Abstract
IMPORTANCE
Few weight loss treatments produce clinically meaningful weight loss outcomes among black women, particularly in the primary care setting. New weight management strategies are necessary for this population. Weight gain prevention might be an effective treatment option, with particular benefits for overweight and class 1 obese black women.
OBJECTIVE
To compare changes in weight and cardiometabolic risk during a 12-month period among black women randomized to a primary care–based behavioral weight gain prevention intervention, relative to usual care.
DESIGN, SETTING, AND PARTICIPANTS
Two-arm randomized clinical trial (the Shape Program). We recruited patients from a 6-site community health center system. We randomized 194 overweight and class 1 obese (body mass index [calculated as weight in kilograms divided by height in meters squared], 25–34.9) premenopausal black women aged 25 to 44 years. Enrollment began on December 7, 2009; 12- and 18-month assessments were completed in February and October 2, 2012.
INTERVENTIONS
The medium-intensity intervention included tailored behavior change goals, weekly self-monitoring via interactive voice response, monthly counseling calls, tailored skills training materials, and a gym membership.
MAIN OUTCOMES AND MEASURES
Twelve-month change in weight and body mass index and maintenance of change at 18 months.
RESULTS
Participants had a mean age of 35.4 years, a mean weight of 81.1 kg, and a mean body mass index of 30.2 at baseline. Most were socioeconomically disadvantaged (79.7% with educational level less than a college degree; 74.3% reporting annual income <$30 000). The 12-month weight change was larger among intervention participants (mean [SD], −1.0 [0.5] kg), relative to usual care (0.5 [0.5] kg; mean difference, −1.4 kg [95%CI, −2.8 to −0.1 kg]; P = .04). At month 12, 62% of intervention participants were at or below their baseline weights compared with 45% of usual-care participants (P = .03). By 18 months, intervention participants maintained significantly larger changes in weight (mean difference, −1.7 kg; 95% CI, −3.3 to −0.2 kg).
CONCLUSIONS AND RELEVANCE
A medium-intensity primary care–based behavioral intervention demonstrated efficacy for weight gain prevention among socioeconomically disadvantaged black women. A “maintain, don’t gain” approach might be a useful alternative treatment for reducing obesity-associated disease risk among some premenopausal black women.
Promoting clinically meaningful weight loss among black women is a particularly vexing clinical challenge.1–3 Across numerous studies, including vanguard clinical weight loss trials,4–6 black women typically demonstrate smaller and less clinically relevant weight losses than white women and men and black men.7 The limited success in promoting clinically meaningful weight loss among this group suggests the need for new weight management approaches.8 For black women who are overweight and class 1 obese (body mass index [BMI; calculated as weight in kilograms divided by height in meters squared], 25–34.9), weight gain prevention might be one such strategy.9,10
There are several reasons that weight gain prevention might be an advantageous treatment option for black women in this BMI range. First, several studies have shown that for black women, overweight and class 1 obesity are less strongly associated with all-cause and cardiovascular disease mortality than in white women.11–15 For example, Calle et al15 found that black women with a BMI between 30 and 35 had an increased mortality risk that ranged up to 17% comparedwith a 30% to 53% increase for white women. Cardiometabolic risk factors similarly exhibit weaker associations with overweight and class 1 obesity in black women than in white women.16–21 For example, Taylor et al16 reported that type 2 diabetes mellitus, hypertension, and low high-density lipoprotein cholesterol were more strongly associated with BMI among whites than among blacks. Stevens et al21 showed that no black women, regardless of BMI, had an incidence of hypertriglyceridemia as high as that of white women with a BMI of 30.
Halting weight gains at the overweight or class 1 obese level might therefore maintain the relative health advantage of black women. However, at approximately 1 kg/y, the mean rate of premenopausal weight gain among black women outpaces the rates among women in other racial or ethnic groups.21,22 By age 40 to 59 years, black women have more than twice the prevalence of class 2 obesity than white women and 3 times the prevalence of class 3 (extreme) obesity.22 The combination of rapid premenopausal weight gain22,23 and extreme obesity22 contributes to disproportionate chronic disease risk among black women. It is possible that preventing premenopausal weight gains and stabilizing weight in the overweight and class 1 obese range would minimize the accumulation of visceral abdominal fat23 and reduce the odds of a host of adverse cardiometabolic outcomes.11–21
Second, black women may be particularly receptive to intervention messages about maintaining weight status. Relative to white women, black women have higher rates of body weight satisfaction, fewer social pressures to lose weight, and sociocultural norms that tolerate heavier body weights.24–26 Thus, prevention of weight gain might also be more acceptable than weight loss for black women in the overweight and class 1 obese weight range.
Finally, weight gain prevention can be achieved at lower treatment intensity than is required for weight loss.27 Such strategies are particularly well suited for delivery using electronic health (eHealth) technologies, which have the potential to reach large, high-risk populations at low cost.28 These features make weight gain prevention ideal for primary care settings, particularly those that serve populations with a disproportionate obesity burden.
We conducted a randomized clinical trial to evaluate the efficacy of a weight gain prevention intervention for overweight and class 1 obese (25–34.9) black female primary care patients in a community health center setting. We hypothesized that, relative to usual care, the intervention would promote weight stability for 12 months that would be maintained 18 months after randomization.
Methods
Study Design
As described elsewhere,29 the Shape Program (hereafter Shape) was a 2-arm parallel-group randomized clinical trial conducted among 194 premenopausal black women with a BMI of 25 to 34.9. Patient enrollment began on December 7, 2009, and 18-month follow-up assessments were completed on October 2, 2012. The Duke University Institutional Review Board and the Piedmont Health Board of Advisors approved all study procedures.
Patients were recruited from 6 community health centers operated by Piedmont Health, a nonprofit, federally qualified community health center system that serves a multi-county service area in central North Carolina. Piedmont's 37 000 registered patients are predominantly racial/ethnic minority (77%) and socioeconomically disadvantaged (98% have a documented household income <200% of the federal poverty level; 59% are uninsured).
