Abstract
Acetazolamide (ACZ) prevents hypoxic pulmonary vasoconstriction (HPV) in isolated lungs, animals, and humans, but not by carbonic anhydrase (CA) inhibition. We studied administration routes in, and certain structural aspects of, ACZ critical to HPV inhibition. Analogs of ACZ during acute hypoxia were tested in unanesthetized dogs. Dogs breathed normoxic gas for 1 h (inspired O2 fraction = 0.21), followed by 10% O2 for 2 h (hypoxia) in these protocols: 1) controls; 2) ACZ intravenously (2 mg·kg−1·h−1); 3) ACZ orally (5 mg/kg, 12 and 1 h before the experiment); 4) inhaled ACZ (750 mg); 5) methazolamide (MTZ) intravenously (3 mg·kg−1·h−1); and 6) N-methyl-acetazolamide (NMA) intravenously (10 mg·kg−1·h−1). In controls, mean pulmonary arterial pressure (MPAP) increased 7 mmHg, and pulmonary vascular resistance (PVR) 224 dyn·s·cm−5 with hypoxia (P < 0.05). With intravenous and inhaled ACZ, MPAP and PVR did not change during hypoxia. With oral ACZ, HPV was only slightly suppressed; MPAP increased 5 mmHg and PVR by 178 dyn·s·cm−5 during hypoxia. With MTZ and NMA, the MPAP rise (4 ± 2 mmHg) was reduced, and PVR did not increase during hypoxia compared with normoxia (MTZ intravenous: 81 ± 77 and 68 ± 82 dyn·s·cm−5 with NMA intravenous). Inhaled ACZ prevents HPV, but not without causing systemic CA inhibition. NMA, a compound lacking CA inhibiting effects by methylation at the sulfonamide moiety, and MTZ, a CA-inhibiting analog methylated at the thiadiazole ring, are only slightly less effective than ACZ in reducing HPV.
Keywords: hypoxic pulmonary vasoconstriction, acetazolamide, N-methyl-acetazolamide, pulmonary arterial pressure, pulmonary vascular resistance
acetazolamide (acz) and other related carbonic anhydrase (CA) inhibitors have long been standard prophylaxis for acute mountain sickness (AMS), a syndrome of poor adaptation of the central nervous system to the hypoxia of high altitude (3, 12).1 Despite four decades of use in AMS, it is unknown whether these drugs might also protect against high-altitude pulmonary edema. This common and acute life threatening maladaptation arises from excessive hypoxic pulmonary vasoconstriction (HPV) and severe pulmonary hypertension, leading to a noncardiac form of hydrostatic interstitial and ultimately hemorrhagic noninflammatory alveolar edema (2, 14, 25). Of note, ACZ has been shown to reduce pulmonary arterial pressure (PAP) and vascular resistance (PVR) during acute alveolar hypoxia in isolated lungs (4), intact animals (8), and humans (10, 26). Our laboratory has also shown that ACZ prevents HPV in unanesthetized dogs in a dosage comparable to the use in humans at high altitude (9) and below the threshold of erythrocyte CA inhibition (15). The typical impact of renal CA inhibition [decrease in plasma bicarbonate (HCO3−) concentration, base excess, and lower systemic pH arising from increased urinary HCO3− excretion] was reduced, but still present, at this low dosage. This left selective pulmonary administration via inhalation as a possible route of administration to avoid the effects of systemic CA inhibition, but yet achieve HPV inhibition.
In addition, we also found a lack of effect on HPV with selective inhibition of extracellular [benzolamide (BZ)] and complete inhibition of extracellular and intracellular [ethoxzolamide (ETZ)] CA isoenzymes, suggesting that CA is not involved in the mediation of HPV (9). In line with these findings, Shimoda et al. (22) showed in rat pulmonary arterial smooth muscle cells (PASMC) that ACZ, but not BZ or ETZ, prevents the initiation of HPV by suppressing the increase in intracellular calcium concentration ([Ca2+]i) on exposure to hypoxia. They also found that N-methyl-acetazolamide (NMA), a molecule lacking CA inhibitory properties through a simple addition of a methyl group to the free amine group of the sulfonamide moiety of ACZ, also abolished the hypoxia-induced increase in [Ca2+]i, which initiates smooth muscle contraction and vasoconstriction. We hypothesize that such a compound may be effective against HPV in vivo without the systemic side effects of CA inhibition.
The aims of the present study were, therefore, twofold. First, we aimed to determine the most effective route of administration with the least possible systemic side effects for ACZ in the reduction of HPV. Hence, we studied the effects of inhaled ACZ on the pulmonary vascular pressure response during acute alveolar hypoxia compared with intravenous (iv) and orally administered ACZ. Second, by using two modified ACZ analogs [NMA and methazolamide (MTZ); Fig. 1] in a separate set of experiments, we have begun to explore what structural aspects of the parent molecule may be critical for its capacity to act on ACZ-sensitive cellular receptors or channels involved in hypoxia-mediated pulmonary vasoconstriction in vivo.
Fig. 1.
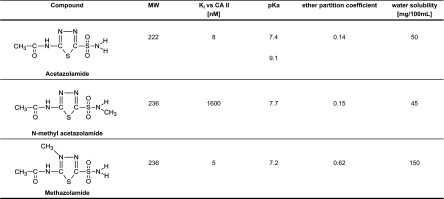
Molecular structure and properties of the carbonic anhydrase (CA)-inhibiting and -noninhibiting sulfonamides. MW, molecular weight; Ki vs. CA II, dissociation constant for inhibitor binding.
MATERIALS AND METHODS
General Procedures
A total of 51 experiments were performed in randomized order on purebred female Beagle dogs [body weight (BW): 14.1 ± 1.3 kg; n = 13] under permission of the Governmental Animal Protection Committee (AZ VB103-G 0084/04). All animals were obtained from the Central Animal Facilities of the Humboldt-University in Berlin.
After the animals were tested for their social behavior, tolerance to urinary bladder catheterization, intravascular cannulae, and acceptance of a specifically fitted face mask, the dogs were trained to lie unrestrained and quietly on their right side on a padded animal table for at least 4 h. Social behavior (less aggressiveness than males) and tolerance to bladder and vascular catheterization while fully awake were the main reasons to only study female animals.
The animals were housed under highly standardized conditions with access to daily outdoor exercise. They were provided a standardized diet (containing 91 ml water, 2.5 mmol sodium, and 3.5 mmol potassium; all values given per kilogram BW per day) 5 days before the experiment, as previously described (8). Health status, body temperature, and BW were checked daily. In addition, animals were monitored for changes in rectal temperature and for absence of menorrhea on a daily basis, and experiments were not performed before or during their menstrual cycle. Via puncture of a foreleg vein, 50 ml of the dog's own blood were collected and stored at 4°C (Biopack, Biotrans, Dreieich, Germany) 8 days before an experiment. This served to replace the blood withdrawn for analysis during the experiments.
After a maximum time of 1 yr in the animal facilities or following completion of the study, all dogs were adopted by local citizens. This policy is the reason why not all dogs were able to go through the entire protocols and explains the variation of group sizes, with an overlap of three dogs in the control and the ACZ iv group in both series.
Procedures During the Experiments
After recording BW and temperature, the animals were instrumented as published previously (9). Briefly, after local anesthesia, the urinary bladder was catheterized, an arterial line (20 G, no. 4235–8, Ohmeda, Erlangen, Germany) was inserted percutaneously and advanced via the femoral artery into the abdominal aorta, and a pulmonary artery catheter (5 F, no. 132F5, Baxter, Unterschleissheim, Germany) was inserted via the right external jugular vein. These catheters were used for continuous systemic and pulmonary blood pressure monitoring, intermittent cardiac output (CO) measurements, and blood sampling. The pressure transducers were adjusted to the level of the right atrium. A custom-made face mask was attached, equipped with a 2.5-cm inner diameter Y-piece to connect it to a ventilator set in continuous positive airway pressure mode (1 cmH2O) (Servo 900 C; Siemens-Elema, Erlangen, Germany). This permitted spontaneous breathing through a low-resistance circuit and measurement of ventilation. Thereafter, the conscious dogs were given 30 min to adjust to the experimental situation.
Two series of experiments were performed.
Series 1: Route of administration of ACZ on HPV.
The groups are as follows: 1) controls (n = 7); 2) ACZ iv (Diamox, Lederle, Wolfratshausen/Germany): a loading dose of 2 mg/kg, followed by a continuous infusion of 2 mg·kg −1·h−1 (n = 7); 3) ACZ orally [ACZ per os (po)]: 5 mg/kg ACZ orally 12 and 1 h before the experiments (n = 7); and 4) ACZ inhaled: 750 mg in 5 ml 0.9% NaCl over 15 min after the initial normoxic measurements (n = 7).
Series 2: Modification of the molecular structure of ACZ on HPV.
