Abstract
Purpose
To examine potential modifying effects of body weight and bilateral oophorectomy on the association of hormone replacement therapy (HRT) with risk of breast cancer, overall and by subtypes according to status of estrogen receptor (ER), progesterone receptor (PR), and human-epidermal-growth-factor receptor 2 (Her2) among postmenopausal women.
Experimental Design
This analysis included 2,510 postmenopausal white women recruited in the Nashville Breast Health Study, a population-based case-control study of breast cancer. Multivariable logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CI) for associations between HRT use and risk of breast cancer overall and by subtypes, adjusted for age and education.
Results
Among women with natural menopause and body-mass-index (BMI) <25 kg/m2, ever-use of HRT was associated with increased breast-cancer risk (OR=1.95, 95% CI=1.32-2.88). Risk was elevated with duration of HRT use (p-for-trend=0.002). Similar association patterns were found for ER+, ER+PR+, and luminal-A cancer subtypes but not ER-, ER-PR-, and triple-negative cancer. In contrast, ever-HRT-use in overweight women (BMI≥25 kg/m2) showed no association with risk of breast cancer overall or by subtypes; interaction tests for modifying effect of BMI were statistically significant. Ever-HRT-use was associated with decreased breast-cancer risk (OR=0.70, 95% CI=0.38-1.31) among women with prior bilateral oophorectomy but elevated risk (OR=1.45, 95% CI=0.92-2.29) among those with hysterectomy without bilateral oophorectomy (p-for-interaction=0.057). Similar associations were seen for virtually all breast-cancer subtypes, although interaction tests were statistically significant for ER+ and luminal A only.
Conclusion
Body weight and bilateral oophorectomy modify associations between HRT use and breast-cancer risk, especially the risk of hormone-receptor-positive tumors.
Introduction
It is widely accepted that female sex hormones, particularly estrogens, play a pivotal role in the etiology of breast cancer. Among postmenopausal women, high body adiposity, typically measured using body mass index (BMI), has been established as a risk factor for breast cancer. This positive association is due to increased endogenous estrogen synthesis in adipose tissues among postmenopausal women (1-3). Exogenous estrogen administration through hormone replacement therapy (HRT) has also been associated with elevated risk of breast cancer (1-3). Numerous studies have reported that the association of overweight/obesity with postmenopausal breast cancer risk is significantly attenuated in women who use HRT (4-11), suggesting that body weight and HRT use may interact in associations with breast-cancer risk among postmenopausal women. Furthermore, a recent analysis of data from the Women's Health Initiative (WHI) randomized clinical trial found that among postmenopausal women with prior hysterectomy, ever-use of estrogen during the intervention phase, compared to the placebo group, was associated with a significantly reduced risk of breast cancer (12). It is unclear, however, if the association may differ by types of surgeries, such as simple hysterectomy or hysterectomy plus bilateral oophorectomy. Nevertheless, these recent findings may challenge existing concepts regarding the association between HRT use and breast-cancer risk (12, 13).
Breast cancer is a complex and heterogeneous disease with a wide spectrum of clinical, histopathologic, and molecular features (14-16). Increasing evidence suggests that breast-cancer subtypes defined by expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (Her2) represent distinct biological entities with distinct clinical profiles (17-19). For example, ER+ tumors are associated with overexpression of genes in the ER signaling pathways and, clinically, have the most favorable prognosis; while triple-negative (ER-PR-Her2-) tumors are most likely to exhibit a basal-like pattern of gene expression and are associated with more aggressive histopathologic features and poor prognosis (16-18). Hormone-related risk factors including obesity and HRT use have been shown to be more closely related to hormone-receptor-positive breast cancer; however, data are very limited regarding possible interactions of HRT, body weight and bilateral oophorectomy in the risk of breast cancer by subtypes.
The Nashville Breast Health Study (NBHS) is a large population-based case-control study of breast cancer with a primary objective to identify genetic and lifestyle risk factors for this common malignancy. Using data from the NBHS, we examined associations between ever-HRT-use and breast-cancer risk by subtype, according to ER, PR, and Her2 status, and further determined whether these associations may be modified by body weight and bilateral oophorectomy.
Methods
The NBHS is a population-based case-control study of incident breast cancer conducted primarily in the Nashville metropolitan area of Tennessee. Eligible cases were women newly diagnosed with primary breast cancer (invasive cancer or ductal carcinoma in situ) between 25 and 75 years old and with no prior history of cancer other than nonmelanoma skin cancer. Most participants (92%) were residents of the Nashville eight-county metropolitan area. From February 1, 2001 through December 31, 2011, 5,078 women were recruited into the study. Breast-cancer cases (n = 2,694) were identified and recruited through a rapid case-ascertainment system established across the major hospitals in Nashville and the Tennessee Cancer Registry. Information on ER, PR, and Her2 status of breast cancer tumors was obtained from pathology records. Controls (n = 2,384) were identified primarily via random-digit dialing (RDD) of households in the Nashville eight-county metropolitan area, and were frequency-matched to cases on five-year age groups, race, and county of residence. Approval for the study was obtained from the Institutional Review Boards of Vanderbilt University Medical Center and each collaborating institution. All study participants signed informed consent to participate in an epidemiologic survey to provide lifestyle and demographic data, release relevant medical information, and provide a saliva sample as a source of genomic DNA for genetic studies of breast cancer.
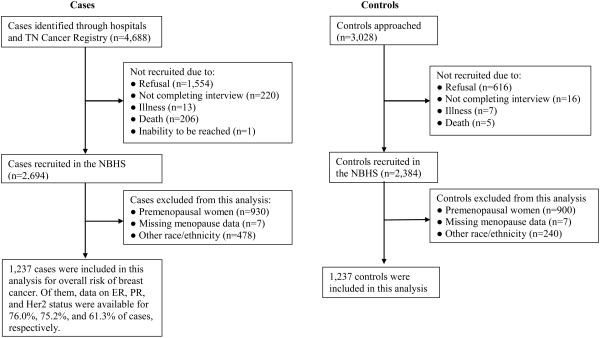
In the NBHS, the median interval from time of breast cancer diagnosis to study enrollment was 10.4 months. Participation rates were approximately 58% for cases and 79% for controls. Reasons for nonparticipation included refusal (n=1,554), not completing the interview (n=220), death (n=206), illness (n=13), and inability to be reached (n=1) among cases; and refusal (n=616), not completing the interview (n=16), illness (n=7), and death (n=5) among controls (Figure 1). Information on socio-demographic characteristics and all major breast cancer risk factors was obtained through telephone interview by trained interviewers using a structured questionnaire. Reference date was defined as date of breast cancer diagnosis for cases and date of interview for controls. Menopause was defined as cessation of menstrual periods, excluding those caused by pregnancy and nursing, for at least 12 months before the reference date. For postmenopausal women, cause of menopause was further assessed, including natural menopause, surgical menopause (hysterectomy with no, one, or two ovaries surgically removed), medication-induced menopause, and unknown reasons. Information on HRT use was collected: Women who indicated ever-use of HRT were asked ages at first and last use, which were used to calculate cumulative duration of HRT. Body mass index (BMI) was defined as weight (kg)/height2 (m2).