Study Participants
Eligibility criteria included age of 25 to 44 years, BMI of 25 to 34.9, at least 1 visit to a Piedmont Health center in the prior 24 months, North Carolina residency, and self-reported English fluency. Exclusion criteria included pregnancy or postpartum status (≤12 months post partum), a history of myocardial infarction or stroke in the prior 2 years, and any history of cognitive, developmental, or psychiatric disorders.
Participant Screening and Recruitment
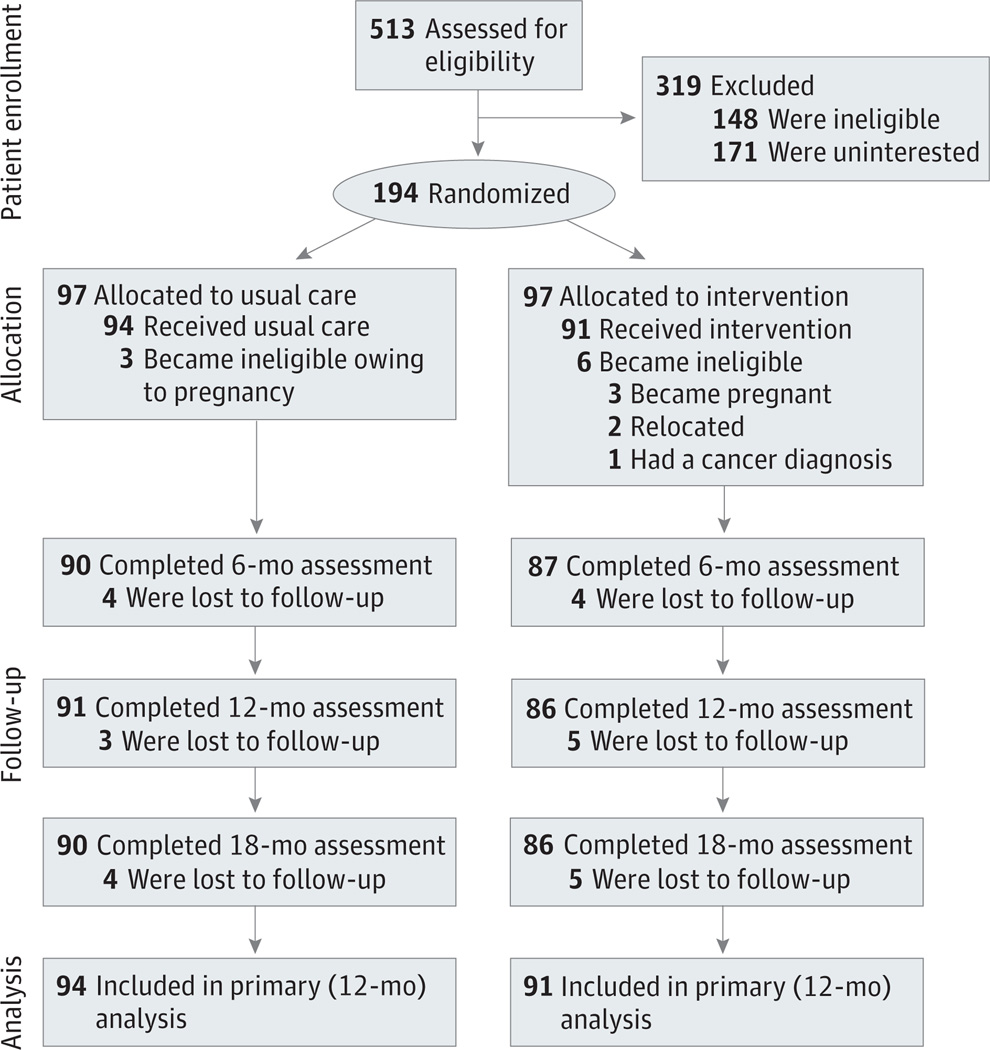
Piedmont Health staff generated lists of potentially eligible patients at each health center. After assessing BMI eligibility using abstracted anthropometric data, research staff sent potential participants study invitation brochures and guidance for opting out of future contact. No patients opted out of recruitment processes. After 1 week, study staff called patients to screen for eligibility. Eligible patients were asked to attend baseline study visits conducted in private rooms within the health centers or study offices. At these visits, participants provided informed consent and completed study assessments (see the CONSORT [Consolidated Standards for Reporting of Trials] flow diagram in the Figure).
Figure.
CONSORT (Consolidated Standards for Reporting of Trials) Flow Diagram
CONSORT flow diagram includes data on patient enrollment, allocation to treatment groups, follow-up, and primary analysis.
After completing baseline assessments, research staff initiated a computer-generated randomization algorithm to allocate participants equally (1:1) across the 2 treatment arms (intervention and usual care); those in the intervention arm were further randomized to 1 of 2 interventionists. The study design precluded blinding patients and interventionists to treatment assignment.
Treatment Arms
Usual Care
Study staff made no attempts to influence the medical treatment provided to those in the usual-care arm. Every 6 months, we sent usual-care participants newsletters that covered general wellness topics but did not discuss weight, nutrition, or physical activity.
Weight Gain Prevention Intervention
The Shape intervention is described more fully elsewhere.29 Briefly, it was a theory-based30 and evidence-based31–33 treatment designed to create a slight (<200 kcal) daily energy deficit to offset 12-month weight gains. Although a small amount of weight loss is advantageous to prevent future gains, we explicitly informed participants that Shape was not a weight loss trial. We did not expect participants to be motivated to lose weight. Instead, we informed participants that Shape was an approach designed to improve their overall well-being and to maintain their current body shape.
The 12-month intervention used the interactive obesity treatment approach (iOTA)31,32 and comprised 5 mutually reinforcing components: (1) tailored behavior change goals; (2) weekly self-monitoring via interactive voice response (IVR) telephone calls; (3) 12 counseling calls delivered monthly by a trained registered dietitian; (4) tailored skills training materials; and (5) a 12-month YMCA membership. Our intervention assigned participants a series of behavior change goals selected from a library of more than 20 goals. If achieved, these goals (eg, no sugar-sweetened beverages, no fast food, replacing energy-dense foods with ≥5 fruits or vegetables per day) will create the intended energy deficit.
