Abstract
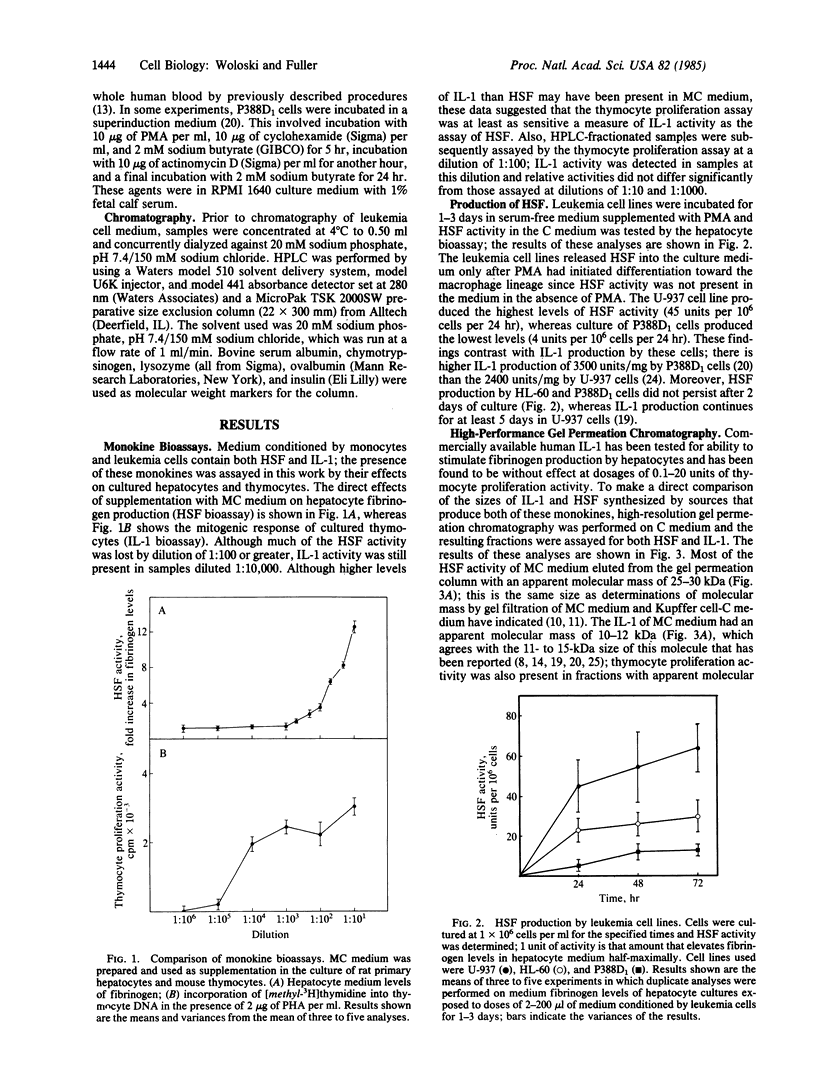
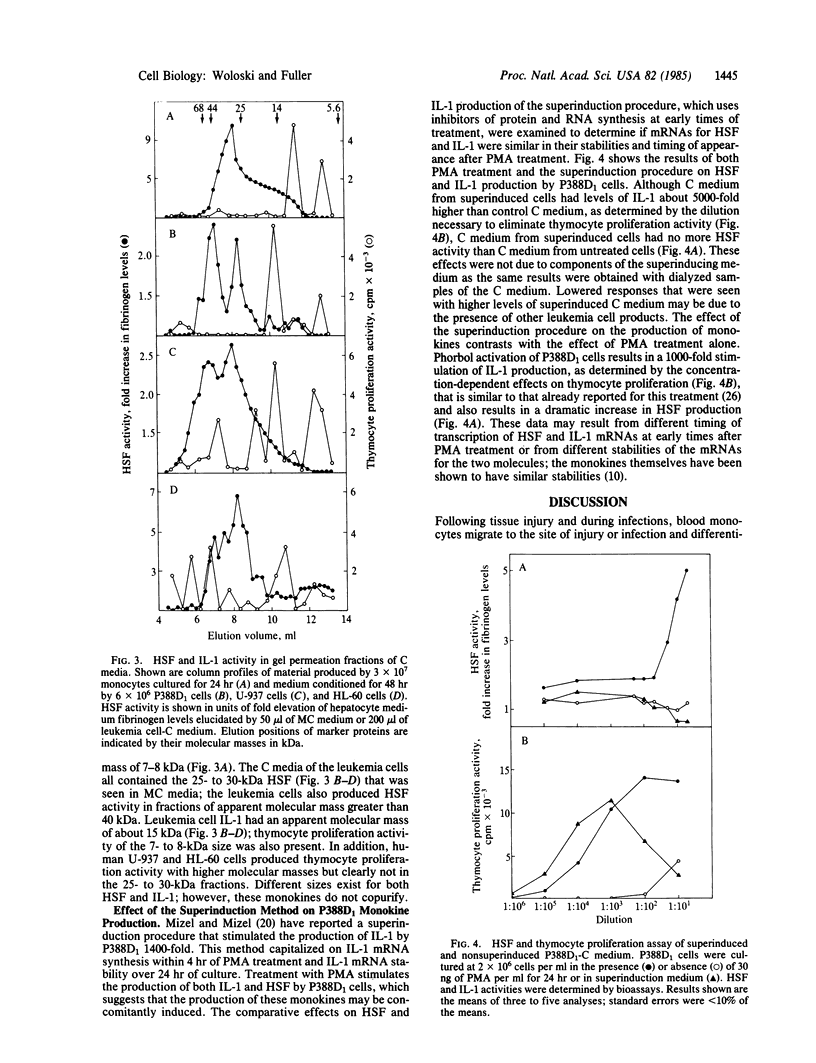
Leukemia cell lines of the monocytic series (HL-60, U-937, and P388D1) produce a hepatocyte-stimulating factor (HSF) following induction of differentiation with phorbol diester. In 24-72 hr, these leukemia cells produce 2-30% the amount of HSF as human peripheral blood monocytes. Cells of the series at earlier stages of differentiation produced greater amounts of HSF. Fractionation of the medium from each cell type by HPLC reveals much of the HSF activity in the 25- to 30-kilodalton range. Under the same culture conditions, interleukin 1 is produced; however, its bioactivity is in the 7- to 15-kilodalton range. Neither monokine shows reciprocal bioactivity. Superinducing culture conditions that greatly increase interleukin 1 production completely eliminate HSF production, suggesting that there is different stability of the mRNA coding for each protein or that there are different temporal events important to the induction of synthesis of these proteins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann H., Jahreis G. P., Sauder D. N., Koj A. Human keratinocytes and monocytes release factors which regulate the synthesis of major acute phase plasma proteins in hepatic cells from man, rat, and mouse. J Biol Chem. 1984 Jun 10;259(11):7331–7342. [PubMed] [Google Scholar]
- Beisel W. R. Effects of infection on nutritional status and immunity. Fed Proc. 1980 Nov;39(13):3105–3108. [PubMed] [Google Scholar]
- Bornstein D. L. Leukocytic pyrogen: a major mediator of the acute phase reaction. Ann N Y Acad Sci. 1982;389:323–337. doi: 10.1111/j.1749-6632.1982.tb22147.x. [DOI] [PubMed] [Google Scholar]
- Fuller G. M., Ritchie D. G. A regulatory pathway for fibrinogen biosynthesis involving an indirect feedback loop. Ann N Y Acad Sci. 1982;389:308–322. doi: 10.1111/j.1749-6632.1982.tb22146.x. [DOI] [PubMed] [Google Scholar]
- Jamieson J. C., Friesen A. D., Ashton F. E., Chou B. Studies on acute phase proteins of rat serum. 1. Isolation and partial characterization of an 1 -acid glycoprotein and an 2 -macroglobulin. Can J Biochem. 1972 Aug;50(8):856–870. doi: 10.1139/o72-121. [DOI] [PubMed] [Google Scholar]
- Kampschmidt R. F., Upchurch H. F., Pulliam L. A. Characterization of a leukocyte-derived endogenous mediator responsible for increased plasma fibrinogen. Ann N Y Acad Sci. 1982;389:338–353. doi: 10.1111/j.1749-6632.1982.tb22148.x. [DOI] [PubMed] [Google Scholar]
