Abstract
Many biomaterials constructed today are complex chemical structures that incorporate biologically active components derived from nature, but the field can still be said to be in its infancy. The need for materials that bring sophisticated properties of structure, dynamics, and function to medical and non-medical applications will only grow. Increasing appreciation of the functionality of biological systems has caused biomaterials researchers to consider nature for design inspiration, and many examples exist of the use of biomolecular motifs. Yet, evolution, nature's only engine for the creation of new designs, has been largely ignored by the biomaterials community. Molecular evolution is an emerging tool that enables one to apply nature's engineering principles to non-natural situations using variation and selection. The purpose of this review is to highlight the most recent advances in the use of molecular evolution in synthetic biology applications for biomaterial engineering, and to discuss some of the areas in which this approach may be successfully applied in the future.
Keywords: synthetic biology, biomaterials, directed evolution, tissue engineering, scaffolds, virus particles, virus-like particles
1. Introduction
Evolution in the Laboratory
Natural selection is nature's way of developing new abilities in response to a changing environment. Enabled by better understanding of its mechanisms and by new analytical tools, scientists have started to bring the power of this process into the laboratory for the development of molecular function. The most straightforward approach has been to coopt biological mechanisms for the production of candidate molecules, and “screen” those candidates by chemical methods, usually their ability to bind to a target. Such methods are now routine in many laboratories, and are typified by phage display for polypeptides [1] and SELEX (Systematic Evolution of Ligands by Exponential Enrichment) for polynucleotides [2]. Many variations of these techniques have been developed.
Nature's preferred method is somewhat different: true selection couples the generation and performance of new candidate molecules with the reproduction of the organism producing them. This type of structure-survival relationship can be far more complex than simple binding, and therefore is more difficult for the laboratory scientist to direct. But, as living systems prove, selection is enormously more powerful in the development of complex, information-rich function. It is our contention that the development of smart materials can and will be revolutionized by these types of evolutionary techniques.
The discovery of new materials by directed evolution is different from traditional materials science in one fundamental respect: it places the greatest burden not on the creation of candidate materials, but rather on the testing of their properties. A single investigator can generate proteins and nucleic acids in astonishing numbers, each differing from all the others in the identity of one or more components of these linear polymers. To take advantage of this synthetic power requires the identification of those members of an evolutionary “library” that have the desired properties. This is by no means a trivial exercise: the success of evolutionary materials discovery, as with all combinatorial methods, requires great attention to the candidate library preparation as well as screening or selection part of the operation. (Readers are referred to two books describing different tools and approaches for candidate library creation and selection [3, 4] as well as two reviews [5, 6] outlining some recent progress in the field.)
For this reason, directed evolution techniques are particularly well suited for biomaterial design and optimization for four reasons. (1) Directed evolution is most easily applied to the phenomenon of binding: “winners” are selected away from “losers” by virtue of enhanced binding to the target, and repeated cycles of mutation and selection may be used to enhance binding kinetics or thermodynamics as desired. Since the construction of materials involves the self-assembly of component pieces, the evolution of specific binding properties can provide a unique advantage. (2) The molecules subjected to directed evolution – polypeptides and polynucleotides – are nanometers in size and highly diverse in structure and dynamics, providing unparalleled diversity in properties. (3) Biological molecules are inherently prone to self-assembly, so that many examples and functional units exist to imitate and coopt. Collagen, hair, and silk are examples of naturally occurring structural materials derived from the self-assembly of relatively simple molecular building blocks. (4) Directed evolution techniques can pair evolvable biomolecules with non-natural components or substrates, such as carbon nanotubes and metallic surfaces.
Current challenges in biomaterials development
Biomaterials are most commonly recognized as scaffolds potentially able to perform useful functions such as (a) promoting cell attachment, survival, proliferation, and differentiation while possessing minimum toxicity in the original and bio-degraded form; (b) allowing the transport or delivery of gases, nutrients, and growth factors; and (c) offering sufficient structural support while being degradable at appropriate rates for tissue regeneration. Readers are directed to detailed reviews describing different biomaterial scaffold properties [7-10]. It is probably safe to assume that the best scaffold for the tissue engineering would be the extracellular matrix (ECM) of the target tissue in its native conformation. Therefore, decellularized organs that retain the ECM [11] present the most common natural scaffold architecture used today, having been incorporated in materials used in heart [12] , lung [13], liver [14], bone [15] and blood vessels [16]. At the same time, decellularized organs have a number of shortcomings that have limited their use in biomaterial applications, including long processing times (increasing the costs of production), limitations on sourcing tissues, and potential immunogenicity. Also, decellularization typically involves exposure to non-physiologic chemical and biologic agents such as detergents, enzymes and physical forces that cause disruption of the associated ECM, potentially stripping the natural scaffold of its inherent bioactivity [11]. Less expensive bioactive materials can be constructed by modifying traditional “bioinert” materials to mimic physicochemical properties of natural materials [17]. Natural ECM materials such as collagen and fibrin gels, or recombinant peptides [18, 19] or proteins that mimic natural ECM materials [20, 21], have been used in this way. Hybrid approaches that combine the best qualities of synthetic materials with biologically active peptides are also the subject of investigation by a number of groups [22-27].
Each of these approaches has their own advantages and limitations. Modification of bioinert materials allows for finer control over material properties however, recapitulating every physicochemical property of a natural material is nearly impossible. Natural materials such as collagen gels are attractive because of their inherent bioactivity, but the complexity and heterogeneity of these materials can cause unpredictable cellular responses. Furthermore, these natural materials can lack the mechanical strength required for certain applications. Peptides or protein fragments that mimic natural ECM materials can form materials by themselves or can be incorporated into other scaffolds to impart biological activity [18, 19, 28-30]. Biological responses to peptides or protein fragments tend to be more predictable than responses to natural ECM material, but such reductionist approaches often cannot achieve the complexity in interactions and stimuli required to achieve a desired response [10]. The use of selection has the potential to overcome the limitations of these current approaches by specifically identifying material components and scaffolds that meet a set of desired criteria.
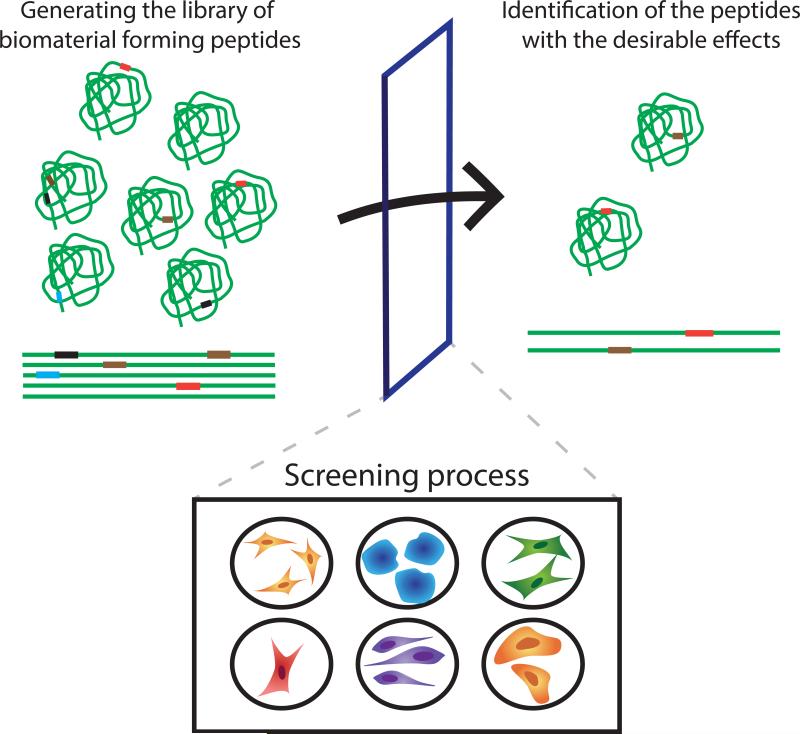
In this review we will focus on methods that harness the power of natural selection to produce new types of biomaterials and on natural building blocks particularly suitable for these approaches. The first section focuses primarily on the scaffolds that are produced with the use of the synthetic capacities of living cells. The second part of the review focuses on viruses and virus-like particles as synthetic scaffolds. The three key steps in molecular evolution – randomization, selection and amplification – ideally make for fast-paced development on the laboratory bench. When applied to biomaterials, “replication” can also mean “manufacturing,” adding further to the speed of the process from discovery to application (Fig.1).