The groups are as follows: 1) controls (n = 9); 2) ACZ iv: 2 mg/kg loading dose, followed by a continuous infusion of 2 mg·kg −1·h−1 (n = 8); 3) MTZ iv (Sigma-Aldrich, Steinheim/Germany): 3 mg/kg loading dose, followed by a continuous infusion of 3 mg·kg−1·h−1 (n = 6); and 4) NMA iv (Prof. Ahamindra Jain, Department of Chemistry, University of California, Berkeley, California): 10 mg/kg loading dose, followed by a continuous infusion of 10 mg·kg−1·h−1 (n = 6).
The interval between experiments on the same dog was at least 14 days to permit complete washout of drugs, restoration of acid-base balance, and resolution of any hypoxic and diuretic effects.
In control experiments, dogs breathed ambient air (21% O2, 79% N2; normoxia) for the 1st h, followed by a gas mixture containing 10% O2 and 90% N2 for 2 h (hypoxia). This time course was the same for all CA inhibitor experiments, but the fraction of inspired O2 was reduced to 0.09–0.085 during the hypoxia period to match the arterial Po2 (PaO2) of the control dogs. This was necessary because of the ventilatory stimulus of the CA inhibitors to maintain an equal stimulus to HPV, as we showed in our earlier publication (8).
Heart rate (HR), mean arterial blood pressure (MAP), central venous pressure (CVP), mean PAP (MPAP), respiratory rate, tidal volume, and minute ventilation (V̇e) were measured continuously, and the data stored on a computer. Additional hemodynamic measurements [CO, pulmonary capillary wedge pressure, calculation of systemic vascular resistance (SVR), and PVR], as well as the withdrawal of blood samples for measurements of arterial and mixed-venous blood gases, actual HCO3−, base excess, plasma electrolytes, and hormones, were performed at hourly intervals, as previously published by our group (8, 9). For analysis of acid-base balance, the hydrogen ion concentrations corresponding to each individual arterial pH value were averaged in each group. Subsequently, the resulting mean H+ concentrations were converted into pH.
Urine flow and urinary concentrations of sodium, potassium, and creatinine were determined for the normoxia and hypoxia periods. To assess glomerular filtration rate (GFR), exogenous creatinine clearance (priming dose 1.4 g over 30 min before the start of experiments, maintenance infusion 4.7 mg/min) was calculated by the standard formula. Urinary and plasma sodium and potassium concentrations were measured by flame photometry (Photometer Eppendorf, Hamburg, Germany). Creatinine concentration was determined with a creatinine analyzer (modified Jaffé reaction; Beckmann Instruments, Brea, Ca).
Blood samples for plasma hormone analysis were cooled and centrifuged at 4°C and stored at −80°C until analysis by commercial kits: plasma renin activity (DiaSorin, Diezenbach, Germany), plasma endothelin-1 concentration (Biomedica, Vienna, Austria), and plasma ACZ concentration [high-performance liquid chromatography (HPLC), Laboratoriumsmedizin, Dortmund, Germany].
Statistical Analysis
All values are given as means ± SD. Statistical analysis was performed using the NCSS software (NCSS2004, Kaysville, UT). After testing for normal distribution of the data, a one-way analysis of variance (between-subjects factor: group) and multiple-comparison tests were applied to quantify the effects of treatment (group) on respiratory, hemodynamic, and blood-gas measurements. All tests were two-tailed and adjusted for multiple comparisons using the Bonferroni procedure. When statistical significance was indicated, post hoc analysis of intragroup differences over time (vs. baseline) and between-group differences (at equal time points) were performed with paired (intragroup) and unpaired (between-groups) t-tests and adjusted for multiple comparisons using the Holm-Bonferroni procedure. Statistical significance was assumed at P < 0.05.
RESULTS
Arterial Blood Gases, Ventilation, and Acid-Base Status
Series 1: Route of administration of ACZ on HPV.
In controls, PaO2 decreased from 93 ± 3 Torr during normoxia to 36 ± 1 Torr during hypoxia (P < 0.01; Table 1). Regardless of the route of administration, PaO2 was higher with ACZ during normoxia compared with controls (range: 101–109 Torr; P < 0.05; Table 1). During hypoxia, PaO2 did not differ between groups (range: 36–39 Torr) (Table 1). The arterial CO2 tension (PaCO2) decreased from 37 ± 3 Torr during normoxia to 28–29 Torr during hypoxia in controls, and from 34–36 to 25–29 Torr during hypoxia with iv and inhaled ACZ (P < 0.05 vs. normoxia). Baseline PaCO2 with oral ACZ was lower (32 ± 2 Torr; P < 0.01 vs. controls) but did not differ from controls during hypoxia (Table 1). V̇e did not differ among groups during normoxia and increased without intergroup differences during hypoxia in all protocols. This increase was not significant with ACZ po due to a higher baseline V̇e in this group (see Table 1).
Table 1.
Arterial blood gases and minute ventilation in controls, with acetazolamide iv, acetazolamide po, inhaled acetazolamide, methazolamide iv, and N-methyl-acetazolamide iv during normoxia and hypoxia
| n | Normoxia 1 h | Hypoxia 1st h | Hypoxia 2nd h | |
|---|---|---|---|---|
| PaO2, Torr | ||||
| Series 1 | ||||
| Controls | 7 | 93 ± 3 | 36 ± 1* | 36 ± 3* |
| ACZ iv | 7 | 109 ± 5† | 38 ± 2* | 39 ± 2* |
| ACZ po | 7 | 108 ± 4† | 37 ± 2* | 37 ± 1* |
| ACZ inhaled | 7 | 101 ± 7† | 39 ± 3* | 37 ± 3* |
| Series 2 | ||||
| Controls | 9 | 95 ± 4 | 35 ± 1* | 36 ± 2* |
| ACZ iv | 8 | 108 ± 4† | 40 ± 3* | 40 ± 1* |
| MTZ iv | 6 | 109 ± 4† | 38 ± 3* | 38 ± 2* |
| NMA iv | 6 | 95 ± 9 | 37 ± 2* | 38 ± 1* |
| PaCO2, Torr | ||||
| Series 1 | ||||
| Controls | 7 | 37 ± 3 | 29 ± 3* | 28 ± 4* |
| ACZ iv | 7 | 34 ± 2 | 28 ± 3* | 27 ± 2* |
| ACZ po | 7 | 32 ± 2† | 28 ± 3 | 26 ± 2* |
| ACZ inhaled | 7 | 36 ± 2 | 26 ± 2* | 25 ± 2* |
| Series 2 | ||||
| Controls | 9 | 36 ± 2 | 29 ± 2* | 26 ± 3* |
| ACZ iv | 8 | 33 ± 2† | 28 ± 1* | 25 ± 1* |
| MTZ iv | 6 | 33 ± 3 | 26 ± 1* | 26 ± 2* |
| NMA iv | 6 | 37 ± 2 | 27 ± 2* | 27 ± 2* |
| V̇e, l/min | ||||
| Series 1 | ||||
| Controls | 7 | 3.5 ± 1.2 | 5.6 ± 1.7* | 5.4 ± 1.6* |
| ACZ iv | 7 | 3.9 ± 0.7 | 6.4 ± 1.4* | 7.1 ± 1.3* |
| ACZ po | 7 | 4.9 ± 0.8 | 6.6 ± 1.1 | 6.5 ± 0.7 |
| ACZ inhaled | 7 | 3.1 ± 0.7 | 5.3 ± 1.2* | 5.6 ± 1.2* |
| Series 2 | ||||
| Controls | 9 | 2.8 ± 0.6 | 4.8 ± 0.6* | 4.8 ± 0.8* |
| ACZ iv | 8 | 4.0 ± 1.0 | 6.2 ± 1.5* | 6.5 ± 1.5* |
| MTZ iv | 6 | 4.3 ± 1.5 | 6.7 ± 1.3*† | 6.7 ± 0.9* |
| NMA iv | 6 | 3.4 ± 1.2 | 5.5 ± 1.8* | 5.6 ± 1.9* |
Values are means ± SD; n, no. of experiments.
PaO2, arterial oxygen tension; PaCO2, arterial carbon dioxide tension; V̇e, expiratory minute ventilation; ACZ, acetazolamide; MTZ, methazolamide; NMA, N-methyl-acetazolamide; iv, intravenous; po, per os.
Significant differences (P < 0.05) vs.
normoxia and
controls at equal time points.
Arterial hydrogen ion concentration decreased and plasma pH increased during hypoxia in all protocols (Table 2). With ACZ iv and po, plasma pH was lower after 2 h of hypoxia compared with control animals (7.414 and 7.416, respectively; controls: 7.458, P < 0.05). Arterial HCO3− and actual base excess were lower with ACZ po, compared with controls at baseline. HCO3− decreased in all protocols where CA inhibitors were applied, reaching statistical significance only for the inhaled ACZ group (see Table 2). After 2 h of hypoxia, actual base excess was lower compared with control animals whenever CA inhibitors were given. With ACZ po, plasma potassium concentration was lower after normoxia compared with control experiments (P < 0.01), but did not differ among groups over time.
