Abstract
Vascular smooth muscle cells (SMCs) are phenotypically diverse. Although most medial SMCs can be classified as “fusiform,” others are of the “epithelioid” phenotype. Proliferation and apoptosis of epithelioid SMCs may contribute importantly to neointimal formation and regression, respectively. Because reactive oxygen species (ROS) are increased in vascular injury and can induce apoptosis of SMCs, we compared the effects of ROS on epithelioid and fusiform SMCs. Epithelioid and fusiform SMC lines were clonally isolated from rat aortic media and studied under similar conditions and passage numbers. H2O2 produced dose- and time-dependent cytotoxicity that was enhanced in epithelioid compared with fusiform cells. After 24-hour exposure to 50 μmol/L H2O2, epithelioid cell numbers were reduced by 34±5% versus a 3±5% (P<0.05) reduction in fusiform cell numbers. Similar results were obtained whether H2O2 was administered to growth-arrested or growing cells or when epithelioid and fusiform cells were exposed to extracellular O2·−. To investigate whether apoptosis contributed to enhanced ROS-induced cytotoxicity in epithelioid SMCs, terminal deoxyribonucleotidyl transferase (TDT)-mediated dUTP-digoxigenin nick-end labeling (TUNEL) staining was performed. The incidence of TUNEL positivity was 5-fold increased in epithelioid versus fusiform SMCs after treatment with 50 μmol/L H2O2 (19±1% epithelioid versus 5±1% fusiform, P<0.05). Enhanced H2O2-induced apoptosis in epithelioid SMCs was confirmed by DNA laddering. Furthermore, when balloon-injured aortas were exposed to H2O2 ex vivo, enhanced apoptosis was observed in neointimal compared with medial SMCs. These results suggest that epithelioid SMCs exhibit enhanced sensitivity to ROS-induced apoptosis, which may play an important role in neointimal regression.
Keywords: smooth muscle cells, apoptosis, reactive oxygen species, hydrogen peroxide, neointima
Recent reports by several groups indicate that smooth muscle cells (SMCs) within the arterial wall comprise a variety of distinct phenotypes that possess unique biochemical and functional properties.1–8 In general, clonally derived SMC lines can be classified as “fusiform” or “epithelioid,” corresponding to the appearance and growth pattern of the cells in culture.2,9 Fusiform SMCs, which are elongated and exhibit the classic “hills-and-valleys” pattern of growth, are predominant in the arterial media of adult animals. Epithelioid SMCs, which are cuboidal and form monolayers, are less abundant in the adult media but migrate and proliferate after endothelial denudation to form the neointima. Epithelioid SMCs possess several properties, including enhanced cytokine-induced migration and secretion of extracellular matrix, which suggest that they may play an important role in healing of diseased blood vessels.
The rate of death of SMCs was reported to be increased in diseased compared with normal blood vessels and may contribute to vascular remodeling and plaque instability.10–12 In theory, this enhanced rate of SMC death could simply be due to the local cellular environment, because diseased blood vessels contain an abundance of inflammatory mediators. Even when removed from the atherosclerotic environment, however, SMCs cultured from atherosclerotic lesions undergo cell death more readily in response to cytokines and P53 than do medial SMCs cultured from the same blood vessels.12,13 Moreover, for several weeks after balloon injury of rat blood vessels, the rate of death is increased in neointimal compared with medial SMCs, even though leukocyte infiltration is not a prominent feature.14–16 These latter observations suggest that neointimal SMCs are inherently more susceptible than are medial SMCs to some cytotoxic stimuli. Because under these conditions the neointimal SMCs are predominately epithelioid, the aforementioned studies raise the possibility that enhanced susceptibility to cytotoxic stimuli could be a feature of the epithelioid phenotype. Putative mechanisms responsible for cytotoxicity of epithelioid SMCs, however, remain to be elucidated.
Among the potential mediators of SMC cytotoxicity are reactive oxygen species (ROS), the levels of which are increased in atherosclerotic and balloon-injured blood vessels.17–20 Exposing SMCs to H2O2 results in induction of apoptotic cell death.21–23 Moreover, ROS have been suggested to be downstream mediators of cell death induced by diverse proapoptotic stimuli, including cytokines and P53.24–27 To our knowledge, however, the effects of ROS on distinct phenotypes of vascular SMCs have not been previously reported.
In this study, we examined the effects of ROS on cell viability and apoptosis in epithelioid and fusiform SMC lines clonally derived from rat aorta. Moreover, aortic segments from balloon-injured rats were treated with H2O2 to examine the effects of ROS on neointimal and medial SMCs in situ. Our results suggest that epithelioid SMC lines and neointimal SMCs in situ exhibit enhanced sensitivity to ROS-induced cytotoxicity and apoptosis. These findings may have significance in regard to understanding the mechanisms responsible for neointimal regression and, perhaps, lesion destabilization.
Methods
Dulbecco’s modified Eagle’s medium (DMEM), minimal essential medium–nonessential amino acid, minimal essential medium–vitamin solution, H2O2, xanthine, xanthine oxidase, mouse monoclonal α-actin antibody, and FITC-conjugated acetylated LDL were obtained from Sigma Chemical Co. HEPES, trypsin, and L-glutamine were obtained from Sigma Chemical Co and prepared for use by the University of Iowa Cancer Center. Fetal calf serum (FCS) was purchased from HyClone Laboratories, and in situ apoptosis detection and DNA ladder isolation kits were purchased from Oncor. The 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethyloxphenyl)-2-(4-sulphenyl)-2H-tetrazolium, inner salt (MTS) test kit was purchased from Promega.
