Abstract
This study evaluated if measures of psychological well-being, including coping style are associated with advance care planning (ACP). Data were from the HEMA-COMM study, a prospective observational study of physician-patient communication in patients with hematologic malignancies. ACP was defined as having a living will, having a health care proxy, discussing life support with family or friends, and discussing life support with a doctor or nurse. 293 patients participated: only 45 (15%) had all the elements of ACP, 215 (73%) had at least 1 element of ACP, while 33 (11%) did not engage in ACP. In multivariate analysis, specific coping styles but not other measures of psychosocial well being were associated with having written ACP. Verbal ACP was associated with patient-reported health and physician estimate of life expectancy. Our study suggests that tailoring ACP discussions to a patient’s coping style may increase engagement in ACP.
Keywords: Advance care planning, Coping, Health Status, Hematological Cancer
INTRODUCTION
Advance care planning (ACP) is a written and verbal process of communicating a patient’s values and preferences to key surrogate decision makers to give guidance in case the patient becomes incapacitated and can no longer make his or her own decisions. Written forms of ACP are living wills or advance directives (AD), designation of a health care proxy (or power of attorney) and documentation of patient end-of-life wishes in the medical record. Verbal methods of ACP include discussions with potential surrogate decision makers and health care providers so that care can be consistent with a patient’s preferences. Advance care planning is intended to protect individuals’ rights, values, and wishes at a time when they cannot speak for themselves. In the absence of ACP, physicians seek guidance from the patient’s next of kin, who may or may not know the patient’s wishes. It is estimated that only 18–36 percent of the adult population has completed ACP despite widespread familiarity with the concept.1,2 The implications of lack of ACP are even more profound among patients with cancer where the chances of incapacity and death are higher than the general population.
Even in cases of life-threatening hematological cancers where patients undergo elective, high-risk procedures such as hematopoietic cell transplantation (HCT), reported completion rates for written plans at the time of transplant were estimated to be only 50%.3,4 One study showed the lack of written plans was associated with a higher adjusted risk of death compared to those who had written plans at the time of HCT, suggesting that those who could have most benefited from having ACP were the very ones without it.4 There is widespread recognition that ACP should be encouraged, and rates of ACP engagement are increasingly considered a measure of quality of care by many organizations.5,6 Thus, there is a need to understand the various factors that would encourage engagement in ACP, especially among cancer patients, so that effective interventions can be developed.
Multiple patient factors are associated with non-completion of ACP: younger age, ethnic minority, lower educational level, strong religious beliefs, deference to family members, emotional distress, and difficulty completing the forms.3,4,7,8 Physician barriers also exist that prevent assistance with ACP: lack of time, perceived low health literacy of the patients, lack of skills, lack of privacy for the discussion, and physicians’ perceptions that some patients are not “sick enough”.9 Another potential reason may be physicians’ concern about the patient’s psychosocial well being although research shows that end-of-life discussions do not make patients feel more depressed.10
This analysis, part of a larger observational study about physician-patient communication, was conducted to determine if coping styles and measures of psychological well-being are associated with ACP. Understanding the associations between psychological factors and completion of ACP may suggest ways to encourage ACP by identifying patient characteristics and barriers that may be interfering with ACP completion.
MATERIALS AND METHODS
Study Cohort
Participants included in this study were part of the Hematology Communications Study (HEMA-COMM), a prospective cohort study evaluating physician-patient communication in patients with hematologic malignancies. Recruitment occurred from September 2003 through June 2007 at Dana-Farber Cancer Institute and Massachusetts General Hospital, Boston, MA, Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance, Seattle, WA, and Massey Cancer Center, Richmond, VA. All recruiting sites obtained Institutional Review Board approval. Participants were at least 18 years of age and able to communicate in the English language. All patients and physicians provided informed consent.
Data Collection
Participants completed a self-administered survey and an interview with a trained research coordinator over the phone prior to their consultations. The consultation was audio-recorded, and physicians completed a self-administered survey about the patient and the consultation. Patients were interviewed within a week after the consultation and again 3 months later. Data used for this study were derived from the patient interviews, patient pre-consultation survey, and the physician questionnaire.