Table 2.
Arterial hydrogen ion concentration, arterial pH, bicarbonate concentration, base excess, and plasma potassium concentration in controls with acetazolamide iv, acetazolamide po, inhaled acetazolamide, methazolamide iv, and N-methyl-acetazolamide iv during normoxia and hypoxia
| n | Normoxia 1 h | Hypoxia 1st h | Hypoxia 2nd h | |
|---|---|---|---|---|
| H+, nM/l | ||||
| Series 1 | ||||
| Controls | 7 | 41.3 ± 1.7 | 35.4 ± 2.6* | 34.8 ± 2.7* |
| ACZ iv | 7 | 41.0 ± 1.7 | 38.0 ± 2.8 | 38.5 ± 2.4*† |
| ACZ po | 7 | 44.3 ± 1.7 | 39.7 ± 1.5*† | 38.4 ± 1.4*† |
| ACZ inhaled | 7 | 42.2 ± 1.6 | 36.9 ± 1.3* | 35.8 ± 1.8* |
| Series 2 | ||||
| Controls | 9 | 41.6 ± 1.7 | 35.0 ± 1.4 | 34.1 ± 1.0 |
| ACZ iv | 8 | 41.2 ± 1.4 | 37.5 ± 1.9 | 38.0 ± 1.5 |
| MTZ iv | 6 | 39.5 ± 1.3 | 35.6 ± 1.9 | 36.0 ± 2.2 |
| NMA iv | 6 | 42.0 ± 1.5 | 34.8 ± 1.6 | 34.4 ± 1.9 |
| pHa | ||||
| Series 1 | ||||
| Controls | 7 | 7.384 | 7.451* | 7.458* |
| ACZ iv | 7 | 7.387 | 7.420 | 7.414*† |
| ACZ po | 7 | 7.353 | 7.402*† | 7.416*† |
| ACZ inhaled | 7 | 7.375 | 7.433* | 7.446* |
| Series 2 | ||||
| Controls | 9 | 7.381 | 7.456* | 7.468* |
| ACZ iv | 8 | 7.385 | 7.425*† | 7.420*† |
| MTZ iv | 6 | 7.404 | 7.448* | 7.444* |
| NMA iv | 6 | 7.376 | 7.458* | 7.463* |
| HCO3−, mM | ||||
| Series 1 | ||||
| Controls | 7 | 21.7 ± 1.7 | 19.2 ± 2.6 | 19.6 ± 3.7 |
| ACZ iv | 7 | 19.8 ± 1.0 | 18.1 ± 1.2 | 16.9 ± 1.0 |
| ACZ po | 7 | 17.5 ± 0.9† | 17.6 ± 1.4 | 16.8 ± 1.7 |
| ACZ inhaled | 7 | 20.5 ± 1.4 | 17.2 ± 1.8* | 16.7 ± 1.4*† |
| Series 2 | ||||
| Controls | 9 | 21.0 ± 1.5 | 20.0 ± 1.6 | 19.5 ± 1.4 |
| ACZ iv | 8 | 19.2 ± 1.1 | 17.9 ± 1.2† | 16.2 ± 1.3*† |
| MTZ iv | 6 | 20.3 ± 1.4 | 18.0 ± 1.2* | 17.4 ± 1.5* |
| NMA iv | 6 | 21.3 ± 1.2 | 19.2 ± 1.5 | 18.9 ± 1.5* |
| BE, mM | ||||
| Series 1 | ||||
| Controls | 7 | −2.3 ± 1.7 | −2.3 ± 2.0 | −2.3 ± 3.1 |
| ACZ iv | 7 | −4.0 ± 1.0 | −4.6 ± 1.0 | −5.8 ± 1.0† |
| ACZ po | 7 | −6.8 ± 0.8† | −5.7 ± 1.0† | −6.0 ± 1.0† |
| ACZ inhaled | 7 | −3.6 ± 1.3 | −5.1 ± 1.7† | −5.2 ± 1.5† |
| Series 2 | ||||
| Controls | 9 | −3.1 ± 1.4 | −2.2 ± 1.5 | −2.9 ± 2.0 |
| ACZ iv | 8 | −4.4 ± 1.0† | −4.6 ± 1.4† | −6.2 ± 1.4† |
| MTZ iv | 6 | −3.1 ± 1.2 | −4.1 ± 1.4 | −4.7 ± 1.7 |
| NMA iv | 6 | −2.9 ± 1.1 | −2.8 ± 1.5 | −3.0 ± 1.6 |
| PK, mM | ||||
| Series 1 | ||||
| Controls | 7 | 3.5 ± 0.2 | 3.3 ± 0.3 | 3.2 ± 0.2 |
| ACZ iv | 7 | 3.0 ± 0.2 | 2.9 ± 0.3 | 2.8 ± 0.3 |
| ACZ po | 7 | 2.9 ± 0.2† | 2.9 ± 0.1 | 2.8 ± 0.2 |
| ACZ inhaled | 7 | 3.2 ± 0.3 | 3.1 ± 0.3 | 3.1 ± 0.3 |
| Series 2 | ||||
| Controls | 9 | 3.5 ± 0.2 | 3.4 ± 0.2 | 3.3 ± 0.2 |
| ACZ iv | 8 | 3.2 ± 0.2 | 3.1 ± 0.3 | 3.0 ± 0.2 |
| MTZ iv | 6 | 3.2 ± 0.2 | 3.0 ± 0.3 | 2.8 ± 0.2† |
| NMA iv | 6 | 3.5 ± 0.4 | 3.3 ± 0.5 | 3.1 ± 0.3 |
Values are mean ± SD; n, no. of experiments.
H+, arterial hydrogen ion concentration; pHa, arterial pH; HCO3−, arterial bicarbonate concentration; BE, base excess; PK, plasma potassium concentration.
Significant differences (P < 0.05) vs.
normoxia and
controls at equal time points.
Series 2: Modification of the molecular structure of ACZ on HPV.
Both ACZ iv- and MTZ iv-treated animals had higher normoxic PaO2 compared with controls. In contrast, NMA iv had no effect on normoxic PaO2. Regardless of the treatment protocol, PaO2 during hypoxia were comparable among all groups during hypoxia (see Table 1). With ACZ iv, PaCO2 was lower after normoxia, but comparable to control animals during hypoxia. In response to hypoxia, V̇e increased in all groups after 2 h of hypoxia (Table 1). After normoxia, plasma pH was comparable among all protocols and increased during hypoxia in all groups. With ACZ and MTZ, the rise in plasma pH was less during hypoxia compared with control animals, while pH in NMA iv-treated animals was entirely comparable to controls at all time points. In line with these data, base excess were lower in ACZ- and MTZ-treated animals compared with controls after 2 h of hypoxia. In contrast, NMA had no effect on base excess or HCO3− compared with control animals (see Table 2).
Pulmonary and Systemic Hemodynamics
Series 1: Route of administration of ACZ on HPV.
Baseline HR and MAP did not differ among protocols and increased during hypoxia in all protocols, although this increase did not reach statistical significance (Table 3). CVP and pulmonary capillary occlusion pressure were comparable among groups during normoxia and hypoxia and did not change compared with baseline (normoxia). CO was 2.7 ± 0.5 l/min in controls at baseline and did not change during hypoxia (2.9 ± 0.6 and 2.7 ± 0.4 l/min, respectively). Of note, CO did not differ between groups at baseline and did not change in response to alveolar hypoxia, regardless of the experimental protocol. At comparable CO and MAP, SVR increased insignificantly without intergroup differences from normoxia (range: 2,880–3,227 to 3,135–3,759 dyn·s·cm−5) after 2 h of hypoxia (see Table 3).
Table 3.