Cell Culture
SMCs were obtained from the thoracic aortas of 250- to 300-g male Sprague-Dawley rats by using the collagenase and elastase digestion method.4,28 Cells were plated in DMEM containing penicillin (100 IU/mL) and streptomycin (100 μg/mL) supplemented with 10% heat-inactivated FCS in a humidified 10% CO2 atmosphere at 37°C. Cloning was performed 48 hours after plating of the primary culture by limiting dilution (0.5 cells per well) into 0.1% gelatin–coated, 24-well plates in DMEM containing 10% FCS and 50% conditioned medium.4 Cloned SMCs were subcultured at subconfluence by trypsinization28 and grown to the third passage in the conditioned medium, after which they were maintained in medium supplemented with 10% FCS. Two separate rat aortas were used to generate cell lines for these studies. In all experiments, epithelioid and fusiform SMC lines were studied under identical conditions and at similar passage numbers (between the sixth and 20th passages).
Cell Proliferation and [3H]Thymidine Incorporation
For evaluation of proliferation by cell numbers, SMCs were plated in DMEM in the presence of 10% FCS at a concentration of 5000 cells/cm2, and the medium was changed every 2 or 3 days. After 7 days, the cells were removed by trypsinization and counted with a hemocytometer. For determination of proliferation by [3H]thymidine incorporation (an index of DNA synthesis), subconfluent SMCs were placed in serum-free medium for 48 hours to induce quiescence, after which the cells were incubated with 1 μCi/mL [3H]thymidine and 10% FCS for 24 hours. After the medium was removed, the cells were washed twice with cold PBS and fixed with 20% trichloroacetic acid for 30 minutes. The cells were lysed with 0.25N NaOH, neutralized with 1N HCl, and analyzed for radioactivity by scintillation counting. Results were expressed as the ratio of total [3H]thy-midine incorporation in FCS-treated cells to total [3H]thymidine incorporation in serum-free controls.
Immunofluorescence Staining
SMCs grown to confluence on Tissue-Tek chamber slides (Nunc) were fixed in absolute methanol at −20°C for 10 minutes and then incubated with monoclonal antibody to α-smooth muscle actin for 1 hour at room temperature. After incubation with 25% FCS in PBS for 30 minutes, FITC-conjugated anti-mouse IgG was applied for 1 hour at room temperature. Stained samples were examined with an inverted fluorescence microscope (Nikon).
In other experiments, cells were grown on chamber slides as described above and then incubated with FITC-conjugated acetylated LDL. After 1-hour incubation at room temperature, cells were examined with an inverted fluorescence microscope. Parallel studies were performed with endothelial cells, which in all instances took up acetylated LDL, to serve as positive controls.
Cell Viability Assay
Cell viability was assessed with the MTS test kit according to the manufacturer’s instructions.29 In brief, the cells were plated in 24-well plates in a final volume of 0.5 mL of culture medium. After treatment with ROS for the indicated times, the cells were washed twice with PBS, and 400 μL of MTS reagent in culture medium was added. The incubation was continued for 2 hours at 37°C, after which optical density was determined at 490 nm.
In Situ Nick-End Labeling and Propidium Iodide Staining
The terminal deoxyribonucleotidyl transferase (TDT)-mediated dUTP-digoxigenin nick-end labeling (TUNEL) assay for detecting DNA fragmentation in situ was performed with a commercially available kit according to the manufacturer’s instructions (ApopTagTM Plus, Oncor). In brief, the samples were preincubated with equilibration buffer for 5 minutes and subsequently incubated with TDT in the presence of digoxigenin-conjugated dUTP for 1 hour at 37°C. The reaction was terminated by incubating the samples in a stopping buffer for 30 minutes. After 3 rinses with PBS, samples were incubated with the fluorescein-labeled anti-digoxigenin antibody for 30 minutes, and 3 rinses with PBS were repeated. Finally, the samples were stained and mounted with Oncor propidium iodide/Antifade and then examined by laser confocal microscopy.
DNA Ladder Analysis
Fragmented DNA was isolated from SMCs after treatment with H2O2 for 24 hours by using a Suicide-Track DNA ladder isolation kit from Oncor according to the manufacturer’s instructions. In brief, cells were lysed in 500 μL of extraction buffer and incubated for 30 minutes on ice. After the high-molecular-weight chromatin was removed, cell lysates were incubated with proteinase K for 60 minutes at 37°C and then incubated with RNase for 60 minutes at 50°C. Fragmented DNA was precipitated with sodium acetate in 2-propanol, washed with ethanol, and then dissolved in 10 mmol/L Tris-HCl and 1 mmol/L EDTA. Finally, the DNA was electrophoresed in 1.5% agarose gel and stained with ethidium bromide.
Determination of ROS-Induced Cytotoxicity in Neointimal and Medial SMCs In Situ by DNA Chromatin Morphology
Male Sprague-Dawley rats (250 to 300 g) subjected to sham or balloon injury of the aorta were purchased from Zivic-Miller Laboratories (Portersville, Pa). The injury was accomplished by using Fogarty 2F arterial embolectomy catheters as previously described.30 Sham-injured animals were treated identically, except that the balloon was not inflated during passage through the aorta. Two weeks after undergoing surgery, the animals were anesthestized with ketamine/xylazine (respectively, 60 mg/kg and 7.5 mg/kg IP), after which the aorta was removed and cut into 5-mm segments. Each segment was placed in an individual well of a 24-well plate containing 1 mL of DMEM and maintained in a humidified 10% CO2 atmosphere at 37°C. Two hours later, 0 to 300 μmol/L H2O2 was added to each well, and the incubation was continued for 15 hours. The segments were then rinsed with cold PBS, fixed with 2% paraformalin for 2 hours, and sectioned in 6-μm-thick slices.