Variables and Measures
Demographic characteristics of patients collected at baseline included: age, sex, race/ethnicity, marital status, educational attainment, annual income, diagnosis, prior cancer treatment, current or planned cancer treatment and Charlson Comorbidity score. Physician estimates of life expectancy and perceptions regarding degree of patient involvement in medical decision making pertaining to care and treatment were collected after the consultation using single item measures.
Psychological well-being of patients was assessed using standardized instruments at the post consultation interview. The degree of anxiety and depression was measured using the 14-item Hospital Anxiety and Depression Scale (HADS).11 In both anxiety and depression scales, scores less than 8 were categorized as normal, 8–10 categorized as borderline and greater than 10 considered indicative of anxiety or depression. Mental and physical functional status were measured using the 36-item Medical Outcomes Study Short Form 36 (SF-36).12 Subscales included: physical functioning, role limitations due to physical problems, bodily pain, social functioning, general mental health, role limitations due to emotional problems, vitality, and general health perceptions. Coping styles were assessed using the Brief Cope.13 This instrument measures 14 subscales of coping and includes the following domains: self-distraction, active coping, denial, substance abuse, use of emotional support, use of instrumental support, behavioral disengagement, venting, positive reframing, self-blame, planning, acceptance, religion and humor. Individual items range from 1–4 and subscales from 2–8. Wilson’s 3 item Denial scale was used to identify patients less likely to gather information about their condition and treatment.14 Responses ranged from 1–4 with higher scores indicating lower denial.
The ACP variables were derived from participants’ response to the following items collected from interviews done 3 months after the consultation: 1) ‘designated a health care proxy’, 2) ‘completed a living will’ 3) ‘discussed wishes regarding life support with family or friends’, and 4) ‘discussed wishes regarding life support with doctor or nurse’. Each item is scored a 0 for a response of ‘no’ and 1 for a response of ‘yes’.3 In this study, we defined ACP in two ways. First, as used in our previous study 4, we ascertained the presence of written plans of ACP as those who responded ‘yes’ to having both a living will and health care proxy, while patients with only one or neither were considered to have no ACP. This definition was chosen to reflect concrete and verifiable measures of engagement in ACP. Second, we also defined verbal ACP based on whether or not patients reported having discussions about life support with their family/friends and medical care team, based on clinical practice which largely defers to orally communicated wishes over written documents.
Statistical Analysis
Univariate comparisons of various demographic and psychosocial factors between participants with or without written plan for ACP were performed using the Wilcoxon test or Chi-square test for continuous or categorical data, respectively. Multivariate logistic regression was used to evaluate which psychosocial factors were associated with having ACP (written plan) while adjusting for statistically significant covariates. Stepwise covariate selection was performed to identify psychosocial domains and patient characteristics (as listed in Table 1) associated with having ACP. Physician estimate of life expectancy was also tested as a covariate in the all model building. A separate logistic model was also constructed to evaluate if the above factors were associated with discussing life support with family and/or physician (verbal plan). Covariates with an alpha of less than or equal to 0.05 were retained in the model. All analyses were performed using SAS for Windows v9.2.
Table 1.