Figure 1. Flow chart of recruitment.
Among the 5,078 NBHS participants, 3,228 were postmenopausal women. Of these, 643 non-Hispanic black women, 31 Hispanic women, and 44 women in other racial/ethnic groups were excluded from this analysis because of small sample size for a separate analysis. Thus, included in this analysis were 2,510 postmenopausal white women (1,273 breast cancer cases and 1,237 healthy controls); 1,185 with natural menopause, 1,127 with surgical menopause, 116 with medication-reduced menopause, and 82 with other reasons (not specified). Among cases, data on ER, PR, and Her2 status were available for 76.0%, 75.2%, and 61.3% of cases, respectively.
In this analysis, breast cancer subtypes were classified by hormone receptor (HR) and Her2 status into the following groups and subgroups: ER status including ER+ and ER-; ER/PR status focusing on ER+PR+ and ER-PR-; and ER/PR/Her2 status including 1) luminal A (ER+ and/or PR+ and Her2-), 2) luminal B (ER+ and/or PR+ and Her2+) and Her2 overexpressing (ER-PR-Her2+), and 3) triple-negative (ER-PR-Her2-) (16, 20).
Statistical analysis
Distributions of demographic characteristics and selected risk factors between cases and controls were compared using t-tests (for continuous variables) or X2 tests (for categorical variables). We used multivariable unconditional logistic regression to estimate odds ratios (OR) and their 95% confidence intervals (CI) for the association between HRT use (ever-use of HRT and duration of use) and overall breast cancer risk. To estimate OR and 95% CI for associations between HRT use and breast-cancer subtypes (ER status, ER/PR status, ER/PR/Her2 status), we used multivariable polytomous unconditional logistic regression that enables simultaneous calculation of association results for multiple outcome categories (21,22). Age was adjusted, along with education, to control for potential influence of socioeconomic status on study results. None of area of residence and known risk factors for breast cancer, including history of breast cancer among first-degree relatives, personal history of benign breast diseases, regular alcohol consumption, physical inactivity, early age at menarche, ever-use of OC, late age at first birth, parity, late age menopause, and long duration of menstruation, were associated with HRT use and thus they are not confounders in this analysis. Therefore, they were not adjusted in the study.
To examine potential modifying effects of body weight on association between HRT use and breast cancer risk, we categorized women into two groups: thin or normal weight women (BMI<25 Kg/m2) and overweight (BMI≥25 Kg/ m2) based on WHO criterion. Among overweight women, additional analyses were performed for pre-obesity (BMI=25-29.9 kg ⁄m2) and obesity (BMI ≥30 kg ⁄m2). For women with surgical menopause, associations of HRT use with breast cancer risk were further examined separately among women who had prior hysterectomy with bilateral oophorectomy (surgical removal of both ovaries) or prior hysterectomy without bilateral oophorectomy (no or only one ovary surgically removed). Tests for trend across categories of duration of HRT use were performed by entering categorical variables as continuous variables in the model. Interaction terms were included in the models to test for interaction between HRT use and BMI. All P-values reported were two-sided. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Table 1 shows characteristics of breast cancer cases and healthy controls among postmenopausal white women in the NBHS, presented overall and by type of menopause (natural or surgical). Overall, compared with controls, cases were slightly older (59.9 years vs. 59.1 years) and were more likely to have family history of breast cancer, personal history of BBD, lower annual household income, and less regular exercise. Other factors, including BMI, were generally comparable. Compared to those with natural menopause (both cases and controls), women with surgical menopause were slightly younger at study enrollment and reported age at menopause about 10 years younger, resulting in total years of menstruation about 10 years shorter. Notably, when women with surgical menopause were further stratified into two groups, prior hysterectomy with bilateral oophorectomy or without bilateral oophorectomy, age, BMI, age at menopause, and years of menstruation were also comparable between cases and controls (data not shown).
Table 1. Characteristics of postmenopausal cases and controls by causes of menopause, the Nashville Breast Health Study.