DNA chromatin morphology was determined by Hoechst 33342 staining, according to previously reported methods.19 In brief, the tissue sections were incubated for 2 hours at 37°C with Hoechst 33342 (Molecular Probes) at a concentration of 5 μg/mL and then viewed under UV microscopy. Some of the fixed tissues were also subjected to TUNEL assay, as described in In Situ Nick-End Labeling and Propidium Iodide Staining.
All procedures on living animals were performed in accordance with guidelines set forth by the Animal Care Committee at the University of Iowa College of Medicine.
Statistical Analyses
All data are expressed as mean±SEM. Differences between mean values of 2 groups were analyzed by Student’s t test for unpaired data. Differences between mean values of multiple groups were analyzed by 1-way ANOVA followed by Bonferroni t testing. Probability values of 0.05 or less were considered to be statistically significant.
Results
Establishment of Epithelioid and Fusiform SMC Lines
Epithelioid and fusiform SMC lines were clonally isolated by using limiting dilution techniques.3,4 Using this method, we established 5 cell lines from 1 rat aorta, 3 of which appeared to be fusiform and 1 epithelioid, according to the criteria published by Bochaton-Piallat et al.4 The fifth cell line had some features of both fusiform and epithelioid cells. Two of the cell lines, 1 fusiform and 1 epithelioid, have been propagated through 20 passages and have retained their distinct morphological characteristics. As shown in Figure 1, the fusiform SMCs appeared elongated and grew in a “hills-and-valleys” pattern (left), while epithelioid SMCs were cuboidal and formed monolayers (right). All 5 cell lines expressed α-actin, and none of the cell lines took up acetylated LDL, thereby confirming their identities as SMCs (data not shown).
Figure 1.
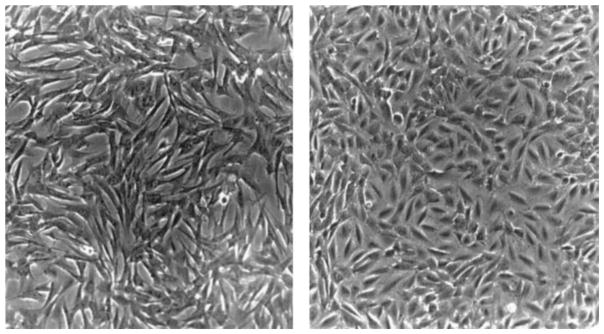
Morphological aspects of fusiform and epithelioid SMC lines. SMCs were isolated from the same aorta, grown in DMEM supplemented with 10% FCS, and examined by phase-contrast microscopy (×200). Fusiform SMCs (left) appeared elongated and grew in a “hills-and-valleys” pattern. Epithelioid SMCs (right) were polygonal and formed monolayers.
Characterization of Cell Proliferation Responses
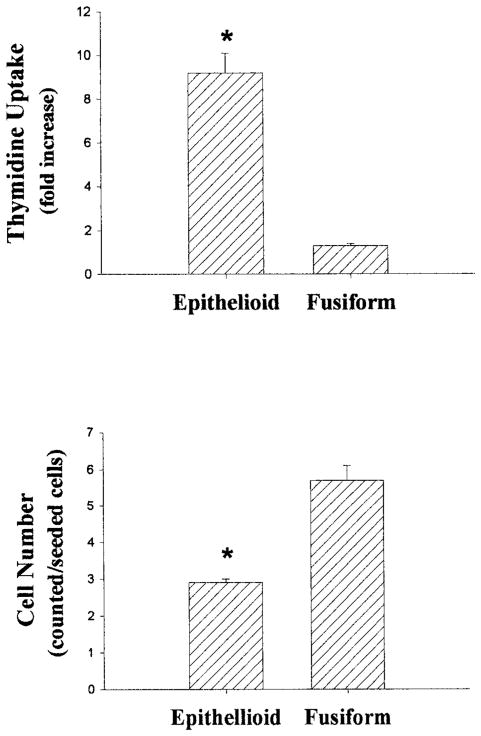
To address whether the fusiform and epithelioid cell lines exhibited distinctive growth responses similar to those reported by Bochaton-Piallat et al,4 we determined rates of DNA synthesis by means of [3H]thymidine incorporation 24 hours after stimulating growth-arrested, subconfluent cultures with FCS. The [3H]thymidine uptake values were expressed as fold increase over control. Although FCS stimulated DNA synthesis in both fusiform and epithelioid SMCs, the increase was much greater (8-fold) for epithelioid compared with fusiform SMCs (Figure 2, upper panel). These results are qualitatively similar to those reported by Bochaton-Piallat et al.4
Figure 2.
Growth responses of fusiform and epithelioid SMC lines. For experiments shown in the upper panel, confluent SMCs were growth-arrested by incubation with serum-free medium for 48 hours. The cells were then incubated with or without 10% FCS for 24 hours, whereas [3H]thymidine uptake (expressed as fold increase) was determined by liquid scintillation counting. For experiments shown in the lower panel, SMCs were passaged into 24-well plates at an initial concentration of 5000 cells/cm2 in medium supplemented with 10% FCS. After 7 days, the cells were removed by trypsinization, and cell numbers (expressed as counted/seeded cells) were determined by using a hemocytometer. Data are expressed as mean±SE, n=3 per group, representative of 2 experiments. *P<0.05 vs fusiform SMCs.