Patient characteristics according to written planning status
| Variables | With Written ACP n=149 | Without Written ACP n=144 | p-value |
|---|---|---|---|
| Median age (range) | 56 (28–80) | 52 (18–79) | 0.001 |
| Sex | 0.94 | ||
| Males | 78 (52) | 76 (53) | |
| Females | 71 (48) | 68 (47) | |
| Race | 0.14 | ||
| White | 138 (93) | 126 (88) | |
| Non-white | 11 (7) | 18 (12) | |
| Marital status | 0.12 | ||
| Married | 123 (82) | 101 (71) | |
| Single | 10 (7) | 18 (12) | |
| Divorced | 12 (8) | 18 (12) | |
| Widowed | 4 (3) | 7 (5) | |
| Current work status | 0.31 | ||
| In school | --- | 2 (1) | |
| Full time working | 57 (38) | 57 (40) | |
| Part time working | 13 (9) | 19 (13) | |
| Homemaker | 6 (4) | 4 (3) | |
| Disabled | 7 (5) | 13 (9) | |
| On medical leave | 21 (14) | 18 (13) | |
| Unemployed | 9 (6) | 9 (6) | |
| Retired | 36 (24) | 21 (15) | |
| Highest education attainment | 0.14 | ||
| Grade school | ---- | 1 (1) | |
| Some high school | 2 (1) | 4 (3) | |
| High school graduate | 14 (10) | 26 (18) | |
| Some college | 32 (21) | 33 (23) | |
| College | 51 (34) | 44 (31) | |
| Post-graduate | 50 (34) | 35 (24) | |
| Annual Income | 0.03 | ||
| Under $15000 | ---- | 7 (5) | |
| $15000 – $24999 | 4 (3) | 8 (6) | |
| $25000 – $49999 | 19 (14) | 26 (20) | |
| $50000 – $74999 | 28 (20) | 22 (17) | |
| $75000 – $99999 | 28 (20) | 18 (14) | |
| $100000 or above | 59 (43) | 51 (39) | |
| Diagnosis | 0.02 | ||
| Lymphoma | 32 (21) | 54 (38) | |
| Acute Leukemia | 35 (23) | 17 (12) | |
| Myelodysplastic syndrome | 30 (20) | 24 (17) | |
| Multiple myeloma | 26 (17) | 23 (16) | |
| Chronic Leukemia | 23 (15) | 24 (17) | |
| Other | 3 (2) | 2 (1) | |
| Have received cancer related treatment | 0.003 | ||
| No | 73 (50) | 94 (65) | |
| Yes | 73 (50) | 50 (35) | |
| Chemotherapy alone | 64 (88) | 36 (72) | 0.008 |
| Radiation ± chemotherapy | 1 (1) | 8 (16) | |
| Stem cell/Bone Marrow transplant | 8 (11) | 6 (12) | |
| Currently receiving or will receive cancer related treatment | 0.85 | ||
| No | 75 (52) | 75 (53) | |
| Yes | 70 (48) | 67 (47) | |
| Chemotherapy alone | 59 (85) | 54 (80) | 0.31 |
| Radiation ± chemotherapy | 8 (11) | 4 (6) | |
| Stem cell/Bone Marrow transplant | 3 (4) | 6 (14) | |
| Median Charlson Comorbidity score (range) | 0 (0–5) | 0 (0–5) | 0.22 |
| Influence of religion/spirituality in daily life | 0.63 | ||
| Not at all | 20 (13) | 19 (13) | |
| Very little | 21 (14) | 19 (13) | |
| Somewhat | 33 (22) | 42 (29) | |
| Quite a bit | 28 (19) | 27 (19) | |
| A great deal | 47 (32) | 36 (25) | |
| Point of view about decision-making regarding medical care and treatment | 0.24 | ||
| Decision left to doctor | 5 (3) | 12 (8) | |
| Decision made by doctor with significant inputs from patient | 29 (20) | 34 (24) | |
| Decision equally shared by doctor and patients | 56 (38) | 54 (38) | |
| Decision made by patient with significant input from doctor | 54 (36) | 40 (28) | |
| Decision left to patient | 4 (3) | 3 (2) |
RESULTS
Participant Characteristics
The HEMA-COMM study screened 913 potential participants of which 770 were found to be eligible. Three hundred sixty four participated in the study for a participation rate of 47% (364/770). Minimal information was available on patients who declined participation or were ineligible. The current analyses are focused on 293 (80%) participants who completed a preconsultation self-administered survey, a preconsultation interview, a postconsultation (after 3 months) interview, and had their consultation successfully audiotaped. Demographic characteristics of those included in the current analysis were similar to those excluded (n=71). Of the 293 participants in the study, 149 (51%) had both a designated health care proxy and a living will, while 144 (49%) were missing one (n=44 missing health care proxy, n=50 missing living will) or both documents (n=50). The characteristics of the study participants according to absence or presence of both written plans for ACP are shown in Table 1. Participants with written plans were older (median age 56 years vs. 52 years, p=0.001) and were also more likely to have higher income, have leukemia or MDS and to have received previous treatments for their cancer. We did not find any significant differences in sex, race, marital status, work status, education level, comorbidity score, and level of religious/spiritual influence in daily life. Participants with or without written plans for ACP were also similar in their preferences for involvement in medical decision making.
