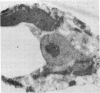
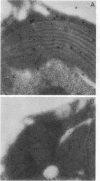
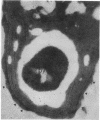
Abstract
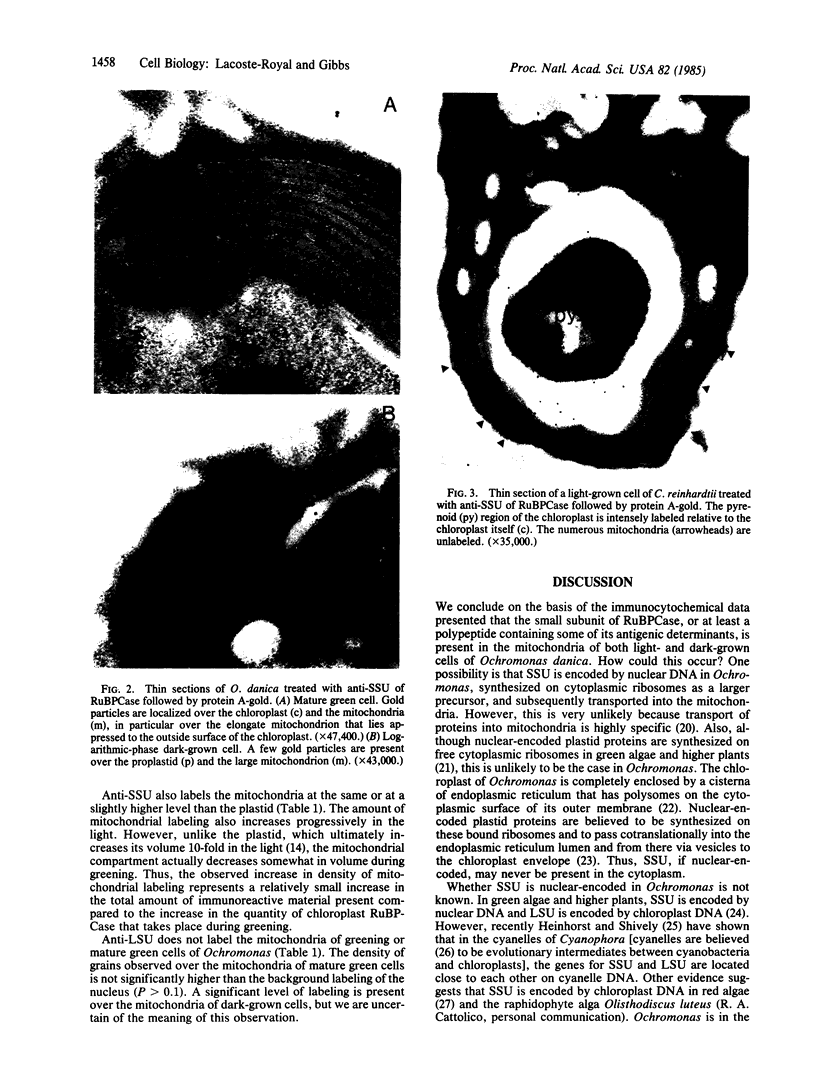
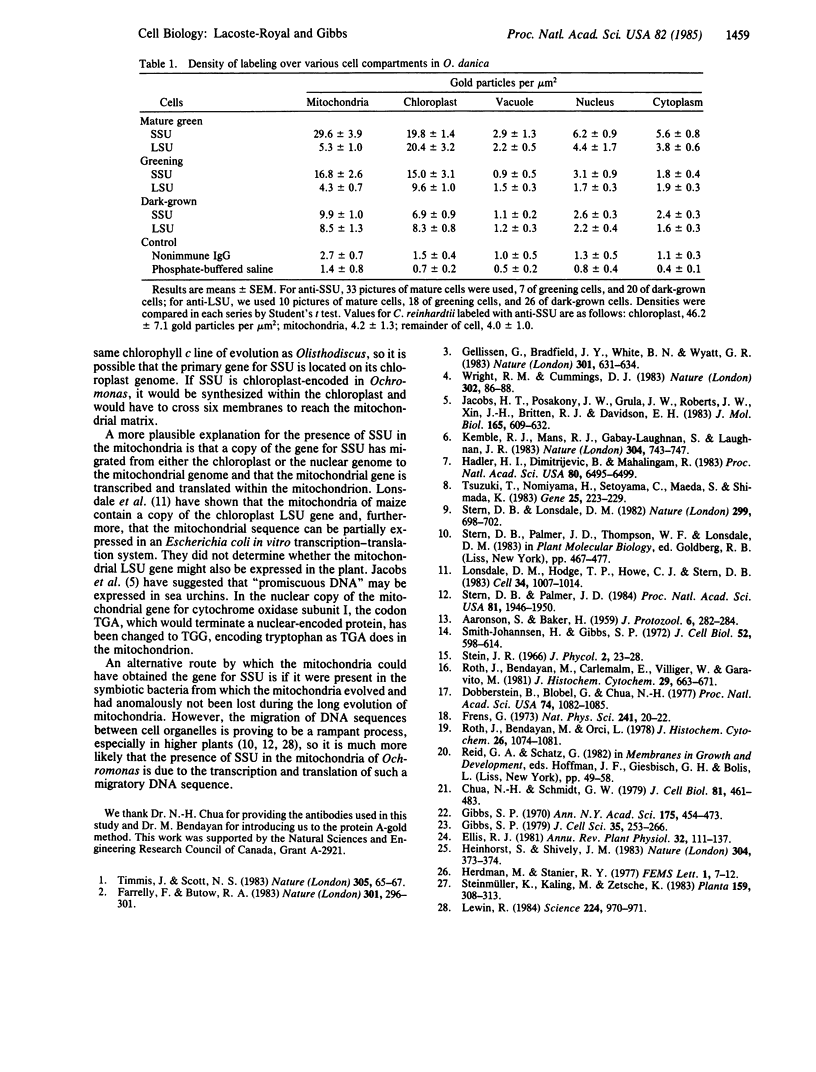
Antibody raised against the small subunit of ribulose-1,5-bisphosphate carboxylase [3-phospho-D-glycerate carboxy-lyase (dimerizing), EC 4.1.1.39] of Chlamydomonas reinhardtii labeled the mitochondria as well as the chloroplast of the chrysophyte alga Ochromonas danica in sections prepared for immunoelectron microscopy by the protein A-gold technique. The same antibody labeled the chloroplast but not the mitochondria of C. reinhardtii. A quantitative study of labeling in dark-grown, greening (32 hr light), and mature green cells of O. danica revealed that anti-small-subunit staining in the mitochondria increased progressively in the light as it does in the plastid. Antibody to the large subunit of the enzyme did not label the mitochondria of either O. danica or C. reinhardtii. In view of the recent demonstrations of homologous DNA sequences in the mitochondrial and chloroplast genomes of higher plants, we suggest that the DNA sequence coding for the small subunit has migrated to the mitochondria from nucleus or chloroplast and is expressed within the organelle.
Keywords: small subunit of ribulose-1,5-bisphosphate carboxylae; immunoelectron microscopy; chrysophycean alga; promiscuous DNA
Full text
PDF
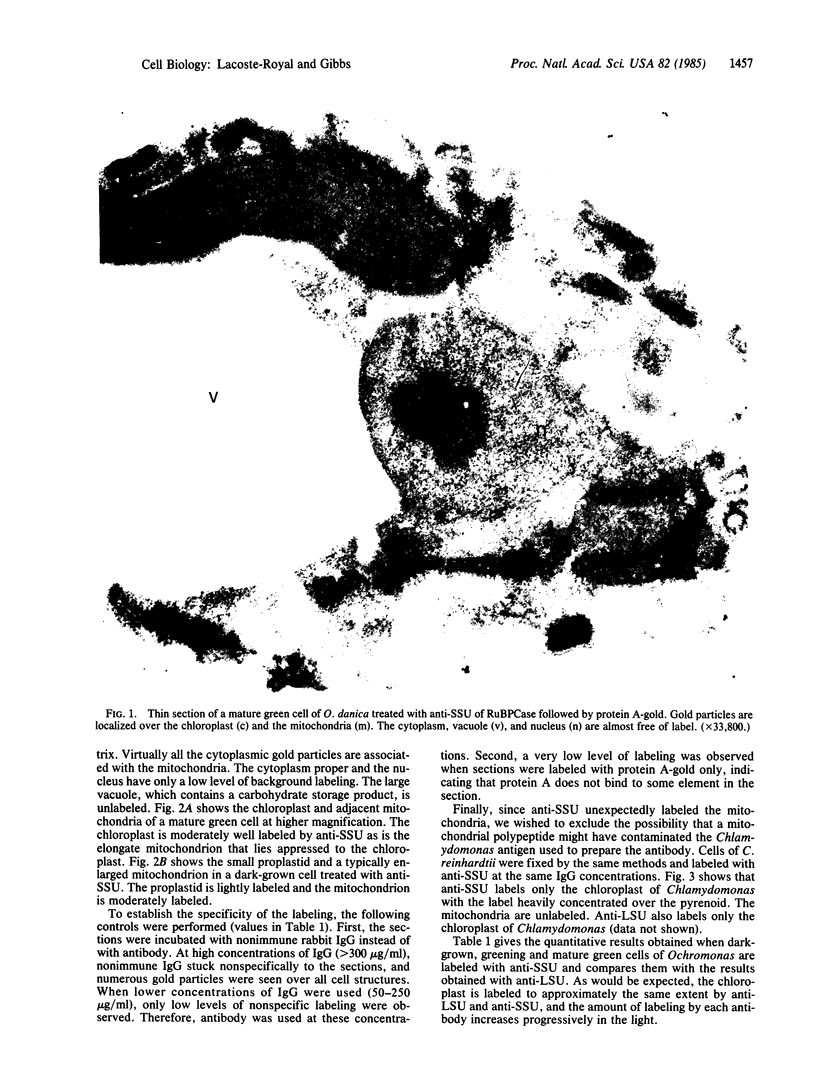
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chua N. H., Schmidt G. W. Transport of proteins into mitochondria and chloroplasts. J Cell Biol. 1979 Jun;81(3):461–483. doi: 10.1083/jcb.81.