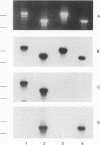
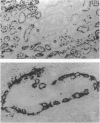
Abstract
Testicular germ cell tumors are the most common form of cancer in young adult males. They result from a derangement of primordial germ cells, and they grow out from a noninvasive carcinoma-in-situ precursor. Since carcinoma in situ can readily be cured by low-dose irradiation, there is a great incentive for non- or minimally invasive methods for detection of carcinoma in situ. We have recently shown that human Tera-2 embryonal carcinoma cells, obtained from a nonseminomatous testicular germ cell tumor, show alternative splicing and alternative promoter use of the platelet-derived growth factor alpha-receptor gene, giving rise to a unique 1.5-kb transcript. In this study we have set up a reverse transcriptase-polymerase chain reaction strategy for characterization of the various transcripts for this receptor. Using this technique, we show that a panel of 18 seminomas and II nonseminomatous testicular germ cell tumors all express the 1.5-kb transcript. In addition, a panel of 27 samples of testis parenchyma with established carcinoma in situ were all found to be positive for the 1.5-kb transcript, while parenchyma lacking carcinoma in situ, placenta, and control semen were all negative. These data show that the 1.5-kb platelet-derived growth factor alpha-receptor transcript can be used as a highly selective marker for detection of early stages of human testicular germ cell tumors.
Full text
PDF
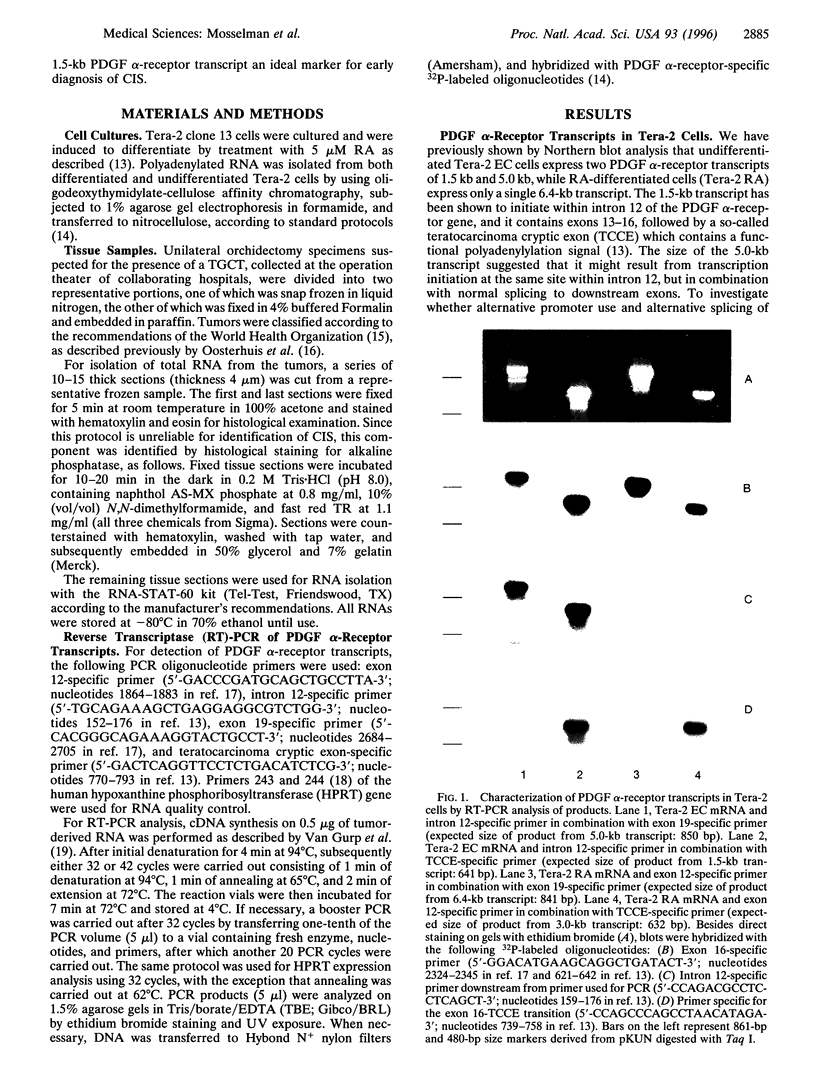

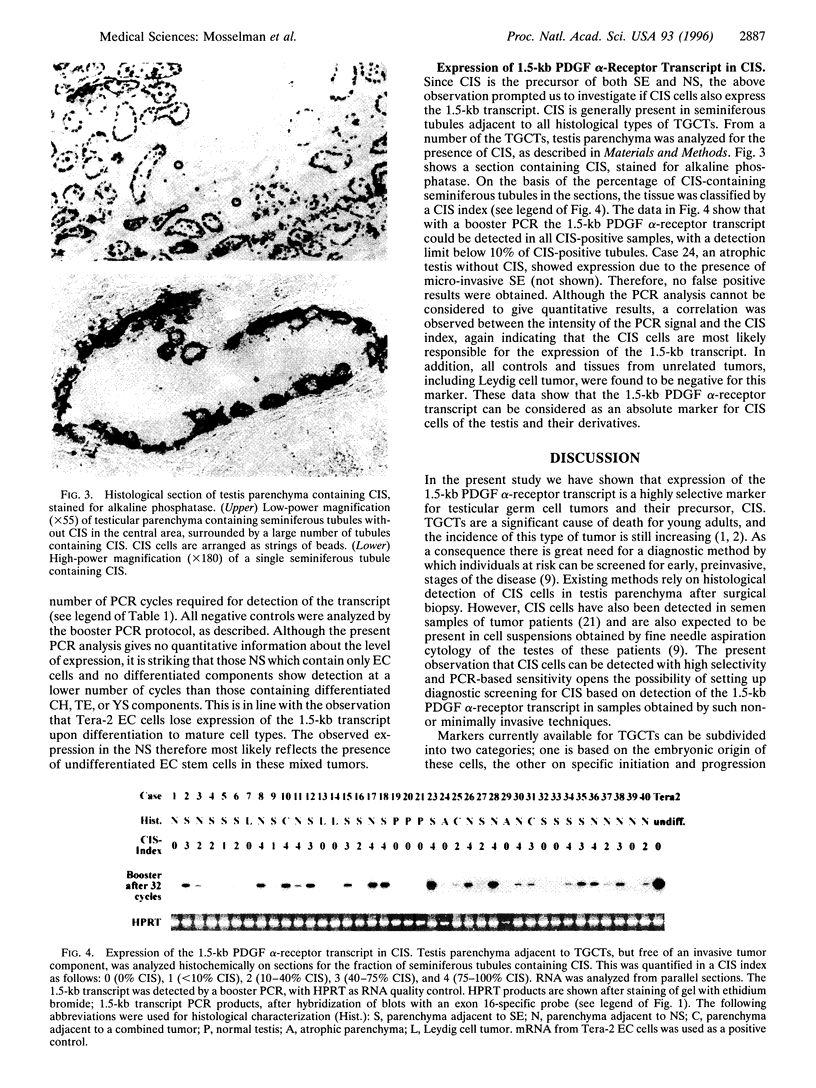

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afink G. B., Nistér M., Stassen B. H., Joosten P. H., Rademakers P. J., Bongcam-Rudloff E., Van Zoelen E. J., Mosselman S. Molecular cloning and functional characterization of the human platelet-derived growth factor alpha receptor gene promoter. Oncogene. 1995 Apr 20;10(8):1667–1672. [PubMed] [Google Scholar]
- Andrews P. W. Human teratocarcinomas. Biochim Biophys Acta. 1988 Aug 3;948(1):17–36. doi: 10.1016/0304-419x(88)90003-0. [DOI] [PubMed] [Google Scholar]