In separate experiments, we performed cell counts 7 days after plating equivalent numbers of both cell lines in culture medium containing 10% FCS. To ensure that plating efficiency and initial viability were similar between the 2 cell lines, some of the cultures were counted 24 hours after plating. At this time, the numbers of cells were similar in both groups [1.36×104±0.09 (fusiform) versus 1.36×104±0.12 (epithelioid); n=3, P>0.05]. As the cell lines proliferated over the ensuing days, however, the growth rate of epithelioid cells slowed, so that 7 days after plating, the number of fusiform cells markedly exceeded that of epithelioid cells (P<0.05, Figure 2, lower panel). Thus, although subconfluent epithelioid cells incorporate more [3H]thymidine than do fusiform SMCs when stimulated by FCS, their growth may be contact-inhibited, as was reported previously.4
Effects of H2O2 on Cell Viability in Epithelioid and Fusiform SMCs
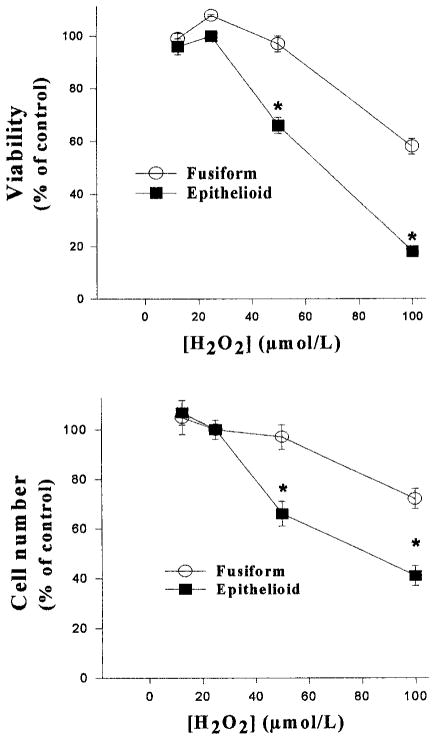
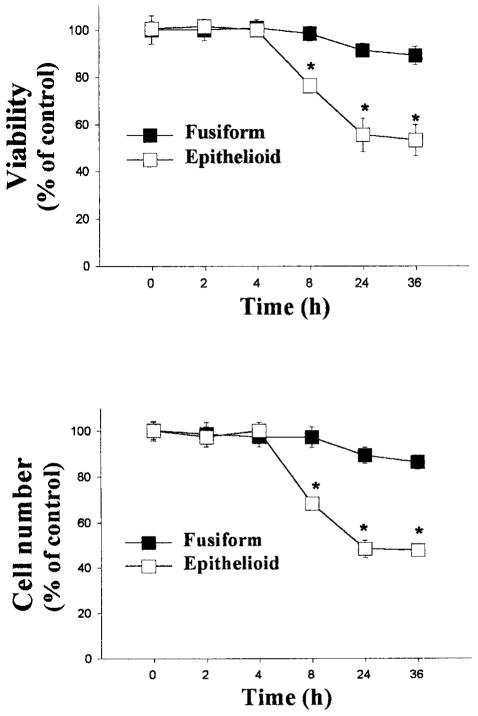
To examine the effects of ROS on the viability of epithelioid and fusiform SMCs, the 2 cell lines were grown to subconfluence, incubated with serum-free medium for 48 hours, and then treated with H2O2 for up to 36 hours. Because the 2 cell lines exhibited different patterns of growth (Figure 2), care was taken to ensure that similar numbers of cells were present in each culture at the time of application of H2O2. In addition, the clonal cell lines were studied at the same passage numbers and under identical conditions to exclude differences other than those attributable to cell phenotype. H2O2 produced dose-dependent decreases in cell numbers and viability in both cell lines (Figure 3). The decreases in cell number and viability, however, were significantly greater in epithelioid compared with fusiform SMCs. Examination of the time course with 50 μmol/L H2O2 indicated that the viability in the epithelioid cell line was decreased by 8 hours, reached its lowest point after incubations of 24 hours, and did not change thereafter (Figure 4).
Figure 3.
Effects of H2O2 on cell viability and numbers in quiescent fusiform and epithelioid SMCs. Confluent SMCs in 24-well plates were growth-arrested in serum free medium for 48 hours, followed by exposure to 0 to 100 μmol/L H2O2 for 24 hours. Cell viability was then determined by MTS assay (upper panel), or the cells were counted by using a hemocytometer (lower panel). The data were normalized to percent of control value (0 μmol/L H2O2). The numbers of cells in the control groups were 9.87±0.86×104 (fusiform) and 9.93±0.87×104 (epithelioid) cells/well. Control values for cell viability (expressed as optical density) were 1.15±0.09 (fusiform) and 1.12±0.09 (epithelioid). Data are expressed as mean±SE, n=3 for each group, representative of 3 experiments. *P<0.05 compared with corresponding fusiform SMC value.
Figure 4.
Time dependence of effects of H2O2 on cell numbers and viability. SMCs were grown to subconfluence in 24-well plates and then treated with 50 μmol/L H2O2 for 0 to 36 hours, after which cell viability (upper panel) and numbers (lower panel) were determined, as described in the legend to Figure 3. The data, expressed as mean±SE, were normalized to percent of control value (0 μmol/L H2O2), n=3 for each group, representative of 2 experiments. *P<0.05 compared with corresponding fusiform SMC value.
To determine whether ROS-induced cytotoxicity depended on the growth state of the cells, experiments were performed with cells that were maintained in medium with 10% FCS before and during incubation with H2O2. Under these conditions, the cells are rapidly growing. Despite this alteration in experimental conditions, similar results were obtained with regard to cytotoxicity (data not shown). Thus, whether administered to growth-arrested or growing cells, H2O2 produced more potent toxicity in epithelioid compared with fusiform SMCs.