- Kaplan H. A., Woloski B. M., Hellman M., Jamieson J. C. Studies on the effect of inflammation on rat liver and serum sialyltransferase. Evidence that inflammation causes release of Gal beta 1 leads to 4GlcNAc alpha 2 leads to 6 sialyltransferase from liver. J Biol Chem. 1983 Oct 10;258(19):11505–11509. [PubMed] [Google Scholar]
- Kimball E. S., Pickeral S. F., Oppenheim J. J., Rossio J. L. Interleukin 1 activity in normal human urine. J Immunol. 1984 Jul;133(1):256–260. [PubMed] [Google Scholar]
- Koeffler H. P. Induction of differentiation of human acute myelogenous leukemia cells: therapeutic implications. Blood. 1983 Oct;62(4):709–721. [PubMed] [Google Scholar]
- Koren H. S., Handwerger B. S., Wunderlich J. R. Identification of macrophage-like characteristics in a cultured murine tumor line. J Immunol. 1975 Feb;114(2 Pt 2):894–897. [PubMed] [Google Scholar]
- Krakauer T., Oppenheim J. J. Interleukin 1 production by a human acute monocytic leukemia cell line. Cell Immunol. 1983 Sep;80(2):223–229. doi: 10.1016/0008-8749(83)90111-9. [DOI] [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Kwan S. W., Fuller G. M. Immunochemical characterization of fibrinogen induction in rat liver. Biochim Biophys Acta. 1977 Apr 19;475(4):659–668. doi: 10.1016/0005-2787(77)90326-4. [DOI] [PubMed] [Google Scholar]
- Kwan S. W., Fuller G. M., Krautter M. A., van Baval J. H., Goldblum R. M. Quantitation of cytoplasmic fibrinogen in rat liver cells. Anal Biochem. 1977 Dec;83(2):589–596. doi: 10.1016/0003-2697(77)90062-8. [DOI] [PubMed] [Google Scholar]
- Lachman L. B. Human interleukin 1: purification and properties. Fed Proc. 1983 Jun;42(9):2639–2645. [PubMed] [Google Scholar]
- Lachman L. B., Metzgar R. S. Characterization of high and low molecular weight lymphocyte-activating factor (interleukin I) from P388D and J774.1 mouse macrophage cell lines. J Reticuloendothel Soc. 1980 Jun;27(6):621–629. [PubMed] [Google Scholar]
- Mizel S. B., Dukovich M., Rothstein J. Preparation of goat antibodies against interleukin 1: use of an immunoadsorbent to purify interleukin 1. J Immunol. 1983 Oct;131(4):1834–1837. [PubMed] [Google Scholar]