Systemic and pulmonary hemodynamic parameters in controls, with acetazolamide iv, acetazolamide po, inhaled acetazolamide, methazolamide iv, and N-methyl-acetazolamide iv during normoxia and hypoxia
| n | Normoxia 1 h | Hypoxia 1st h | Hypoxia 2nd h | |
|---|---|---|---|---|
| HR, 1/min | ||||
| Series 1 | ||||
| Controls | 7 | 78 ± 12 | 108 ± 24* | 101 ± 20 |
| ACZ iv | 7 | 71 ± 11 | 98 ± 16 | 95 ± 20 |
| ACZ po | 7 | 86 ± 6 | 109 ± 16 | 100 ± 16 |
| ACZ inhaled | 7 | 85 ± 16 | 85 ± 20 | 97 ± 23 |
| Series 2 | ||||
| Controls | 9 | 75 ± 8 | 111 ± 17* | 110 ± 14* |
| ACZ iv | 8 | 75 ± 10 | 100 ± 20* | 102 ± 18* |
| MTZ iv | 6 | 74 ± 15 | 96 ± 6 | 93 ± 12 |
| NMA iv | 6 | 72 ± 11 | 112 ± 17* | 110 ± 15* |
| MAP, mmHg | ||||
| Series 1 | ||||
| Controls | 7 | 95 ± 8 | 106 ± 11 | 105 ± 25 |
| ACZ iv | 7 | 89 ± 10 | 104 ± 15 | 108 ± 15 |
| ACZ po | 7 | 96 ± 4 | 109 ± 13 | 108 ± 17 |
| ACZ inhaled | 7 | 93 ± 10 | 105 ± 16 | 106 ± 15 |
| Series 2 | ||||
| Controls | 9 | 94 ± 11 | 103 ± 11 | 100 ± 18 |
| ACZ iv | 8 | 88 ± 8 | 101 ± 7 | 103 ± 12 |
| MTZ iv | 6 | 90 ± 10 | 105 ± 20 | 103 ± 18 |
| NMA iv | 6 | 89 ± 10 | 109 ± 10 | 103 ± 16 |
| CO, l/min | ||||
| Series 1 | ||||
| Controls | 7 | 2.7 ± 0.5 | 2.9 ± 0.6 | 2.7 ± 0.4 |
| ACZ iv | 7 | 2.3 ± 0.5 | 2.5 ± 0.4 | 2.3 ± 0.3 |
| ACZ po | 7 | 2.5 ± 0.6 | 2.8 ± 0.6 | 2.4 ± 0.4 |
| ACZ inhaled | 7 | 2.6 ± 0.4 | 2.4 ± 0.4 | 2.5 ± 0.3 |
| Series 2 | ||||
| Controls | 9 | 2.3 ± 0.6 | 2.8 ± 0.6 | 2.7 ± 0.5 |
| ACZ iv | 8 | 2.2 ± 0.9 | 2.5 ± 0.6 | 2.5 ± 0.6 |
| MTZ iv | 6 | 2.2 ± 0.6 | 2.8 ± 0.8 | 2.6 ± 0.9 |
| NMA iv | 6 | 2.7 ± 0.7 | 3.3 ± 1.0 | 3.3 ± 0.9 |
| SVR, dyn·s·cm−5 | ||||
| Series 1 | ||||
| Controls | 7 | 2,896 ± 659 | 2,907 ± 680 | 3,135 ± 793 |
| ACZ iv | 7 | 3,101 ± 838 | 3,354 ± 708 | 3,759 ± 844 |
| ACZ po | 7 | 3,227 ± 849 | 3,147 ± 818 | 3,633 ± 848 |
| ACZ inhaled | 7 | 2,880 ± 515 | 3,519 ± 618 | 3,400 ± 572 |
| Series 2 | ||||
| Controls | 9 | 3,351 ± 874 | 2,940 ± 648 | 3,004 ± 587 |
| ACZ iv | 8 | 3,470 ± 1,049 | 3,223 ± 660 | 3,369 ± 1,021 |
| MTZ iv | 6 | 2,541 ± 614 | 2,294 ± 401 | 2,538 ± 309 |
| NMA iv | 6 | 2,735 ± 899 | 2,751 ± 779 | 2,532 ± 501 |
| CVP, mmHg | ||||
| Series 1 | ||||
| Controls | 7 | 2 ± 1 | 2 ± 1 | 2 ± 1 |
| ACZ iv | 7 | 2 ± 1 | 2 ± 1 | 2 ± 1 |
| ACZ po | 7 | 2 ± 1 | 2 ± 1 | 2 ± 1 |
| ACZ inhaled | 7 | 3 ± 1 | 2 ± 1 | 2 ± 1 |
| Series 2 | ||||
| Controls | 9 | 2 ± 1 | 2 ± 1 | 2 ± 1 |
| ACZ iv | 8 | 3 ± 1 | 2 ± 1 | 3 ± 0 |
| MTZ iv | 6 | 2 ± 1 | 3 ± 1 | 2 ± 1 |
| NMA iv | 6 | 3 ± 1 | 2 ± 0 | 3 ± 1 |
| MPAP, mmHg | ||||
| Series 1 | ||||
| Controls | 7 | 13 ± 2 | 20 ± 2* | 20 ± 3* |
| ACZ iv | 7 | 12 ± 2 | 14 ± 2† | 13 ± 2† |
| ACZ po | 7 | 13 ± 2 | 17 ± 2* | 17 ± 3* |
| ACZ inhaled | 7 | 13 ± 2 | 15 ± 2† | 15 ± 3† |
| Series 2 | ||||
| Controls | 9 | 11 ± 2 | 18 ± 2* | 18 ± 2* |
| ACZ iv | 8 | 11 ± 3 | 14 ± 3† | 13 ± 3† |
| MTZ iv | 6 | 11 ± 1 | 15 ± 3* | 14 ± 3 |
| NMA iv | 6 | 13 ± 2 | 17 ± 3 | 16 ± 4 |
| PVR, dyn·s·cm−5 | ||||
| Series 1 | ||||
| Controls | 7 | 265 ± 52 | 463 ± 110* | 489 ± 87* |
| ACZ iv | 7 | 284 ± 81 | 354 ± 80 | 350 ± 74† |
| ACZ po | 7 | 292 ± 35 | 385 ± 107 | 470 ± 145 |
| ACZ inhaled | 7 | 284 ± 47 | 378 ± 88 | 386 ± 91 |
| Series 2 | ||||
| Controls | 9 | 256 ± 70 | 405 ± 88* | 444 ± 85* |
| ACZ iv | 8 | 269 ± 66 | 305 ± 70 | 300 ± 87† |
| MTZ iv | 6 | 244 ± 49 | 329 ± 90 | 325 ± 81 |
| NMA iv | 6 | 262 ± 62 | 321 ± 86 | 330 ± 85 |
| PCWP, mmHg | ||||
| Series 1 | ||||
| Controls | 7 | 4 ± 1 | 3 ± 0 | 4 ± 1 |
| ACZ iv | 7 | 4 ± 1 | 3 ± 1 | 3 ± 1 |
| ACZ po | 7 | 4 ± 1 | 4 ± 1 | 4 ± 1 |
| ACZ inhaled | 7 | 4 ± 1 | 4 ± 1 | 4 ± 0 |
| Series 2 | ||||
| Controls | 9 | 4 ± 0 | 4 ± 1 | 4 ± 1 |
| ACZ iv | 8 | 4 ± 1 | 4 ± 1 | 4 ± 1 |
| MTZ iv | 6 | 5 ± 1 | 4 ± 1 | 4 ± 1 |
| NMA iv | 6 | 4 ± 1 | 4 ± 1 | 4 ± 1 |
Values are mean ± SD; n, no. of experiments.
HR, heart rate; MAP, mean arterial pressure; CO, cardiac output; SVR, systemic vascular resistance; CVP, central venous pressure; MPAP, mean pulmonary artery pressure; PVR, pulmonary vascular resistance; PCWP, pulmonary capillary wedge pressure.
Significant differences (P < 0.05) vs.
normoxia and
controls at equal time points.