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobberstein B., Blobel G., Chua N. H. In vitro synthesis and processing of a putative precursor for the small subunit of ribulose-1,5-bisphosphate carboxylase of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1082–1085. doi: 10.1073/pnas.74.3.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrelly F., Butow R. A. Rearranged mitochondrial genes in the yeast nuclear genome. Nature. 1983 Jan 27;301(5898):296–301. doi: 10.1038/301296a0. [DOI] [PubMed] [Google Scholar]
- Gellissen G., Bradfield J. Y., White B. N., Wyatt G. R. Mitochondrial DNA sequences in the nuclear genome of a locust. Nature. 1983 Feb 17;301(5901):631–634. doi: 10.1038/301631a0. [DOI] [PubMed] [Google Scholar]
- Gibbs S. P. The route of entry of cytoplasmically synthesized proteins into chloroplasts of algae possessing chloroplast ER. J Cell Sci. 1979 Feb;35:253–266. doi: 10.1242/jcs.35.1.253. [DOI] [PubMed] [Google Scholar]
- Hadler H. I., Dimitrijevic B., Mahalingam R. Mitochondrial DNA and nuclear DNA from normal rat liver have a common sequence. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6495–6499. doi: 10.1073/pnas.80.21.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs H. T., Posakony J. W., Grula J. W., Roberts J. W., Xin J. H., Britten R. J., Davidson E. H. Mitochondrial DNA sequences in the nuclear genome of Strongylocentrotus purpuratus. J Mol Biol. 1983 Apr 25;165(4):609–632. doi: 10.1016/s0022-2836(83)80270-8. [DOI] [PubMed] [Google Scholar]
- Lewin R. No Genome Barriers to Promiscuous DNA: The movement of DNA sequences between mitochondrial, chloroplast and nuclear genomes is even more prolific than had been expected. Science. 1984 Jun 1;224(4652):970–971. doi: 10.1126/science.224.4652.970. [DOI] [PubMed] [Google Scholar]