As a group, of the 293 participants, 185 (63%) had a living will, 199 (68%) had a healthcare proxy, 231 (79%) discussed life support with family, while only 62 (21%) discussed life support with their healthcare providers. Only 45 (15%) had all the elements of ACP (living will, healthcare proxy, and discussion of wishes about life support with their family/friends and health providers). However, 215 (73%) had at least 1 element of ACP, while 33 (11%) did not have any written planning or discussion about life support with family or health providers.
Discussions about Life Support and Estimates of Cure and Life Expectancy
Figure 1 shows the distribution of participants according to presence or absence of written plans and discussion about life support. Participants with written plans were more likely to discuss their wishes with their medical care team (30% vs. 10%). Conversely, 33% of participants without written plans did not discuss their wishes with anyone (including family) compared to 8% of participants with written plans for ACP.
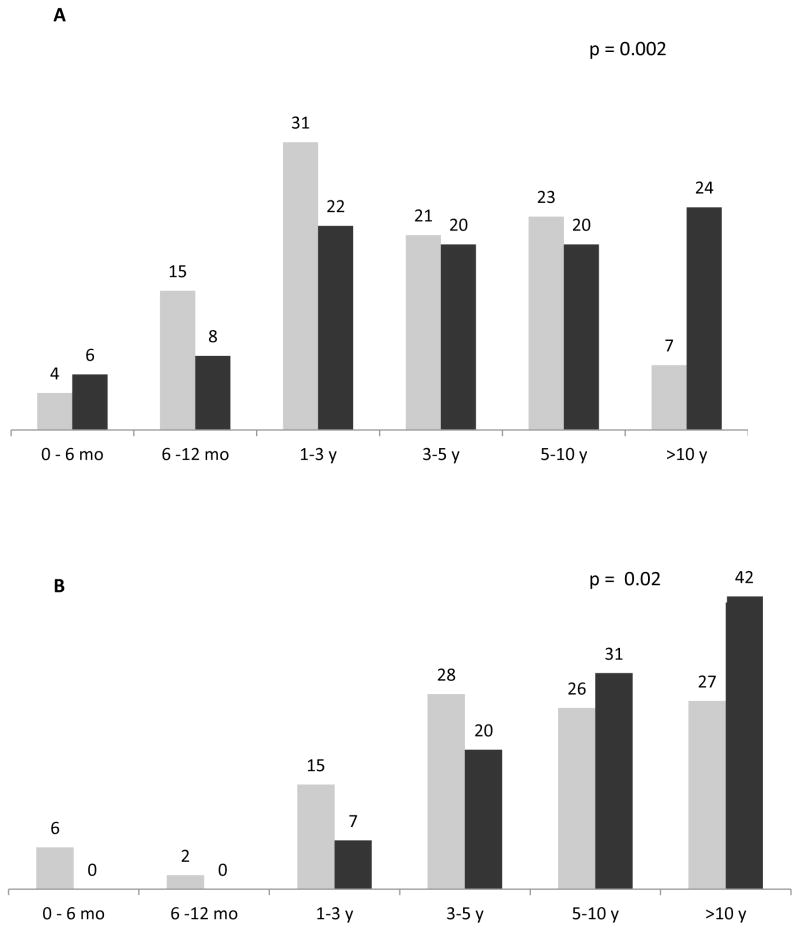
Figure 1.