Fig.1.
Directed evolution processes for biological and synthetic materials.
2. Protein scaffolds and their selection
A growing body of data has demonstrated that cellular phenotype can be tightly linked to biomaterial parameters such as material mechanics, biochemistry, nanostructure and degradation rate. Protein-based biomaterials are capable of imparting rich biochemical information to direct cell fate, in addition to providing structural support, therefore development of such materials has seen tremendous growth [8, 20, 21]. Historically, protein-based scaffolds are obtained in three different ways: decellularization of existing tissues [11, 14], precipitation of natural protein based fibers [31], or creation and use of recombinant proteins or peptides [32], often with additional modifications. In this section we focus on the design of novel engineered biomaterials that are coded by natural amino acid sequences. The notable advances at the intersection of synthetic biology and biomaterial engineering that are discussed here convey some of the promise of the coming era in which biomolecular engineers will be able to precisely formulate properties of biomaterials to serve specific function.
The availability of gene sequences and modern techniques of molecular cloning and protein expression has led to the widespread use of recombinant proteins in place of natural ones for biomaterials design. Recombinant protein engineering offers many advantages, including (a) significant increases in protein yield and batch-to-batch consistency over that which can be achieved by extraction of a native protein from animal tissues, (b) the ability to use human amino acid sequences, therefore avoiding adverse immunological responses, and (c) the ability to modify scaffold design by manipulation of the sequence of coded genes in combination with detailed knowledge of the protein structure and function, or by combinatorial trial-and-error. Recombinant proteins were described almost four decades ago, with insulin [21], collagen [33], and elastin [34] being among the most significant. Recombinant silk-like and elastomeric proteins became available only in the beginning of this century [35, 36]. Rational design modifications have been made to a multitude of matrix proteins, including fibronectin, for a diverse range of applications. Our group has utilized this approach to create protein fragments with specific point mutations within the integrin binding region of fibronectin (FnIII9-10) that enhance integrin-driven cell attachment for the specific cellular phenotypes [28, 37].
Overall, however, the rational design of novel proteins is not yet a general solution to materials problems. Detailed knowledge of protein structure is often unavailable, and structural proteins often exhibit low solubility and poor process characteristics. In such circumstances, the knowledge of a structure may not be a good predictor of how a particular mutation will affect the function of the protein. There is reason for some optimism on this front, however, as theory and simulation grow in their ability to predict protein structure and function. For example, some of the principles defining how primary sequence of the protein determines tertiary conformation have been recently demystified [38].
Increasingly diverse protein-based materials can be created by fusing multiple protein domains. These protein-engineered biomaterials are typically designed to be repeating sequences from different naturally occurring protein scaffolds [39], computationally derived sequences [40], or sequences selected through high-throughput screening methods such as phage display [41] or peptide arrays [42]. This can potentially lead to the achievement of highly desirable properties such as biocompatibility, biodegradability, and information density. One particularly interesting example from Martino et al. featured the fusion of growth factor domains VEGF-A, BMP-2 and PDGF-BB with the fibronectin cell binding domains [43]. The approach led to significant enhancement of the regenerative effects of the growth factors in vivo for skin and bone repair. The close proximity of these two domains was found to enhance tissue regeneration through synergistic signaling of the integrin and the growth factor receptor [43].
Since the basic blocks for protein-based biomaterials are naturally occurring amino acids, concerns over toxicity of the bulk material and degradation products are low. Genetic elements responsible for the formation of different types of biomaterials in natural hosts are sometimes not known and may be identified through sequencing of the host genome and gene mutagenesis. Then, using genetic engineering techniques the identified genes can be transferred to the expression host of interest. Subsequently, the recombinant proteins production can be tuned to be batch-to-batch consistent and also cost-effective. Because this approach combines the elements of design with the power of natural selection through the incorporation of genetic libraries [44], it can be superior to the use of proteins from natural sources or synthetic polymers. For example, Banta S. et al. [44] reported the design of artificial multidomain proteins composed of two associative leucine zipper end-blocks and a random coil midblock to build hydrogels with desirable self-assembly properties. These domains fold into the amphiphilic α-helices, and the resulting hydrophobic interactions drive association of the proteins into oligomeric clusters [45]. Also, self-assembly of the leucine zipper domains of the AC10A protein leads to a network, in which oligomer bundles serve as junction points [46]. Due to the introduced variation, the hydrophilic midblock lacks a regular secondary structure and prevents precipitation of the chain under conditions that favor leucine zipper aggregation. Introduction of the variable domain is the first one to our knowledge that has been reported in the field of biomaterials [46]. This study is not a typical directed evolution study since just one selection cycle has been utilized to pick winners. Nevertheless, it opens the road to the use of selection for the purpose of the biomaterial construction.
Natural protein evolution is thought to occur with the guidance of functional rather than structural influences. By this reasoning, non-native amino acid sequences are beginning to be explored to create scaffolds that are often otherwise unavailable. In one example, high throughput screening was used to identify various non-native sequences recognized by a protease for a degradation [47, 48]. Incorporation of these protease sensitive domains into elastin-like polypeptide yielded a family of cell adhesive polypeptides with tunable degradation rates spanning two orders of magnitude despite 97% sequence homology to the original sequences [49].
Proper selection of such libraries against the immune system of the host may eliminate the potential immunogenicity problem. Some other advantages of this technological approach include the easy protein purification of uniform and predictable quality and quantity. For tissue engineering applications, well-defined natural scaffolds can be modified via directed evolution that represents the next logical step in tunable biomaterial design.
In summary, recombinant protein engineering at the molecular (DNA) level is a useful technique for generating large amounts of protein polymers with versatile control over sequence specificity. Furthermore, the field of synthetic biology will likely provide numerous additional tools for development of increasingly advanced recombinant protein production strategies. In the next section, we will elaborate on the application of synthetic biology in design of non-natural, synthetic polymeric biomaterials.
3. The power of selection and synthetic polymeric scaffolds
Unlike most man-made materials, materials used in living systems are frequently multifunctional and dynamic, and are built using ‘bottom-up’ fabrication methods [50]. Both the materials themselves and the biophysical processes involved in their formation are inspiring the design and synthesis of new types of synthetic material that are potentially useful in a wide range of medical and non-medical applications [51-54]. One of the driving forces behind the use of the synthetic materials is to simplify independent changes to the biochemical and biomechanical properties. For example, the degree of crosslinking in polymer systems can be easily modified to create materials with specific desired mechanical properties [55, 56]. Independently from mechanical properties, these materials can be feathered with biologically active peptides that mimic the biochemical properties of the ECM. This widening of biomaterials applications demands an intellectual shift in how such synthetic materials are created and should include the identification of synthetic biology as a useful tool for biomaterials scientists. Distinct aspects of the use of synthetic biology approaches in the biomaterials field and the potential impact on medicine and other industries are our focus here.
The concept of “synthetic biology” is extremely broad and encompasses all aspects of designing biological systems, from modifying natural materials to creating complex synthetic materials or processes that are based on a naturally occurring counterpart. While naturally occurring protein-based materials have numerous inherent advantageous properties, such as appropriate size, specificity and affinity for their specific application, they are often difficult and expensive to manufacture and lack stability. Artificial components, such as polymers, offer a unique alternative as they are often easy to manufacture, low-cost and display high stability over a range of conditions. Advances in the field of nanotechnology have opened many doors for the creation of completely synthetic materials that recapitulate the features and functions of naturally occurring materials. One particularly promising method to recapitulate the molecular recognition feature of naturally occurring antibodies is through the technique of molecular imprinting. This technique involves the polymerization of monomers in the presence of a desired molecular target, which results in the formation of complementary binding sites. Molecular imprinting has been described using bulk materials, films, beads, membranes nanofibers, soluble micro/nano-gels, nanoparticles and dendrimers [57-65]. Recent and particularly promising examples have been provided by Shea and coworkers in the creation of “plastic antibodies” through solid phase synthesis or polymerization, and with the use of the evolutionary approach [66]. Molecularly imprinted polymers can be stored for years at room temperature, easily sterilized through autoclaving or UV irradiation, produced at low cost, and remain stable at temperatures in excess of 100 °C. In contrast, naturally occurring antibodies are typically only stable for 6-12 months, must be stored frozen, cannot be easily sterilized, cost between $1-1000/mg and denature at approximately 70 degrees C. The ability of “plastic” antibodies to recognize ligands in vitro have been demonstrated in numerous studies and recently the ability of molecularly imprinted polymers to successfully target ligands in vivo has been described [67, 68]. These examples highlight the promise of such synthetic materials to effectively target in vivo and could be used for a multitude of imaging and or therapeutic purposes.