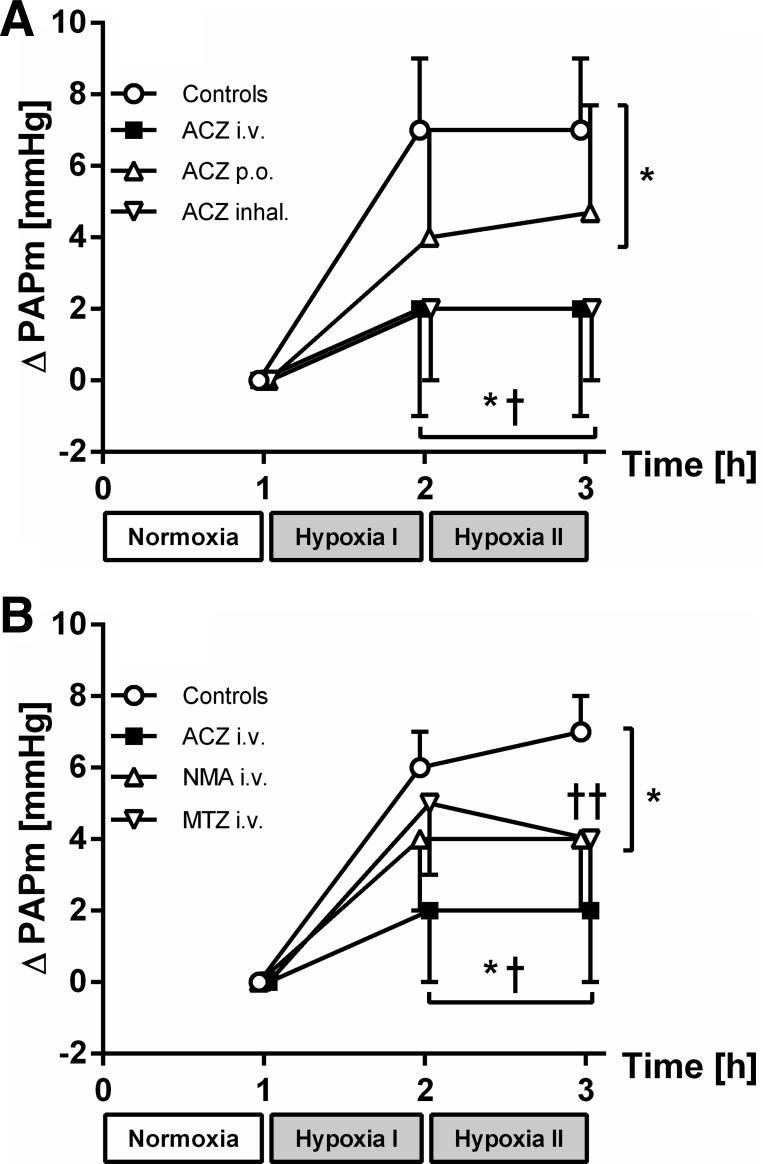
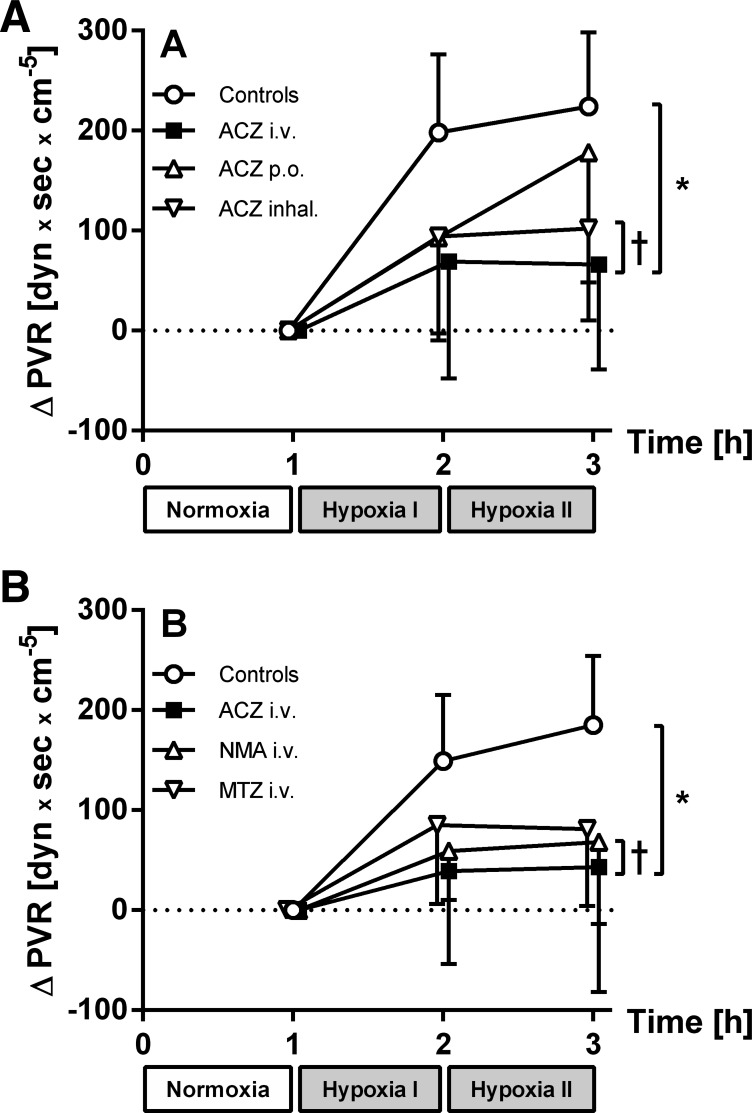
Baseline MPAP was comparable after 1 h of normoxia in all protocols (Table 3 and Fig. 2A). In controls, MPAP increased from 13 ± 2 mmHg after normoxia to 20 ± 3 mmHg after 2 h of hypoxia, while PVR increased from 265 ± 52 dyn·s·cm−5 (normoxia) to 489 ± 87 dyn·s·cm−5 at the end of the protocol. With iv and inhaled ACZ, MPAP did not change compared with baseline during normoxia and was significantly lower during hypoxia compared with control animals (Table 3 and Fig. 2A). When comparing the change in PVR with these inhibitors, PVR did not increase in response to alveolar hypoxia and was lower with ACZ iv compared with controls (P < 0.01, see Fig. 3A). Despite this impact on MPAP and PVR with iv and inhaled administration, the increase in MPAP (5 ± 3 mmHg vs. normoxia) and pulmonary arterial resistance (178 ± 130 dyn·s·cm−5) during hypoxia was not fully prevented with orally administered ACZ (see Table 3 and Figs. 2A and 3A). This reduction in MPAP and PVR was not statistically significant except for MPAP after 1 h of hypoxia.
Fig. 2.
A: change (Δ) in mean pulmonary arterial pressure (PAPm) in series 1. Differences are shown in values obtained after normoxia and the 1st and 2nd h of hypoxia in controls, intravenous (iv) acetazolamide (ACZ), per os (po) ACZ, and inhaled ACZ. B: ΔPAPm in series 2. Differences are shown in values obtained after normoxia and the 1st and 2nd h of hypoxia in controls, iv ACZ, N-methyl-acetazolamide (NMA), and methazolamide (MTZ). Values are means ± SD; n = 7 (A) and 6–9 (B). *P < 0.05 vs. normoxia. †P < 0.05 vs. controls.
Fig. 3.
A: Δpulmonary vascular resistance (PVR) in series 1. Differences are shown in values obtained after normoxia and the 1st and 2nd h of hypoxia in controls, iv ACZ, po ACZ, and inhaled acetazolamide. B: ΔPVR in series 2. Differences are shown in values obtained after normoxia and the 1st and 2nd h of hypoxia in controls, iv ACZ, NMA, and MTZ. Values are means ± SD; n = 7 (A) and 6–9 (B). *P < 0.05 vs. normoxia. †P < 0.05 vs. controls.
Series 2: Modification of the molecular structure of ACZ on HPV.
In line with the data from series 1, HR, MAP, CVP, and pulmonary arterial occlusion pressure did not change over time and did not differ between the treatment protocols and the control experiments. Despite an increase of ∼0.5 l/min, CO did not change significantly during hypoxia in control experiments (normoxia: 2.5 ± 0.6 l/min vs. 2.8 ± 0.6 and 2.7 ± 0.5 l/min during hypoxia) or CA-inhibited animals compared with baseline values. Also, no differences in CO were measured between treatment groups compared with the control group (Table 3). There were no significant differences with regards to SVR between groups and no significant changes in SVR within groups over time.
MPAP increased by 7 ± 2 mmHg in control experiments (normoxia: 11 ± 2 and 18 ± 2 mmHg after 2 h of hypoxia) and PVR increased by 149 ± 66 dyn·s·cm−5 (hypoxia I) and 185 ± 69 dyn·s·cm−5 (hypoxia II, see Figs. 2B and 3B and Table 3). Again, with ACZ iv, this increase in MPAP (2 ± 3 mmHg) and PVR (43 ± 125 dyn·s·cm−5) was virtually inhibited (P < 0.01 vs controls) after 2 h of hypoxia. With MTZ and NMA iv, PAP increased by 4 ± 2 mmHg after 2 h of hypoxia (P < 0.05 vs. normoxia), and thus the change in MPAP was reduced with these inhibitors compared with control experiments (P < 0.01). In addition, PVR did not increase during hypoxia compared with normoxia (MTZ iv: 81 ± 77 and 68 ± 82 dyn·s·cm−5 with NMA iv), and the change in PVR was lower compared with control experiments with NMA iv (P < 0.05 respectively, see Table 3 and Fig. 3B).
Renal Function and Urinary Excretion Data
Series 1: Route of administration of ACZ on HPV.
Urine output, GFR, and sodium and potassium excretion in control experiments did not change after 2 h of hypoxia. With ACZ iv, urine output was increased compared with controls during the first 2 h of the experiment. After inhalation of ACZ, urine output transiently increased during the 1st h of hypoxia. GFR was not altered by CA inhibition, regardless of the route of administration (Table 4). Urinary excretion of sodium and potassium was increased after 2 h of hypoxia with inhaled and iv ACZ, while oral administration had no effect on electrolyte excretion.
Table 4.