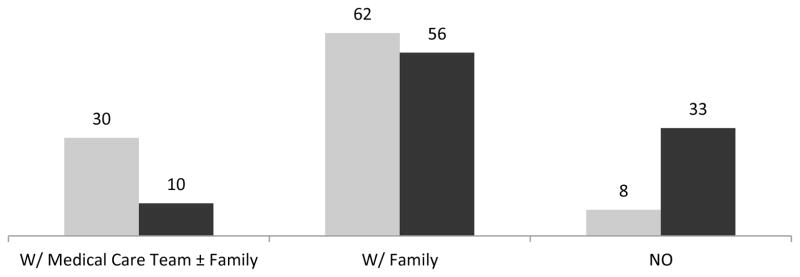
Proportion (%) of patients discussing life support according to ACP; (Grey = With Written Plans for ACP Positive, Black = Without Written Plans for ACP)
Figures 2A and 2B show the distribution of participants according to having written plans and physician or patient estimate of life expectancy. There was a statistically significant difference with regards to physician estimate of life expectancy according to having written plans. Participants with written plans were less likely to have physician estimated life expectancy that is greater than 10 years (7% versus 24%), while 50% of the participants with written plans have a physician estimated life expectancy between 0 to 3 years, overall p=0.002. There was also a statistically significant difference in patient estimate of life expectancy according to having written plans. Participants with written plans were less likely to estimate their life expectancy to be greater than 10 years (27% versus 42%) and more likely to estimate their life expectancy to be less than 5 years (73% versus 58%), overall p=0.02. Neither physician nor patient-reported life expectancy was associated with written ACP in multivariate analysis.
Figure 2.
A) Physician estimate of life expectancy, B) Patient estimate of life expectancy; (Grey = With Written Plans for ACP Positive, Black = Without Written Plans for ACP)
Coping, Psychosocial Well Being and ACP
Table 2 shows the psychosocial profile of participants according to whether or not they have written plans. We did not observe any differences in use of denial, levels of depression or anxiety, measures of social support and quality of life between the two groups. However, participants differed in their use of coping patterns. Having written plans for ACP was associated with active coping, emotional support, instrumental support, reframing, and planning as coping mechanisms. Table 3A shows the multivariate analysis evaluating psychosocial factors associated with having written plans adjusted for age and whether or not prior cancer treatment was received. Analyses showed that none of the psychosocial factors were associated with the odds of having written plans except for coping styles. Specifically, instrumental support (Odds Ratio [OR] 1.32, 95% confidence interval [CI] 1.14–1.53, p<0.001); active coping (OR 1.23, 95% CI 1.06–1.42, p=0.005); use of emotional support (OR 1.21, 95% CI 1.04–1.40), p=0.01); planning (OR 1.19, 95% CI 1.03–1.37, p=0.02), and positive reframing (OR 1.14, 95% CI 1.00–1.29), p=0.05) were all associated with having written plans. Older age, and having received prior cancer treatment were also significantly associated with having written plans for ACP. As for factors associated with discussions about life support with family/friends and/or health providers (verbal plans), Table 3B shows that lower physical component score of the SF-36 (OR 0.98, 95% CI 0.96–0.99, p=0.03), lower score on general health (OR 0.98, 95% CI 0.97–0.99, p=0.007), and lower physician estimate of life expectancy (OR 0.82, 95% CI 0.67–0.99, p=0.04) were the only factors associated with having discussed life support with family/friends and/or health providers.
Table 2.
Psychosocial well-being and quality of life according to advance care planning status
| Domain | With Written ACP n=149 | Without Written ACP n=144 | p-value |
|---|---|---|---|
| A. Coping (BRIEF-COPE Scale) | |||
| Self-distraction | 5 (2–8) | 5 (2–8) | 0.14 |
| Active coping | 7 (2–8) | 6 (2–8) | 0.01 |
| Denial | 2 (2–8) | 2 (2–8) | 0.46 |
| Substance abuse | 2 (2–8) | 2 (2–7) | 0.31 |
| Use of emotional support | 7 (2–8) | 6 (2–8) | 0.03 |
| Use of instrumental support | 6 (2–8) | 5 (2–8) | <0.001 |
| Behavioral disengagement | 2 (2–7) | 2 (2–6) | 0.85 |
| Venting | 4 (2–8) | 4 (2–8) | 0.75 |
| Positive reframing | 5 (2–8) | 5 (2–8) | 0.15 |
| Self–blame | 2 (2–6) | 2 (2–8) | 0.42 |
| Planning | 7 (2–8) | 6 (2–8) | 0.02 |
| Acceptance | 7 (2–8) | 7 (2–8) | 0.33 |