| Subject characteristics | All postmenopausal women | Women with natural menopause | Women with surgical menopause | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (n=1,237) % |
Case (n=1,273) % |
p value | Control (n=587) % |
Case (n=598) % |
p value | Control (n=558) % |
Case (n=569) % |
p value | |
| Age (mean ± SD) | 59.1 ± 8.3 | 59.9 ± 7.8 | 0.008 | 60.4 ± 6.9 | 60.6 ± 6.9 | 0.602 | 58.4 ± 9.2 | 59.4 ± 8.6 | 0.049 |
| Education | |||||||||
| ≤High school | 33.4 | 36.2 | 26.9 | 32.1 | 41.2 | 41.7 | |||
| Some college | 32.8 | 30.5 | 0.277 | 34.4 | 27.4 | 0.022 | 31.4 | 3.30 | 0.692 |
| ≥College | 33.8 | 33.3 | 38.7 | 40.5 | 27.4 | 25.3 | |||
| Income per annum (US$) | |||||||||
| ≤20,000 | 8.5 | 11.5 | 6.6 | 10.0 | 10.3 | 13.7 | |||
| 20,001-40,000 | 18.1 | 20.0 | 18.6 | 20.5 | 18.5 | 19.3 | |||
| 40,001-60,000 | 26.8 | 23.7 | 0.026 | 25.9 | 21.6 | 0.077 | 27.9 | 26.0 | 0.334 |
| >60,000 | 46.6 | 44.8 | 48.9 | 47.9 | 43.3 | 41.0 | |||
| Body mass index (kg/m2) | |||||||||
| < 25 | 40.1 | 38.8 | 43.1 | 41.8 | 36.8 | 34.3 | |||
| ≥ 25 | 59.9 | 61.2 | 0.502 | 56.9 | 58.2 | 0.646 | 63.2 | 65.7 | 0.386 |
| Regularly consumed alcohol | |||||||||
| No | 79.3 | 82.5 | 76.5 | 81.9 | 83.3 | 83.8 | |||
| Yes, 1-10 years | 7.7 | 6.5 | 9.6 | 7.0 | 5.0 | 5.3 | |||
| 11-20 years | 5.2 | 4.8 | 0.202 | 5.8 | 4.2 | 0.128 | 4.1 | 5.1 | 0.595 |
| >20 years | 7.8 | 6.1 | 8.4 | 6.9 | 7.6 | 5.8 | |||
| Parity and number of live births | |||||||||
| 0 | 13.6 | 13.8 | 13.8 | 17.1 | 11.3 | 10.7 | |||
| 1 | 16.2 | 17.2 | 17.7 | 17.7 | 14.9 | 16.7 | |||
| 2 | 37.6 | 36.6 | 0.894 | 38.0 | 36.0 | 0.472 | 38.0 | 36.0 | 0.799 |
| ≥ 3 | 32.6 | 32.4 | 30.5 | 29.3 | 35.8 | 36.6 | |||
| Age at menarche (yrs, mean ± SD) | 12.6 ± 1.5 | 12.6 ± 1.6 | 0.528 | 12.6 ± 1.5 | 12.7 ± 1.4 | 0.720 | 12.5 ± 1.7 | 12.5 ± 1.6 | 0.379 |
| Age at menopause (yrs, mean ± SD) | 45.0 ± 8.3 | 45.5 ± 8.2 | 0.179 | 50.1 ± 4.8 | 50.1 ± 4.2 | 0.973 | 39.2 ± 7.6 | 39.7 ± 7.8 | 0.279 |
| Years of menstruation (yrs, mean ± SD) | 32.3 ± 9.2 | 32.8 ± 8.6 | 0.120 | 37.3 ± 6.3 | 37.3 ± 5.8 | 0.916 | 26.5 ± 8.7 | 37.3 ± 7.9 | 0.138 |
| Ever use of OC | 77.7 | 77.1 | 0.751 | 78.0 | 76.2 | 0.459 | 76.2 | 76.6 | 0.885 |
| Ever use of HRT | 72.6 | 75.5 | 0.102 | 62.7 | 67.3 | 0.097 | 83.1 | 83.5 | 0.882 |
| Regular exercise | 55.8 | 51.7 | 0.036 | 58.6 | 54.2 | 0.125 | 52.4 | 47.9 | 0.128 |
| aFamily history of breast cancer | 15.6 | 22.1 | < 0.001 | 18.2 | 21.4 | 0.170 | 13.4 | 22.3 | < 0.001 |
| bPersonal history of BBD | 39.1 | 53.3 | < 0.001 | 37.6 | 48.3 | < 0.001 | 41.4 | 57.3 | < 0.001 |
Family history: first-degree blood relatives with breast cancer
BBD: benign breast diseases
As shown in Table 2, among women with natural menopause and BMI<25 kg/m2, ever-use of HRT was associated with significantly elevated overall risk of breast cancer (OR=1.95, 95% CI=1.32-2.88). Risk increased with increasing total duration of HRT use (OR=1.67, 95% CI=0.99-2.81, for use <5 years; OR=1.93, 95% CI=1.13-3.31, for use for 5-9 years; and OR=2.13, 95% CI=1.26-3.59, for use ≥10 years; p for trend =0.002). In contrast, among women with BMI ≥25 kg/m2, no association of ever-HRT-use or duration of use was observed with overall risk of breast cancer (OR=0.91, 95% CI=0.67-1.25; p for trend =0.706). Significant interaction between BMI and ever HRT use (p for interaction =0.001) or between BMI and duration of HRT use (p for interaction =0.012) was detected. These two interaction tests remained statistically significant after adjusting for multiple comparisons (adjusted p value <0.025 for two comparisons). Adjusting for additional variables, including area of residence and know breast cancer risk factors including history of breast cancer among first-degree relatives, personal history of benign breast diseases, regular alcohol consumption, physical inactivity, age at menarche, parity, and years of menstruation did not materially change study results (data not shown).
Table 2. Associations of HRT ever-use and duration of use with breast cancer risk by BMI among women with natural menopause, the Nashville Breast Health Study.
| Variables | BMI < 25 | BMI ≥ 25 | |||
|---|---|---|---|---|---|
| Case/control | aOR (95%CI) | Case/control | aOR (95%CI) | bp for interaction | |
| HRT use | |||||
| Never | 63/99 | 1.00 (ref.) | 131/119 | 1.00 (ref.) | |
| Ever | 186/152 | 1.95 (1.32-2.88) | 213/214 | 0.91 (0.67-1.25) | 0.001 |
| Duration of use | |||||
| < 5 years | 47/48 | 1.67 (0.99-2.81) | 63/75 | 0.76 (0.50-1.16) | |
| 5-9 years | 47/37 | 1.93 (1.13-3.31) | 60/48 | 1.14 (0.72-1.80) | 0.012 |
| ≥ 10 years | 71/51 | 2.13 (1.26-3.59) | 61/56 | 1.03 (0.65-1.62) | |
| p for trend | P = 0.002 | P = 0.706 | |||
Adjusted for age and education.
Interaction between BMI (<25 or ≥25) and HRT ever-use or duration of use for overall risk of breast cancer.
We further evaluated associations of ever-use of HRT and duration of use with risk of breast cancer defined by ER, PR and Her2 status among women with natural menopause. As shown in Tables 3 and 4, among women with BMI <25 kg/m2, positive associations with HRT use were most pronounced for breast cancer defined as ER+, ER+PR+, and luminal A, while more modestly elevated risks associated with HRT use were seen for ER-, ER-PR-, luminal B/Her2 overexpression or triple-negative tumors. With the exception of the triple-negative subtype, interaction tests between BMI and HRT use or duration of HRT use were statistically significant at p<0.05 and remained mostly statistically significant even after Bonferroni correction for multiple comparisons.