Renal excretion parameters in controls, with acetazolamide iv, acetazolamide po, inhaled acetazolamide, methazolamide iv, and N-methyl-acetazolamide iv during normoxia and hypoxia
| n | Normoxia 1 h | Hypoxia 1st h | Hypoxia 2nd h | |
|---|---|---|---|---|
| UV, μl·min−1·kg−1 | ||||
| Series 1 | ||||
| Controls | 7 | 33 ± 24 | 49 ± 37 | 32 ± 18 |
| ACZ iv | 7 | 104 ± 28† | 123 ± 63† | 63 ± 28 |
| ACZ po | 7 | 40 ± 14 | 76 ± 44 | 51 ± 28 |
| ACZ inhaled | 7 | 35 ± 23 | 93 ± 44* | 87 ± 41 |
| Series 2 | ||||
| Controls | 9 | 30 ± 26 | 43 ± 34 | 25 ± 18 |
| ACZ iv | 8 | 67 ± 31 | 73 ± 54 | 68 ± 32 |
| MTZ iv | 6 | 31 ± 28 | 80 ± 53 | 74 ± 27 |
| NMA iv | 6 | 42 ± 22 | 108 ± 76† | 53 ± 58 |
| UNAV, μmol·min−1·kg−1 | ||||
| Series 1 | ||||
| Controls | 7 | 0.3 ± 0.3 | 0.4 ± 0.5 | 0.8 ± 1.3 |
| ACZ iv | 7 | 5.1 ± 2.1† | 5.2 ± 2.0† | 6.6 ± 2.5† |
| ACZ po | 7 | 0.8 ± 0.5 | 1.3 ± 0.7 | 1.3 ± 0.5 |
| ACZ inhaled | 7 | 0.4 ± 0.4 | 7.2 ± 2.8†* | 5.1 ± 2.2†* |
| Series 2 | ||||
| Controls | 9 | 0.2 ± 0.1 | 0.4 ± 0.4 | 0.9 ± 1.1 |
| ACZ iv | 8 | 5.6 ± 2.1† | 6.6 ± 2.1† | 7.9 ± 1.9†* |
| MTZ iv | 6 | 1.4 ± 0.5 | 3.5 ± 1.2† | 5.3 ± 1.5†* |
| NMA iv | 6 | 0.5 ± 0.5 | 1.4 ± 1.1 | 1.8 ± 1.2 |
| UKV, μmol·min−1·kg−1 | ||||
| Series 1 | ||||
| Controls | 7 | 0.3 ± 0.3 | 0.4 ± 0.3 | 0.9 ± 1.4 |
| ACZ iv | 7 | 5.1 ± 3.0† | 4.6 ± 2.4† | 5.4 ± 3.5† |
| ACZ po | 7 | 1.8 ± 1.0 | 2.1 ± 1.2 | 2.1 ± 1.3 |
| ACZ inhaled | 7 | 0.5 ± 0.4 | 7.5 ± 2.1†* | 5.1 ± 1.5†* |
| Series 2 | ||||
| Controls | 9 | 0.4 ± 0.5 | 1.0 ± 1.3 | 1.9 ± 1.5 |
| ACZ iv | 8 | 5.5 ± 2.6† | 5.2 ± 2.0† | 5.9 ± 3.0† |
| MTZ iv | 6 | 4.4 ± 2.4† | 4.8 ± 2.1† | 5.3 ± 2.1† |
| NMA iv | 6 | 0.2 ± 0.1 | 0.6 ± 0.5 | 0.9 ± 0.7 |
| GFR, ml·min−1·kg−1 | ||||
| Series 1 | ||||
| Controls | 7 | 3.9 ± 0.5 | 3.8 ± 0.3 | 3.8 ± 0.5 |
| ACZ iv | 7 | 3.2 ± 0.2 | 3.1 ± 0.5 | 3.3 ± 0.6 |
| ACZ po | 7 | 3.0 ± 0.4 | 3.3 ± 0.6 | 3.2 ± 0.3 |
| ACZ inhaled | 7 | 3.9 ± 1.0 | 3.6 ± 0.9 | 3.6 ± 0.8 |
| Series 2 | ||||
| Controls | 9 | 3.6 ± 0.8 | 3.8 ± 0.7 | 3.7 ± 0.7 |
| ACZ iv | 8 | 2.8 ± 0.3 | 2.9 ± 0.6 | 3.0 ± 0.6 |
| MTZ iv | 6 | 3.3 ± 0.2 | 3.3 ± 0.4 | 3.3 ± 0.5 |
| NMA iv | 6 | 4.1 ± 1.1 | 3.9 ± 1.1 | 3.7 ± 0.9 |
Values are mean ± SD; n, no. of experiments.
UV, urinary volume; UNAV, urinary sodium excretion; UKV, urinary potassium excretion; GFR, glomerular filtration rate.
Significant differences (P < 0.05) vs.
normoxia and
controls at equal time points.
Series 2: Modification of the molecular structure of ACZ on HPV.
Again, GFR, urine output, as well as sodium and potassium excretion did not change in control experiments after 2 h of hypoxia (Table 4). With ACZ iv, sodium and potassium excretion were increased during normoxia compared with controls and increased both with ACZ and MTZ compared with controls after 2 h of hypoxia. In contrast, NMA caused none of these well-known effects of renal CA inhibition on sodium and potassium excretion (Table 4). While there was an increased urine output after the 1st h of hypoxia with NMA, this was not sustained in the 2nd h. NMA had also no further impact on urine output or GFR.
ACZ plasma concentration.
Representative ACZ plasma concentrations were measured via HPLC in individual dogs in series 1 and 2. After ACZ iv, plasma concentrations were comparable after normoxia (series 1: 6.9 ± 3.2 μg/ml, n = 3; series 2: 5.0 ± 1.0 μg/ml, n = 3) and provided stable ACZ plasma concentrations over the experimental time course (series 1: 8.0 ± 2.6 and 7.9 ± 1.9 μg/ml during the 1st and 2nd h of hypoxia, n = 3; series 2: 6.9 ± 1.4 and 7.4 ± 0.4 μg/m during hypoxia I and II, respectively, n = 3). In series 1, ACZ plasma concentrations were lower with oral administration during hypoxia compared with the iv administration (4.6 ± 1.1 and 3.7 ± 0.6 μg/ml during hypoxia I and II, respectively, n = 2). With inhaled ACZ, plasma concentrations were 4.03 ± 2.2 and 3.3 ± 1.7 μg/ml during hypoxia I and II, respectively (n = 6). No ACZ was detected with NMA iv during the experimental time course (n = 6).
DISCUSSION
Our results can be summarized as follows: 1) inhaled ACZ prevents HPV, but this route of administration does not avoid the accompanying effects of systemic CA inhibition; 2) NMA, a compound lacking any CA inhibiting property, mitigates the pulmonary vascular pressure response to acute alveolar hypoxia in fully awake dogs, but not as effectively as ACZ at the doses we studied; and 3) MTZ, an analog of ACZ that is methylated at the thiadiazole ring and is an equally potent inhibitor of CA, was only slightly less effective than ACZ in reducing HPV.
Routes and Effectiveness of Different Forms of ACZ Administration
We compared different routes of administration of ACZ and their respective effects on the pulmonary vascular response to acute alveolar hypoxia. We show that iv and inhaled ACZ both reduce HPV, with a greater reduction in MPAP and pulmonary arterial resistance with ACZ iv. The major stimulus for HPV is the decrease in oxygen tension in the PASMC, determined by the O2 tension in the alveolar gas (PaO2). By adjusting the fraction of inspired O2 to control for the respiratory stimulant effects of CA inhibition and subsequent increases in PaO2, we show that reduction of PAP and PVR was not due to differences in PaO2. In addition, the reduction in PVR was not due to influences of ACZ on CO, whereby increases in CO at equal MPAP could lead to a reduction in PVR via mathematical coupling of PVR and CO (see Table 3). The greater reduction with ACZ iv may be due to the higher ACZ plasma concentrations achieved with iv administration compared with inhaled ACZ. This is in line with the findings of Shimoda et al. (22) in rat PASMCs, who found a steep dose-response relationship over the range of 10–100 μM ACZ on hypoxia-mediated increases in [Ca2+]i, equivalent to the concentration differences achieved in our in vivo study.
As to the effects of CA inhibitors, we have shown that mitigation of HPV in vivo is not due to CA inhibition, because BZ and ETZ, both potent inhibitors but structurally quite different from ACZ, fail to reduce HPV (9). Our results were parallel to those found in vitro by Shimoda et al. (22) using the same selective intra- and extracellular CA inhibitors in rat PASMCs. In their study, both agents failed to suppress the increase in [Ca2+]i upon exposure to hypoxia. In addition, ACZ might abrogate HPV via possible effects on the pulmonary vascular endothelium and the release of vasoactive substances from CA-containing pulmonary endothelial cells. In this regard, our laboratory (8) has previously shown that inhibition of HPV with ACZ is not correlated with changes in plasma catecholamine, endothelin-1, or angiotensin II levels, hormonal mediators that themselves alter pulmonary hemodynamics.
