Abstract
Vascular endothelial growth factor (VEGF) is a potent angiogenic stimulator. Controlled release of such stimulators may enhance and guide the vascularization process, and when applied in a nerve conduit may play a role in nerve regeneration. We report the fabrication and in vitro characterization of VEGF encapsulating poly-lactic-co-glycolic acid (PLGA) microspheres and the in vivo application of nerve conduits supplemented with VEGF-containing microspheres. PLGA microspheres containing VEGF were prepared by the double emulsion-solvent evaporation technique. This yielded 83.16% of the microspheres with a diameter < 53 µm. VEGF content measured by ELISA indicated 93.79 ±10.64% encapsulation efficiency. Release kinetics were characterized by an initial burst release of 67.6±8.25% within the first 24 hours, followed by consistent release of approximately 0.34% per day for 4 weeks. Bioactivity of the released VEGF was tested by human umbilical vein endothelial cell (HUVEC) proliferation assay. VEGF released at all time points enhanced HUVEC proliferation confirming that VEGF retained its bioactivity through the 4-week time period. When the microsphere delivery system was placed in a biosynthetic nerve scaffold, robust nerve regeneration was observed. This study established a novel system for controlled release of growth factors and enables in vivo studies of nerve conduits conditioned with this system.
Keywords: microsphere, poly-lactic co-glycolic acid, vascular endothelial growth factor, bioactivity, biodegradation, nerve guide
1. INTRODUCTION
Nerve regeneration requires cells, extracellular matrix, growth factors and the complex interplay between the three. When a nerve defect is repaired with a single lumen conduit these components are not present. Introduction of growth factors (GFs) or cells into a conduit would encourage axon growth in tissue engineering implants [1]. Most studies have focused on application of GFs with neurotrophic effects. Since in the process of nerve regeneration, especially that after nerve graft repair or conduit repair, revascularization precedes regeneration, the current study aims at delivery of pro-angiogenic GF to the regenerating nerve site. Pro-angiogenic GFs promote angiogenesis leading to increased transportation of oxygen and nutrients to the nerve tissue. One important pro-angiogenic GF is vascular endothelial growth factor (VEGF). VEGF is a homodimeric glycoprotein that increases microvascular permeability, stimulates the proliferation and migration of endothelial cells, and promotes angiogenesis [2–4]. VEGF also directly affects neurons and glial cells by inhibition of apoptosis, promotion of survival and stimulation of neurogenesis. VEGF’s angiogenic effect on vascular remodeling may play an important indirect role in nerve regeneration.
The mode and timing of GF delivery is as important as the GFs themselves. Two major strategies have been used to administer angiogenic growth factors: application of exogenous recombinant human proteins or by gene transfer to induce endogenous secretion of the target growth factors [2–5]. There has been some success in pre-clinical animal models and initial clinical trials of pro-angiogenic GF delivery [6–8]. However, double-blinded clinical trials with large cohorts of patients failed to show efficacy of intravenous infusions of recombinant human VEGF and VEGF gene transfer therapy [9–12]. This lack of effect has been attributed to the requirement for a high level of biological activity (in pico- to nanomolar range), pleiotrophic effects (acting on variety of targets) and short biological half-life [13]. To solve these problems, many delivery systems that control the release of therapeutic agents have been developed. Examples include protein encapsulation or covalent linkage to an implant, protein encapsulation in microspheres, subcutaneous implantion of osmotic minipumps [14]. Each delivery system has its advantages and disadvantages. Proteins encapsulated or covalently linked to implants are easily degraded and exhibit reduced bioactivity. Implanted osmotic pumps and injection devices require a second operation for device removal. Microsphere delivery systems are of increasing interest because the localized delivery systems release bioactive molecules directly to the desired site. This mitigates the risk of premature enzymatic degradation and allows for sustained release of the therapeutic agents [15].