At baseline and at the 6-month assessment, a computer algorithm assigned each participant 3 behavior change goals based on her need for change, self-efficacy, and readiness. Participants self-monitored their daily adherence to the behavior change goals during IVR calls (2–4 min) that were issued weekly by our computer systems. Brief tailored feedback and short skills training tips were provided after entry of self-monitoring data. Every 2 months, we provided participants with personalized progress reports and replaced 2 of their assigned goals based on their baseline and 6-month survey responses.
Piedmont Health registered dietitians (“Shape coaches”), who were trained in motivational interviewing principles,34 led monthly 20-minute counseling calls. Coaches reviewed patient self-monitoring data, provided skills training and social support, and used goal setting and problem-solving strategies to enhance behavior change self-efficacy. Study staff provided Shape coaches with a 2-day baseline training session, weekly supervision, and refresher trainings every 6 months. Study staff reviewed 5% of coaching calls for protocol adherence.
At baseline and every 2 months thereafter, participants were provided with a set of printed tailored skills training materials (designed for low-literacy audiences).
Measurements
The trial’s primary outcomes were change in weight and BMI at 12 months. We also examined maintenance of weight change at 18 months. Participants changed into hospital gowns for physical assessments. Trained staff measured participant heights to the nearest 0.1 cm using a calibrated wall-mounted stadiometer (Seca 214)35; weights were measured to the nearest 0.1 kg with an electronic scale (Seca Model 876).35 Secondary measures included waist circumference, blood pressure, and fasting glucose, triglyceride, and cholesterol levels, assessed using methods described elsewhere.29 All measurements were collected at baseline and at 6-, 12-, and 18-month follow-ups. We provided reimbursements of $50 each at baseline and at all follow-up study visits. We examined several measures of intervention engagement. These included IVR call completion, defined as the proportion of weekly IVR calls (52 total) resulting in the complete transmission of all self-monitoring data. We also examined the proportion of completed coaching calls (12 total) and use of the provided YMCA membership.
Data Analysis
The primary intent-to-treat analyses were based on the mean difference in weight and BMI between treatment arms at 12 months after adjustment for health center. We used mixed-effects regression models, which included all 185 participants who remained eligible at 12 months, to test a time × treatment interaction on absolute change in weight and BMI. The mixed-effects regression models used a random intercept and an unstructured covariance matrix. Participants with missing visits were treated as missing at random. Analyses were conducted using Proc MIXED in SAS, version 9.2 (SAS Institute). Similar modeling was estimated to examine maintenance of weight change 18 months after randomization, as well as change in cardiometabolic risk factors. We examined the proportion of participants who were at or below their baseline weight and tested for differences by software intervention group, controlling for site by using Cochran-Mantel-Haenszel statistic tests for general associations. We also compared blood pressure control (systolic blood pressure <140 mm Hg and diastolic blood pressure <90 mm Hg) between treatment arms across time using generalized estimating equation models with Proc GLIMMIX in SAS software, with a logistic link function, an unstructured covariance matrix, and a random subject effect.
We compared the findings from our intent-to-treat analyses with those from (1) per-protocol models that included only data collected within the window (4 weeks after the 6-, 12-, and 18-month study visits) and (2) models using multiple imputation to replace missing and out-of-window data. Multiple imputation models were based on the assumption of arbitrary missing patterns and used Markov chain Monte Carlo methods, which assume multivariate normality to impute missing values. These analyses followed procedures described in the SAS OnlineDoc (version 8; http://v8doc.sas.com)for multiple imputation using 10 generated data sets. Outcomes from these models were in line with the primary intent-to-treat analyses. This trial was designed to have 80% power to detect significant BMI differences of 1.03 between treatment groups 12 months after baseline.
Results
Baseline Characteristics
Participants had a mean (SD) baseline weight of 81.1 (8.8) kg, a mean BMI of 30.2 (2.5) (Table 1), and a mean age of 35.4 (5.5) years. Most participants (79.7%) had an educational level less than a college degree and were currently employed (71%). Many (74.3%) reported an annual income less than $30 000. Just less than one-third (30.8%) met criteria for the metabolic syndrome, and 21.5% reported depressive symptoms consistent with major depression. There were no baseline differences between treatment arms in weight or sociodemographic characteristics (Table 1).
Table 1.