We postulated that inhaled ACZ might prevent HPV without the unwanted systemic side effects of CA inhibition. Our data show that, although HPV was reduced with this route of administration, ACZ is absorbed adequately via the pulmonary epithelium. At this dose (750 mg), there is also sufficient absorption systemically to inhibit renal CA and cause the typical fall in actual HCO3− concentration and base excess (see Table 2). Thus it does not appear possible to selectively affect HPV by inhalation of ACZ without systemic side effects, unless the molecule can be modified to limit uptake across the lung. Yet, despite the effects of systemic absorption on acid-base status, inhaled ACZ still reduced HPV and exerted its well-known ventilatory stimulant effects, the latter being advantageous for acclimatization to high altitude and in prevention of AMS. While several studies investigated the inhibiting effects of inhaled ACZ on another smooth muscle response in the lungs, that of bronchoconstriction (7, 18, 23), to our knowledge this is the first report of the pulmonary vascular effects of inhaled ACZ during acute hypoxic exposure.
Concordant with our previous findings, we found a reduction, but not abolishment, of HPV with orally administered ACZ at this same dose regime (5 mg/kg twice a day, 12 and 1 h before hypoxia) (9). Although in this series the effect of oral ACZ was only borderline significant, when the data from this study are combined with the data of our earlier work using the same dosing and hypoxia exposure (9), the effect of oral ACZ is statistically significant. The dosage and time of ACZ administration are similar to those used by mountaineers during ascent to high altitude. A possible explanation for the reduced effect on HPV at these oral doses compared with the iv and inhaled doses we studied is lower effective drug concentrations at the level of the pulmonary vascular smooth muscle at the time of hypoxia exposure. This is suggested by the drug levels we measure with oral ACZ (4.6 and 3.7 μg/ml during hypoxia I and II, respectively). This question can only be resolved with more extensive pharmacodynamics studies. Another possibility is the physiological impact of renal CA inhibition: greater renal HCO3− loss, more pronounced negative base excess values, and lower arterial pH (see Table 2). These are the well-known metabolic effects of systemic CA inhibition, which, in general, are known to augment HPV (11, 24) and could have acted to diminish the extent of HPV inhibition. However, in the oral ACZ group, the acidosis and HCO3− losses were comparable after 2 h of hypoxia to those of the ACZ iv group, in whom HPV was fully prevented. Furthermore, we have shown in the same animal model (8) that HPV suppression occurs—despite even greater systemic acidosis developing—with 10-fold higher ACZ dosing and complete inhibition of all CA isoenzymes. Thus, although always present with prolonged administration of ACZ, metabolic acidosis cannot explain the lesser effect with oral ACZ dosing. Although the drug plasma concentration with oral ACZ was nearly comparable to inhaled ACZ, the local tissue concentration of ACZ in the pulmonary vasculature was probably much higher after inhalation of the drug directly before hypoxia. These findings, taken together, point to crucial dose-, time-, and route-dependent effects for ACZ in the prevention of HPV and ultimately the need for better correlation of physiological effect with drug levels in plasma and the lung vasculature. Because ACZ does not reduce HPV by inhibition of CA, and we do not know its target, any correspondence of drug levels vis-à-vis CA inhibition likely will not provide guidance.
Analogs of ACZ and HPV
We hypothesized that the addition of a methyl group on the thiadiazole ring structure (and thus eliminating one dissociable proton from ACZ) might impinge on its ability to act on an ACZ-specific cellular receptor or channel and, therefore, alter the capability to prevent HPV. We therefore used MTZ, a more lipophilic compound providing slightly stronger CA inhibition coupled with higher diffusivity into tissues (15). While absolute PAP and pulmonary arterial resistance did not increase during hypoxia, the difference compared with controls only reached statistical significance when analyzing the relative changes of MPAP and PVR (see Fig. 3, A and B). Thus, although MTZ is effective against HPV in vivo, the addition of one methyl group to the heterocyclic ring structure of ACZ slightly impairs its capability to prevent HPV. Despite near complete similarity in the molecular structure, differences in the pharmacological effects of ACZ and MTZ aside from CA inhibition have been described previously. For example, ACZ is a specific opener of rat skeletal muscle large-conductance calcium-stimulated potassium (BKCa) channels, while MTZ has no effect on these channels (27). Identifying the possible ion channels involved in reduction of HPV by CA inhibitors was not the main focus of our study, but our view is that it is highly improbable that BKCa channels play an important role in the mediation of HPV and its prevention by ACZ. First, Roth et al. (21) showed that HPV was not altered in perfused lungs of mice deficient in BKCa channels. Second, we have demonstrated in studies of isolated rat PASMC that ACZ does not alter resting membrane potential or act to repress depolarization by preventing the inhibition of voltage-gated K+ channels in these cells (22). Third, indirect evidence stems from the fact that, while lacking any effect on BKCa channels in other models, MTZ is effective against HPV in our recent experiments.
We next tested whether a molecule lacking any CA inhibiting activity, but which in most other respects (size, molecular weight, ionization constants, aromatic ring structure, and intramolecular electrostatic charge distribution; see Fig. 1) is equivalent to ACZ could still be effective in prevention of HPV in vivo. We, therefore, synthesized NMA, a compound in which one of the amine hydrogens of the sulfonamide moiety (SO2NH2) necessary for high-affinity drug binding to the active site of CA is replaced with a methyl group (SO2NHCH3), yielding a dissociation constant for inhibitor binding (Ki) vs. CA isoenzyme II (CA II) of 1,600 nM compared with the Ki of ACZ against CA II of 8 nM (16). Using this compound, the PAP and pulmonary arterial resistance did not increase in response to alveolar hypoxia compared with breathing 21% oxygen, with the efficacy of NMA against HPV being somewhat lower compared with ACZ (see Table 3 and Fig. 3, A and B). One possible explanation for the effect on HPV in vivo would be conversion of NMA to the original compound ACZ. Using HPLC, we did not detect any conversion to ACZ in dog plasma after administration of NMA. In earlier work, Maren (16) found a 2% conversion of NMA to ACZ in dogs. Of note, the detection threshold for ACZ concentrations in plasma with HPLC is 1 μg/ml, and thus we cannot exclude the possibility that some conversion of NMA to ACZ occurred below the detection threshold of our test. In addition, NMA had no influence on baseline or hypoxic ventilatory responses, acid-base status, or renal function, while the typical effects of CA inhibition were present in all other experiments where potent CA inhibitors were applied (see Tables 1, 2, and 4). These findings indicate that conversion of NMA to ACZ was not the underlying mechanism of the pulmonary vascular effects of NMA. Another possible explanation for the effects of NMA on HPV could be that changes in intracellular pH (pHi) with this inhibitor may influence the magnitude of HPV. Madden et al. (13) have shown that hypoxia causes an increase in pHi that is associated with smooth muscle cell contraction. In support, HPV was enhanced in isolated lung of rats when pHi was increased, whereas decreasing pHi blunted HPV in this animal model (20). In vitro, ACZ causes a small but significant acid shift in basal PASMC pHi from 7.26 to 7.22 (22), yet Farrukh et al. (6) demonstrated that the pHi needs to be altered >0.5 pH units to induce the typical [Ca2+]i changes observed with hypoxia. Moreover, although greater CA inhibition with ETZ caused a more pronounced decrease in pHi, ETZ had no effect on Ca2+ increase in hypoxic rat PASMCs (22) and did not alter HPV in vivo in the fully awake beagle (9). In the same PASMCs (22), NMA had no effect on pHi, but, nonetheless, prevented the hypoxia-induced increase in [Ca2+]i. Taken together, these results suggest that inhibition of HPV with ACZ or NMA in vitro and in vivo is not due to changes in pHi (24).
Study Limitations and Implications
Our results have several physiological and clinical implications and limitations.
First, one limitation of our data is the inability at present to infer the underlying mechanisms of inhibition of HPV by ACZ and its analogs. Although we ruled out differences in PaO2, release of pulmonary vasoconstrictors (endothelin-1, angiotensin II levels, or catecholamines) or CA inhibition as the underlying mechanism, more studies are needed to identify the molecular pathways of ACZ in prevention of HPV. Of note, Aamand et al. (1) recently proposed that CA, when inhibited by ACZ and another CA inhibiting sulfonamide, dorzolamide, acts as a nitrite reductase to generated nitric oxide (NO). However, if ACZ were working in this fashion, one might expect to observe a rise in NO production, such as an increase in exhaled NO. In the isolated, perfused lung treated with ACZ and exposed to hypoxia, we showed no change in exhaled NO as a surrogate for increased NO formation, despite a reduction in PAP (5).
Our finding that NMA reduces the PAP and pulmonary arterial resistance during acute hypoxia may offer new strategies in the treatment of high-altitude pulmonary edema and other hypoxic forms of pulmonary arterial hypertension without the burden of side effects from systemic CA inhibition. Thus our recent findings add to the evidence that the sulfonamide inhibitors ACZ and MTZ not only act as inhibitors of CA, but exert important physiological effects aside from CA inhibition.