To determine whether enhanced ROS-induced cytotoxicity would be observed in epithelioid SMCs when a different oxidant species was applied, the effects of treatment with the combination of xanthine plus xanthine oxidase (which generates extracellular O2·.−) were examined. Similar to the results obtained with H2O2, xanthine plus xanthine oxidase produced more potent toxicity in epithelioid compared with fusiform SMCs (after treatment with 0.1 mmol/L xanthine + 0.25 mU/mL xanthine oxidase, viability expressed as percent of control cells treated with 0.1 mmol/L xanthine alone was 75±3% for epithelioid cells, P<0.05 versus 101±2% viability for fusiform cells, n=3 per group).
To further investigate whether the enhanced ROS-induced cytotoxicity in our epithelioid SMCs was a function of cell phenotype, we tested the cells against a different fusiform cell line clonally isolated from the same rat aorta. H2O2-induced cytotoxicity was similarly enhanced in the epithelioid SMCs compared with the other fusiform cell line (data not shown). Furthermore, similar results were observed when H2O2-induced cytotoxicity was compared in different epithelioid and fusiform lines derived from an additional rat aorta (data not shown). To summarize, these results suggest that under a variety of experimental conditions, ROS-induced cytotoxicity was enhanced among epithelioid compared with fusiform SMC lines.
Effects of H2O2 on Apoptosis of Epithelioid and Fusiform SMCs
Apoptosis of vascular SMCs has been suggested to play an important role in atherosclerosis and restenosis after angioplasty and can be induced in vitro by application of H2O2.20 To examine whether increased apoptosis contributes to the enhanced susceptibility to H2O2-induced cytotoxicity in epithelioid SMCs, TUNEL staining was performed 24 hours after treatment with H2O2. In the absence of exposure to H2O2, the incidence of TUNEL-positive cells was similar in epithelioid and fusiform SMC lines (Figure 5, left). After treatment with 50 μmol/L H2O2, however, there was a much greater increase in the number of TUNEL-positive epithelioid cells compared with fusiform cells, suggesting an enhanced rate of apoptosis.
Figure 5.
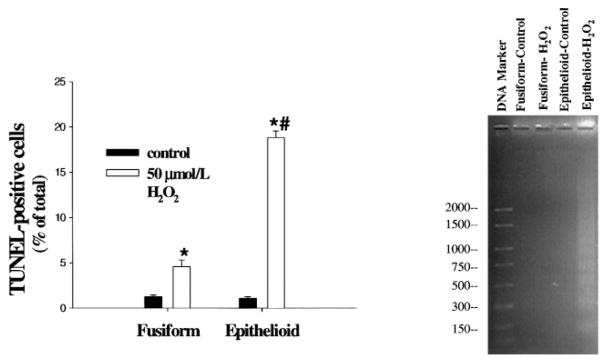
Effects of H2O2 on apoptosis in fusiform and epithelioid SMCs. After treatment with 0 (control) or 50 μmol/L H2O2 for 24 hours as described in the legend to Figure 2, the TUNEL assay was performed (left panel), or DNA fragments were extracted and subjected to agarose gel electrophoresis for assessment of laddering (right panel); the values to the left of the DNA marker refer to the numbers of base pairs. TUNEL-positive cells were quantified and expressed as the percentage of total cells in each cell line. Values are expressed as mean±SE, n=3, representative of 2 experiments. *P<0.05 vs corresponding control value. #P<0.05 vs fusiform SMC value at 50 μmol/L H2O2.
To confirm that apoptosis was enhanced in epithelioid compared with fusiform SMCs, agarose gel electrophoresis was used to examine DNA laddering, a sign of fragmentation of nuclear DNA into oligonucleosomal subunits. Substantial DNA laddering was detected in epithelioid SMCs treated with 50 μmol/L H2O2, whereas little or no laddering was detected in untreated epithelioid and fusiform SMCs or in fusiform SMCs treated with 50 μmol/L H2O2 (Figure 5, right).
Effects of H2O2 on Neointimal and Medial SMCs In Situ
After balloon injury of the rat aorta, epithelioid SMCs from the media migrate and proliferate to form the neointima.3–9,15 To compare the effects of H2O2 on neointimal versus medial SMCs in situ, the thoracic aorta was removed 15 days after balloon or sham injury. Segments of aorta were incubated with 0 to 300 μmol/L H2O2 for 15 hours in culture medium, stained with Hoechst 33342, and then examined by UV microscopy.