Baseline Characteristics of Study Participants
| Characteristic | Total (N = 185) |
Usual Care (n = 94) |
Intervention (n = 91) |
|
|---|---|---|---|---|
| Age, mean (SD), y | 35.4 (5.5) | 35.2 (5.5) | 35.6 (5.5) | |
| Weight, mean (SD), kg | 81.1 (8.8) | 81.0 (8.8) | 81.3 (8.8) | |
| Body mass index, mean (SD)a | 30.2 (2.5) | 30.2 (2.4) | 30.1 (2.7) | |
| Waist circumference, mean (SD), cm | 97.8 (8.2) | 97.3 (8.0) | 98.2 (8.5) | |
| Blood pressure, mean (SD), mm Hg | ||||
| Systolic | 123.1 (14.8) | 122.9 (14.5) | 123.2 (15.3) | |
| Diastolic | 80.7 (10.9) | 80.4 (11.3) | 80.9 (10.7) | |
| Lipids, mean (SD), mg/dL | ||||
| Total cholesterol | 178.9 (37.5) | 181.3 (38.6) | 176.4 (36.4) | |
| Triglycerides | 102.5 (47.6) | 105.0 (54.6) | 99.6 (38.1) | |
| HDL cholesterol | 53.9 (16.1) | 53.9 (16.4) | 53.7 (15.8) | |
| LDL cholesterol | 107.0 (34.3) | 106.8 (34.3) | 107.3 (34.6) | |
| Glucose, mean (SD), mg/dL | 104.4 (43.2) | 105.4 (49.8) | 103.4 (35.5) | |
| Educational level, No. (%)b | ||||
| Less than high school | 19 (10.4) | 10 (10.8) | 9 (10.1) | |
| High school | 44 (24.2) | 22 (23.7) | 22 (24.7) | |
| Vocational or trade school after high school | 16 (8.8) | 6 (6.5) | 10 (11.2) | |
| Some college | 66 (36.3) | 34 (36.6) | 32 (36.0) | |
| College or above | 37 (20.3) | 21 (22.6) | 16 (18.0) | |
| Medical conditions, No. (%) | ||||
| Hypertensionc | 67 (36.4) | 34 (36.6) | 33 (36.3) | |
| Diabetes mellitusc | 12 (6.5) | 5 (5.3) | 7 (7.7) | |
| Metabolic syndromed | 57 (30.8) | 29 (30.9) | 28 (30.8) | |
| Depressione | 38 (21.5) | 18 (19.8) | 20 (23.3) | |
| Employment status, No. (%)b | ||||
| Employed | 130 (71.4) | 66 (70.2) | 64 (72.7) | |
| Not employed | 52 (28.6) | 28 (29.8) | 24 (27.3) | |
| Household income/y, No. (%)b | ||||
| <$10 000 | 38 (20.8) | 19 (20.7) | 19 (20.9) | |
| $10 000–19 999 | 52 (28.4) | 32 (34.8) | 20 (22.0) | |
| $20 000–29 999 | 46 (25.1) | 23 (25.0) | 23 (25.3) | |
| >$30 000 | 47 (25.7) | 18 (19.6) | 29 (31.9) | |
| Poverty, No. (%)b,f | ||||
| Yes | 60 (33.0) | 33 (35.9) | 27 (30.0) | |
| Borderline | 53 (29.1) | 27 (29.4) | 26 (28.9) | |
| No | 69 (37.9) | 32 (34.8) | 37 (41.1) | |
| Tried to lose weight in past 12 mo, No. (%)b | ||||
| Yes | 133 (72.3) | 70 (75.3) | 63 (69.2) | |
| No | 51 (27.7) | 23 (24.7) | 28 (30.8) | |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein.
SI conversion factors: To convert HDL, LDL, and total cholesterol values to millimoles per liter, multiply by 0.0259; to convert triglycerides to millimoles per liter, multiply by 0.0113; to convert glucose values to millimoles per liter, multiply by 0.0555.
Calculated as weight in kilograms divided by height in meters squared.
Numbers may not sum to 185 because of missing data.
Self-reported.
The criteria for metabolic syndrome were based on the guidelines developed by the National Cholesterol Education Program’s Adult Treatment Panel III report.36 Metabolic syndrome was defined as the presence of ≥3 of the following risk determinants: (1) increased waist circumference (>102 cm [>40 in] for men, >88 cm [>35 in] for women); (2) elevated triglycerides (≥150 mg/dL); (3) low HDL cholesterol (<40 mg/dL in men, <50 mg/dL in women); (4) hypertension (≥130/≥85 mm Hg); and (5) impaired fasting glucose (≥110 mg/dL).
Depression score ≥10 on the Patient Health Questionnaire–8 measure.37
Poverty thresholds were based on the US Census Bureau 2009 poverty thresholds.38
As shown in the Figure, we enrolled and randomized 194 participants. During the trial, 9 participants became ineligible (3 in the usual-care and 6 in the intervention arm). Of the remaining 185 participants, 177 (95.7%), 177 (95.7%), and 176 (95.1%) completed the 6-, 12-, and 18-month visits, respectively; 169 (91.4%) completed all 4 study visits. Participant attrition did not differ by treatment arm.
Intervention Engagement
Eligible intervention participants completed 81.9% of counseling calls during the 12-month intervention period. Five of 91 participants (5.5%) requested cessation of the counseling calls but continued to use other intervention components. Excluding these participants, the counseling call completion rate averaged 84.3% during the 12-month intervention period. The IVR call completion rate ranged between 65.2% and 89.5% per week, with a mean (SD) of 72% (28%). Of the intervention participants, 64 (70.3%) initiated their free YMCA membership during the 12-month study period, and 37 (40.7%) of them visited the YMCA more than once.
Weight Gain Prevention
In intent-to-treat analyses (Table 2),36–38 the mean (SD) 12-month weight change was significantly larger in the intervention arm (−1.0 [0.5] kg), relative to usual care (0.5 [0.5] kg; mean difference, −1.4 kg; 95%CI, −2.8 to −0.1 kg; P = .04). The mean (SD) 12-month change in BMI was similarly larger for intervention participants (−0.3 [0.2]) than for those receiving usual care (0.3 [0.2]; mean difference, −0.6; 95%CI, −1.1 to −0.1; P = .02). At 12 months, a significantly larger proportion of intervention participants (62.1%) were at or below their baseline weight compared with those in usual care (45.4%; P = .03).
Table 2.
Change in Weight and Body Mass Indexa
| Mean (SE) Change | ||||
|---|---|---|---|---|
| Measure | Usual Care (n = 94) |
Intervention (n = 91) |
Difference, Mean (95% CI) | |
| Weight, kg | ||||
| Month 6 | 0.1 (0.4) | −1.0 (0.4) | −1.1 (−2.3 to 0.04) | |
| Month 12 | 0.5 (0.5) | −1.0 (0.5) | −1.4 (−2.8 to −0.1) | |
| Month 18b | 0.8 (0.6) | −0.9 (0.6) | −1.7 (−3.3 to −0.2) | |
| Body mass indexc | ||||
| Month 6 | 0.1 (0.2) | −0.3 (0.2) | −0.4 (−0.8 to 0.03) | |
| Month 12 | 0.3 (0.2) | −0.3 (0.2) | −0.6 (−1.1 to −0.1) | |
| Month 18b | 0.4 (0.2) | −0.2 (0.2) | −0.6 (−1.2 to −0.1) | |
Denominators vary because of missing data.
The sample included 184 participants at month 18; 1 intervention participant became ineligible owing to pregnancy between 12 and 18 months.