The creation of biological mimics from synthetic components is also an active field with many potential applications. Another avenue of exploration that seeks to marry biology with synthetic components is the synthesis of polymeric materials through the use of biological processes, such as DNA translation [69]. DNA translation allows for the production of complex biopolymers from a nucleic acid template in a highly controllable manner, while synthetic polymer polymerization is difficult to control at the molecular level. Though the development of molecular imprinting has allowed for more precise control over the nanostructure of polymeric materials, control over the precise polymer length, sequence and molecular weight distribution have remained a difficult endeavor. Attempts to utilize DNA templates for the creation of sequence defined synthetic polymers has recently been described as a method to circumvent these limitations. Niu et al. described the development of an enzyme-free DNA-templated translation system that through peptide nucleic acid adapters recognizes a specific DNA sequence and binds specific polymer building blocks, allowing for precise control over polymer synthesis [70]. This method successfully utilized PEG, alpha-(D)-peptides and beta-peptides as building blocks for polymer chain synthesis. The use of DNA-templated synthesis to create polymeric materials that closely resemble natural nucleic acids, modified DNA, peptide nucleic acid, threoses nucleic acid, hexitol nucleic acid, and non-natural peptides have also been described [71-78]. This technique could allow for the generation of a number of polymer libraries that could be rapidly screened for biocompatibility, mechanical properties as well as utility for a particular application. Furthermore, this approach could allow for the selection of biomaterial features at the length scale of individual molecules and cells (tens of nanometers to tens of micrometers). Material properties at this length scale, such as surface topology, have significant effects on how cells perceive, interact and ingest the material, which affects the efficacy of materials used as drug carriers or vehicles targeting specific cells and tissues in the body [79-82].
In summary, bottom-up approaches have a number of advantages for the creation of novel synthetic materials relative to traditional polymeric synthesis methods, including control of materials properties from the molecular to the bulk level. The ability to specifically select for properties at a variety of scales provides biomaterials scientists with a powerful method for creating materials with precise control over their effects on cellular phenotype.
4. Materials based on Viruses and Virus-Like Particles
Proteins and peptides have naturally received a great deal of attention as components of higher-order materials for medicinal applications [83]. Here we highlight the special case of viruses and virus-like building blocks in this role, represented schematically in Figure 2. Whether or not they bear a lipid coating, viruses and related structures contain a shell made from multiple copies of a limited number of coat protein polypeptides, often intimately associated with packaged nucleic acid. Ranging in diameter from approximately 15 nm (for the iron-storage protein ferritin) [84] to more than 1 micron [85], these scaffolds provide extraordinary examples of the power of self-assembly in the construction of supramolecular materials [86]. Virus particles are also sufficiently stable to be building blocks for larger architectures, with predictable mechanical properties related to their three-dimensional structure [87]. They can be produced in quantity, either as natural virions or expressed by standard methods in the non-replicative form of virus-like particles, containing only protein and nucleic acid, and lacking genomic or protein elements required for infectivity. Lastly, viruses evolve rapidly in vivo, including changing residues and structural motifs of their capsids in response to adaptation by their host organisms [88]. Viral coat proteins are therefore more tolerant of mutational change than many other proteins. This combination of efficiency in production and plasticity in sequence makes viruses and VLPs building blocks of great promise for biomaterials development. We refer the reader to two excellent recent reviews of this field by Wang [89] and Harris [90]; here we highlight important cases and update these reports with some recent examples. Note that the use of viruses and VLPs as functional entities themselves (such as for vaccines, drug delivery, or gene delivery) is extensive and not considered here; we focus on their use as building blocks in higher-order biomaterial structures.
Fig. 2.
Current and anticipated development of evolutionary biomaterials based on viruses and virus-like particles. Genetically-programmable rod-shaped and icosahedral particles can be self-assembled in various ways into different surface and three-dimensional morphologies (step A). Interactions of these materials with cells leads to controlled adhesion, growth, and proliferation (step B). The eventual goal is to guide the development of functioning higher-order structures such as organs by programmed cell differentiation (step C). At each stage, it may be possible to create feedback mechanisms to allow the selection of desired properties from the directed evolution of the building blocks (black arrows).
From a materials perspective, viruses come in two main flavors: rod-shaped (filamentous) structures such as tobacco mosaic virus (TMV) and spherical (usually icosahedral) capsids such as cowpea mosaic virus (CPMV). Many viruses are, of course, more topologically complex than these, featuring desymmetrizing components usually associated with recognition and attachment to host cells or delivery of packaged polynucleotide, but simpler highly-symmetric variants have so far been the building blocks of choice. The past several years have seen much more activity with filamentous virus structures than icosahedral ones in materials applications.
Virus scaffold construction
A good deal of recent effort has gone into variations on the assembly of the rod-shaped virus-like particle architecture. In its simplest incarnation, TMV virus-like particles were stabilized by the introduction of one cysteine point mutation in the capsid protein (T103C), resulting in much greater resistance to pH variation and reductive denaturation [91]. The cell-free expression technology of Schwartz was also used to control intra-particle disulfide formation [92].
Manipulation of the packaged polynucleotide offers other opportunities for the control of particle assembly. Thus, Yi and coworkers prepared TMV virus-like particles of well-defined length using synthetic mRNA as a template [93]. A similar bottom-up approach for the assembly of two-dimensional virus-like particles arrays was described by Azucena, et al. in which RNA was first attached to silica or poly(dimethylsiloxane) surfaces, followed by addition of purified subunit protein of TMV [94]. In this case, rod-shaped structures were formed identical in width but different in length compared to natural TMV virions, even though RNA of the same length as the TMV genome was used. The conversion of brush-motif polynucleotide to nucleoprotein coatings offers the opportunity to change surface properties dramatically and in a modular manner.
The fundamental factors affecting viral assembly have long been a subject of study by both molecular modelers and engineers. Zlotnick, et al. recently provided a simple but powerful conceptual framework for the polynucleotide-guided assembly of virions [95]. A set of three physical parameters (capsid protein association to nucleic acid, protein-protein interaction energetics, and the work required to package nucleic acid) were evaluated in the context of well-characterized virus examples to provide insights into the combinations that allow assembly of higher order structures. The interplay between these factors was evident in the assembly of a novel trifunctional peptide-polymer building block around viral DNA to make artificial virus-like structures, developed by Stupp and coworkers [96].
Two-dimensional capsid assemblies
When placed at an air-liquid or liquid-liquid interface, Wang, Russell, and coworkers found CPMV[97] and TMV particles [98] to self-assemble into large-scale two-dimensional arrays in order to minimize interfacial energy. Nanoparticle self-assembly at interfaces has long been known, but the use of viruses and VLPs allow for modulation of the phenomenon by genetic- and chemically-controlled change in particle surface charge and hydrophobicity. An excellent example was provided several years ago by the groups of Belcher and Hammond [99]. In this work, rod-shaped M13 viruses were used to report on the interfacial properties and dynamics of layer-by-layer assemblies of charged polyelectrolyte materials. This application highlights the monodisperse nature of virus-based building blocks: in many respects, their properties can be more controllable and reliable in their composition than any non-biological nanostructure. The self-aggregation behavior of viral nanoparticles at interfaces was later manipulated by extrusion to give larger-scale, continuous films of ordered nanoparticles, as demonstrated by Chung with the filamentous M13 capsid [100]. In both of these studies, the genetic mutability of the phage was used to introduce functional cell-binding peptides, rather than to change the assembly properties or the resulting material structure.