- Claesson-Welsh L., Eriksson A., Westermark B., Heldin C. H. cDNA cloning and expression of the human A-type platelet-derived growth factor (PDGF) receptor establishes structural similarity to the B-type PDGF receptor. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4917–4921. doi: 10.1073/pnas.86.13.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R. A., Nguyen P. N., McBride L. J., Koepf S. M., Caskey C. T. Identification of mutations leading to the Lesch-Nyhan syndrome by automated direct DNA sequencing of in vitro amplified cDNA. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1919–1923. doi: 10.1073/pnas.86.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giwercman A. Carcinoma-in-situ of the testis: screening and management. Scand J Urol Nephrol Suppl. 1992;148:1–47. [PubMed] [Google Scholar]
- Giwercman A., Hopman A. H., Ramaekers F. C., Skakkebaek N. E. Carcinoma in situ of the testis. Detection of malignant germ cells in seminal fluid by means of in situ hybridization. Am J Pathol. 1990 Mar;136(3):497–502. [PMC free article] [PubMed] [Google Scholar]
- Giwercman A., Skakkebaek N. E. Carcinoma in situ of the testis: biology, screening and management. Eur Urol. 1993;23 (Suppl 2):19–21. doi: 10.1159/000474694. [DOI] [PubMed] [Google Scholar]
- Giwercman A., von der Maase H., Berthelsen J. G., Rørth M., Bertelsen A., Skakkebaek N. E. Localized irradiation of testes with carcinoma in situ: effects on Leydig cell function and eradication of malignant germ cells in 20 patients. J Clin Endocrinol Metab. 1991 Sep;73(3):596–603. doi: 10.1210/jcem-73-3-596. [DOI] [PubMed] [Google Scholar]
- Jørgensen N., Rajpert-De Meyts E., Graem N., Müller J., Giwercman A., Skakkebaek N. E. Expression of immunohistochemical markers for testicular carcinoma in situ by normal human fetal germ cells. Lab Invest. 1995 Feb;72(2):223–231. [PubMed] [Google Scholar]
- Ma Y. G., Rosfjord E., Huebert C., Wilder P., Tiesman J., Kelly D., Rizzino A. Transcriptional regulation of the murine k-FGF gene in embryonic cell lines. Dev Biol. 1992 Nov;154(1):45–54. doi: 10.1016/0012-1606(92)90046-j. [DOI] [PubMed] [Google Scholar]
- Mosselman S., Claesson-Welsh L., Kamphuis J. S., van Zoelen E. J. Developmentally regulated expression of two novel platelet-derived growth factor alpha-receptor transcripts in human teratocarcinoma cells. Cancer Res. 1994 Jan 1;54(1):220–225. [PubMed] [Google Scholar]