Calculated as weight in kilograms divided by height in meters squared.
These weight changes were maintained at 18 months. Intervention participants exhibited a mean (SD) 18-month weight loss of −0.9 (0.6) kg, a significant loss compared with usual-care participants who gained a mean of 0.8 (0.6) kg (mean difference, −1.7 kg; 95% CI, −3.3 to −0.2 kg; P = .03). The intervention produced a significantly larger mean (SD) 18-month change in BMI (−0.2 [0.2]), relative to usual care (0.4 [0.2]; mean difference, −0.6; 95% CI, −1.2 to −0.1; P = .03). By 18 months, more intervention participants (53.2%) than control participants (38.5%) had weights at or below baseline values (P = .04).
Change in Cardiometabolic Risk Factors
We observed no differences between treatment arms in change in waist circumference, blood pressure, blood pressure control, glucose, or lipid levels at any time point (Table 3).
Table 3.
Change in Cardiometabolic Risk Factorsa
| Mean (SE) Change | ||||
|---|---|---|---|---|
| Measure | Usual Care (n = 94) | Intervention (n = 91) | Difference, Mean (95% CI) | |
| Systolic blood pressure, mm Hg | ||||
| Month 6 | −1.2 (1.3) | −1.7 (1.3) | −0.5 (−4.2 to 3.2) | |
| Month 12 | −1.6 (1.5) | −1.6 (1.5) | 0.01 (−4.1 to 4.2) | |
| Month 18b | 0.8 (1.3) | −3.0 (1.4) | −3.8 (−7.6 to 0.2) | |
| Diastolic blood pressure, mm Hg | ||||
| Month 6 | −1.3 (1.0) | −2.5 (1.0) | −1.1 (−4.0 to 1.7) | |
| Month 12 | −1.6 (1.1) | −2.3 (1.2) | −0.7 (−3.9 to 2.5) | |
| Month 18b | −1.0 (1.1) | −1.9 (1.1) | −0.9 (−3.9 to 2.2) | |
| Total cholesterol, mg/dL | ||||
| Month 6 | 0.9 (2.6) | −1.5 (2.6) | −2.4 (−9.7 to 4.8) | |
| Month 12 | −2.4 (2.6) | −4.9 (2.7) | −2.5 (−9.8 to 4.9) | |
| Month 18b | −4.5 (2.7) | −4.3 (2.8) | 0.1 (−7.6 to 7.8) | |
| Triglycerides, mg/dL | ||||
| Month 6 | 16.2 (6.8) | 8.9 (7.2) | −7.4 (−26.8 to 12.1) | |
| Month 12 | 4.2 (5.9) | 6.1 (6.4) | 1.8 (−15.3 to 18.9) | |
| Month 18b | 1.8 (5.3) | 0.2 (5.7) | 1.6 (−16.9 to 13.8) | |
| HDL cholesterol, mg/dL | ||||
| Month 6 | −3.2 (1.1) | −3.2 (1.1) | −0.03 (−3.1 to 3.0) | |
| Month 12 | −1.4 (1.2) | −1.6 (1.2) | −0.2 (−3.4 to 3.1) | |
| Month 18b | −1.6 (1.2) | −1.2 (1.2) | 0.4 (−3.0 to 3.8) | |
| LDL cholesterol, mg/dL | ||||
| Month 6 | 2.6 (2.9) | −0.8 (3.1) | −3.4 (−11.7 to 4.9) | |
| Month 12 | 0.1 (2.8) | −5.2 (3.1) | −5.4 (−13.7 to 2.9) | |
| Month 18b | −1.6 (2.9) | −3.3 (3.1) | −1.7 (−10.0 to 6.7) | |
| Waist circumference, cm | ||||
| Month 6 | −0.8 (0.6) | −1.4 (0.7) | −0.6 (−2.4 to 1.2) | |
| Month 12 | 0.3 (0.7) | −1.0 (0.7) | −1.3 (−3.1 to 0.5) | |
| Month 18b | −0.2 (0.8) | −1.4 (0.8) | −1.2 (−3.4 to 1.0) | |
| Fasting glucose, mg/dL | ||||
| Month 6 | 5.7 (3.6) | 5.8 (3.7) | 0.1 (−10.2 to 10.3) | |
| Month 12 | −5.1 (2.9) | −1.6 (3.0) | 3.5 (−4.7 to 11.7) | |
| Month 18b | −7.4 (3.1) | −3.1 (3.2) | 4.3 (−4.5 to 13.1) | |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein.
SI conversion factors: To convert HDL, LDL, and total cholesterol values to millimoles per liter, multiply by 0.0259; to convert triglycerides to millimoles per liter, multiply by 0.0113; to convert glucose values to millimoles per liter, multiply by 0.0555.
Denominators vary because of missing data.
The sample included 184 participants at month 18; 1 intervention participant became ineligible owing to pregnancy between 12 and 18 months.
Intervention Engagement and Weight Change
The IVR call completion rate was significantly correlated with 12-month weight loss (Spearman r= −0.2; P = .04); however, 12-month weight loss was not significantly correlated with completion of Shape counseling calls (Spearman r= −0.2; P = .16).
Adverse Events
Six serious adverse events were reported among participants in the intervention arm, including gynecological surgery in 2 participants and knee replacement, breast abscess, musculoskeletal injury, and cancer diagnosis in 1 participant each; all except the patient with the cancer diagnosis required hospitalization. We could not conclusively determine whether reported events resulted from study participation.
Discussion
Weight gain prevention has potential as a first-line weight management strategy.39–41 By shifting weight gain trajectories, this “maintain, don’t gain” approach may be particularly useful to combat the average 1-kg/y weight gain (and rapid entry into extreme obesity) typically observed among black women. Our findings demonstrate that weight gain can be prevented by using a medium-intensity42 eHealth intervention that is easily integrated into the primary care setting. Indeed, we found that the Shape intervention prevented weight gain as intended through 18-month follow-up, and even achieved slight reductions in weight and BMI.