Recent work by Wang, Lee, and colleagues suggests that interfacial assembly is not necessary for forming higher-order structures [101]. Polysaccharides were used to modulate the osmotic pressure-related “depletion interaction” between tobacco mosaic virus particles. Again, the long, thin shape of these virions served to maximize a physical interaction, resulting in micron-scale structures with structures controlled by the nature of the depletion agent. These assemblies take the form of gel-like materials at the macroscale that are able to undergo temperature- and hydration-dependent sol-gel transitions.
Surfaces for cell growth and differentiation
In recent years, a wide variety of techniques have been used to establish interesting connections between the micro- and nanoscale topographical features of surfaces and the adherence, growth, and proliferation of cells on those surfaces. Such studies are important in understanding and controlling the properties of cells in applications such as wound healing, tissue regeneration, and implantation of medical devices. Virus particles represent interesting building blocks for such surfaces since their monodisperse and repetitive structures allow for precise control of topology, assuming that they can be self-assembled in a coherent manner.
Wang and workers have employed three methods for the coverage of surfaces with well-aligned rod-shaped particles for this purpose. In a simple “convective” technique, a high concentration of M13 or TMV virus was physically pushed across a chemically-prepared substrate such as glass, creating a dense monolayer of aligned particles that can be crosslinked in place [102]. The nanostructural features of the resulting surface are controlled by the molecular structure of the rod-shaped particle, in principle allowing for the control of materials properties at the genomic level. Fibroblasts were found to respond to the oriented nature of the surface, exhibiting ordered growth and deposition of ordered extracellular matrices [103].
The aforementioned deposition of particles from the air-water interface has also proven fruitful. A combination of surface and interfacial interactions were found to come into play in a study of TMV self-assembly from evaporating solutions placed inside glass capillary tubes, also by the Wang laboratory [104]. Different patterns of mono- and multi-layered particles were observed depending on parameters such as particle concentration and solution ionic strength. Furthermore, the growth and differentiation of smooth muscle cells cultured inside virus-coated tubes varied with the deposition pattern of the underlying rod-shaped nanoparticles. A related method for the spontaneous formation of different patterns of ordered M13 phage particles on two-dimensional templates was recently published by Lee and coworkers, and the resulting surfaces shown to have interesting optical, biomineralization, and cell-binding properties [100].
Most recently, the simple pressure-induced flowing of solutions through capillary tubes of varying surface composition has been reported to provide aligned surface monolayers of a variety of different rod-shaped particles, including gold nanoparticles and several virus-derived building blocks [105]. Myoblast cells were observed to consistently adhere, grow, and differentiate on the protein particle-based substrates.
Mao and coworkers have started to take fuller advantage of the genetic tenability of phage particles in the context of mammalian cell growth. Deposition of M13 variants displaying different cell-binding peptides resulted in nanostructured films in which controlled surface morphology and chemical signaling could be used to influence the adhesion, growth, and differentiation of mesenchymal stem cells [106]. The resulting observations continue a march toward the development of substrate materials that mirror the complexity and multifunctional nature of natural surfaces from which cells take important cues that determine their fate.
Electronic and photochemical materials
The use of filamentous phage as guides or components of nanowires [107, 108] continues to be a popular application of virus structures in materials science. A common and powerful theme is the installation of peptide sequences and the tendency of rod-shaped phage to adopt collinear aggregates, both of which can help to induce the templated deposition of the desired substance. Examples include metallic silver [109], palladium (with no added reducing agent, characterized by small-angle x-ray scattering) [110], and silicon oxide (by sol-gel synthesis to make millimeter-length fibers) [111]. Gold and iron oxide nanoparticles were also arrayed on TMV by simple charge complementation, the surface electrostatic properties of the scaffold being modulated simply by changing the solution pH, whereas the metal and metal oxide nanoparticles were relatively insensitive to this parameter [112]. Photoresponsive M13 particles were created by Mao and coworkers by the simple reaction of engineered surface tyrosine residues with aromatic diazonium salts [113]. While ensemble materials were not explored, the photochemistry of the attached azo dyes was found to be influenced by the unique functional group density of the protein scaffold.
In addition to these primary reports, two useful reviews to the field have recently appeared. An overview of mineralization and metal deposition techniques for nanomaterials was provided by Faramarzi and Sadighi [114], and Adamcik and Mezzenga contributed a long-overdue assessment of protein-based fiber structures from a polymer physics perspective [115]. The authors of the latter paper discussed the formation, intermolecular forces, structures, and properties of such nanowires, with concepts and insights applicable to virus-based systems.
The most sophisticated use of virus components in electronic materials has been achieved by the Belcher-Hammond team at MIT. These investigators used the high aspect ratio of the M13 particle (approximately 900 nm long and 7 nm in diameter) to excellent effect in the layer-by-layer construction of dye-sensitized solar cells [116]. Embedding the bionanoparticle in a polyelectrolyte matrix, virus-templated growth of semiconducting TiO2, and bake-out of the organic/protein scaffold resulted in a porous, ordered photoanode material and strong device performance. Incorporation of gold nanoparticles to give added plasmon-derived light harvesting ability highlights the great potential inherent in the controlled chemistry and assembly provided by virus particles [117].
Other applications
While mineralization has been perhaps the most popular materials-focused use of virus capsids [118], and the immunogenicity of virus particles represents an important application in biomedical science [119], a novel combination of these two features was recently described by Tang, Qin, and colleagues [120]. In order to improve the stability and shelf life of the icosahedral enterovirus type 71, a particle used for attenuated live virus vaccines, the virion was genetically modified to display a peptide motif known to nucleate the deposition of calcium phosphide (CaP) mineral. The key biological features of the particle – infectivity, genetic stability upon repeated passage, and immunogenicity – were all unchanged, while the modified structure was imbued with the ability to nucleate the formation of a CaP shell around itself. The resulting biomineralized particles were shown to be both significantly more stable and more immunogenic than the non-mineralized precursors, the latter presumably because CaP can be degraded under physiological conditions to reveal the antigenic polypeptide shell. The Finn group has similarly observed dramatically enhanced thermal stabilities of organic polymer-coated particles [121], but immunogenicity has not been tested. A review of efforts to encase biomolecular scaffolds in inorganic material shells, in which virus particles play a strong role, has recently appeared [122].
As described above and in more extensive reviews elsewhere [89, 90], it is clear that viruses can be unique scaffolds for the construction of functional materials. So far, however, the chemical, structural, and mechanical properties of viruses have claimed the lion's share of attention. Little advantage has been taken of the genetic mutability of such building blocks, other than the relatively simple installation of cysteine or short peptide units for additional covalent and noncoavlent connection capabilities. Because the field hasn't yet taken advantage of the full power of evolution hinted at in early contributions [123, 124], the development of virus-based materials can be said to still be in its infancy. It is our conviction that the marriage of methods for natural selection with functional tests of materials properties and performance – still on the horizon for all but a few such functions – has the power to revolutionize the pace and scope of materials discovery and optimization.
5. Conclusion: Evolving Biomaterials
Synthetic biology is the design and construction of biological systems, which inevitably gains its greatest inspiration from the mechanisms by which life generates and manages functional information. As a consequence, the field of synthetic biology is quite diverse, ranging from the creation of gene circuits to build specific responsiveness into synthetic organisms to the use of evolution in the creation of new molecular species with optimized function. These natural tools for the creation of dynamic and adaptive systems have also been applied to synthetic biology approaches to new and useful biomaterials. The emphasis thus far has been on technological approaches, including molecular biomimicry, directed evolution, and natural self-assembly. It is obvious that we have only scratched the surface with respect to the complexity of systems (e.g. VLPs) being explored. However, it is our opinion that the next levels of complexity and function will require the further use of evolution as the driving force. Few have explored methods in evolution-based design for biomaterials and even fewer have fully capitalized on its potential.