| Religion | 5 (2–8) | 5 (2–8) | 0.15 |
| Humor | 3 (2–8) | 4 (2–8) | 0.42 |
| B. Denial (Wilson’s Denial Scale) | 4 (2–4) | 4 (2–4) | 0.64 |
| C. Anxiety and Depression (Hospital Anxiety and Depression Scale) | |||
| HADS – Anxiety | 0 (0–2) | 0 (0–2) | 0.72 |
| HADS – Depression | 0 (0–2) | 0 (0–2) | 0.14 |
| D. Social Support (MOS – Social Support Scale) | |||
| Emotional and informational support | 84 (25–100) | 84 (0–100) | 0.66 |
| Tangible support | 88 (19–100) | 82 (0–100) | 0.21 |
| Affection | 100 (8–100) | 100 (0–100) | 0.12 |
| Positive social interactions | 92 (25–100) | 83 (0–100) | 0.49 |
| Overall support | 88 (29–100) | 86 (0–100) | 0.36 |
| E. Quality of Life (SF-36) | |||
| Physical Composite score | 47 (13–66) | 46 (15–64) | 0.99 |
| Mental Composite score | 50 (7–66) | 51 (21–65) | 0.45 |
| Physical functioning | 80 (5–100) | 80 (5–100) | 0.70 |
| Role limitations due to physical problems | 50 (0–100) | 50 (0–100) | 0.77 |
| Bodily Pain | 84 (12–100) | 84 (12–100) | 0.60 |
| Social functioning | 75 (0–100) | 75 (0–100) | 0.88 |
| General mental health | 80 (0–100) | 76 (20–100) | 0.46 |
| Role limitations due to emotional problems | 100 (0–100) | 100 (0–100) | 0.65 |
| Vitality | 50 (0–100) | 50 (0–90) | 0.48 |
| General health perceptions | 57 (5–100) | 58 (0–100) | 0.85 |
Table 3.
| Table 3A. Multivariate analysis evaluating psychosocial factors associated with having written plans for advance care planning | |||
|---|---|---|---|
| Variables | Parameter Estimate | Odds Ratio (95% Confidence Interval) | p-value |
| Use of instrumental supporta | 0.2785 | 1.32 (1.14–1.53) | <0.001 |
| Active Copinga | 0.2083 | 1.23 (1.06–1.42) | 0.005 |
| Use of emotional supporta | 0.1904 | 1.21 (1.04–1.40) | 0.01 |
| Planninga | 0.1710 | 1.19 (1.03–1.37) | 0.02 |
| Reframinga | 0.1279 | 1.14 (1.00–1.29) | 0.05 |
| Ageb | 0.6278 | 1.87 (1.28–2.74) | 0.001 |
| Prior cancer treatmentc | 0.8008 | 2.23 (1.36–3.65) | 0.002 |
| Table 3B. Multivariate analysis evaluating psychosocial factors associated with verbal advance care planning about life support | |||
|---|---|---|---|
| Variables | Parameter Estimate | Odds Ratio (95% Confidence Interval) | p-value |
| Physical Composite Scored | −0.0221 | 0.98 (0.96–0.99) | 0.03 |
| General Healthd | −0.0145 | 0.98 (0.97–0.99) | 0.007 |
| Physician estimate of life expectancye | −0.2037 | 0.82 (0.67–0.99) | 0.04 |
All factors are domains from Brief-COPE
Age categorized as <40, 40 – 60, > 60
Prior cancer treatment categorized as No, Yes;
All factors are domains from SF-36 quality of life scale
Physician estimate of life expectancy categorized as: >10 years, 5–10 years, 3–5 years, 1–3 years, 6–12 months, 0–6 months
DISCUSSION
The process of engaging in ACP is a means for the patient, family and care team to discuss the patient’s life values and hopes for treatment. This is particularly important when major complications or disease recurrence occurs and the succession of treatments provided is unsuccessful. Definitions of ACP have evolved from just the completion of a living will and designation of a health care proxy, to include discussion and verbal clarification of wishes with loved ones and health care providers. Regardless of how ACP is defined, the fact that a minority of the general population or at most half of patients with hematologic malignancies have ACP,3,4 suggests that current approaches to engage patients in ACP could be improved. Recently, arguments have been put forth that the objective of ACP should be to prepare patients and surrogates to make the best possible in-the-moment medical decisions with their health care team, rather than to have treatment wishes documented in written advance directives.15 While the focus is away from written documents, thought and discussion about these issues, whether stimulated by completion of documents or conversations with surrogate decision makers over time, seem necessary to prepare for these decisions.