Table 3. Associations of HRT ever-use and duration of use with risk of breast cancer subtypes defined by ER or ER/PR status among women with natural menopause, the Nashville Breast Health Study.
| Variables | Ever use of HRT | Duration of HRT use | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nonusers | Ever-users | < 5 years | 5-9 years | ≥ 10 years | |||||||
| Case/control | aOR (95%CI) | Case/control | a OR (95%CI) | Case/control | aOR (95%CI) | Case/control | aOR (95%CI) | Case/control | aOR (95%CI) | p for trend | |
| ER status | |||||||||||
| ER+ | |||||||||||
| BMI<25 | 32/99 | 1.00 (ref.) | 122/152 | 2.52 (1.56-4.05) | 31/48 | 2.210(1.19-4.05) | 30/37 | 2.41 (1.28-4.55) | 47/51 | 2.82 (1.52-5.22) | < 0.001 |
| BMI≥25 | 76/119 | 1.00 (ref.) | 120/214 | 0.87 (0.60-1.25) | 40/75 | 0.82 (0.51-1.33) | 30/48 | 0.97 (0.56-1.66) | 36/56 | 0.98 (0.58-1.56) | 0.940 |
| bp for interaction < 0.001 | cp for interaction < 0.001 | ||||||||||
| ER- | |||||||||||
| BMI<25 | 15/99 | 1.00 (ref.) | 26/152 | 1.12 (0.55-2.25) | 6/48 | 0.82 (0.30-2.27) | 6/37 | 1.08 (0.39-3.01) | 12/51 | 1.60 (0.64-4.02) | 0.341 |
| BMI≥25 | 27/119 | 1.00 (ref.) | 35/214 | 0.78 (0.45-1.37) | 11/75 | 0.72 (0.33-1.55) | 9/48 | 0.87 (0.38-2.01) | 9/56 | 0.86 (0.37-2.04) | 0.693 |
| bp for interaction =0.231 | cp for interaction = 0.092 | ||||||||||
| ER/PR status | |||||||||||
| ER+PR+ | |||||||||||
| BMI<25 | 23/99 | 1.00 (ref.) | 94/152 | 2.69 (1.58-4.60) | 19/48 | 1.90 (0.93-3.84) | 21/37 | 2.35 (1.15-4.78) | 42/51 | 3.50 (1.78-6.86) | < 0.001 |
| BMI≥25 | 61/119 | 1.00 (ref.) | 97/214 | 0.88 (0.59-1.30) | 32/75 | 0.82 (0.49-1.37) | 24/48 | 0.96 (0.54-1.72) | 30/56 | 1.02 (0.58-1.79) | 0.951 |
| bp for interaction < 0.001 | cp for interaction <0.001 | ||||||||||
| ER-PR- | |||||||||||
| BMI<25 | 13/99 | 1.00 (ref.) | 24/152 | 1.15 (0.55-2.41) | 5/48 | 0.81 (0.27-2.43) | 5/37 | 1.00 (0.33-3.03) | 12/51 | 1.68 (0.66-4.32) | 0.315 |
| BMI≥25 | 27/119 | 1.00 (ref.) | 34/214 | 0.77 (0.44-1.36) | 10/75 | 0.65 (0.30-1.44) | 9/48 | 0.87 (0.38-2.01) | 9/56 | 0.87 (0.37-2.05) | 0.704 |
| bp for interaction = 0.173 | cp for interaction = 0.058 | ||||||||||
Adjusted for age and education.
Interaction between HRT ever-use and BMI (<25 or ≥25) for risk of ER+, ER-, ER+PR+, and ER-PR- tumors, respectively.
Interaction between duration of HRT use and BMI (<25 or ≥25) for risk of ER+, ER-, ER+PR+, and ER-PR- tumors, respectively.
Table 4. Associations of HRT ever-use and duration of use with risk of breast cancer subtypes defined by ER/PR/Her2 status among women with natural menopause, the Nashville Breast Health Study.
| Variables | Ever use of HRT | Duration of HRT use | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nonusers | Ever-users | < 5 years | 5-9 years | ≥ 10 years | |||||||
| Case/control | aOR (95%CI) | Case/control | a OR (95%CI) | Case/control | aOR (95%CI) | Case/control | a OR (95%CI) | Case/control | aOR (95%CI) | p for trend | |
| ER/PR/Her2 | |||||||||||
| Luminal A | |||||||||||
| BMI<25 | 19/99 | 1.00 (ref.) | 87/152 | 2.89 (1.64-5.10) | 22/48 | 2.59 (1.27-5.29) | 19/37 | 2.48 (1.18-5.26) | 34/51 | 3.05 (1.50-6.21) | 0.002 |
| BMI≥25 | 48/119 | 1.00 (ref.) | 75/214 | 0.83 (0.54-1.28) | 22/75 | 0.69 (0.38-1.25) | 19/48 | 0.95 (0.50-1.79) | 26/56 | 1.06 (0.59-1.92) | 0.840 |
| bp for interaction < 0.001 | cp for interaction = 0.019 | ||||||||||
| Luminal B/Her2 overexpression | |||||||||||
| BMI<25 | 9/99 | 1.00 (ref.) | 22/152 | 1.71 (0.74-3.93) | 5/48 | 1.19 (0.38-3.79) | 7/37 | 2.18 (0.75-5.38) | 9/51 | 2.24 (0.76-6.59) | 0.092 |
| BMI≥25 | 26/119 | 1.00 (ref.) | 23/214 | 0.52 (0.28-0.96) | 11/75 | 0.66 (0.31-1.44) | 3/48 | 0.29 (0.09-1.02) | 6/56 | 0.65 (0.24-1.73) | 0.109 |
| bp for interaction = 0.009 | cp for interaction = 0.004 | ||||||||||
| Triple negative | |||||||||||
| BMI<25 | 7/99 | 1.00 (ref.) | 12/152 | 1.12 (0.42-3.00) | 2/48 | 0.58 (0.11-2.91) | 2/37 | 0.79 (0.16-4.04) | 6/51 | 1.79 (0.51-6.36) | 0.442 |
| BMI≥25 | 11/119 | 1.00 (ref.) | 20/214 | 1.12 (0.51-2.44) | 3/75 | 0.51 (0.14-1.89) | 7/48 | 1.61 (0.58-4.44) | 5/56 | 1.08 (0.34-3.42) | 0.572 |
| bp for interaction = 0.862 | cp for interaction = 0.637 | ||||||||||
Adjusted for age and education.
Interaction between HRT ever-use and BMI (<25 or ≥25) for risk of for risk of luminal A, luminal B/Her2 overexpression, and triple-negative subtypes, respectively.