- Mizel S. B. Interleukin 1 and T cell activation. Immunol Rev. 1982;63:51–72. doi: 10.1111/j.1600-065x.1982.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Mizel D. Purification to apparent homogeneity of murine interleukin 1. J Immunol. 1981 Mar;126(3):834–837. [PubMed] [Google Scholar]
- Mizel S. B. Studies on the purification and structure-functional relationships of murine lymphocyte activating factor (Interleukin 1). Mol Immunol. 1980 May;17(5):571–577. doi: 10.1016/0161-5890(80)90155-8. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Palacios R., Ivhed I., Sideras P., Nilsson K., Sugawara I., Fernandez C. Accessory function of human tumor cell lines. I. Production of interleukin 1 by the human histiocytic lymphoma cell line U-937. Eur J Immunol. 1982 Oct;12(10):895–899. doi: 10.1002/eji.1830121018. [DOI] [PubMed] [Google Scholar]
- Ritchie D. G., Fuller G. M. An in vitro bioassay for leukocytic endogenous mediator(s) using cultured rat hepatocytes. Inflammation. 1981 Dec;5(4):275–287. doi: 10.1007/BF00911093. [DOI] [PubMed] [Google Scholar]
- Ritchie D. G., Fuller G. M. Hepatocyte-stimulating factor: a monocyte-derived acute-phase regulatory protein. Ann N Y Acad Sci. 1983 Jun 27;408:490–502. doi: 10.1111/j.1749-6632.1983.tb23268.x. [DOI] [PubMed] [Google Scholar]
- Rupp R. G., Fuller G. M. The effects of leucocytic and serum factors on fibrinogen biosynthesis in cultured hepatocytes. Exp Cell Res. 1979 Jan;118(1):23–30. doi: 10.1016/0014-4827(79)90579-2. [DOI] [PubMed] [Google Scholar]
- Sanders K. D., Fuller G. M. Kupffer cell regulation of fibrinogen synthesis in hepatocytes. Thromb Res. 1983 Oct 15;32(2):133–145. doi: 10.1016/0049-3848(83)90025-7. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Seglen P. O. Preparation of rat liver cells. 3. Enzymatic requirements for tissue dispersion. Exp Cell Res. 1973 Dec;82(2):391–398. doi: 10.1016/0014-4827(73)90357-1. [DOI] [PubMed] [Google Scholar]
- Tatsuta E., Sipe J. D., Shirahama T., Skinner M., Cohen A. S. Different regulatory mechanisms for serum amyloid A and serum amyloid P synthesis by cultured mouse hepatocytes. J Biol Chem. 1983 May 10;258(9):5414–5418. [PubMed] [Google Scholar]
- Woloski B. M., Kaplan H. A., Gospodarek E., Jamieson J. C. Studies on rat cytokines as mediators of the acute phase response. Biochem Biophys Res Commun. 1983 Apr 15;112(1):14–19. doi: 10.1016/0006-291x(83)91790-4. [DOI] [PubMed] [Google Scholar]