Of the general steps in a molecular evolution approach, the creation of candidate libraries is critical but relatively straightforward (as in peptide design and display for enhanced binding) or at least conceptually simple if laborious (as in the synthesis of synthetic polymer libraries). We have highlighted recent work employing DNA-templated synthesis that could, along with other advances, dramatically improve the generation of materials diversity. The second step, selection of desired function, is much more complex, because one would like to emulate nature's approach of functional selection, rather than molecular screening. However, as in the evolution of functional small molecules, the development of more high-throughput systems for the analysis of complex functional outcomes can be a powerful enabler of screening-based approaches. The final step in directed evolution is modification, iteration, and replication. If the field can overcome the obvious challenges in this completion of the evolutionary cycle, what should emerge are unfathomable biomolecular and polymeric species whose creation has been intimately linked to function. Since functional behavior is likely to involve many interactions of weak or transient nature, this evolution-driven approach should lead to new insights that could not have been designed a priori, and new appreciations of structure-function relationships in complex materials.
While the field of biomaterials began with the goal of creating bioinert structures such as titanium and polyethylene oxide polymers, it is clear that “smart” materials – those that dynamically interact with (and perhaps adapt to) surrounding tissue and cellular processes – have many more potential applications than traditional ones. Nature, the master architect of biomaterials, uses evolution to produce matchless responsive systems. If natural biomaterials are the inspiration for our targets in this field, natural mechanisms can be an effective inspiration for their creation. As shown by the above discussion of the building blocks and principles that can be used to produce biomaterials in the laboratory, if any endeavor can be said to have “only scratched the surface,” it is this one.
Acknowledgements
This work was funded in part by the NIH (R21EB013743, R01EB011566, R21EB015663), the Georgia Tech Center for Bioengineering for Soldier Survivability Seed Grant (DoD, W81XWH1110306), and an American Heart Association Postdoctoral Fellowship to ACB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith GP, Petrenko VA. Phage Display. Chem Rev. 1997;97:391–410. doi: 10.1021/cr960065d. [DOI] [PubMed] [Google Scholar]
- 2.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–10. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 3.Robertson DE, Noel JP. Protein Engineering. Academic Press; 2004. [Google Scholar]
- 4.Arnold FH, Georgiou G. Directed Evolution Library Creation. Humana Press; Totowa, New Jersey: 2003. [Google Scholar]
- 5.Ruff AJ, Dennig A, Schwaneberg U. To get what we aim for--progress in diversity generation methods. FEBS J. 2013;280:2961–78. doi: 10.1111/febs.12325. [DOI] [PubMed] [Google Scholar]
- 6.Goldsmith M, Tawfik DS. Enzyme engineering by targeted libraries. Methods Enzymol. 2013;523:257–83. doi: 10.1016/B978-0-12-394292-0.00012-6. [DOI] [PubMed] [Google Scholar]
- 7.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–92. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 8.Mallick KK, Cox SC. Biomaterial scaffolds for tissue engineering. Front Biosci. 2013;5:341–60. doi: 10.2741/e620. [DOI] [PubMed] [Google Scholar]
- 9.Edalat F, Sheu I, Manoucheri S, Khademhosseini A. Material strategies for creating artificial cell-instructive niches. Curr Opin Biotechnol. 2012;23:820–5. doi: 10.1016/j.copbio.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker TH. The role of ECM proteins and protein fragments in guiding cell behavior in regenerative medicine. Biomaterials. 2011;32:4211–4. doi: 10.1016/j.biomaterials.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 11.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–43. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 13.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–41. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–20. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grayson WL, Frohlich M, Yeager K, Bhumiratana S, Chan ME, Cannizzaro C, et al. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci U S A. 2010;107:3299–304. doi: 10.1073/pnas.0905439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quint C, Kondo Y, Manson RJ, Lawson JH, Dardik A, Niklason LE. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci U S A. 2011;108:9214–9. doi: 10.1073/pnas.1019506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baino F, Vitale-Brovarone C. Three-dimensional glass-derived scaffolds for bone tissue engineering: current trends and forecasts for the future. J Biomed Mater Res A. 2011;97:514–35. doi: 10.1002/jbm.a.33072. [DOI] [PubMed] [Google Scholar]
- 18.Collier JH, Segura T. Evolving the use of peptides as components of biomaterials. Biomaterials. 2011;32:4198–204. doi: 10.1016/j.biomaterials.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gasiorowski JZ, Collier JH. Directed intermixing in multicomponent self-assembling biomaterials. Biomacromolecules. 2011;12:3549–58. doi: 10.1021/bm200763y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiMarco RL, Heilshorn SC. Multifunctional materials through modular protein engineering. Adv Mater. 2012;24:3923–40. doi: 10.1002/adma.201200051. [DOI] [PubMed] [Google Scholar]
- 21.Werkmeister JA, Ramshaw JA. Recombinant protein scaffolds for tissue engineering. Biomed Mater. 2012;7:012002. doi: 10.1088/1748-6041/7/1/012002. [DOI] [PubMed] [Google Scholar]
- 22.Huang X, Zauscher S, Klitzman B, Truskey GA, Reichert WM, Kenan DJ, et al. Peptide interfacial biomaterials improve endothelial cell adhesion and spreading on synthetic polyglycolic acid materials. Ann Biomed Eng. 2010;38:1965–76. doi: 10.1007/s10439-010-9986-5. [DOI] [PubMed] [Google Scholar]
- 23.Stabenfeldt SE, Gourley M, Krishnan L, Hoying JB, Barker TH. Engineering fibrin polymers through engagement of alternative polymerization mechanisms. Biomaterials. 2012;33:535–44. doi: 10.1016/j.biomaterials.2011.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soon AS, Smith MH, Herman ES, Lyon LA, Barker TH. Development of self-assembling mixed protein micelles with temperature-modulated avidities. Adv Healthc Mater. 2013;2:1045–55. doi: 10.1002/adhm.201200330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wojtowicz AM, Shekaran A, Oest ME, Dupont KM, Templeman KL, Hutmacher DW, et al. Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair. Biomaterials. 2010;31:2574–82. doi: 10.1016/j.biomaterials.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim TG, Park TG. Biomimicking extracellular matrix: cell adhesive RGD peptide modified electrospun poly(D,L-lactic-co-glycolic acid) nanofiber mesh. Tissue Eng. 2006;12:221–33. doi: 10.1089/ten.2006.12.221. [DOI] [PubMed] [Google Scholar]
- 27.Wang PY, Wu TH, Tsai WB, Kuo WH, Wang MJ. Grooved PLGA films incorporated with RGD/YIGSR peptides for potential application on skeletal muscle tissue engineering. Colloids Surf B Biointerfaces. 2013;110:88–95. doi: 10.1016/j.colsurfb.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Markowski MC, Brown AC, Barker TH. Directing epithelial to mesenchymal transition through engineered microenvironments displaying orthogonal adhesive and mechanical cues. J Biomed Mater Res A. 2012;100:2119–27. doi: 10.1002/jbm.a.34068. [DOI] [PubMed] [Google Scholar]