Interaction between duration of HRT ever-use and BMI (<25 or ≥25) for risk of luminal A, luminal B/Her2 overexpression, and triple-negative subtypes, respectively.
Table 5 shows associations between ever-use of HRT and breast cancer risk among postmenopausal women with prior hysterectomy with or without bilateral oophorectomy. Among women with prior hysterectomy without bilateral oophorectomy, ever-use of HRT was associated with a marginally significant increase in overall risk of breast cancer (OR=1.45, 95% CI=0.92-2.29). In contrast, among women with prior hysterectomy with bilateral oophorectomy, ever-use of HRT was associated with reduced risk of breast cancer (OR=0.70, 95% CI=0.38-1.31). This pattern of association was seen for virtually all breast cancer subtypes, although interaction tests were statistically significant for ER+ and luminal A tumors only (p=0.034 and 0.017, respectively). No statistically significant interactions between HRT use and BMI were detected among women with prior hysterectomy with or without bilateral oophorectomy; however, sample size for these analyses was small.
Table 5. Association of HRT use with risk of breast cancer overall and subtypes defined by ER, PR, and Her2 status among postmenopausal women with prior hysterectomy (with or without bilateral oophorectomy), the Nashville Breast Health Study.
| Variables ER status | Hysterectomy without bilateral oophorectomy | Hysterectomy with bilateral oophorectomy | bp for interaction | ||
|---|---|---|---|---|---|
| Case/control | aOR (95%CI) | Case/control | aOR (95%CI) | ||
| Overall risk | |||||
| Never use of HRT | 65/74 | 1.00 (ref.) | 24/19 | 1.00 (ref.) | |
| Ever-use of HRT | 148/103 | 1.45 (0.92-2.29) | 312/352 | 0.70 (0.38-1.31) | 0.057 |
| ER status | |||||
| ER+ | |||||
| Never use of HRT | 37/74 | 1.00 (ref.) | 17/19 | 1.00 (ref.) | |
| Ever-use of HRT | 82/103 | 1.28 (0.74-2.21) | 163/352 | 0.51 (0.26-1.01) | 0.034 |
| ER- | |||||
| Never use of HRT | 14/74 | 1.00 (ref.) | 4/19 | 1.00 (ref.) | |
| Ever-use of HRT | 29/103 | 1.44 (0.66-3.15) | 63/352 | 0.89 (0.29-2.70) | 0.449 |
| ER/PR status | |||||
| ER+PR+ | |||||
| Never use of HRT | 34/74 | 1.00 (ref.) | 13/19 | 1.00 (ref.) | |
| Ever-use of HRT | 72/103 | 1.22 (0.70-2.16) | 127/352 | 0.52 (0.25-1.09) | 0.067 |
| ER-PR- | |||||
| Never use of HRT | 14/74 | 1.00 (ref.) | 4/19 | 1.00 (ref.) | |
| Ever-use of HRT | 28/103 | 1.39 (0.63-3.04) | 59/352 | 0.83 (0.27-2.53) | 0.416 |
| ER/PR/Her2 status | |||||
| Luminal A | |||||
| Never use of HRT | 21/74 | 1.00 (ref.) | 12/19 | 1.00 (ref.) | |
| Ever-use of HRT | 58/103 | 1.36 (0.72-2.60) | 100/352 | 0.44 (0.21-0.95) | 0.017 |
| Luminal B/Her2 over-expression | |||||
| Never use of HRT | 12/74 | 1.00 (ref.) | 3/19 | 1.00 (ref.) | |
| Ever-use of HRT | 15/103 | 0.87 (0.35-2.17) | 39/352 | 0.67 (0.19-2.40) | 0.652 |
| Triple negative | |||||
| Never use of HRT | 8/74 | 1.00 (ref.) | 3/19 | 1.00 (ref.) | |
| Ever-use of HRT | 20/103 | 2.02 (0.76-5.35) | 30/352 | 0.58 (0.16-2.08) | 0.159 |
Adjusted for age and education.
Interaction between HRT use and type of hysterectomy (hysterectomy with or without bilateral oophorectomy) for overall risk of breast cancer and risk of ER+, ER-, ER+PR+, ER-PR-, luminal A, luminal B/Her2 overexpression, and triple-negative subtypes, respectively.
Discussion
It has been hypothesized that risk factors most closely associated with postmenopausal ER+PR+ breast tumors may operate through mechanisms related to estrogen and progesterone exposure, whereas the etiology of ER-PR- breast cancer may be independent of hormonal exposure (23). Many studies have examined associations between HRT use and risk of breast cancer stratified by hormone-receptor status. Results, although not entirely consistent, suggest that positive association with HRT use is restricted to, or stronger, among women with ER+ or ER+PR+ tumors compared with ER- or ER-PR- tumors (3, 11, 24-31). The Nurses' Health Study (NHS) reported a stronger association between past use of HRT and ER+ tumors compared to ER- tumors (24). Li and colleagues reported that HRT use was associated with a 2-fold increased risk of ER+ tumors only, with higher risk for current long-term use (OR, 2.9; 95% CI, 1.8-4.8) (26). In our study, as expected, ever-HRT-use showed a stronger, approximately 2.7-fold increased risk of ER+PR+ tumors, with a 3.4-fold increased risk with long-term HRT use, compared with a 1.5-fold increased risk of ER-PR- tumors in postmenopausal women with normal weight.
Cumulative evidence suggests that HRT use may interact with adiposity for breast cancer risk (4-11, 26, 29, 32). The NHS reported a positive association of both waist-to-hip ratio (WHR) and waist circumference with breast cancer risk, but only in postmenopausal women who had never received HRT (4). The WHI Observational Study (6) reported that compared to slimmer women (BMI <22.6 kg/m2), heavier women (BMI >31.1 kg/m2) had an elevated risk of postmenopausal breast cancer (RR=2.52; 95% CI=1.62-3.93), which was only apparent among HRT non-users. Similar findings were reported in the Million Women Study conducted in England and Scotland (8) and the European Prospective Investigation into Cancer and Nutrition (EPIC) (7). In a pooled analysis of more than 50 epidemiologic studies (Collaborative Group on Hormonal Factors in Breast Cancer), association of HRT use with breast cancer was found among women with BMI <25 kg/m2, but not among heavier women (29).