In this study, we fabricated PLGA microspheres containing VEGF121 by double emulsion-solvent evaporation. Degradation of PLGA microspheres and the release kinetics of VEGF were characterized. The bioactivity of released VEGF from PLGA microspheres was verified by the HUVEC proliferation assay. These VEGF containing microspheres were then implanted into the lumen of polycaprolactone fumarate (PCLF) conduit to bridge a 1-cm gap in rat sciatic nerve.
2. MATERIALS AND METHODS
2.1. Materials
PLGA (5050 DLG 4A, Lakeshore Biomaterials, Birmingham, AL) possessed 50:50 lactic acid to glycolic acid ratio, 0.35 – 0.45 dL/g inherent viscosity and 29 kDa molecular weight. Recombinant human VEGF (isoform 121) was purchased from R&D systems (Minneapolis, MN). Quantikine VEGF ELISA kit was also obtained from R&D systems and used according to manufacture’s instructions. Bovine serum albumin (BSA), poly (vinyl alcohol) (PVA, 87–89% hydrolyzed) and isopropyl alcohol were from Sigma-Aldrich (St. Louis, MO). Dulbecco’s phosphate buffered saline (DPBS) was obtained from Invitrogen (Grand Island, N.Y.). Methylene chloride was purchased from Fisher Scientific (Pittsburgh, PA). The human umbilical vein endothelial cell (HUVEC) line and endothelial cell growth medium-2 (EGM-2) used in the bioactivity studies were both purchased from Clonetics (Walkersville, MD). CellTiter 96 Q non-radioactive cell proliferation assay kit was obtained from Promega (Madison, WI) for the MTS assay and used according to manufacturer’s instructions. Syringe filters (Supor® Membrane, low protein binding, 0.2 µm) were purchased from Acrodisc (Port Washington, NY).
2.2. Preparation of VEGF-containing Microspheres
A water-in-oil-in-water (w1-o-w2) double emulsion-solvent evaporation technique (L1) was applied to fabricate PLGA microspheres containing VEGF (0.1 µg/mg of microspheres). Briefly, VEGF stock solution (250 µg/mL) was prepared under sterile conditions with PBS containing 0.25% (w/v) BSA per manufacturer’s recommendation. 100 µL of VEGF stock solution was emulsified in a solution of 250 mg PLGA in 1mL of methylene chloride for 30 seconds on a vortex device (Vortex Genie, Fisher, Pittsburgh, PA) at setting 9. The mixture was re-emulsified at the same vortex speed for another 30 seconds in 2 mL of 2% (w/v) PVA solution to create the double emulsion. The content was then added to 100 mL of 0.3% (w/v) PVA solution and 100 mL of 2% (w/v) isopropyl alcohol solution and stirred for at least 1 hour to evaporate the methylene chloride. The microspheres were collected and washed 3 times with distilled water by centrifugation at 2000 rpm for 3 minutes. Free flowing powder of microspheres was prepared by freezing them at −80°C for 1 hour and vacuum-drying overnight (Savant Speedvac Systems, NY). In order to obtain smaller and more even microspheres, a sieve (53 µm, Humboldt) was used. Two batches of VEGF-PLGA microspheres were produced. Another three batches of PLGA microspheres without VEGF (plain microspheres) were produced for microsphere characterization. To investigate the effect of BSA on VEGF preservation and encapsulation, microspheres containing VEGF and an excess of BSA (denoted as VEGF-BSA(2000)-containing microspheres) were fabricated. The fabrication procedure was identical to the VEGF-containing microspheres with the exception that in addition to the 100µL of VEGF a 2000:1 fold excess of BSA:VEGF was emulsified in a solution of 250 mg PLGA during the fabrication process.