Several characteristics of Shape should be considered to contextualize our study outcomes. First, nationally, more than half (51.2%) of overweight and obese black women fall within the 25 to 34.9 BMI range.22 This population has elevated risk for future weight gain, extreme obesity, and obesity-associated chronic disease. Our findings may have major clinical and public health significance. Preventing weight gain in this population over time might help maintain the population’s lower relative risk of obesity-associated chronic disease and mortality. Second, Shape participants were recruited from a socioeconomically disadvantaged population that has been underrepresented in obesity intervention trials. Socioeconomic factors are critical drivers of obesity risk behaviors and environments43 and may pose a particular challenge to obesity interventions tested among racial or ethnic minority populations. Third, Shape was conducted in the primary care setting but without primary care providers delivering intervention content. This was an intentional design decision that reflects the challenges facing primary care providers in busy and underresourced community health center settings. We also used inexpensive eHealth technologies to enhance the intervention's reach, accessibility, and scalability. Our findings—particularly the high rates of IVR call engagement and their correlation with greater weight losses—demonstrate that eHealth intervention strategies can be effectively implemented in socioeconomically disadvantaged patient populations that are increasingly adopting such technologies.44,45
Relatively few studies have aimed to prevent weight gain among patients not first exposed to a weight loss intervention, especially in the US primary care setting. Our findings may be most comparable to those of the Groningen Overweight and Lifestyle study (GOAL), which randomized primary care patients (with hypertension and/or dyslipidemia) to usual care or to a weight gain prevention intervention.41 The intervention comprised 4 sessions of individual counseling and a telephone-based feedback session with a nurse practitioner. The GOAL intervention produced significant weight change of −1.4 kg and BMI change of −0.5 at 12 months; like Shape, the GOAL intervention did not produce significant change in blood pressure or lipid levels. However, because GOAL participants were much older and had more comorbid conditions than Shape participants, we suspect that they may have been more motivated to maintain their weight to control their chronic health conditions.
The primary treatment goal of Shape was to create a slight energy deficit sufficient to offset weight gain. Shape was not a weight loss intervention, and participants were fully and frequently informed to this effect. Weight loss, although welcome, was unintended. Interestingly, however, the magnitude of 12-and 18-month weight and BMI change outcomes with Shape exceed those of several behavioral weight loss interventions conducted among black populations,31,46–48 particularly those in the primary care setting.31 Our findings show that similar levels of weight change can be produced at lower treatment intensity with an eHealth approach and with intervention content focused on weight gain prevention as opposed to weight loss.
The rates of cardiometabolic risk (hypertension, 36.3%; and metabolic syndrome, 30.8%) among Shape participants are concerning; without intervention, this group will probably bear additional chronic disease burden. Weight loss is certainly indicated as a primary treatment approach, particularly given its well-demonstrated ability to produce improvements in cardiometabolic risk.49 However, there is little evidence that weight gain prevention will promote reductions in cardiometabolic risk.50 Indeed, Shape did not produce changes in cardiometabolic risk factors, although this outcome is perhaps unsurprising given the length of study follow-up and the low magnitude of weight change. Although weight gain prevention might seem controversial given current national guidelines,51 considering the difficulty in achieving clinically meaningful long-term weight loss in black women,3 using weight gain prevention to stabilize cardiometabolic parameters might be a reasonably desirable alternative treatment outcome.
Several considerations limit the interpretations drawn from our findings. First, 18 months of follow-up may not have been sufficient to demonstrate the magnitude of our findings; trials of similar interventions with longer follow-up are desirable. Second, our intervention included multiple components and our trial design did not allow us to examine their independent effects. Finally, our trial design did not control for attention; this may have affected outcomes among those randomized to the intervention arm.
Several issues may affect the generalizability of these findings. The Shape intervention was targeted to a large population (51.2% of the overweight and class 1 obese black women). We conducted Shape in a community health center system that has a large, socioeconomically disadvantaged patient population. Although this is a major strength of the trial, our findings may not extend to patient populations in dissimilar settings. Finally, we deemed more than half (53.6%) of those excluded as “uninterested” in participation. More than two-thirds of this group comprised “passive” disinterest. Thus, we have no data on which to base judgments about their reasons for nonparticipation. However, randomized participants did not differ in either age or BMI from those who were uninterested.
In summary, we found that a primary care–based behavioral intervention stabilized weight over 12 and 18 months among overweight and class 1 obese black women. Weight gain prevention has important health benefits for this population and prevention messages have sociocultural salience. Promoting weight loss is a challenge in all populations, but it has been consistently and disproportionately more onerous among black women. It is clear that new treatment approaches, such as weight gain prevention, are necessary to contend with the considerable challenge of obesity in this population.
Acknowledgments
Funding/Support: This trial is funded by grant R01DK078798 from the National Institute for Diabetes and Digestive and Kidney Diseases. Dr Emmons was supported by grant K05CA124415 from the National Cancer Institute.
Role of the Sponsors: The National Institute for Diabetes and Digestive and Kidney Diseases had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Study concept and design: Bennett, Foley, Levine, Whiteley, Steinberg, Batch, Greaney, Miranda, Holder.
Acquisition of data: Bennett, Foley, Levine, Miranda, Wroth, Holder, Emmons.
Analysis and interpretation of data: Bennett, Foley, Askew, Steinberg, Batch, Emmons, Puleo.
Drafting of the manuscript: Bennett, Foley, Greaney, Puleo.
Critical revision of the manuscript for important intellectual content: Foley, Levine, Whiteley, Askew, Steinberg, Batch, Greaney, Miranda, Wroth, Holder, Emmons.
Statistical analysis: Puleo.
Obtained funding: Whiteley.
Administrative, technical, and material support: Bennett, Foley, Levine, Askew, Batch, Greaney, Miranda, Wroth, Holder, Emmons.
Study supervision: Bennett, Foley, Miranda.
Conflict of Interest Disclosures: None reported.