Data on whether adiposity modifies the association of HRT use with risk of breast cancer by hormone receptor subtypes are very limited. Recently, the EPIC study reported that current use of HRT, compared to never-use of HRT, was significantly associated with increased risk of both ER+ and ER- tumors, although more strongly with the former. Associations with HRT were significantly stronger in leaner women (BMI ≤22.5 kg/m2) than overweight women (BMI ≥25.9kg/m2), and HRs were statistically significant in leaner women for both ER+PR+ (HR=2.33; 95% CI=1.84-2.92) and ER-PR- (HR=1.74; 95% CI=1.15-2.63) breast cancer (11). These estimates are remarkably similar to the corresponding ORs for HRT use among lean women in our study. Results from a case-control study in the Seattle-Puget Sound metropolitan area (26) were not entirely consistent. A positive association between estrogen plus progestin hormone therapy and ER+PR+ breast cancer was observed regardless of BMI. It was strongest among lean women (BMI<25 kg/m2), although the interaction was not statistically significant. Moreover, in contrast to the results of our study and the EPIC report, HRT was not associated with the risk of ER-PR- breast cancer, regardless of BMI. Our study has extended previous results by including for the first time Her2 status in the classification of breast cancer subtypes. When Her2 status was considered, associations between HRT use and risk of HR+Her2- and HR+/HR-Her2+ subtypes were much stronger than the association between HRT use and risk of HR-Her2- (triple negative breast cancer). Such associations were only seen in women with BMI <25 kg/m2, and not in overweight women (BMI ≥25 kg/m2). Thus, our results provide additional evidence that the relationship between ever-use of HRT and risk of breast cancer subtypes defined by HR and Her2 status is modified by body weight.
The biological mechanisms underlying this interaction have been postulated but remain unclear. The positive relationship between body weight and breast cancer risk among postmenopausal women is generally attributed to increased conversion of adrenal androgens to estrogens by aromatase within the larger adipose stores of overweight women, as well as decreased circulating sex hormone-binding globulin (SHBG) which leads to more bioavailable estrogens (33-35). Thus, in general, overweight postmenopausal women have higher circulating estrogen levels than normal-weight women. Among HRT users, however, the proportional increase in circulating estrogen levels from exogenous estrogen among postmenopausal women may be relatively smaller in overweight/obese women compared with normal-weight women (36, 37). In addition, overweight women tend to have a higher prevalence of insulin resistance and hyperinsulinemia. Insulin itself is a mitogenic agent, and chronic hyperinsulinemia is also associated with down-regulation of SHBG and insulin-like growth factor binding protein-1 and -2, leading to increased levels of bioavailable estrogen, insulin-like growth factor-1 (IGF-1), and testosterone (38). Long-term treatment with low estrogen doses among overweight women may improve insulin resistance and reduce elevated insulin levels, thereby attenuating the stimulatory effects of insulin on tumor growth and resulting in a reduction of breast cancer risk compared with HRT treatment in leaner women.
A few studies have found that HRT use was associated with reduced breast cancer risk in women with surgically induced menopause (12, 39, 40). In a case-control study of 472 postmenopausal women with a BRCA1 mutation, Eisen et al. found that among women with surgically induced menopause, ever-HRT users had a lower breast cancer risk compared to nonusers (OR=0.48, 95% CI=0.19-1.21) (39). The WHI study recently reported that among postmenopausal women with prior hysterectomy, ever-use of estrogen during the 5.9 year intervention phase was associated with decreased breast cancer risk after follow-up of more than 10 years (HR=0.77; 95% CI=0.62-0.95) (12). In line with these previous findings, our study also observed significantly reduced breast cancer risk associated with HRT use among women with surgically induced menopause, but only among women who had a hysterectomy with both ovaries removed. Among those who had a hysterectomy with no or one ovary removed, ever-use of HRT was associated with a non-significant increase in breast cancer risk.
Some in vitro experiments have shown that estrogen deprivation of hormone-dependent MCF-7 breast cancer cells causes them to undergo adaptive changes in which estrogen switches from being a proliferative agent to paradoxically inhibiting growth and inducing apoptosis (42). Thus, it has been postulated that administration of estrogen through HRT use may induce apoptosis of breast cancer cells that are present among women with bilateral oophorectomy, and thus reduce breast cancer risk. However, it is possible that HRT use among women with bilateral oophorectomy is an indicator of early age at oophorectomy and thus shorter duration of endogenous estrogen exposure since postmenopausal women with bilateral oophorectomy typically do not use HRT. Indeed, in our study, women with bilateral oophorectomy had an early age of menopause. Furthermore, bilateral oophorectomy itself is also reported to be associated with reduced breast cancer risk (41). Therefore, the reduced risk associated with HRT use among women with bilateral oophorectomy may not be due to a protective effect of exogenous estrogen exposure among these women as suggested by in vitro experiments. Instead, this association could be due to confounding effects that cannot be controlled in our study.
Several limitations of our study should be acknowledged. First, approximately 30% of breast cancer cases in our study did not have information available regarding receptor status, which might introduce selection bias. However, in our study sample, prevalence rates of ER+, PR+, and Her2+ were 77.4%, 62.6%, and 23.8%, respectively, consistent with many previous large-scale studies conducted among white women (16, 20, 24). Second, as with all case-control studies, our study is subject to recall bias, especially as we relied on self-reported information on reproductive factors and HRT use. Several studies, however, have shown generally consistent agreement between self-report and medical records regarding reproductive factors and hormone use in postmenopausal women (46-48). Furthermore, the stronger association of exogenous estrogen use with ER+ tumors observed in this study argues against the effect of recall bias on our study results. Third, breast cancer intrinsic subtypes in our study were defined based on ER, PR, and HER2 status without information on Ki-67 (a proliferation marker). Since luminal B tumors include two subgroups (ER+ and/or PR+, Her2+, any Ki-67 and ER+ and/or PR+, Her2-, Ki-67 high), it is likely that some cases included in the luminal A group (ER+ and/or PR+, Her2-, Ki-67 low) may actually represent luminal B tumors (ER+ and/or PR+, Her2-, Ki-67 high). Fourth, multiple ORs were estimated in each analysis of interaction between HRT use (or duration of HRT use) and breast cancer risk, overall or by subtypes. Some ORs were statistically significant perhaps due to multiple comparisons. However, the focus of this study is to identify potential interaction, for which only a limited number of tests were performed, and virtually all significant interactions identified in this study remained significant after adjusting for multiple comparisons.
