similarly for the high stressor and the control sessions (session × time, P = 0.14) for both men and women (session × sex, P = 0.78). MAP was greater for men compared with women (sex effect, P < 0.001), and men had greater rates of increase throughout the fatiguing contraction for both the control and stressor sessions (time × sex, P < 0.001; Fig. 4B). The change in MAP from the start of the contraction to task failure was not associated with the stressor-induced reduction in time to failure (P > 0.05).
Rate-pressure product.
To understand the cardiac workload during the fatiguing contraction, rate-pressure product was quantified (heart rate multiplied by MAP), as we have done before (53). The rate-pressure product increased (time effect, P < 0.001) from the start of the task to task failure for the high-stressor and the control sessions similarly (session × time, P = 0.17) for men and women for both sessions (session × time × sex, P = 0.87; Fig. 4C).
Psychological Levels of Stress
STAI (state) increased from baseline to after the high-stressor task (30.3 ± 7.3 to 39.6 ± 10.2) compared with the control session (33.2 ± 10.4 to 37.0 ± 11.2, session × time, P = 0.04) with no overall session effect (P = 0.90). STAI was similar for both men and women (sex effect, P = 0.53) with no sex differences across time (time × sex, P = 0.69).
Perceived anxiety was also measured with the VAS throughout the session. Anxiety levels were greater for women than men at baseline for the high-stressor session (1.5 ± 1.4 vs. 0.64 ± 0.35, respectively; P = 0.04) but were similar for the control session (1.5 ± 1.3 vs. 0.94 ± 0.65, respectively; P = 0.14). Perceived anxiety increased after the high-stressor task (106 ± 108 vs. −13 ± 38%, session × time after stress, P < 0.001) and after the fatiguing contraction (180 ± 192 vs. 49 ± 79%, session × fatigue, P = 0.03), similarly for men and women, compared with quiet sitting during the control session (session × fatigue × sex, P = 0.23). Women had elevated levels of anxiety at baseline and after the high-stressor task and quiet sitting (control session; sex effect, P = 0.04). Hence, from baseline, men had a 40% increase, and women had a 28% increase in anxiety after exposure to the cognitive stressor. Anxiety (VAS) returned to baseline levels during recovery (time effect during recovery, P < 0.001), similarly for the men and women (sex effect, P > 0.05).
Study Two: Low Stressor vs. Control
Time to failure, maximal strength, and reduction in strength were similar between the control and low-stressor sessions (P > 0.05; Table 2). Voluntary activation was reduced after the fatiguing contraction, similarly for men and women for control (96 ± 4% to 80 ± 13%) and low-stressor sessions (96 ± 4% to 81 ± 13%, P > 0.05).
The eRT was reduced similarly for the control (19.6 ± 6.7 Nm to 16.3 ± 6.8 Nm) and low-stressor sessions (18.9 ± 6.8 Nm to 16.0 ± 5.8 Nm, P = 0.001), with a main effect of sex (P < 0.001), because twitch amplitudes were lower for women compared with men. Peak relaxation rates were faster after the fatiguing contraction for both the control (−11.2 ± 2.3 s−1 to −11.8 ± 2.7 s−1) and low-stressor sessions (−11.2 ± 2.2 s−1 to −12.2 ± 4.0 s−1, P > 0.05), with a main effect of sex (P < 0.001), because women had slower relaxation rates than men for both sessions.
M wave was similar for control and low-stressor sessions throughout the fatiguing contraction (P > 0.05). The biceps brachii MEP area increased from baseline to after the fatiguing contraction (fatigue effect, P < 0.001) for both men and women (P > 0.05), similarly for the control and low-stressor sessions (P > 0.05). The silent period was elevated at baseline in the low-stressor session (227 ± 80 ms) compared with the control session (206 ± 70 ms, P < 0.05) but did not increase further after the fatiguing contraction. The silent-period duration during the 20% fatiguing contraction was not influenced by the low-stressor task (P > 0.05).
There was no effect of the low-stressor task on heart rate during the fatiguing contraction compared with the control session (session effect, P = 0.53). Men had greater increases in heart rate during the fatiguing contraction (time × sex, P = 0.007) and greater heart rates for both control and low-stressor sessions overall (sex effect, P = 0.01). MAP did not differ between the control and low-stressor sessions (session effect, P = 0.32), but MAP was greater for men than women for both sessions (sex effect, P = 0.004).