Our study showed that measures of anxiety, depression, denial, social support and quality of life were not associated with written ACP, consistent with prior research.16 Instead, we found that ACP was associated with reported coping patterns that were problem-focused, as opposed to emotion-focused. Problem-focused coping or active coping strategies generally address stress by trying to change the situation.17 These coping strategies are often used when a person feels something constructive can be done to affect the outcome of events. In contrast, emotion-focused coping strategies are used when individuals feel they have little control over the situation, so they cope by simply trying to endure the stress.18,19 People can simultaneously use both types of coping strategies, and interventions can train people or encourage preferential use of one coping strategy over the other.20 Our findings support a two-pronged approach where people with problem-focused coping can be given the tools to engage in ACP and encouraged to participate as a means of contributing to the discussion if they become seriously ill. Emotion-focused copers may respond better to other approaches, such as presenting ACP as a routine part of oncologic care, or may need more active efforts to help them manage the difficult emotions (anxiety, sadness) that come with serious illness. Previous studies have shown that tailoring of information can improve psychological, behavioral, and physiologic outcomes when the information patients receive about their medical condition is adjusted for their coping styles.21 Miller described patients with monitoring-style coping (individuals who are alert for and sensitized to the negative, potentially painful, and dangerous aspects of information and experience) do better when given more information, while those with blunting-style coping (individuals who distract themselves from information) do better with less information.
Our study also showed that the predictors for verbal forms of ACP such as discussions about life support with family and/or health providers differ from predictors of written forms of ACP. We found that a discussion between the patient and heath provider about end-of-life decisions is more likely if the patient is in poorer health with a shorter life expectancy. Other studies suggest that before then, physicians may not even be aware that their patients have thought about ACP. 22 These results are consistent with a culture in which ACP engagement occurs when either the physician or the patient feels there is a need for these discussions. The concern is that this perception, that discussion with physicians about issues related to end-of-life care such as life support is not relevant or necessary unless there exists some belief that they are likely to be needed soon, further increases the emotional stakes of such discussions. ACP should be encouraged early and repeatedly for all patients diagnosed with life-threatening illnesses. If such policies were followed routinely, there may be less discomfort from physicians and patients about whether initiation of discussions means that the patient is imminently dying.
Our study has several limitations. The study recruited participants in large cancer centers which may differ demographically as well as in the characteristics of their disease. For instance, the level of education and income of the study participants may be higher than those treated in the community. If so, then the rate of ACP in the wider community may actually be lower than the rates found in our study. Another limitation of our study is that we did not precisely define some measures of ACP engagement. Similarly, the question about discussions with family and healthcare providers about life support did not measure degree or type of communication about these issues. The associations we found may vary depending on the quality of the discussions with healthcare providers.
Since our study suggests that a patient’s coping style is associated with written plans for ACP while poorer health is associated with verbal ACP, future studies should evaluate whether tailoring of ACP discussions based on coping styles improves written ACP engagement rates, and whether changing the focus from imminent planning to “way in advance” care planning increases verbal ACP rates.