In summary, our study clearly shows that the relationship between HRT use and breast cancer risk among postmenopausal women is modified by body weight and prior bilateral oophorectomy. Among women having natural menopause, ever-HRT-use was significantly associated with increased risk of breast cancer, especially ER+, ER+PR+, luminal A, and luminal B and Her2 overexpression subtypes in women of normal weight (BMI <25 kg/m2). Such associations, however, were not seen among overweight women (BMI ≥25 kg/m2). Among women with prior bilateral oophorectomy, ever-HRT-use was associated with reduced breast cancer risk for ER+, ER+PR+, and luminal A tumors. These results may be helpful in recommending HRT use among postmenopausal women and identifying high-risk women among HRT users. Further investigation and clarification of underlying mechanisms contributing to these effects are warranted.
Statement of Translational Relevance.
Our study shows that the association between HRT use and breast cancer risk among postmenopausal women is modified by body weight and prior bilateral oophorectomy. Ever-HRT-use was significantly associated with increased risk of breast cancer, especially ER+, ER+PR+, luminal A, and luminal B and Her2 overexpression subtypes, in women with normal weight (BMI <25 kg/m2) and natural menopause. Such associations, however, were not seen among overweight women (BMI ≥25 kg/m2). These results may be helpful in recommending HRT use among postmenopausal women and identifying high-risk women among HRT users.
Acknowledgments
This work was supported by a research grant (R01CA100374) from the US National Cancer Institute. The authors would like to thank study participants and research staff of The Nashville Breast Health Study (NBHS) for their support of this research, and Mary Jo Daly for technical assistance in manuscript preparation. Surveys for this study were conducted by the Biospecimen and Survey Shared Resource, supported in part by P30CA68485.
Footnotes
Conflicts of interest: None of the authors declare any conflicts of interest.
References
- 1.Narod SA. Hormone replacement therapy and the risk of breast cancer. Nat Rev Clin Oncol. 2011;8:669–76. doi: 10.1038/nrclinonc.2011.110. [DOI] [PubMed] [Google Scholar]
- 2.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–82. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 3.Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13:1558–68. [PubMed] [Google Scholar]
- 4.Huang Z, Willett WC, Colditz GA, Hunter DJ, Manson JE, Rosner B, et al. Waist circumference, waist: hip ratio, and risk of breast cancer in the Nurses' Health Study. Am J Epidemiol. 1999;150:1316–24. doi: 10.1093/oxfordjournals.aje.a009963. [DOI] [PubMed] [Google Scholar]
- 5.van den Brandt PA, Spiegelman D, Yaun, Adami HO, Beeson L, Folsom AR, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152:514–27. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 6.Morimoto LM, White E, Chen Z, Chlebowski RT, Hays J, Kuller L, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women's Health Initiative (United States) Cancer Causes Control. 2002;13:741–51. doi: 10.1023/a:1020239211145. [DOI] [PubMed] [Google Scholar]
- 7.Lahmann PH, Hoffmann K, Allen N, van Gils CH, Khaw KT, Tehard B, et al. Body size and breast cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC) Int J Cancer. 2004;111:762–71. doi: 10.1002/ijc.20315. [DOI] [PubMed] [Google Scholar]
- 8.Reeves GK, Beral V, Green J, Gathani T, Bull D Million Women Study Collaborators. Hormonal therapy for menopause and breast-cancer risk by histological type: a cohort study and meta-analysis. Lancet Oncol. 2006;7:910–18. doi: 10.1016/S1470-2045(06)70911-1. [DOI] [PubMed] [Google Scholar]
- 9.Howell A, Chapman M, Harvie M. Energy restriction for breast cancer prevention. Recent Results Cancer Res. 2009;181:97–111. doi: 10.1007/978-3-540-69297-3_11. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki R, Orsini N, Saji S, Key TJ, Wolk A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status--a meta-analysis. Int J Cancer. 2009;124:698–712. doi: 10.1002/ijc.23943. [DOI] [PubMed] [Google Scholar]
- 11.Ritte R, Lukanova A, Berrino F, Dossus L, Tjønneland A, Olsen A, et al. Adiposity, hormone replacement therapy use and breast cancer risk by age and hormone receptor status: a large prospective cohort study. Breast Cancer Res. 2012;14:R76. doi: 10.1186/bcr3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, et al. Health risks and benefits 5 years after stopping randomized treatment with conjugated equine estrogens in postmenopausal women with prior hysterectomy. JAMA. 2011;305:1305–14. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chlebowski RT, Anderson GL. Changing Concepts: Menopausal Hormone Therapy and Breast Cancer. J Natl Cancer Inst. 2012;104:517–27. doi: 10.1093/jnci/djs014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weigelt B, Reis-Filho JS. Histological and molecular types of breast cancer: is there a unifying taxonomy? Nat Rev Clin Oncol. 2009;6:718–30. doi: 10.1038/nrclinonc.2009.166. [DOI] [PubMed] [Google Scholar]
- 15.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 17.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–23. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7:4–13. doi: 10.3121/cmr.2009.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phipps AI, Chlebowski RT, Prentice R, McTiernan A, Stefanick ML, Wactawski-Wende J, et al. Body size, physical activity, and risk of triple-negative and estrogen receptor-positive breast cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:454–63. doi: 10.1158/1055-9965.EPI-10-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parise CA, Bauer KR, Brown MM, Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor2 (HER2) among women with invasive breast cancer in California, 1999-2004. The Breast Journal. 2009;15:593–602. doi: 10.1111/j.1524-4741.2009.00822.x. [DOI] [PubMed] [Google Scholar]
- 21.Greene WH. Econometric Analysis. fifth. Prentice Hall; 1993. pp. 720–3. [Google Scholar]