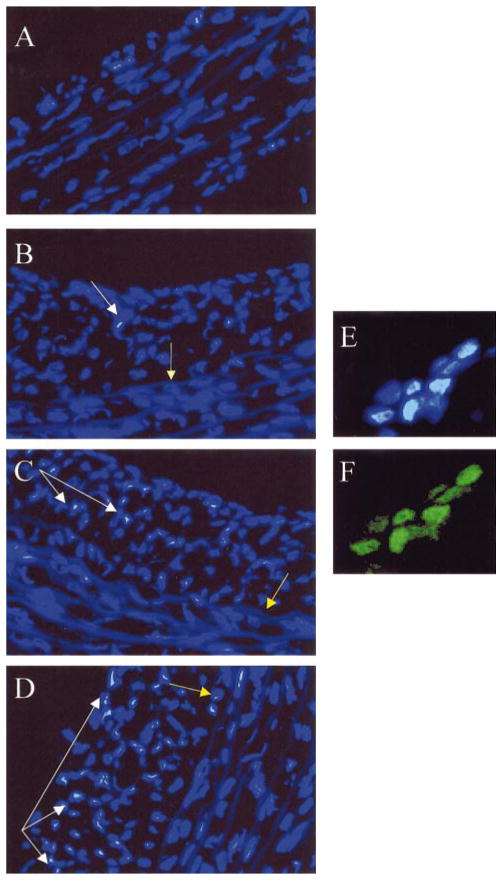
In sham-injured aorta, no significant neointima was observed, and medial SMCs with chromatin condensation were infrequently detected, even after treatment with 300 μmol/L H2O2 (Figure 6A). In balloon-injured vessels, a prominent neointima was observed (Figures 6B through 6D), comprising almost exclusively SMCs [identified by positive staining for α-actin (not shown)]. In the absence of treatment with H2O2, SMCs with chromatin condensation were occasionally detected in the neo-intima but not in the media (Figure 6B). Treatment with 100 or 300 μmol/L H2O2 resulted in dose-dependent increases in the numbers of neointimal SMCs with chromatin condensation (Figures 6C and 6D). The neointimal SMCs that showed chromatin condensation by Hoechst 33342 staining were also TUNEL-positive, thus confirming apoptosis (Figures 6E and 6F). In contrast, even after treatment with 300 μmol/L H2O2, very few of the underlying medial SMCs were apoptotic (Figure 6D). Although these studies in freshly harvested blood vessels used higher concentrations of H2O2 for a shorter period of time, the results in vessels are nevertheless consistent with the findings in regard to enhanced H2O2-induced toxicity in epithelioid versus fusiform cell lines in tissue culture.
Figure 6.
Enhanced sensitivity to H2O2-induced apoptosis in neointimal compared with medial SMCs. Fifteen days after balloon injury of the rat thoracic aorta, segments of sham-injured (A) or balloon-injured (B through D) aorta were incubated ex vivo with 0 (B), 100 (C), or 300 (A and D) μmol/L H2O2, stained with Hoechst 33342, and observed under UV microscopy (×200). All cell nuclei appear blue. White arrows show chromatin condensation characteristic of apoptotic nuclei, and yellow arrows indicate location of the internal elastic lamina. Panels E and F show colocalization of chromatin condensation detected by Hoechst 33342 staining (E) with TUNEL-positive nuclei (F). Each figure is representative of multiple sections taken from a single aortic segment in each treatment group, and similar results were obtained with aortic segments from 2 additional balloon-injured rats.
Discussion
There are 2 major findings of the present study: first, H2O2-induced cytotoxicity was enhanced in epithelioid compared with fusiform SMCs and second, the cytotoxicity was, at least in part, mediated by apoptosis. These results were extended to SMCs in situ by showing that H2O2-induced apoptosis was enhanced in neointimal compared with medial SMCs after balloon injury of the aorta. Because the fusiform and epithelioid cell lines were derived from the same rat aorta and studied under identical conditions and passage numbers, our results suggest that the phenomenon is related to phenotypic rather than genetic or environmental differences between cell lines. The enhanced sensitivity to ROS-induced cytotoxicity in epithelioid SMCs may help to explain the higher rate of SMC death observed in vascular lesions under some circumstances.
It is becoming increasingly accepted that the normal arterial media contains diverse phenotypes of SMCs. Although the majority of medial SMCs in adult animals can be classified as fusiform, epithelioid SMCs have been derived from the media of blood vessels of several species of animals and maintained in culture through multiple passages.3–8 These cell lines exhibit distinct morphological features, display unique profiles of SMC proteins, and possess characteristic growth patterns. Moreover, epithelioid SMCs may play an important role in neointimal formation. After balloon-induced endothelial denudation, epithelioid SMCs within the media are believed to migrate into the area of injury, where subsequent clonal amplification leads to progressive neointimal thickening.3–9,15 Although this migration and proliferation of SMCs may lead to pathological consequences, such as restenosis after balloon angioplasty,14,15 the presence of neointimal SMCs may also have beneficial effects, including enhancement of lesion stability.11 Thus, factors that influence the death of neointimal SMCs might modulate diverse processes such as vascular regression and plaque rupture.
In this study, we successfully isolated fusiform and epithelioid SMC clones from the same adult rat aortic media. Clear differences were noted in the morphological appearances and growth patterns of the 2 cell lines (Figures 1 and 2), which closely resembled the epithelioid and fusiform SMC lines originally isolated by Bochaton-Piallat et al,4 who used similar methods. Because oxidative stress has been suggested to play a very important role in SMC death, we tested the effects of H2O2 on cell viability and cell numbers. We found that H2O2 induced dose- and time-dependent cytotoxicity that was enhanced in epithelioid compared with fusiform SMCs. Because the cell lines were derived from uninjured aorta and studied under virtually identical circumstances, the differences in ROS-induced cytotoxicity most likely were not the result of factors extrinsic to the cells themselves. Consequently, our results suggest that epithelioid SMCs differ from fusiform SMCs not only in regard to patterns of growth but also in terms of their intrinsic susceptibility to death.
Apoptosis was reported to be the major form of cell death resulting from exposure of cultured vascular SMCs to ROS.21–23 Moreover, ROS have been suggested to be downstream mediators of cell death induced by proapoptotic factors prevalent in athero-sclerotic lesions, such as cytokines and p53.24–27 Therefore, we examined whether the enhanced H2O2-induced cytotoxicity in epithelioid SMC lines was mediated by apoptosis. Using 2 different methods, we showed that after treatment with H2O2, there was a much greater increase in the number of apoptotic epithelioid cells compared with fusiform cells. ROS can affect many aspects of cell function that modulate apoptosis. For example, application of ROS has been shown to activate mitogen-activated protein kinases and the Janus kinases signal transducer and activator of transcription factors (JAK-STAT) pathway, which are important upstream regulators of apoptosis.31,32 ROS have also been shown to directly activate caspases, the downstream mediators of most types of apoptosis.33 Finally, susceptibility to exogenously applied ROS depends on the cellular redox status, which was recently reported to be a determinant of apoptosis after balloon injury.19 Thus, a number of mechanisms, including alterations in upstream or downstream apoptosis signaling pathways or in antioxidant systems, could account for the enhanced sensitivity to ROS-induced apoptosis observed in epithelioid SMCs.