STAI (state) increased from baseline to after the low-stressor task and quiet sitting (control session; time effect, P = 0.006). There was no sex difference (P = 0.66) in STAI and no interactions (P > 0.05). Anxiety (VAS) increased similarly after the low-stressor task and fatiguing contraction for the low-stressor and control sessions (P < 0.05) similarly (P > 0.05).
DISCUSSION
These studies found that weaker individuals exhibit greater fatigability during low-force contractions with the elbow flexor muscles when a difficult cognitive stressor was imposed. A strength-mediated mechanism rather than the sex of the subject was the dominant factor for the greater fatigability with the high cognitive stressor. The relative decline in strength was similar across sessions in both men and women despite the decrease in time to failure during the high-stressor session, demonstrating that the reduction in time to failure with the high stressor was not due to premature termination of the sustained contraction. Corticospinal and muscular mechanisms contributed similarly to the decreased MVC after the high-stressor and control sessions. Furthermore, the reduction in time to failure with the difficult mental-math task (high stressor) was not due to distraction, because a low-stressor task (simple mental math) did not alter the time to failure. These studies indicate that weaker individuals—both men and women—are susceptible to high stressor-induced fatigability during low-intensity fatiguing contractions that are often performed during ergonomic tasks, and this is due to a strength-related mechanism.
Strength Is a Primary Predictor of Greater Fatigability with the Imposed High Cognitive Stressor
In this study, men were stronger than women, but the time to failure was reduced more for the women (19.4% reduction) than the men (9.5%), similar to what we had found before (53). Our data, however, indicate that initial strength, rather than sex alone, was the primary predictor of the reduced time-to-task failure when a high-stressor task was imposed, because when the time to failure covaried for initial strength, the sex difference disappeared. In addition, initial strength was the only predictor of the difference between the control and high-stressor session in time to failure. Only in the men, whose strength levels varied considerably among individuals, was there an association between initial strength and difference in time to failure. This association could be driven by the greater range of strength among men (56–117 Nm, i.e., 61 Nm) compared with women (33–55 Nm, i.e., 22 Nm). To demonstrate further the importance of initial strength to the time-to-failure difference when exposed to the high stressor, we separated the subjects by their strength, showing that the weakest participants (all women) had the greatest decrement in time to failure (24%), and the strongest individuals (men) had the least difference in time to failure (6%). Stronger men, therefore, are protected against the greater fatigue when exposed to a high stressor.
An important finding was that the strength differences among participants before the fatiguing contraction were not due to a difference in voluntary activation among the weaker and stronger participants. The strength-related mechanism, therefore, could be mediated by an increase in sympathetic activation that affects fatigability during the submaximal contraction: 1) fiber types and 2) stressor-induced changes in blood perfusion that may have greater effects in weaker subjects. An increase in psychosocial stress (arousal) can result in activation of the sympathetic nervous system with subsequent release of neuromodulators and hormones throughout the central and peripheral nervous system (29), causing elevations of heart rate, blood pressure, and cortisol levels (53). This study indicated that perceived anxiety (VAS and STAI-state), heart rate, and blood pressure were all elevated during the high-stressor task relative to quiet sitting (control session), demonstrating that sympathetic activation was likely elevated.
Sympathetic activation can modulate fiber-type activity. The second study investigated whether the peak relaxation rates, which may reflect fiber-type proportional area, mediated the changes in time to failure with the high stressor. Contractile force can be potentiated in type II fast-twitch fibers and relaxation rates reduced in type I slow-twitch fibers in animal and human muscle when sympathetic activation is high (1, 40), predisposing muscle with more type I fibers to greater fatigability with increased sympathetic activation. We found that men exhibited greater eRT amplitudes and faster peak relaxation rates than women, before and after the fatiguing contraction. Faster relaxation of evoked contractions is associated with greater proportional area of type II fibers and faster calcium uptake into the sarcoplasmic reticulum in human muscle (14). Furthermore, men have a larger cross-sectional area of type II muscle fibers in biceps compared with women (5, 32). Any stressor-induced increase in sympathetic activation that may have occurred during the fatiguing contractions, however, did not alter the peak relaxation rates of the men or women (see Fig. 3C). Accordingly, the peak relaxation rates were associated with time to failure for the control session, but there was no association between these two variables for the high-stressor session. Furthermore, there was no association between initial peak rates of relaxation or the change in peak rates of relaxation with fatigue and the difference in time-to-task failure between sessions. Thus the change in time to failure with the high stressor was likely not due to a differential activation of types I and II fibers in participants of different strength. Although peak relaxation rates increased for both men and women throughout recovery for both sessions, as seen before (19, 24), this was likely due to increased muscle temperature during the fatiguing contraction.