2.3. Microsphere Morphology
The morphology of microspheres was visualized using scanning electron microscopy (SEM, Hitachi S4700, Pleasanton, CA). A small quantity of plain microspheres was sprinkled on the SEM stubs and coated by SEM coating system (Bio-rad, polaron division) with gold palladium under argon atmosphere using a gold palladium sputter module in a vacuum evaporator. Samples were then observed for their surface morphology with SEM and photographs were taken under 180X magnification. One stub was made for each batch and seven pictures were taken for each stub. Image J software (NIH) was used to measure the diameter of the microspheres after the pictures were taken. All the microspheres (n=1758) included in the pictures were measured and the diameter of the microspheres was expressed as mean ± SD.
2.4. Encapsulation Efficiency
The encapsulation efficiency of sieved VEGF-PLGA microspheres was determined by extracting and quantifying the encapsulated VEGF. 10 mg sieved VEGF-PLGA microspheres were dissolved in 2 mL methylene chloride at 37°C. VEGF was extracted from the organic layer with 2 mL distilled water three times and combined. A total of 6 mL supernatant was collected and VEGF content was measured by enzyme-linked immunosorbent assay (ELISA), according to the manufacturer’s procedures. Briefly, 50 µL of assay diluent and 200 µL of VEGF standard solution or samples were added to wells of a microplate coated with a monoclonal antibody against VEGF and incubated for 2 hours at room temperature. The microplates were then washed three times with wash buffer. After complete removal of wash buffer, 200 µL of VEGF conjugate was added and incubated for another 2 hours in room temperature. After washing, 200 µL substrate solution was added and incubated for 20 minutes at room temperature in a light-proof container. Then 50 µL stop solution was added and following color development, the optical density (OD) was determined at 450 nm corrected at 540 nm or 570 nm within 30 minutes through a microplate reader (Spectramas Plus 384, Molecular Devices). The measured VEGF content was divided by the loaded VEGF content (0.1 µg/mg X 10mg) to calculate the encapsulation efficiency. Encapsulation efficiency of VEGF in VEGF-BSA(2000)-containing microspheres was measured the same way as that of VEGF-containing microspheres was measured.
2.5. Degradation of PLGA Microspheres
Both mass loss and molecular weight loss were determined in our experiment to delineate the degradation fate of PLGA microspheres. 20 mg sieved plain microspheres were added to a 1.5 mL microcentrifuge tube and weighed on an electronic balance to obtain the initial mass weight. Then, 500 µL PBS was added into the microcentrifuge tube which was incubated at 37 °C. The medium was changed every week by centrifugation and refreshing the PBS. Six time points were assigned to determine the changes in mass weight at 1, 2, 3, 4, 5 and 6 weeks of incubation. Three samples per time point were allocated. At each time point, the microspheres were washed three times with distilled water and freeze-dried. The microcentrifuge tubes with the degrading samples were weighed again. The difference between the weight as this point and the initial weight was the mass loss that incurred until this time point. The initial microspheres and degrading samples were subject to molecular weight measurement at the same time points as well. The molecular weight of the initial microspheres and degrading samples were determined using gel permeation chromatography (GPC). The GPC system consists of Waters 515 HPLC isocratic pump, Waters 717 Plus autosample, Waters 2410 refractive index detector. Tetrahydrofuran was used as the eluent at 1 mL/min. Polystyrene standards of molecular weight 474, 1060, 2950, 6690, 10800, 18600, 39200 g/mol were used to construct the calibration curve and the number averaged molecular weight was reported [16].
2.6. In vitro Release Kinetics
The in vitro release of VEGF from the PLGA microspheres was carried out in PBS solution at 37 °C. Briefly, 40 mg sieved VEGF-PLGA microspheres were suspended in 1.5 mL microcentrifuge tube filled with 1 mL PBS and placed in a 37°C incubator. After 6 hours and every 24 hours thereafter till day 40, samples were centrifuged at 5000 rpm for 5 minutes at room temperature. The supernatant was collected and replaced with the same volume of fresh PBS. Before the tube was put back into the incubator, it was vortexed to evenly suspend the microspheres. The supernatant was divided into two parts, one for release kinetics and another for VEGF bioassay. The supernatant collected at each time point was fast frozen by liquid nitrogen and kept at −80 °C for later assessment. The amount of VEGF released into the supernatant at each time point was measured by ELISA.