Although H2O2 is known to induce death of vascular SMCs,21–23 recent reports also indicate that H2O2 can stimulate SMC proliferation.34,35 Although the effects of ROS on cells can vary widely depending on dose, the divergent results reported in these studies are not readily explained by differences in the amounts or methods of application of H2O2. Furthermore, differences in genotype are not likely to be responsible, since the same types of cell (rat aortic SMCs) were used in all of the studies. Under the conditions described in the present study, we found that H2O2 induced death in both epithelioid and fusiform rat aortic SMC lines, without demonstrably stimulating cell proliferation. These findings suggest that phenotypic differences between populations of SMCs are not likely to explain the discrepant reports in the literature regarding the effects of H2O2 on SMC proliferation and viability. Taken together, these results suggest that the effects of ROS on SMC proliferation and viability vary, depending on yet-to-be defined factors related either to the cells themselves or to the experimental conditions.
In summary, we have demonstrated that epithelioid SMC lines and neointimal SMCs in situ exhibit enhanced sensitivity to ROS-induced apoptosis compared with fusiform or medial SMCs from the same blood vessel. These findings may have important implications in regard to neointimal regression and, perhaps, lesion destabilization.
Acknowledgments
This work was supported by grants HL49264 (to A.A.S. and N.L.W.) and CA66081 (to A.A.S. and L.W.O.) from the National Institutes of Health, by an American Heart Association Clinician-Scientist Award (to N.L.W.), and by an American Heart Association Postdoctoral Fellowship Award (to W.-G.L.).
References
- 1.Bochaton-Piallat ML, Gabbiani F, Ropraz P, Gabbiani G. Age influences the replicative activity and the differentiation features of cultured rat aortic smooth muscle cell populations and clones. Arterioscler Thromb. 1993;13:1449–1455. doi: 10.1161/01.atv.13.10.1449. [DOI] [PubMed] [Google Scholar]
- 2.Lemire JM, Covin CW, White S, Giachelli CM, Schwartz SM. Characterization of cloned aortic smooth muscle cells from young rats. Am J Pathol. 1994;144:1068–1084. [PMC free article] [PubMed] [Google Scholar]
- 3.Villaschi S, Nicosia RF, Smith MR. Isolation of a morphologically and functionally distinct smooth muscle cell type from the intimal aspect of the normal rat aorta: evidence for smooth muscle cell heterogeneity. In Vitro Cell Dev Biol. 1994;30A:585–595. doi: 10.1007/BF02631257. [DOI] [PubMed] [Google Scholar]
- 4.Bochaton-Piallat ML, Ropraz P, Gabbiani F, Gabbiani G. Phenotypic heterogeneity of rat arterial smooth muscle cell clones: implications for the development of experimental intimal thickening. Arterioscler Thromb Vasc Biol. 1996;16:815–820. doi: 10.1161/01.atv.16.6.815. [DOI] [PubMed] [Google Scholar]
- 5.Holifield B, Helgaso T, Jemelka S, Taylor A, Navran S, Allen J, Seidel C. Differentiated vascular myocytes: are they involved in neointimal formation? J Clin Invest. 1996;97:814–825. doi: 10.1172/JCI118481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frid MG, Aldashev AA, Dempsey EC, Stenmark KR. Smooth muscle cells isolated from discrete compartments of the mature vascular media exhibit unique phenotypes and distinct growth capabilities. Circ Res. 1997;81:940–952. doi: 10.1161/01.res.81.6.940. [DOI] [PubMed] [Google Scholar]
- 7.Shanahan CM, Weissberg PL. Smooth muscle cell heterogeneity: patterns of gene expression in vascular smooth muscle cells in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1998;18:333–338. doi: 10.1161/01.atv.18.3.333. [DOI] [PubMed] [Google Scholar]
- 8.Neuville P, Yan ZQ, Gidlof A, Pepper MS, Hansson GK, Gabbiani, Sirsjo A. Retinoic acid regulates arterial smooth muscle cell proliferation and phenotypic feature in vivo and in vitro through an RARα-dependent signaling pathway. Arterioscler Thromb Vasc Biol. 1999;19:1430–1436. doi: 10.1161/01.atv.19.6.1430. [DOI] [PubMed] [Google Scholar]
- 9.Bochaton-Piallat ML, Gabbiani G, Pepper MS. Plasminogen activator expression in rat arterial smooth muscle cells depends on their phenotype and is modulated by cytokines. Circ Res. 1998;82:1086–1093. doi: 10.1161/01.res.82.10.1086. [DOI] [PubMed] [Google Scholar]
- 10.Kockx MM, Guido RY, Meyer D, Muhring J, Jacob W, Bult H, Herman AG. Apoptosis and related proteins in different stages of human atherosclerotic plaques. Circulation. 1998;97:2307–2315. doi: 10.1161/01.cir.97.23.2307. [DOI] [PubMed] [Google Scholar]
- 11.Kockx MM, Herman AG. Apoptosis in atherogenesis: implications for plaque destabilization. Eur Heart J. 1998;19(suppl G):G23–G28. [PubMed] [Google Scholar]