- 29.Chaisri P, Chingsungnoen A, Siri S. Repetitive RGD peptide as cell-stimulating agent on electrospun PCL scaffold for tissue engineering. Biotechnol J. 2013 doi: 10.1002/biot.201300191. [DOI] [PubMed] [Google Scholar]
- 30.Carson AE, Barker TH. Emerging concepts in engineering extracellular matrix variants for directing cell phenotype. Regen Med. 2009;4:593–600. doi: 10.2217/rme.09.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reichl S. Films based on human hair keratin as substrates for cell culture and tissue engineering. Biomaterials. 2009;30:6854–66. doi: 10.1016/j.biomaterials.2009.08.051. [DOI] [PubMed] [Google Scholar]
- 32.Hsiong SX, Huebsch N, Fischbach C, Kong HJ, Mooney DJ. Integrin-adhesion ligand bond formation of preosteoblasts and stem cells in three-dimensional RGD presenting matrices. Biomacromolecules. 2008;9:1843–51. doi: 10.1021/bm8000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ala-Kokko L, Hyland J, Smith C, Kivirikko KI, Jimenez SA, Prockop DJ. Expression of a human cartilage procollagen gene (COL2A1) in mouse 3T3 cells. J Biol Chem. 1991;266:14175–8. [PubMed] [Google Scholar]
- 34.Indik Z, Abrams WR, Kucich U, Gibson CW, Mecham RP, Rosenbloom J. Production of recombinant human tropoelastin: characterization and demonstration of immunologic and chemotactic activity. Arch Biochem Biophys. 1990;280:80–6. doi: 10.1016/0003-9861(90)90521-y. [DOI] [PubMed] [Google Scholar]
- 35.Lazaris A, Arcidiacono S, Huang Y, Zhou JF, Duguay F, Chretien N, et al. Spider silk fibers spun from soluble recombinant silk produced in mammalian cells. Science. 2002;295:472–6. doi: 10.1126/science.1065780. [DOI] [PubMed] [Google Scholar]
- 36.Elvin CM, Carr AG, Huson MG, Maxwell JM, Pearson RD, Vuocolo T, et al. Synthesis and properties of crosslinked recombinant pro-resilin. Nature. 2005;437:999–1002. doi: 10.1038/nature04085. [DOI] [PubMed] [Google Scholar]
- 37.Brown AC, Rowe JA, Barker TH. Guiding epithelial cell phenotypes with engineered integrin-specific recombinant fibronectin fragments. Tissue Eng Part A. 2011;17:139–50. doi: 10.1089/ten.tea.2010.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koga N, Tatsumi-Koga R, Liu G, Xiao R, Acton TB, Montelione GT, et al. Principles for designing ideal protein structures. Nature. 2012;491:222–7. doi: 10.1038/nature11600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–3. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 40.Tong AH, Drees B, Nardelli G, Bader GD, Brannetti B, Castagnoli L, et al. A combined experimental and computational strategy to define protein interaction networks for peptide recognition modules. Science. 2002;295:321–4. doi: 10.1126/science.1064987. [DOI] [PubMed] [Google Scholar]
- 41.Sidhu SS, Bader GD, Boone C. Functional genomics of intracellular peptide recognition domains with combinatorial biology methods. Curr Opin Chem Biol. 2003;7:97–102. doi: 10.1016/s1367-5931(02)00011-x. [DOI] [PubMed] [Google Scholar]
- 42.Okada H, Uezu A, Soderblom EJ, Moseley MA, 3rd, Gertler FB, Soderling SH. Peptide array X-linking (PAX): a new peptide-protein identification approach. PLoS One. 2012;7:e37035. doi: 10.1371/journal.pone.0037035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martino MM, Tortelli F, Mochizuki M, Traub S, Ben-David D, Kuhn GA, et al. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci Transl Med. 2011;3:100ra89. doi: 10.1126/scitranslmed.3002614. [DOI] [PubMed] [Google Scholar]
- 44.Banta S, Wheeldon IR, Blenner M. Protein engineering in the development of functional hydrogels. Annu Rev Biomed Eng. 2010;12:167–86. doi: 10.1146/annurev-bioeng-070909-105334. [DOI] [PubMed] [Google Scholar]
- 45.O'Shea EK, Klemm JD, Kim PS, Alber T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science. 1991;254:539–44. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- 46.Petka WA, Harden JL, McGrath KP, Wirtz D, Tirrell DA. Reversible hydrogels from self-assembling artificial proteins. Science. 1998;281:389–92. doi: 10.1126/science.281.5375.389. [DOI] [PubMed] [Google Scholar]
- 47.Harris JL, Backes BJ, Leonetti F, Mahrus S, Ellman JA, Craik CS. Rapid and general profiling of protease specificity by using combinatorial fluorogenic substrate libraries. Proc Natl Acad Sci U S A. 2000;97:7754–9. doi: 10.1073/pnas.140132697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Backes BJ, Harris JL, Leonetti F, Craik CS, Ellman JA. Synthesis of positional-scanning libraries of fluorogenic peptide substrates to define the extended substrate specificity of plasmin and thrombin. Nat Biotechnol. 2000;18:187–93. doi: 10.1038/72642. [DOI] [PubMed] [Google Scholar]
- 49.Straley K, Heilshorn SC. Designer protein-based scaffolds for neural tissue engineering. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:2101–2. doi: 10.1109/IEMBS.2009.5334310. [DOI] [PubMed] [Google Scholar]
- 50.Barish RD, Schulman R, Rothemund PW, Winfree E. An information-bearing seed for nucleating algorithmic self-assembly. Proc Natl Acad Sci U S A. 2009;106:6054–9. doi: 10.1073/pnas.0808736106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan H, Park SH, Finkelstein G, Reif JH, LaBean TH. DNA-templated self-assembly of protein arrays and highly conductive nanowires. Science. 2003;301:1882–4. doi: 10.1126/science.1089389. [DOI] [PubMed] [Google Scholar]
- 52.Gartner ZJ, Tse BN, Grubina R, Doyon JB, Snyder TM, Liu DR. DNA-templated organic synthesis and selection of a library of macrocycles. Science. 2004;305:1601–5. doi: 10.1126/science.1102629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Douglas SM, Dietz H, Liedl T, Hogberg B, Graf F, Shih WM. Self-assembly of DNA into nanoscale three-dimensional shapes. Nature. 2009;459:414–8. doi: 10.1038/nature08016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu LQ, Payne GF. Biofabrication: using biological materials and biocatalysts to construct nanostructured assemblies. Trends Biotechnol. 2004;22:593–9. doi: 10.1016/j.tibtech.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 55.Best JP, Cui J, Mullner M, Caruso F. Tuning the mechanical properties of nanoporous hydrogel particles via polymer cross-linking. Langmuir. 2013;29:9824–31. doi: 10.1021/la402146t. [DOI] [PubMed] [Google Scholar]
- 56.Nielsen LE. Cross-Linking–Effect on Physical Properties of Polymers. J Macromol Sci Polym Rev. 1969;3:69–103. [Google Scholar]
- 57.Ji Y, Yin J, Xu Z, Zhao C, Huang H, Zhang H, et al. Preparation of magnetic molecularly imprinted polymer for rapid determination of bisphenol A in environmental water and milk samples. Anal Bioanal Chem. 2009;395:1125–33. doi: 10.1007/s00216-009-3020-5. [DOI] [PubMed] [Google Scholar]
- 58.Poma A, Guerreiro Antonio, Whitcombe Michael J., Piletska Elena V., Turner Anthony P. F., Piletsky Sergey A. Solid-Phase Synthesis of Molecularly Imprinted Polymer Nanoparticles with a Reusable Template–“Plastic Antibodies”. Adv Funct Mater. 2013 doi: 10.1002/adfm.201202397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, Yang HH, You QH, Zhuang ZX, Wang XR. Protein recognition via surface molecularly imprinted polymer nanowires. Anal Chem. 2006;78:317–20. doi: 10.1021/ac050802i. [DOI] [PubMed] [Google Scholar]