- Mostofi F. K., Sesterhenn I. A., Davis C. J., Jr Immunopathology of germ cell tumors of the testis. Semin Diagn Pathol. 1987 Nov;4(4):320–341. [PubMed] [Google Scholar]
- Rosnet O., Birnbaum D. Hematopoietic receptors of class III receptor-type tyrosine kinases. Crit Rev Oncog. 1993;4(6):595–613. [PubMed] [Google Scholar]
- Schöler H. R., Dressler G. R., Balling R., Rohdewohld H., Gruss P. Oct-4: a germline-specific transcription factor mapping to the mouse t-complex. EMBO J. 1990 Jul;9(7):2185–2195. doi: 10.1002/j.1460-2075.1990.tb07388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakkebaek N. E., Berthelsen J. G., Giwercman A., Müller J. Carcinoma-in-situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int J Androl. 1987 Feb;10(1):19–28. doi: 10.1111/j.1365-2605.1987.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Strohmeyer T., Peter S., Hartmann M., Munemitsu S., Ackermann R., Ullrich A., Slamon D. J. Expression of the hst-1 and c-kit protooncogenes in human testicular germ cell tumors. Cancer Res. 1991 Apr 1;51(7):1811–1816. [PubMed] [Google Scholar]
- Swerdlow A. J. The epidemiology of testicular cancer. Eur Urol. 1993;23 (Suppl 2):35–38. doi: 10.1159/000474700. [DOI] [PubMed] [Google Scholar]
- Ueno H., Escobedo J. A., Williams L. T. Dominant-negative mutations of platelet-derived growth factor (PDGF) receptors. Inhibition of receptor function by ligand-dependent formation of heterodimers between PDGF alpha- and beta-receptors. J Biol Chem. 1993 Oct 25;268(30):22814–22819. [PubMed] [Google Scholar]
- de Jong B., Oosterhuis J. W., Castedo S. M., Vos A., te Meerman G. J. Pathogenesis of adult testicular germ cell tumors. A cytogenetic model. Cancer Genet Cytogenet. 1990 Sep;48(2):143–167. doi: 10.1016/0165-4608(90)90115-q. [DOI] [PubMed] [Google Scholar]
- van Gurp R. J., Oosterhuis J. W., Kalscheuer V., Mariman E. C., Looijenga L. H. Biallelic expression of the H19 and IGF2 genes in human testicular germ cell tumors. J Natl Cancer Inst. 1994 Jul 20;86(14):1070–1075. doi: 10.1093/jnci/86.14.1070. [DOI] [PubMed] [Google Scholar]