Alternatively, the strength-related reduction in time to failure with the stressor may have been mediated by perfusion differences between strong and weaker subjects that in turn, accelerated both central and muscular mechanisms. Women, who were weaker in this study, typically have lower blood pressure, a blunted vasoconstrictor response to α-adrenergic stimulation in the brachial artery, and lower muscle sympathetic nerve activity (MSNA) (6, 20). During a submaximal contraction in the absence of psychosocial stress (control), perfusion of the muscle and the time-to-task failure can depend on muscle size and absolute strength. Weaker individuals, for example, can have greater perfusion during a submaximal contraction compared with stronger individuals (13) and therefore, a longer time-to-task failure under control conditions. Accordingly, women who are usually weaker than men have a slower rate of rise in MAP during a submaximal contraction, which likely reflects less of a buildup of metabolites (less metaboreflex) in women than men (11). Increased arousal, however, can increase sympathetic activation, possibly altering the balance of vasoconstriction and vasodilation (46). This balance in perfusion potentially differs in response to arousal between strong and weak subjects, because weaker subjects have greater perfusion at baseline. Indirect indicators of sympathetic activation (MAP and heart rate) were elevated during the stressor session compared with the control session. The mechanism for the greater fatigability, therefore, may be due to the stressor-induced sympathetic activation and increased vasoconstriction to the weaker muscles when exposed to the high stressor. Although we may have expected the difference in time to failure to be associated with changes in the heart rate or MAP, the autonomic nervous system is highly efficient in regulating cardiovascular responses to stress and exercise in healthy individuals (21, 26). Furthermore, as the increase in sympathetic activity results in elevations in heart rate and MAP, there is evidence of a dissociation between MAP and MSNA during stress (3). The increase in blood pressure and heart rate with exposure to the high stressor during the fatiguing contraction also resulted in increased cardiac load, which was indicated by the rate-pressure product. The rate-pressure product was greater for the men than the women for both the control and high-stressor session, but both sexes had increased cardiac load with the stressor. These findings can have significant clinical implications for both men and women who perform low-force, sustained contractions for prolonged periods under stressful work conditions.
Voluntary Activation and Corticomotor Excitability
Voluntary activation measured with superimposition of a stimulus at the cortex during a maximal effort reflects the ability of the motor cortex to drive the distal nervous system and muscle maximally. Any deficit in voluntary activation indicates that the recruitment of motor units and/or discharge frequencies were not optimal during the maximal effort (7). Before the fatiguing contraction, voluntary activation for men and women was near maximal (96% for both sessions) and was similar for the sexes and across the sessions. This is an important finding, because the time to failure and changes in time to failure across sessions were not related to differences in voluntary activation before fatigue. Thus the subjects were activating a similar portion of their muscles during each of the fatiguing contractions across the sessions.
Voluntary activation elicited with TMS, however, was reduced to ∼79% for both men and women and after each low-intensity fatiguing contraction as seen before (19, 42, 54). The fall in voluntary activation was similar at task failure, but this was accelerated during the high-stressor sessions for both men and women, because the time to failure was briefer during the high-stressor sessions. Voluntary activation also remained depressed, even 20 min after termination of the sustained contraction, and was similar at 20-min recovery for both the control and high-stressor sessions. Certainly, the increase in physiological stress response can be centrally mediated and different for men and women (50). Here, we showed that increased arousal during a low-force task appeared to accelerate the loss of neural drive to the motor cortex, assessed during maximal efforts immediately after a low-force fatiguing contraction or during recovery.
Corticomotor excitability was quantified with the MEP during voluntary contractions. The MEP is the EMG response elicited from the TMS and represents the net excitation in the corticomotor tract (45). Biceps brachii and brachioradialis MEP area increased during and immediately after the fatiguing contraction, indicating an increase in the excitability within the central nervous system. This increase was similar for both men and women and across sessions. The compound muscle-action potential (M wave) was similar throughout the fatiguing contraction and across sessions. Consequently, propagation of the action potential at the neuromuscular junction (43) did not change with muscle fatigability or arousal in men and women. This is important, because changes observed in the MEP can be due to mechanisms at the neuromuscular junction, which would be reflected in an altered M wave (43). The increase in MEP in these findings were due to an increase in cortical excitability or motoneuron excitability, because both the MEP and responses to stimulation of descending tracts at the cervicomedullary level increase with fatigue during submaximal contractions of the elbow flexor muscles (25). Nevertheless, the increase in corticomotor excitability, measured during voluntary contractions, was similar across sessions.