- 60.Poma A, Turner AP, Piletsky SA. Advances in the manufacture of MIP nanoparticles. Trends Biotechnol. 2010;28:629–37. doi: 10.1016/j.tibtech.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 61.Zeng Z, Hoshino Y, Rodriguez A, Yoo H, Shea KJ. Synthetic polymer nanoparticles with antibody-like affinity for a hydrophilic peptide. ACS Nano. 2010;4:199–204. doi: 10.1021/nn901256s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zimmerman SC, Wendland MS, Rakow NA, Zharov I, Suslick KS. Synthetic hosts by monomolecular imprinting inside dendrimers. Nature. 2002;418:399–403. doi: 10.1038/nature00877. [DOI] [PubMed] [Google Scholar]
- 63.Turiel E, Martin-Esteban A. Molecularly imprinted polymers for sample preparation: a review. Anal Chim Acta. 2010;668:87–99. doi: 10.1016/j.aca.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 64.Nishino H, Huang CS, Shea KJ. Selective protein capture by epitope imprinting. Angew Chem Int Ed. 2006;45:2392–6. doi: 10.1002/anie.200503760. [DOI] [PubMed] [Google Scholar]
- 65.Hoshino Y, Kodama T, Okahata Y, Shea KJ. Peptide imprinted polymer nanoparticles: a plastic antibody. J Am Chem Soc. 2008;130:15242–3. doi: 10.1021/ja8062875. [DOI] [PubMed] [Google Scholar]
- 66.Hoshino Y, Shea KJ. The evolution of plastic antibodies. J Mater Chem A. 2011;21:3517–21. [Google Scholar]
- 67.Hoshino Y, Koide H, Urakami T, Kanazawa H, Kodama T, Oku N, et al. Recognition, neutralization, and clearance of target peptides in the bloodstream of living mice by molecularly imprinted polymer nanoparticles: a plastic antibody. J Am Chem Soc. 2010;132:6644–5. doi: 10.1021/ja102148f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoshino Y, Koide H, Furuya K, Haberaecker WW, 3rd, Lee SH, Kodama T, et al. The rational design of a synthetic polymer nanoparticle that neutralizes a toxic peptide in vivo. Proc Natl Acad Sci U S A. 2012;109:33–8. doi: 10.1073/pnas.1112828109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Storhoff JJ, Mirkin CA. Programmed Materials Synthesis with DNA. Chem Rev. 1999;99:1849–62. doi: 10.1021/cr970071p. [DOI] [PubMed] [Google Scholar]
- 70.Niu J, Hili R, Liu DR. Enzyme-free translation of DNA into sequence-defined synthetic polymers structurally unrelated to nucleic acids. Nat Chem. 2013;5:282–92. doi: 10.1038/nchem.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leitzel JC, Lynn DG. Template-directed ligation: from DNA towards different versatile templates. Chem Rec. 2001;1:53–62. doi: 10.1002/1528-0691(2001)1:1<53::AID-TCR8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 72.Ura Y, Beierle JM, Leman LJ, Orgel LE, Ghadiri MR. Self-assembling sequence-adaptive peptide nucleic acids. Science. 2009;325:73–7. doi: 10.1126/science.1174577. [DOI] [PubMed] [Google Scholar]
- 73.Li X, Zhan ZY, Knipe R, Lynn DG. DNA-catalyzed polymerization. J Am Chem Soc. 2002;124:746–7. doi: 10.1021/ja017319j. [DOI] [PubMed] [Google Scholar]
- 74.Schrum JP, Ricardo A, Krishnamurthy M, Blain JC, Szostak JW. Efficient and rapid template-directed nucleic acid copying using 2′-amino-2′,3′-dideoxyribonucleoside-5′-phosphorimidazolide monomers. J Am Chem Soc. 2009;131:14560–70. doi: 10.1021/ja906557v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Henry AA, Romesberg FE. The evolution of DNA polymerases with novel activities. Curr Opin Biotechnol. 2005;16:370–7. doi: 10.1016/j.copbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 76.Josephson K, Hartman MC, Szostak JW. Ribosomal synthesis of unnatural peptides. J Am Chem Soc. 2005;127:11727–35. doi: 10.1021/ja0515809. [DOI] [PubMed] [Google Scholar]
- 77.Datta B, Schuster GB. DNA-directed synthesis of aniline and 4-aminobiphenyl oligomers: programmed transfer of sequence information to a conjoined polymer nanowire. J Am Chem Soc. 2008;130:2965–73. doi: 10.1021/ja0726106. [DOI] [PubMed] [Google Scholar]
- 78.McKee ML, Milnes PJ, Bath J, Stulz E, O'Reilly RK, Turberfield AJ. Programmable one-pot multistep organic synthesis using DNA junctions. J Am Chem Soc. 2012;134:1446–9. doi: 10.1021/ja2101196. [DOI] [PubMed] [Google Scholar]
- 79.Chung BG, Kang L, Khademhosseini A. Micro- and nanoscale technologies for tissue engineering and drug discovery applications. Expert Opin Drug Discov. 2007;2:1653–68. doi: 10.1517/17460441.2.12.1653. [DOI] [PubMed] [Google Scholar]
- 80.Ravichandran R, Liao S, Ng C, Chan CK, Raghunath M, Ramakrishna S. Effects of nanotopography on stem cell phenotypes. World J Stem Cells. 2009;1:55–66. doi: 10.4252/wjsc.v1.i1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mukhatyar VJ, Salmeron-Sanchez M, Rudra S, Mukhopadaya S, Barker TH, Garcia AJ, et al. Role of fibronectin in topographical guidance of neurite extension on electrospun fibers. Biomaterials. 2011;32:3958–68. doi: 10.1016/j.biomaterials.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Charest JL, Garcia AJ, King WP. Myoblast alignment and differentiation on cell culture substrates with microscale topography and model chemistries. Biomaterials. 2007;28:2202–10. doi: 10.1016/j.biomaterials.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 83.Hosseinkhani H, Hong PD, Yu DS. Self-assembled proteins and peptides for regenerative medicine. Chem Rev. 2013;113:4837–61. doi: 10.1021/cr300131h. [DOI] [PubMed] [Google Scholar]
- 84.Lawson DM, Artymiuk PJ, Yewdall SJ, Smith JMA, Livingstone JC, Treffry A, et al. Solving the Structure of Human H-Ferritin by Genetically Engineering Intermolecular Crystal Contacts. Nature. 1991;349:541–4. doi: 10.1038/349541a0. [DOI] [PubMed] [Google Scholar]
- 85.Philippe N, Legendre M, Doutre G, Coute Y, Poirot O, Lescot M, et al. Pandoraviruses: Amoeba Viruses with Genomes Up to 2.5 Mb Reaching That of Parasitic Eukaryotes. Science. 2013;341:281–6. doi: 10.1126/science.1239181. [DOI] [PubMed] [Google Scholar]
- 86.Fraenkel-Conrat H, Williams RC. Reconstitution of Active Tobacco Mosaic Virus from Its Inactive Protein and Nucleic Acid Components. Proc Natl Acad Sci U S A. 1955;41:690–8. doi: 10.1073/pnas.41.10.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ivanovska IL, Miranda R, Carrascosa JL, Wuite GJL, Schmidt CF. Discrete fracture patterns of virus shells reveal mechanical building blocks. Proc Natl Acad Sci USA. 2011;108:12611–6. doi: 10.1073/pnas.1105586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Siebenga JJ, Vennema H, Renckens B, de Bruin E, van der Veer B, Siezen RJ, et al. Epochal evolution of GGII.4 norovirus capsid proteins from 1995 to 2006. J Virol. 2007;81:9932–41. doi: 10.1128/JVI.00674-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Z, Qiao J, Niu Z, Wang Q. Natural supramolecular building blocks: from virus coat proteins to viral nanoparticles. Chem Soc Rev. 2012;41:6178–94. doi: 10.1039/c2cs35108k. [DOI] [PubMed] [Google Scholar]
- 90.Lee SY, Lim JS, Harris MT. Synthesis and application of virus-based hybrid nanomaterials. Biotechnol Bioeng. 2012;109:16–30. doi: 10.1002/bit.23328. [DOI] [PubMed] [Google Scholar]
- 91.Zhou K, Li F, Dai G, Meng C, Wang Q. Disulfide bond: dramatically enhanced assembly capability and structural stability of tobacco mosaic virus nanorods. Biomacromolecules. 2013;14:2593–600. doi: 10.1021/bm400445m. [DOI] [PubMed] [Google Scholar]