- 12.Bennett MR, Evan GI, Schwartz SM. Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J Clin Invest. 1995;95:2266–2274. doi: 10.1172/JCI117917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett MR, Littlewood TD, Schwartz SM, Weissberg PL. Increased sensitivity of human vascular smooth muscle cells from atherosclerotic plaques to P-53–mediated apoptosis. Circ Res. 1997;81:591–599. doi: 10.1161/01.res.81.4.591. [DOI] [PubMed] [Google Scholar]
- 14.Bochaton-Piallat ML, Gabbiani F, Redard M, Desmouliere A, Gabbiani G. Apoptosis participates in cellularity regulation during rat aortic intimal thickening. Am J Pathol. 1995;147:267–277. [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz SM, DeBlois D, O’Brien ERM. The intima: soil for atherosclerosis and restenosis. Circ Res. 1995;77:445–465. doi: 10.1161/01.res.77.3.445. [DOI] [PubMed] [Google Scholar]
- 16.Han D, Haudenschild C, Hong M. Evidence for apoptosis in human atherosclerosis and in a rat vascular injury model. Am J Pathol. 1995;147:267–277. [PMC free article] [PubMed] [Google Scholar]
- 17.Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993;91:2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller FJ, Jr, Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res. 1998;82:1298–1305. doi: 10.1161/01.res.82.12.1298. [DOI] [PubMed] [Google Scholar]
- 19.Pollman MJ, Hall JL, Gibbons GH. Determinants of vascular smooth muscle cell apoptosis after balloon angioplasty injury. Circ Res. 1999;84:113–121. doi: 10.1161/01.res.84.1.113. [DOI] [PubMed] [Google Scholar]
- 20.Nunes GL, Robinson K, Kalynuch A, King SB, III, Sgoutas DS, Berk BC. Vitamins C and E inhibit O2− production in the pig coronary artery. Circulation. 1997;96:3593–3601. doi: 10.1161/01.cir.96.10.3593. [DOI] [PubMed] [Google Scholar]
- 21.Li PF, Dietz R, von Harsdorf R. Reactive oxygen species induce apoptosis of vascular smooth muscle cell. FEBS Lett. 1997;404:249–252. doi: 10.1016/s0014-5793(97)00093-8. [DOI] [PubMed] [Google Scholar]
- 22.Li PF, Dietz R, Harsdorf VR. Differential effects of hydrogen peroxide and superoxide anion on apoptosis and proliferation of vascular smooth muscle cells. Circulation. 1997;96:3602–3604. doi: 10.1161/01.cir.96.10.3602. [DOI] [PubMed] [Google Scholar]
- 23.Fiorani M, Cantoni O, Tasinato A, Boscoboinik D, Azzi A. Hydrogen peroxide- and fetal bovine serum-induced DNA synthesis in vascular smooth muscle cells: positive and negative regulation by protein kinase C isoforms. Biochim Biophys Acta. 1995;1269:98–104. doi: 10.1016/0167-4889(95)00109-6. [DOI] [PubMed] [Google Scholar]
- 24.Tan S, Sagara Y, Liu Y, Maher P, Schubert D. The regulation of reactive oxygen species production during programmed cell death. J Cell Biol. 1998;141:1423–1432. doi: 10.1083/jcb.141.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bank K, Hutter E, Gonchoroff NJ, Perl A. Molecular ordering in HIV-induced apoptosis. J Biol Chem. 1998;273:11944–11953. doi: 10.1074/jbc.273.19.11944. [DOI] [PubMed] [Google Scholar]
- 26.Manna SK, Zhang HJ, Yan T, Oberley LW, Aggarwal BB. Overexpression of manganese superoxide dismutase suppresses tumor necrosis factor-induced apoptosis and activation of nuclear transcription factor-β and activated protein-1. J Biol Chem. 1998;273:13245–13254. doi: 10.1074/jbc.273.21.13245. [DOI] [PubMed] [Google Scholar]
- 27.Johnson TM, Yu ZX, Ferrans VJ, Lowenstein RA, Finkel T. Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc Natl Acad Sci U S A. 1996;93:11848–11852. doi: 10.1073/pnas.93.21.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang X, Kaduce TL, Weintraub NL, Van Rollins M, Spector AA. Functional implications of a newly characterized pathway of 11,12-epoxyeicosatrienoic acid metabolism in arterial smooth muscle cells. Circ Res. 1996;79:784–793. doi: 10.1161/01.res.79.4.784. [DOI] [PubMed] [Google Scholar]
- 29.Riss TL, Moravec RA. Comparison of MTT, XTT, and a novel tetrazolium compound MTS for in vitro proliferation and chemosensitivity assays. Mol Biol Cell. 1992;(suppl 3):184a–188a. [Google Scholar]
- 30.Clowes AW, Reidy MA, Clowes MM. Mechanisms of stenosis after arterial injury. Lab Invest. 1983;49:208–215. [PubMed] [Google Scholar]
- 31.Wang X, Martindale JL, Liu Y, Holbrook NJ. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem J. 1998;333:291–300. doi: 10.1042/bj3330291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon AR, Rai U, Fanburg BL, Cochran BH. Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol. 1998;275:C1640–C1652. doi: 10.1152/ajpcell.1998.275.6.C1640. [DOI] [PubMed] [Google Scholar]
- 33.McConkey DJ, Orrenius S. Signal transduction pathways in apoptosis. Stem Cells. 1996;14:619–631. doi: 10.1002/stem.140619. [DOI] [PubMed] [Google Scholar]
- 34.Rao GN, Berk BC. Reactive oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res. 1992;18:775–794. doi: 10.1161/01.res.70.3.593. [DOI] [PubMed] [Google Scholar]
- 35.Sundaresan M, Yu XZ, Victor JF, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:297–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]