- 22.Biesheuvel CJ, Vergouwe Y, Steyerberg EW, Grobbee DE, Moons KG. Polytomous logistic regression analysis could be applied more often in diagnostic research. J Clin Epidemiol. 2008;61:125–34. doi: 10.1016/j.jclinepi.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Gapstur SM, Morrow M, Sellers TA. Hormone replacement therapy and risk of breast cancer with a favorable histology: results of the Iowa Women's Health Study. JAMA. 1999;28:2091–7. doi: 10.1001/jama.281.22.2091. [DOI] [PubMed] [Google Scholar]
- 24.Colditz GA, Rosner BA, Chen WY, Holmes MD, Hankinson SE. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst. 2004;96:218–28. doi: 10.1093/jnci/djh025. [DOI] [PubMed] [Google Scholar]
- 25.Li CI, Malone KE, Porter PL, Weiss NS, Tang MT, Cushing-Haugen KL, et al. Relationship between long durations and different regimens of hormone therapy and risk of breast cancer. JAMA. 2003;289:3254–63. doi: 10.1001/jama.289.24.3254. [DOI] [PubMed] [Google Scholar]
- 26.Li CI, Malone KE, Daling JR. Interactions between body mass index and hormone therapy and postmenopausal breast cancer risk (United States) Cancer Causes Control. 2006;17:695–703. doi: 10.1007/s10552-005-0001-7. [DOI] [PubMed] [Google Scholar]
- 27.Ma H, Bernstein L, Pike MC, Ursin G. Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res. 2006;8:R43. doi: 10.1186/bcr1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Setiawan VW, Monroe KR, Wilkens LR, Kolonel LN, Pike MC, Henderson BE. Breast cancer risk factors defined by estrogen and progesterone receptor status: the multiethnic cohort study. Am J Epidemiol. 2009;169:1251–9. doi: 10.1093/aje/kwp036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 1997;350:1047–59. [PubMed] [Google Scholar]
- 30.Rosenberg LU, Einarsdóttir K, Friman EI, Wedrén S, Dickman PW, Hall P, et al. Risk factors for hormone receptor-defined breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2006;15:2482–8. doi: 10.1158/1055-9965.EPI-06-0489. [DOI] [PubMed] [Google Scholar]
- 31.Beral V, Reeves G, Bull D, Green J Million Women Study Collaborators. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst. 2011;103:296–305. doi: 10.1093/jnci/djq527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ursin G, Tseng CC, Paganini-Hill A, Enger S, Wan PC, Formenti S, et al. Does menopausal hormone replacement therapy interact with known factors to increase risk of breast cancer? J Clin Oncol. 2002;20:699–706. doi: 10.1200/JCO.2002.20.3.699. [DOI] [PubMed] [Google Scholar]
- 33.Kaaks R, Rinaldi S, Key TJ, Berrino F, Peeters PH, Biessy C, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocrin Relat Cancer. 2005;12:1071–82. doi: 10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- 34.Baglietto L, English DR, Hopper JL, MacInnis RJ, Morris HA, Tilley WD, et al. Circulating steroid hormone concentrations in postmenopausal women in relation to body size and composition. Breast Cancer Res Treat. 2009;115:171–9. doi: 10.1007/s10549-008-0069-3. [DOI] [PubMed] [Google Scholar]
- 35.Hankinson SE, Colditz GA, Hunter DJ, Manson JE, Willett WC, Stampfer MJ, et al. Reproductive factors and family history of breast cancer in relation to plasma estrogen and prolactin levels in postmenopausal women in the Nurses' Health Study (United States) Cancer Causes Control. 1995;6:217–24. doi: 10.1007/BF00051793. [DOI] [PubMed] [Google Scholar]
- 36.Marsdon J. Hormone-replacement therapy and breast cancer. Lancet Oncol. 2002;3:303–311. doi: 10.1016/s1470-2045(02)00732-5. [DOI] [PubMed] [Google Scholar]
- 37.Karim R, Mack WJ, Hodis HN, Roy S, Stanczyk FZ. Influence of age and obesity on serum estradiol, estrone, and sex hormone binding globulin concentrations following oral estrogen administration in postmenopausal women. J Clin Endocrinol Metab. 2009;94:4136–43. doi: 10.1210/jc.2009-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Looper MH, Howell AH. Central obesity and breast cancer risk: a systematic review. Obesity. 2003;4:157–173. doi: 10.1046/j.1467-789x.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 39.Eisen A, Lubinski J, Gronwald J, Moller P, Lynch HT, Klijn J, et al. Hormone therapy and the risk of breast cancer in BRCA1 mutation carriers. J Natl Cancer Inst. 2008;100:1361–7. doi: 10.1093/jnci/djn313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rebbeck TR, Friebel T, Wagner T, Lynch HT, Garber JE, Daly MB, et al. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2005;23:7804–10. doi: 10.1200/JCO.2004.00.8151. [DOI] [PubMed] [Google Scholar]
- 41.Lewis-Wambi JS, Jordan VC. Estrogen regulation of apoptosis: how can one hormone stimulate and inhibit? Breast Cancer Res. 2009;11:206. doi: 10.1186/bcr2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis JS, Meeke K, Osipo C, Ross EA, Kidawi N, Li T, et al. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. J Natl Cancer Int. 2005;97:1746–59. doi: 10.1093/jnci/dji400. [DOI] [PubMed] [Google Scholar]
- 43.Jordan VC, Ford LG. Paradoxical clinical effects of estrogen on breast cancer risk: a “new” biology of estrogen-induced apoptosis. Cancer Prev Res. 2011;4:633–7. doi: 10.1158/1940-6207.CAPR-11-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nichols HB, Visvanathan K, Newcomb PA, Hampton JM, Egan KM, Titus-Ernstoff L, et al. Bilateral oophorectomy in relation to risk of postmenopausal breast cancer: confounding by nonmalignant indications for surgery? Am J Epidemiol. 2011;173:1111–20. doi: 10.1093/aje/kwq510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chlebowski RT, Chen Z, Anderson GL, Rohan T, Aragaki A, Lane D, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl cancer Inst. 2005;97:439–48. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Diamant AL, Thind A, Maly RC. Validity of self-reports of breast cancer treatment in low-income, medically underserved women with breast cancer. Breast Cancer Res Treat. 2010;119:745–51. doi: 10.1007/s10549-009-0447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schootman M, Jeffe DB, West MM, Aft R. Self-report by elderly breast cancer patients was an acceptable alternative to surveillance, epidemiology, and end results (SEER) abstract data. J Clin Epidemiol. 2005;58:1316–19. doi: 10.1016/j.jclinepi.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Phillips KA, Milne RL, Buys S, Friedlander ML, Ward JH, McCredie MR, et al. Agreement between self-reported breast cancer treatment and medical records in a population-based Breast Cancer Family Registry. J Clin Oncol. 2005;23:4679–86. doi: 10.1200/JCO.2005.03.002. [DOI] [PubMed] [Google Scholar]