The silent period with fatigue has traditionally been thought to reflect inhibition in the spinal cord (early phase) (15) and also cortical inhibition from interneurons (mainly GABAB mediated) in the latter phase (16). More recent studies demonstrate that the increase in inhibition may be mediated primarily by spinal mechanisms during maximal (31) and low-intensity submaximal contractions (30). The silent-period duration, elicited during the MVC, was greater at baseline for the cognitive sessions compared with control, indicating greater inhibition at the start of the stressor session. Anticipation may have played some role in the longer silent period, although baseline measurements of the silent period occurred before the participants had knowledge of which session they were attending (at least for their first session). Thus some participants may have been aware that a stressor session was to occur before the measurements. Despite reaching similar silent-period durations at task failure, the silent period remained elevated in early and mid-recovery for the high-stressor session relative to the control session. Difficult mental activity or anticipation of the task may have resulted in some corticomotor inhibition. The longer silent-period duration at baseline and during recovery for the cognitive sessions is not understood and deserves exploration in future studies. The silent-period duration during the low-intensity fatiguing contraction, however, did not change from the start of the task to task failure.
A similar decrease in the eRT amplitude after the briefer fatiguing contraction in the high-stressor session compared with the control session indicated that the decreased force capacity of the muscle was accelerated during the high-stressor session. Thus taken together with the similar reductions in voluntary activation for the high-stressor and control sessions, both neural and muscular mechanisms of fatigability contributed to the decline in MVC force and were accelerated with imposition of the high stressor, because the time to failure was briefer than the control session.
Conclusions
These studies indicate that in young adults, the increased fatigability that occurs with exposure to a high cognitive stressor was associated with initial strength and not restricted to women but involved both men and women, who exerted lower levels of absolute target force during the submaximal, sustained fatiguing contraction. Furthermore, the greater fatigability was not due to a lack of voluntary activation in initial strength. Although there were similar reductions in voluntary activation and peak-twitch amplitudes for the high-stressor and control sessions at task failure, both mechanisms were accelerated when the high stressor was imposed during the fatiguing contraction and contributed to a similar decrease in MVC force at task failure in the control and high-stressor sessions. Although these mechanisms were accelerated during the high-stressor session, they were likely secondary to a strength-mediated mechanism for the earlier task failure. Mental distraction or a low-level stressor did not alter fatigability in young men and women. These studies suggest that weaker individuals, who perform low-intensity, sustained contractions during ergonomic tasks with the upper limb, are predisposed to greater fatigability and accelerated declines in voluntary activation and peak relaxation rates when a challenging cognitive stressor is imposed.
GRANTS
Support for this research was provided by grants #3 T42 OH008672 from the National Institute for Occupational Safety and Health and 1R15AG039697 from the National Institute on Aging to S. K. Hunter.
DISCLOSURES
There are no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Author contributions: M.L.K-R., H.M.P., T.Y., B.S-D., and S.K.H. conception and design of research; M.L.K-R., H.M.P., J.P., T.Y., and B.S-D. performed experiments; M.L.K-R., H.M.P., J.P., T.Y., B.S-D., and K.A.N. analyzed data; M.L.K-R., H.M.P., K.A.N., and S.K.H. interpreted results of experiments; M.L.K-R. and H.M.P. prepared figures; M.L.K-R. drafted manuscript; M.L.K-R. and S.K.H. edited and revised manuscript; M.L.K-R., H.M.P., J.P., T.Y., B.S-D., K.A.N., and S.K.H. approved final version of manuscript.