- 92.Bundy BC, Swartz JR. Efficient disulfide bond formation in virus-like particles. J Biotechnol. 2011;154:230–9. doi: 10.1016/j.jbiotec.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 93.Rego JM, Lee JH, Lee DH, Yi H. Biologically inspired strategy for programmed assembly of viral building blocks with controlled dimensions. Biotechnol J. 2013;8:237–46. doi: 10.1002/biot.201100504. [DOI] [PubMed] [Google Scholar]
- 94.Azucena C, Eber FJ, Trouillet V, Hirtz M, Heissler S, Franzreb M, et al. New approaches for bottom-up assembly of tobacco mosaic virus-derived nucleoprotein tubes on defined patterns on silica- and polymer-based substrates. Langmuir. 2012;28:14867–77. doi: 10.1021/la302774h. [DOI] [PubMed] [Google Scholar]
- 95.Zlotnick A, Porterfield JZ, Wang JCY. To Build a Virus on a Nucleic Acid Substrate. Biophys J. 2013;104:1595–604. doi: 10.1016/j.bpj.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ruff Y, Moyer T, Newcomb CJ, Demeler B, Stupp SI. Precision Templating with DNA of a Virus-like Particle with Peptide Nanostructures. J Am Chem Soc. 2013;135:6211–9. doi: 10.1021/ja4008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Russell JT, Lin Y, Boker A, Su L, Carl P, Zettl H, et al. Self-assembly and cross-linking of bionanoparticles at liquid-liquid interfaces. Angew Chem Int Ed. 2005;44:2420–6. doi: 10.1002/anie.200462653. [DOI] [PubMed] [Google Scholar]
- 98.He J, Niu Z, Tangirala R, Wang JY, Wei X, Kaur G, et al. Self-assembly of tobacco mosaic virus at oil/water interfaces. Langmuir. 2009;25:4979–87. doi: 10.1021/la803533n. [DOI] [PubMed] [Google Scholar]
- 99.Yoo PJ, Zacharia NS, Doh J, Nam KT, Belcher AM, Hammond PT. Controlling surface mobility in interdiflusing polyelectrolyte multilayers. Acs Nano. 2008;2:561–71. doi: 10.1021/nn700404y. [DOI] [PubMed] [Google Scholar]
- 100.Chung WJ, Oh JW, Kwak K, Lee BY, Meyer J, Wang E, et al. Biomimetic self-templating supramolecular structures. Nature. 2011;478:364–8. doi: 10.1038/nature10513. [DOI] [PubMed] [Google Scholar]
- 101.Li T, Zan XJ, Winans RE, Wang Q, Lee B. Biomolecular Assembly of Thermoresponsive Superlattices of the Tobacco Mosaic Virus with Large Tunable Interparticle Distances. Angew Chem Int Ed. 2013;52:6638–42. doi: 10.1002/anie.201209299. [DOI] [PubMed] [Google Scholar]
- 102.Rong J, Lee LA, Li K, Harp B, Mello CM, Niu Z, et al. Oriented cell growth on self-assembled bacteriophage M13 thin films. Chem Commun (Camb) 2008:5185–7. doi: 10.1039/b811039e. [DOI] [PubMed] [Google Scholar]
- 103.Wu L, Lee LA, Niu Z, Ghoshroy S, Wang Q. Visualizing cell extracellular matrix (ECM) deposited by cells cultured on aligned bacteriophage M13 thin films. Langmuir. 2011;27:9490–6. doi: 10.1021/la201580v. [DOI] [PubMed] [Google Scholar]
- 104.Lin Y, Balizan E, Lee LA, Niu Z, Wang Q. Self-assembly of rodlike bio-nanoparticles in capillary tubes. Angew Chem Int Ed. 2010;49:868–72. doi: 10.1002/anie.200904993. [DOI] [PubMed] [Google Scholar]
- 105.Zan X, Feng S, Balizan E, Lin Y, Wang Q. Facile method for large scale alignment of one dimensional nanoparticles and control over myoblast orientation and differentiation. ACS Nano. 2013;7:8385–96. doi: 10.1021/nn403908k. [DOI] [PubMed] [Google Scholar]
- 106.Wang J, Wang L, Li X, Mao C. Virus activated artificial ECM induces the osteoblastic differentiation of mesenchymal stem cells without osteogenic supplements. Sci Rep. 2013;3:1242. doi: 10.1038/srep01242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tseng RJ, Tsai CL, Ma LP, Ouyang JY. Digital memory device based on tobacco mosaic virus conjugated with nanoparticles. Nat Nanotechnol. 2006;1:72–7. doi: 10.1038/nnano.2006.55. [DOI] [PubMed] [Google Scholar]
- 108.Maeda Y, Matsui H. Genetically engineered protein nanowires: unique features in site-specific functionalization and multi-dimensional self-assembly. Soft Matter. 2012;8:7533–44. [Google Scholar]
- 109.Nam KT, Lee YJ, Krauland EM. Peptide-mediated reduction of silver ions on engineered biological scaffolds. ACS Nano. 2008;2:1480–6. doi: 10.1021/nn800018n. [DOI] [PubMed] [Google Scholar]
- 110.Freer AS, Guarnaccio L, Watford K, Smith J, Steilberg J, Culver JN, et al. SAXS characterization of genetically engineered tobacco mosaic virus nanorods coated with palladium in the absence of external reducing agents. J Colloid Interf Sci. 2013;392:213–8. doi: 10.1016/j.jcis.2012.09.072. [DOI] [PubMed] [Google Scholar]
- 111.Grelet E, Moreno A, Backov R. Hybrid Macroscopic Fibers from the Synergistic Assembly Between Silica and Filamentous Viruses. Langmuir. 2011;27:4334–8. doi: 10.1021/la200743n. [DOI] [PubMed] [Google Scholar]
- 112.Khan AA, Fox EK, Gorzny ML, Nikulina E, Brougham DF, Wege C, et al. pH Control of the Electrostatic Binding of Gold and Iron Oxide Nanoparticles to Tobacco Mosaic Virus. Langmuir. 2013;29:2094–8. doi: 10.1021/la3044126. [DOI] [PubMed] [Google Scholar]
- 113.Murugesan M, Abbineni G, Nimmo SL, Cao B, Mao C. Virus-based photo-responsive nanowires formed by linking site-directed mutagenesis and chemical reaction. Sci Rep. 2013;3:1820. doi: 10.1038/srep01820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Faramarzi MA, Sadighi A. Insights into biogenic and chemical production of inorganic nanomaterials and nanostructures. Adv Colloid Interf Sci. 2013;189:1–20. doi: 10.1016/j.cis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 115.Adamcik J, Mezzenga R. Proteins Fibrils from a Polymer Physics Perspective. Macromolecules. 2012;45:1137–50. [Google Scholar]
- 116.Chen PY, Ladewski R, Miller R, Dang XN, Qi JF, Liau F, et al. Layer-by-layer assembled porous photoanodes for efficient electron collection in dye-sensitized solar cells. J Mater Chem A. 2013;1:2217–24. [Google Scholar]
- 117.Chen PY, Dang X, Klug MT, Qi J, Dorval Courchesne NM, Burpo FJ, et al. Versatile three-dimensional virus-based template for dye-sensitized solar cells with improved electron transport and light harvesting. ACS Nano. 2013;7:6563–74. doi: 10.1021/nn4014164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Korkmaz N, Kim YJ, Nam CH. Bacteriophages as templates for manufacturing supramolecular structures. Macromol Biosci. 2013;13:376–87. doi: 10.1002/mabi.201200290. [DOI] [PubMed] [Google Scholar]
- 119.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787–96. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 120.Wang G, Cao RY, Chen R, Mo L, Han JF, Wang X, et al. Rational design of thermostable vaccines by engineered peptide-induced virus self-biomineralization under physiological conditions. Proc Natl Acad Sci U S A. 2013;110:7619–24. doi: 10.1073/pnas.1300233110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Manzenrieder F, Luxenhofer R, Retzlaff M, Jordan R, Finn MG. Stabilization of Virus-Like Particles with Poly(2-oxazoline)s. Angew Chem Int Ed. 2011;50:2601–5. doi: 10.1002/anie.201006134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Patil AJ, Li M, Mann S. Integrative self-assembly of functional hybrid nanoconstructs by inorganic wrapping of single biomolecules, biomolecule arrays and organic supramolecular assemblies. Nanoscale. 2013;5:7161–74. doi: 10.1039/c3nr02796a. [DOI] [PubMed] [Google Scholar]
- 123.Mao C, Solis DJ, Reiss BD, Kottmann ST, Sweeney RY, Hayhurst A, et al. Virus-Based Toolkit for the Directed Synthesis of Magnetic and Semiconducting Nanowires. Science. 2004;303:213–7. doi: 10.1126/science.1092740. [DOI] [PubMed] [Google Scholar]
- 124.Lee S-W, Mao C, Flynn CE, Belcher AM. Ordering of Quantum Dots Using Genetically Engineered Viruses. Science. 2002;296:892–5. doi: 10.1126/science.1068054. [DOI] [PubMed] [Google Scholar]