In summary, we found that inhaled ACZ prevents HPV, but with relevant effects of systemic CA inhibition. Next, we showed that a compound lacking any CA inhibiting property (NMA) mitigates the pulmonary vascular pressure response to acute alveolar hypoxia in fully awake dogs. In addition, we found that an analog of ACZ that is methylated at the thiadiazole ring and is an equally potent inhibitor of CA (MTZ) is only slightly less effective than ACZ in reducing HPV.
GRANTS
This study was supported by a grant from the Deutsche Forschungsgemeinschaft to C. Höhne (Ho 3295/2-1), to P. Pickerodt and W. Boemke (Pi 795/2-1), and by a grant from the National Heart, Lung, and Blood Institute to E. R. Swenson (HL-24163).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.A.P., C.H., W.B., and E.R.S. conception and design of research; P.A.P., R.C.F., F.N., and S.T. performed experiments; P.A.P., S.T., W.B., and E.R.S. analyzed data; P.A.P., R.C.F., C.H., F.N., S.T., W.B., and E.R.S. interpreted results of experiments; P.A.P. prepared figures; P.A.P., R.C.F., C.H., W.B., and E.R.S. drafted manuscript; P.A.P., R.C.F., C.H., F.N., S.T., W.B., and E.R.S. edited and revised manuscript; P.A.P., R.C.F., C.H., F.N., S.T., W.B., and E.R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are indebted to Rainer Mohnhaupt for excellent help with the statistics, and to Birgit Brandt, Sabine Molling, and to the late Daniela Bayerl for expert technical assistance.
Part of this work was presented at the “Hypoxia Symposium 2007 & 2009” meeting, Lake Louise, Canada.
Footnotes
This article is the topic of an Invited Editorial by Luc J. Teppema (25a).
REFERENCES
- 1.Aamand R, Dalsgaard T, Jensen FB, Simonsen U, Roepstorff A, Fago A. Generation of nitric oxide from nitrite by carbonic anhydrase: a possible link between metabolic activity and vasodilation. Am J Physiol Heart Circ Physiol 297: H2068–H2074, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Bärtsch P, Mairbäurl H, Maggiorini M, Swenson ER. Physiological aspects of high-altitude pulmonary edema. J Appl Physiol 98: 1101–1110, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Bärtsch P, Swenson ER. Acute high-altitude illnesses. N Engl J Med 368: 2294–2302, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Deem S, Hedges RG, Kerr ME, Swenson ER. Acetazolamide reduces hypoxic pulmonary vasoconstriction in isolated perfused rabbit lungs. Respir Physiol 123: 109–119, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Deem S, Min JH, Moulding JD, Eveland R, Swenson ER. Red blood cells prevent inhibition of hypoxic pulmonary vasoconstriction by nitrite in isolated, perfused rat lungs. Am J Physiol Heart Circ Physiol 292: H963–H970, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Farrukh IS, Hoidal JR, Barry WH. Effect of intracellular pH on ferret pulmonary arterial smooth muscle cell calcium homeostasis and pressure. J Appl Physiol 80: 496–505, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Foresi A, Cavigioli G, Pelucchi A, Mastropasqua B, Marazzini L. Effect of acetazolamide on cough induced by low-chloride-ion solutions in normal subjects: Comparison with furosemide. J Allergy Clin Immunol 97: 1093–1099, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Hohne C, Krebs MO, Seiferheld M, Boemke W, Kaczmarczyk G, Swenson ER. Acetazolamide prevents hypoxic pulmonary vasoconstriction in conscious dogs. J Appl Physiol 97: 515–521, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Hohne C, Pickerodt PA, Francis RC, Boemke W, Swenson ER. Pulmonary vasodilation by acetazolamide during hypoxia is unrelated to carbonic anhydrase inhibition. Am J Physiol Lung Cell Mol Physiol 292: L178–L184, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Ke T, Wang J, Swenson ER, Zhang X, Hu Y, Chen Y, Liu M, Zhang W, Zhao F, Shen X, Yang Q, Chen J, Luo W. Effect of acetazolamide and gingko biloba on the human pulmonary vascular response to an acute altitude ascent. High Alt Med Biol 14: 162–167, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lejeune P, Brimioulle S, Leeman M, Hallemans R, Melot C, Naeije R. Enhancement of hypoxic pulmonary vasoconstriction by metabolic acidosis in dogs. Anesthesiology 73: 256–264, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Luks AM, Swenson ER. Medication and dosage considerations in the prophylaxis and treatment of high-altitude illness. Chest 133: 744–755, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Madden JA, Ray DE, Keller PA, Kleinmann JG. Ion exchange activity in pulmonary artery smooth muscle cells: the response to hypoxia. Am J Physiol Lung Cell Mol Physiol 280: L264–L271, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Maggiorini M, Melot C, Pierre S, Pfeiffer F, Greve I, Sartori C, Lepori M, Hauser M, Scherrer U, Naeije R. High-altitude pulmonary edema is initially caused by an increase in capillary pressure. Circulation 103: 2078–2083, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Maren TH. Carbonic anhydrase: chemistry, physiology, inhibition. Physiol Rev 47: 595–781, 1967 [DOI] [PubMed] [Google Scholar]
- 16.Maren TH. Carbonic anhydrase inhibition. V. N5-sibstituted 2-acetylamino-1,3,4-thiadiazole-5 sulfonamides: metabolic conversion and use as control substances. J Pharmacol Exp Ther 117: 385–401, 1956 [PubMed] [Google Scholar]
- 17.McNaughton NCL, Davies CH, Randall A. Inhibition of alpha(1E) Ca(2+) channels by carbonic anhydrase inhibitors. J Pharm Sci 95: 240–247, 2004 [DOI] [PubMed] [Google Scholar]
- 18.O'Connor BJ, Yeo CT, Chen-Worsdall YM, Barnes PJ, Chung KF. Effects of acetazolamide and amiloride against metabisulphite-induced bronchoconstriction in mild asthma. Thorax 49: 1096–1098, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pickkers P, Hughes AD, Russel FGM, Thien T, Smits P. In vivo evidence for KCa channel opening properties of acetazolamide in the human vasculature. Br J Pharmacol 132: 443–450, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raffestin B, McMurtry IF. Effects of intracellular pH on hypoxic vasoconstriction in rat lungs. J Appl Physiol 63: 2524–2531, 1987 [DOI] [PubMed] [Google Scholar]
- 21.Roth M, Rupp M, Hofmann S, Mittal M, Fuchs B, Sommer N, Parajuli N, Quanz K, Schubert D, Dony E, Schermuly RT, Ghofrani HA, Sausbier U, Rutschmann K, Wilhelm S, Seeger W, Ruth P, Grimminger F, Sausbier M, Weissmann N. Heme oxygenase-2 and large-conductance Ca2+-activated K+ channels: lung vascular effects of hypoxia. Am J Respir Crit Care Med 180: 353–364, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Shimoda LA, Luke T, Sylvester JT, Shih HW, Jain A, Swenson ER. Inhibition of hypoxia-induced calcium responses in pulmonary arterial smooth muscle by acetazolamide is independent of carbonic anhydrase inhibition. Am J Physiol Lung Cell Mol Physiol 292: L1002–L1012, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Spicuzza L, Ciancio N, Pellegrino R, Bellofiore S, Polosa R, Ricciardolo FL, Brusasco V, Di Maria GU. The effect of inhaled furosemide and acetazolamide on bronchoconstriction induced by deep inspiration in asthma. Monaldi Arch Chest Dis 59: 150–154, 2003 [PubMed] [Google Scholar]
- 24.Swenson ER. Carbonic anhydrase inhibitors and hypoxic pulmonary vasoconstriction. Respir Physiol Neurobiol 151: 209–216, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Swenson ER, Maggiorini M, Mongovin S, Gibbs JS, Greve I, Mairbäurl H, Bärtsch P. Pathogenesis of high-altitude pulmonary edema: Inflammation is not an etiologic factor. J Am Med Assoc 287: 2228–2235, 2002 [DOI] [PubMed] [Google Scholar]
- 25a.Teppema LJ. Multifaceted clinical effects of acetazolamide: will the underlying mechanisms please stand up? J Appl Physiol; 10.1152/japplphysiol.00141.2014 [DOI] [PubMed] [Google Scholar]
- 26.Teppema LJ, Balanos GM, Steinback CD, Brown AD, Foster GE, Duff HJ, Leigh R, Poulin MJ. Effects of acetazolamide on ventilatory, cerebrovascular, and pulmonary vascular responses to hypoxia. Am J Respir Crit Care Med 175: 277–281, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Tricarico D, Barbieri M, Mele A, Carbonara G, Camerino DC. Carbonic anhydrase inhibitors are specific openers of skeletal muscle BK channel of K+-deficient rats. FASEB J 18: 760–761, 2004 [DOI] [PubMed] [Google Scholar]