Acknowledgments
Funding Source: National Cancer Institute - NCI CA098486
Footnotes
Financial Disclosures: The authors have no financial disclosures
References
- 1.U.S. Department of Health and Human Services. ASPE/DALTCP Publication No. HHS-100-03-0023. Rand Corporation; 2008. Advance directives and advance care planning: Report to congress. [Google Scholar]
- 2.Fried TR, Drikamer M. Garnering support for advance care planning. Journal of the American Medical association. 2010;303(3):269–270. doi: 10.1001/jama.2009.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joffe S, Mello M, Cook EF, Lee SJ. Advance care planning in patients undergoing hematopoietic cell transplant. Biology of Blood and Marrow. 2007;13:65–73. doi: 10.1016/j.bbmt.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 4.Ganti AK, Lee SJ, Vose JM, et al. Outcomes after hematopoietic Stem-cell transplantation for hematologic malignancies in patients with or without advance care planning. Journal of Clinical Oncology. 2007;25(35):5643–5648. doi: 10.1200/JCO.2007.11.1914. [DOI] [PubMed] [Google Scholar]
- 5.Walling A, Lorentz KA, Dy SM, et al. Evidence-based recommendations for information and care planning in cancer care. Journal of Clinical Oncology. 2004;29(23):3896–902. doi: 10.1200/JCO.2007.15.9509. [DOI] [PubMed] [Google Scholar]
- 6.Fargerlin A, Schnieder CE. Enough: the failure of the living will. Hastings Center Report. 2007;34:30–42. [PubMed] [Google Scholar]
- 7.Ramsaroop SD, Reid MC, Adelman RD. Completing and advance directive in the primary care setting: What do we need for success? Journal of the American Geriatric Society. 2007;55:277–283. doi: 10.1111/j.1532-5415.2007.01065.x. [DOI] [PubMed] [Google Scholar]
- 8.Mezey MD, Leitman R, Mitty EL, Bottrell MM, Ramsey GC. Why hospital patients do and do not execute an advance directive. Nursing Outlook. 2000;48:165–167. doi: 10.1067/mno.2000.101772. [DOI] [PubMed] [Google Scholar]
- 9.Tung EE, North FN. Advance care planning in the primary care settings: A comparison of attending staff and resident barriers. American Journal of Hospice & Palliative Medicine. 2009;26(6):456–463. doi: 10.1177/1049909109341871. [DOI] [PubMed] [Google Scholar]
- 10.Wright AA, Zhang B, Ray A, et al. Associations between end-od-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. Journal of the American Medical association. 2008;300(14):1665–73. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 12.Ware JE, Snow KK, Kosinski M, et al. SF-36 Health Survey: a manual and interpretation guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 13.Carver CS. You want to measure coping but your protocol’s too long: consider the brief COPE. Int J Behav Med. 1997;4:92–100. doi: 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- 14.Daltroy LH, Morlino CI, Eaton H, et al. Preoperative education for total hip and knee replacement patients. Arthritis Care Res. 1998;11:469–478. doi: 10.1002/art.1790110607. [DOI] [PubMed] [Google Scholar]
- 15.Sudore RL, Fried TR. Redefining the ‘planning’ in advance care planning: preparing for end-of-life decision making. Ann Intern Med. 2010;153:256–261. doi: 10.1059/0003-4819-153-4-201008170-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spencer R, Nilsson M, Wright A, Pirl W, Prigerson H. Anxiety disorders in advanced cancer patients: correlate and predictors of end-of-life outcomes. Cancer. 2010;116(7):1810–19. doi: 10.1002/cncr.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazarus RS, DeLongis A, Folkman S, Gruen R. Stress and adaptational outcomes: the problem of confounded measures. Am J Psychol. 1985;40(7):770–85. [PubMed] [Google Scholar]
- 18.Silveira MJ, Kim SYH, Langa KM. Advance directives and outcomes of surrogate decision making before death. New England Journal of Medicine. 2010;362(13):1211–1218. doi: 10.1056/NEJMsa0907901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folkman S, Lazarus RS, Dunkel-Schetter C, DeLongis A, Gruen R. Dynamics of a stressful encounter: Cognitive appraisal, coping, and encounter outcomes. Journal of Personality and Social Psychology. 1986;50:992–1003. doi: 10.1037//0022-3514.50.5.992. [DOI] [PubMed] [Google Scholar]
- 20.Rose JH, Radziewicz R, Bowman KF, O’Toole EE. A coping and communications support intervention tailored to older patients diagnosed with late-stage cancer. Clin Interv Aging. 2008;3(1):77–95. doi: 10.2147/cia.s1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller SM. Monitoring versus blunting styles of coping with cancer influence the information patients want and need about their disease. Cancer. 1995;76(2):167–77. doi: 10.1002/1097-0142(19950715)76:2<167::aid-cncr2820760203>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 22.Ozanne EM, Partridge A, Moy B, Ellis KJ, Sepucha KR. Doctor-patient communication about advance directives in metastatic breast cancer. J Palliat Med. 2009;12(6):547–53. doi: 10.1089/jpm.2008.0254. [DOI] [PubMed] [Google Scholar]