Additional Contributions: We express deep gratitude to the administration and staff of Piedmont Health for their continued collaboration and participation in the Shape Program. In particular, we thank Ashley Brewer, RD, LDN, Greg Wheatley, MPH, RD, LDN, Kristen Norton, MA, RD, LDN, and the staff of Piedmont Health’s health centers for their support. We appreciate the tireless work of research assistants Daniel Dix, Michele Lanpher, Veronica Lett, and Jade Miller. We are grateful to Martha Zorn, MS, at the University of Massachusetts, Amherst for her assistance with data analysis. Finally, we would like to especially thank the women participating in Shape.
REFERENCES
- 1.Yancey AK, Kumanyika SK, Ponce NA, et al. Population-based interventions engaging communities of color in healthy eating and active living: a review [published online December 15, 2003] Prev Chronic Dis. 2004;1(1):A09. [PMC free article] [PubMed] [Google Scholar]
- 2.Kumanyika SK. Obesity treatment in minorities. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. 3rd ed. New York, NY: Guilford Publications; 2002. pp. 416–446. [Google Scholar]
- 3.Osei-Assibey G, Kyrou I, Adi Y, Kumar S, Matyka K. Dietary and lifestyle interventions for weight management in adults from minority ethnic/non-white groups: a systematic review. Obes Rev. 2010;11(11):769–776. doi: 10.1111/j.1467-789X.2009.00695.x. [DOI] [PubMed] [Google Scholar]
- 4.Hollis JF, Gullion CM, Stevens VJ, et al. Weight Loss Maintenance Trial Research Group. Weight loss during the intensive intervention phase of the Weight-Loss Maintenance trial. Am J Prev Med. 2008;35(2):118–126. doi: 10.1016/j.amepre.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wing RR, Hamman RF, Bray GA, et al. Diabetes Prevention Program Research Group. Achieving weight and activity goals among Diabetes Prevention Program lifestyle participants. Obes Res. 2004;12(9):1426–1434. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svetkey LP, Erlinger TP, Vollmer WM, et al. Effect of lifestyle modifications on blood pressure by race, sex, hypertension status, and age. J Hum Hypertens. 2005;19(1):21–31. doi: 10.1038/sj.jhh.1001770. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgibbon ML, Tussing-Humphreys LM, Porter JS, Martin IK, Odoms-Young A, Sharp LK. Weight loss and African-American women: a systematic review of the behavioural weight loss intervention literature. Obes Rev. 2012;13(3):193–213. doi: 10.1111/j.1467-789X.2011.00945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumanyika SK, Gary TL, Lancaster KJ, et al. Achieving healthy weight in African-American communities: research perspectives and priorities. Obes Res. 2005;13(12):2037–2047. doi: 10.1038/oby.2005.251. [DOI] [PubMed] [Google Scholar]
- 9.Gill TP. Key issues in the prevention of obesity. Br Med Bull. 1997;53(2):359–388. doi: 10.1093/oxfordjournals.bmb.a011618. [DOI] [PubMed] [Google Scholar]
- 10.Wareham NJ, van Sluijs EMF, Ekelund U. Physical activity and obesity prevention: a review of the current evidence. Proc Nutr Soc. 2005;64(2):229–247. doi: 10.1079/pns2005423. [DOI] [PubMed] [Google Scholar]
- 11.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289(2):187–193. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein EA, Brown DS, Wrage LA, Allaire BT, Hoerger TJ. Individual and aggregate years-of-life-lost associated with overweight and obesity. Obesity (Silver Spring) 2010;18(2):333–339. doi: 10.1038/oby.2009.253. [DOI] [PubMed] [Google Scholar]
- 13.Stevens J. Obesity and mortality in Africans-Americans. Nutr Rev. 2000;58(11):346–353. doi: 10.1111/j.1753-4887.2000.tb01832.x. [DOI] [PubMed] [Google Scholar]
- 14.Stevens J, Juhaeri, Cai J, Jones DW. The effect of decision rules on the choice of a body mass index cutoff for obesity: examples from African American and white women. Am J Clin Nutr. 2002;75(6):986–992. doi: 10.1093/ajcn/75.6.986. [DOI] [PubMed] [Google Scholar]
- 15.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341(15):1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 16.Taylor HA, Jr, Coady SA, Levy D, et al. Relationships of BMI to cardiovascular risk factors differ by ethnicity. Obesity (Silver Spring) 2010;18(8):1638–1645. doi: 10.1038/oby.2009.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durazo-Arvizu RA, McGee DL, Cooper RS, Liao Y, Luke A. Mortality and optimal body mass index in a sample of the US population. Am J Epidemiol. 1998;147(8):739–749. doi: 10.1093/oxfordjournals.aje.a009518. [DOI] [PubMed] [Google Scholar]
- 18.Dustan HP. Obesity and hypertension in blacks. Cardiovasc Drugs Ther. 1990;4(suppl 2):395–402. doi: 10.1007/BF02603183. [DOI] [PubMed] [Google Scholar]
- 19.Pan WH, Flegal KM, Chang HY, Yeh WT, Yeh CJ, Lee WC. Body mass index and obesity-related metabolic disorders in Taiwanese and US whites and blacks: implications for definitions of overweight and obesity for Asians. Am J Clin Nutr. 2004;79(1):31–39. doi: 10.1093/ajcn/79.1.31. [DOI] [PubMed] [Google Scholar]
- 20.Colin Bell A, Adair LS, Popkin BM. Ethnic differences in the association between body mass index and hypertension. Am J Epidemiol. 2002;155(4):346–353. doi: 10.1093/aje/155.4.346. [DOI] [PubMed] [Google Scholar]
- 21.Stevens J, Juhaeri, Cai J, Jones DW. The effect of decision rules on the choice of a body mass index cutoff for obesity: examples from African American and white women. Am J Clin Nutr. 2002;75(6):986–992. doi: 10.1093/ajcn/75.6.986. [DOI] [PubMed] [Google Scholar]
- 22.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Coady S, Carr JJ, Hoffman U, Taylor HA, Fox CS. Differential associations of abdominal visceral, subcutaneous adipose tissue with cardiometabolic risk factors between African and European Americans [published online February 14, 2013] Obesity (Silver Spring) 2013 doi: 10.1002/oby.20307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flynn KJ, Fitzgibbon M. Body images and obesity risk among black females: a review of the literature. Ann Behav Med. 1998;20(1):13–24. doi: 10.1007/BF02893804. [DOI] [PubMed] [Google Scholar]