REFERENCES
- 1.Bowman WC, Goldberg AA, Raper C. A comparison between the effects of sympathomimetic amines on fast- and slow-contracting mammalian muscles. Br J Pharmacol Chemother 19: 464–484, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray SR, Graham JD, Martin Ginis KA, Hicks AL. Cognitive task performance causes impaired maximum force production in human hand flexor muscles. Biol Psychol 89: 195–200, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Carter JR, Ray CA. Sympathetic neural responses to mental stress: responders, nonresponders and sex differences. Am J Physiol Heart Circ Physiol 296: H847–H853, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christou EA, Jakobi JM, Critchlow A, Fleshner M, Enoka RM. The 1- to 2-Hz oscillations in muscle force are exacerbated by stress, especially in older adults. J Appl Physiol 97: 225–235, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Edström L, Nyström B. Histochemical types and sizes of fibres in normal human muscles. A biopsy study. Acta Neurol Scand 45: 257–269, 1969 [DOI] [PubMed] [Google Scholar]
- 6.Ettinger SM, Silber DH, Collins BG, Gray KS, Sutliff G, Whisler SK, McClain JM, Smith MB, Yang QX, Sinoway LI. Influences of gender on sympathetic nerve responses to static exercise. J Appl Physiol 80: 245–251, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Herd JA. Cardiovascular response to stress. Physiol Rev 71: 305–330, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10: 361–374, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Hess CW, Mills KR, Murray NM. Magnetic stimulation of the human brain: facilitation of motor responses by voluntary contraction of ipsilateral and contralateral muscles with additional observations on an amputee. Neurosci Lett 71: 235–240, 1986 [DOI] [PubMed] [Google Scholar]
- 11.Hunter SK. Sex differences in human fatigability: mechanisms and insight to physiological responses. Acta Phys (Oxf). 10.1111/PMID:24433272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter SK, Butler JE, Todd G, Gandevia SC, Taylor JL. Supraspinal fatigue does not explain the sex difference in muscle fatigue of maximal contractions. J Appl Physiol 101: 1036–1044, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Hunter SK, Schletty JM, Schlachter KM, Griffith EE, Polichnowski AJ, Ng AV. Active hyperemia and vascular conductance differ between men and women for an isometric fatiguing contraction. J Appl Physiol 101: 140–150, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Hunter SK, Thompson MW, Ruell PA, Harmer AR, Thom JM, Gwinn TH, Adams RD. Human skeletal sarcoplasmic reticulum Ca2+ uptake and muscle function with aging and strength training. J Appl Physiol 86: 1858–1865, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol 466: 521–534, 1993 [PMC free article] [PubMed] [Google Scholar]
- 16.Inghilleri M, Berardelli A, Marchetti P, Manfredi M. Effects of diazepam, baclofen and thiopental on the silent period evoked by transcranial magnetic stimulation in humans. Exp Brain Res 109: 467–472, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Johansen-Berg H, Matthews PM. Attention to movement modulates activity in sensori-motor areas, including primary motor cortex. Exp Brain Res 142: 13–24, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 31: 151–178, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Keller ML, Pruse J, Yoon T, Schlinder-Delap B, Harkins A, Hunter SK. Supraspinal fatigue is similar in men and women for a low-force fatiguing contraction. Med Sci Sports Exerc 43: 1873–1883, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol 36: 1233–1238, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Korthuis R. Skeletal Muscle Circulation. San Rafel, CA: Morgan & Claypool Life Sciences, 2011 [PubMed] [Google Scholar]
- 22.Krantz G, Forsman M, Lundberg U. Consistency in physiological stress responses and electromyographic activity during induced stress exposure in women and men. Integr Physiol Behav Sci 39: 105–118, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Kriska AM, Knowler WC, LaPorte RE, Drash AL, Wing RR, Blair SN, Bennett PH, Kuller LH. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care 13: 401–411, 1990 [DOI] [PubMed] [Google Scholar]
- 24.Kuchinad RA, Ivanova TD, Garland SJ. Modulation of motor unit discharge rate and H-reflex amplitude during submaximal fatigue of the human soleus muscle. Exp Brain Res 158: 345–355, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Levenez M, Garland SJ, Klass M, Duchateau J. Cortical and spinal modulation of antagonist coactivation during a submaximal fatiguing contraction in humans. J Neurophysiol 99: 554–563, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Littell EH. Neural regulation of cardiovascular response to exercise: part II. Phys Ther 61: 1411–1418, 1981 [DOI] [PubMed] [Google Scholar]
- 27.Lorist MM, Kernell D, Meijman TF, Zijdewind I. Motor fatigue and cognitive task performance in humans. J Physiol 545: 313–319, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn 65: 209–237, 2007 [DOI] [PubMed] [Google Scholar]
- 29.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res 886: 172–189, 2000 [DOI] [PubMed] [Google Scholar]