- Lonsdale D. M., Hodge T. P., Howe C. J., Stern D. B. Maize mitochondrial DNA contains a sequence homologous to the ribulose-1,5-bisphosphate carboxylase large subunit gene of chloroplast DNA. Cell. 1983 Oct;34(3):1007–1014. doi: 10.1016/0092-8674(83)90558-5. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Carlemalm E., Villiger W., Garavito M. Enhancement of structural preservation and immunocytochemical staining in low temperature embedded pancreatic tissue. J Histochem Cytochem. 1981 May;29(5):663–671. doi: 10.1177/29.5.6166664. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Smith-Johannsen H., Gibbs S. P. Effects of chloramphenicol on chloroplast and mitochondrial ultrastructure in Ochromonas danica. J Cell Biol. 1972 Mar;52(3):598–614. doi: 10.1083/jcb.52.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. B., Lonsdale D. M. Mitochondrial and chloroplast genomes of maize have a 12-kilobase DNA sequence in common. Nature. 1982 Oct 21;299(5885):698–702. doi: 10.1038/299698a0. [DOI] [PubMed] [Google Scholar]
- Stern D. B., Palmer J. D. Extensive and widespread homologies between mitochondrial DNA and chloroplast DNA in plants. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1946–1950. doi: 10.1073/pnas.81.7.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki T., Nomiyama H., Setoyama C., Maeda S., Shimada K. Presence of mitochondrial-DNA-like sequences in the human nuclear DNA. Gene. 1983 Nov;25(2-3):223–229. doi: 10.1016/0378-1119(83)90226-3. [DOI] [PubMed] [Google Scholar]
- Wright R. M., Cummings D. J. Integration of mitochondrial gene sequences within the nuclear genome during senescence in a fungus. Nature. 1983 Mar 3;302(5903):86–88. doi: 10.1038/302086a0. [DOI] [PubMed] [Google Scholar]