Abstract
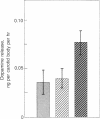
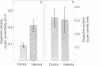
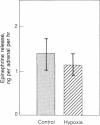
Chemoreceptors for oxygen reside within the carotid body, but it is not known which cells actually sense hypoxia and by what mechanisms they transduce this information into afferent signals in the carotid sinus nerve. We have developed systems for the growth of glomus cells of the carotid body in dissociated cell culture. Here we demonstrate that, as in vivo, these cells contain the putative neurotransmitters dopamine, serotonin, and norepinephrine. Oxygen tension regulates the rate of dopamine secretion from the glomus cells. Similar to chemically stimulated catecholamine secretion from other adrenergic cells this hypoxia-stimulated release requires extracellular calcium. These results are compatible with the suggestion that the glomus cells of the carotid body are chemoreceptor cells and that they signal hypoxia by regulated secretion of dopamine.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biscoe T. J. Carotid body: structure and function. Physiol Rev. 1971 Jul;51(3):437–495. doi: 10.1152/physrev.1971.51.3.437. [DOI] [PubMed] [Google Scholar]
- Chiocchio S. R., Biscardi A. M., Tramezzani J. H. 5-hydroxytryptamine in the carotid body of the cat. Science. 1967 Nov 10;158(3802):790–791. doi: 10.1126/science.158.3802.790. [DOI] [PubMed] [Google Scholar]
- Davidson J. T., Whipp B. J., Wasserman K., Koyal S. N., Lugliani R. Role of the carotid bodies in breath-holding. N Engl J Med. 1974 Apr 11;290(15):819–822. doi: 10.1056/NEJM197404112901502. [DOI] [PubMed] [Google Scholar]
- Eyzaguirre C., Fidone S. J. Transduction mechanisms in carotid body: glomus cells, putative neurotransmitters, and nerve endings. Am J Physiol. 1980 Nov;239(5):C135–C152. doi: 10.1152/ajpcell.1980.239.5.C135. [DOI] [PubMed] [Google Scholar]
- Fidone S., Gonzalez C., Yoshizaki K. Effects of hypoxia on catecholamine synthesis in rabbit carotid body in vitro. J Physiol. 1982 Dec;333:81–91. doi: 10.1113/jphysiol.1982.sp014440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidone S., Gonzalez C., Yoshizaki K. Effects of low oxygen on the release of dopamine from the rabbit carotid body in vitro. J Physiol. 1982 Dec;333:93–110. doi: 10.1113/jphysiol.1982.sp014441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman M. C., Schaffner A. E. Carotid body cell culture and selective growth of glomus cells. Am J Physiol. 1984 Jan;246(1 Pt 1):C106–C113. doi: 10.1152/ajpcell.1984.246.1.C106. [DOI] [PubMed] [Google Scholar]
- JOELS N., NEIL E. The excitation mechanism of the carotid body. Br Med Bull. 1963 Jan;19:21–24. doi: 10.1093/oxfordjournals.bmb.a069999. [DOI] [PubMed] [Google Scholar]
- Lahiri S. Chemical modification of carotid body chemoreception by sulfhydryls. Science. 1981 May 29;212(4498):1065–1066. doi: 10.1126/science.6262913. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M. The ontogeny of the neural crest in avian embryo chimaeras. Nature. 1980 Aug 14;286(5774):663–669. doi: 10.1038/286663a0. [DOI] [PubMed] [Google Scholar]
- Mefford I. N. Application of high performance liquid chromatography with electrochemical detection to neurochemical analysis: measurement of catecholamines, serotonin and metabolites in rat brain. J Neurosci Methods. 1981 Feb;3(3):207–224. doi: 10.1016/0165-0270(81)90056-x. [DOI] [PubMed] [Google Scholar]
- Pearse A. G., Polak J. M., Rost F. W., Fontaine J., Le Lièvre C., Le Douarin N. Demonstration of the neural crest origin of type I (APUD) cells in the avian carotid body, using a cytochemical marker system. Histochemie. 1973;34(3):191–203. doi: 10.1007/BF00303435. [DOI] [PubMed] [Google Scholar]
- Zapata P., Stensaas L. J., Eyzaguirre C. Axon regeneration following a lesion of the carotid nerve: electrophysiological and ultrastructural observations. Brain Res. 1976 Aug 27;113(2):235–253. doi: 10.1016/0006-8993(76)90939-2. [DOI] [PubMed] [Google Scholar]