- 30.McNeil CJ, Giesebrecht S, Gandevia SC, Taylor JL. Behaviour of the motoneurone pool in a fatiguing submaximal contraction. J Physiol 589: 3533–3544, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNeil CJ, Martin PG, Gandevia SC, Taylor JL. The response to paired motor cortical stimuli is abolished at a spinal level during human muscle fatigue. J Physiol 587: 5601–5612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller AE, MacDougall JD, Tarnopolsky MA, Sale DG. Gender differences in strength and muscle fiber characteristics. Eur J Appl Physiol Occup Physiol 66: 254–262, 1993 [DOI] [PubMed] [Google Scholar]
- 33.Molenaar JP, McNeil CJ, Bredius MS, Gandevia SC. Effects of aging and sex on voluntary activation and peak relaxation rate of human elbow flexors studied with motor cortical stimulation. Age (Dordr) 35: 1327–1337, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mottram CJ, Hunter SK, Rochette L, Anderson MK, Enoka RM. Time to task failure varies with the gain of the feedback signal for women, but not for men. Exp Brain Res 174: 575–587, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Noteboom JT, Barnholt KR, Enoka RM. Activation of the arousal response and impairment of performance increase with anxiety and stressor intensity. J Appl Physiol 91: 2093–2101, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Noteboom JT, Fleshner M, Enoka RM. Activation of the arousal response can impair performance on a simple motor task. J Appl Physiol 91: 821–831, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 9: 97–113, 1971 [DOI] [PubMed] [Google Scholar]
- 38.Passatore M, Roatta S. Influence of sympathetic nervous system on sensorimotor function: whiplash associated disorders (WAD) as a model. Eur J Appl Physiol 98: 423–449, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Porter MM, Stuart S, Boij M, Lexell J. Capillary supply of the tibialis anterior muscle in young, healthy, and moderately active men and women. J Appl Physiol 92: 1451–1457, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Roatta S, Arendt-Nielsen L, Farina D. Sympathetic-induced changes in discharge rate and spike-triggered average twitch torque of low-threshold motor units in humans. J Physiol 586: 5561–5574, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simoneau JA, Bouchard C. Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am J Physiol Endocrinol Metab 257: E567–E572, 1989 [DOI] [PubMed] [Google Scholar]
- 42.Smith JL, Martin PG, Gandevia SC, Taylor JL. Sustained contraction at very low forces produces prominent supraspinal fatigue in human elbow flexor muscles. J Appl Physiol 103: 560–568, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Taylor JL, Butler JE, Gandevia SC. Altered responses of human elbow flexors to peripheral-nerve and cortical stimulation during a sustained maximal voluntary contraction. Exp Brain Res 127: 108–115, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Taylor JL, Gandevia SC. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J Appl Physiol 104: 542–550, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Taylor JL, Gandevia SC. Transcranial magnetic stimulation and human muscle fatigue. Muscle Nerve 24: 18–29, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Thomas GD, Segal SS. Neural control of muscle blood flow during exercise. J Appl Physiol 97: 731–738, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Todd G, Taylor JL, Butler JE, Martin PG, Gorman RB, Gandevia SC. Use of motor cortex stimulation to measure simultaneously the changes in dynamic muscle properties and voluntary activation in human muscles. J Appl Physiol 102: 1756–1766, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Todd G, Taylor JL, Gandevia SC. Measurement of voluntary activation of fresh and fatigued human muscles using transcranial magnetic stimulation. J Physiol 551: 661–671, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Todd G, Taylor JL, Gandevia SC. Reproducible measurement of voluntary activation of human elbow flexors with motor cortical stimulation. J Appl Physiol 97: 236–242, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol 583: 194–203, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wasmund WL, Westerholm EC, Watenpaugh DE, Wasmund SL, Smith ML. Interactive effects of mental and physical stress on cardiovascular control. J Appl Physiol 92: 1828–1834, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Wust RC, Morse CI, de Haan A, Jones DA, Degens H. Sex differences in contractile properties and fatigue resistance of human skeletal muscle. Exp Physiol 93: 843–850, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Yoon T, Keller ML, De-Lap BS, Harkins A, Lepers R, Hunter SK. Sex differences in response to cognitive stress during a fatiguing contraction. J Appl Physiol 107: 1486–1496, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoon T, Schlinder-Delap B, Keller ML, Hunter SK. Supraspinal fatigue impedes recovery from a low-intensity sustained contraction in old adults. J Appl Physiol 112: 849–858, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]