- 25.Blixen CE, Singh A, Xu M, Thacker H, Mascha E. What women want: understanding obesity and preferences for primary care weight reduction interventions among African-American and Caucasian women. J Natl Med Assoc. 2006;98(7):1160–1170. [PMC free article] [PubMed] [Google Scholar]
- 26.Ard JD. Unique perspectives on the obesogenic environment. J Gen Intern Med. 2007;22(7):1058–1060. doi: 10.1007/s11606-007-0243-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitz MKH, Jeffery RW. Public health interventions for the prevention and treatment of obesity. Med Clin North Am. 2000;84(2):491–512. doi: 10.1016/s0025-7125(05)70233-9. viii. [DOI] [PubMed] [Google Scholar]
- 28.Bennett GG, Glasgow RE. The delivery of public health interventions via the Internet: actualizing their potential. Annu Rev Public Health. 2009;30:273–292. doi: 10.1146/annurev.publhealth.031308.100235. [DOI] [PubMed] [Google Scholar]
- 29.Foley P, Levine E, Askew S, et al. Weight gain prevention among black women in the rural community health center setting: the Shape Program. BMC Public Health. 2012;12(1):305. doi: 10.1186/1471-2458-12-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bandura A. Self-Efficacy: The Exercise of Control. New York, NY: Freeman; 1997. [Google Scholar]
- 31.Bennett GG, Warner ET, Glasgow RE, et al. Be Fit, Be Well Study Investigators. Obesity treatment for socioeconomically disadvantaged patients in primary care practice. Arch Intern Med. 2012;172(7):565–574. doi: 10.1001/archinternmed.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett GG, Herring SJ, Puleo E, Stein EK, Emmons KM, Gillman MW. Web-based weight loss in primary care: a randomized controlled trial. Obesity (Silver Spring) 2010;18(2):308–313. doi: 10.1038/oby.2009.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greaney ML, Quintiliani LM, Warner ET, et al. Weight management among patients at community health centers: the “Be Fit, Be Well” study. Obes Weight Management. 2009;5(5):222–228. [Google Scholar]
- 34.Miller W, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behaviors. New York, NY: Guilford Press; 1993. [Google Scholar]
- 35.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey Protocol. Hyattsville, MD: US Dept of Health and Human Services, Centers for Disease Control and Prevention; 2007. [Google Scholar]
- 36.National Institutes of Health. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): Executive summary. Bethesda, MD: National Institutes of Health; 2001. [Google Scholar]
- 37.Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1–3):163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 38.US Census Bureau. Poverty thresholds 2009. [Accessed May 7, 2012]; http://www.census.gov/hhes/www/poverty/data/threshld/thresh09.html.
- 39.Serdula MK, Khan LK, Dietz WH. Weight loss counseling revisited. JAMA. 2003;289(14):1747–1750. doi: 10.1001/jama.289.14.1747. [DOI] [PubMed] [Google Scholar]
- 40.Lean M, Lara J, Hill JO. ABC of obesity: strategies for preventing obesity. BMJ. 2006;333(7575):959–962. doi: 10.1136/bmj.333.7575.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ter Bogt NCW, Bemelmans WJE, Beltman FW, Broer J, Smit AJ, van der Meer K. Preventing weight gain: one-year results of a randomized lifestyle intervention. Am J Prev Med. 2009;37(4):270–277. doi: 10.1016/j.amepre.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 42.McTigue KM, Harris R, Hemphill B, et al. Screening and interventions for obesity in adults: summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2003;139(11):933–949. doi: 10.7326/0003-4819-139-11-200312020-00013. [DOI] [PubMed] [Google Scholar]
- 43.Giskes K, van Lenthe F, Avendano-Pabon M, Brug J. A systematic review of environmental factors and obesogenic dietary intakes among adults: are we getting closer to understanding obesogenic environments? Obes Rev. 2011;12(5):e95–e106. doi: 10.1111/j.1467-789X.2010.00769.x. [DOI] [PubMed] [Google Scholar]
- 44.Smith A. Mobile Access 2010. Washington, DC: Pew Internet & American Life Project; 2010. [Google Scholar]
- 45.Smith A. Technology Trends Among People of Color. Washington, DC: Pew Internet & American Life Project; 2010. [Google Scholar]
- 46.Kumanyika SK, Wadden TA, Shults J, et al. Trial of family and friend support for weight loss in African American adults. Arch Intern Med. 2009;169(19):1795–1804. doi: 10.1001/archinternmed.2009.337. [DOI] [PubMed] [Google Scholar]
- 47.Fitzgibbon ML, Stolley MR, Schiffer L, Sharp LK, Singh V, Dyer A. Obesity Reduction Black Intervention Trial (ORBIT): 18-month results. Obesity (Silver Spring) 2010;18(12):2317–2325. doi: 10.1038/oby.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin PD, Dutton GR, Rhode PC, Horswell RL, Ryan DH, Brantley PJ. Weight loss maintenance following a primary care intervention for low-income minority women. Obesity (Silver Spring) 2008;16(11):2462–2467. doi: 10.1038/oby.2008.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wing RR, Lang W, Wadden TA, et al. Look AHEAD Research Group. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Truesdale KP, Stevens J, Lewis CE, Schreiner PJ, Loria CM, Cai J. Changes in risk factors for cardiovascular disease by baseline weight status in young adults who maintain or gain weight over 15 years: the CARDIA study. Int J Obes (Lond) 2006;30(9):1397–1407. doi: 10.1038/sj.ijo.0803307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moyer VA US Preventive Services Task Force. Screening for and management of obesity in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(5):373–378. doi: 10.7326/0003-4819-157-5-201209040-00475. [DOI] [PubMed] [Google Scholar]