2.7. Bioactivity of Released VEGF
The bioactivity of VEGF released from the microspheres was evaluated by determining the proliferative capacity of HUVECs after VEGF treatment in an in vitro system. HUVECs were cultured in T-25 cell culture flasks (Falcon, BD, Franklin Lakes, NJ). The culture medium, endothelial cell growth medium-2, is a proprietary basal media supplemented with the GFs, like human recombinant epidermal growth factor, human basic fibroblast growth factor, VEGF, ascorbic acid, hydrocortisone, human recombinant insulin-like growth factor, heparin, 5% fetal bovine serum, gentamicin, and amphotericin. The HUVECs were incubated at 37 °C in a 5% CO2/95% air, 95% humidity incubator. Culture media were changed every other day and the cells were subcultured to 80% confluency. Cells at passage 2 – 6 were used in this bioassay study.
To observe the best conditions for testing the biofunction of VEGF, including cell numbers, culture medium and culture duration, HUVECs proliferation assay was carried out under various conditions. Different number of cells added to each well of the 96-well plate of the non-radioactive cell proliferation assay kit was 1000, 3000 and 5000, respectively. To determine the culture medium, 3000/well HUVECs were cultured for two days in base medium plus all the supplement components including serum and growth factors (denoted as “full medium”) and in base medium plus serum only (denoted as “GF- medium”). To determine the optimal culture duration, 3000/well HUVECs were cultured in GF- medium for 1, 2 or 3 days. After the pilot study, GF- medium, 3000 cells/well and culturing for 2 days were determined and bioassay of VEGF effect on HUVECs was carried on using this combination of culture conditions. Briefly, a basal medium just supplemented with 5% fetal bovine serum by excluding the supplied GFs from the medium was used. HUVECs were suspended in this medium and plated into tissue culture-grade 96-well plates at a density of 3000 cells/50 µL/well. After cell attachment, 50 µL of supernatant from each time point was added to the wells. Same volume of fresh VEGF aliquoted into the corresponding concentrations of released VEGF as determined by ELISA was added to the wells as positive control, while same volume of PBS was added into the wells that served as negative control. The supernatants collected at each time point up to day 28 were used for the assay. They were sterilized by membrane filtration (0.2 µm) before they were added to the wells. The plate stayed in the incubator for 2 days followed by the addition of 20 µL MTS reagent to each well. After additional incubation for 2 hours, optical density (OD) values of the wells were read at 490nm wavelength. This assay was done in 8 repeats.
2.8. Implantation of Nerve Conduits Supplemented with VEGF-containing Microspheres
In-house developed PCLF conduits [17] were used. The conduits had an inner diameter of 1.6 mm and a length of 12 mm. Growth factor reduced basement membrane matrix Matrigel (BD MatrigelTM, Sparks, MD) was used to suspend the microspheres. 1 mg of PLGA microspheres containing VEGF (0.1µg/mg) were evenly distributed into Matrigel that was pre-diluted 1:3 with PBS. After Matrigel cured inside the conduit, a 1 mm space formed at both ends of the conduit. These conduits were then used to repair a 1 cm defect of the sciatic nerve in Lewis rats (n=8). The animal study was approved by the Institutional Animal Care and Use Committee and guidelines for care and use of laboratory animals were followed. The left sciatic nerve was exposed via a posterior thigh approach. A 5 mm segment of the nerve was removed at the mid-thigh level. With nerve retraction, this created a 1 cm gap between the nerve ends. The conduit with Matrigel suspended microspheres was placed between the two nerve ends, which were sutured 1 mm into the open ends of the conduit with 9–0 nylon sutures. Nerve conduits containing blank microspheres were implanted in the same fashion and served as controls (n=6).
2.9 Evaluations of Postoperative Nerve Regeneration
Nerve conduction study was done before sacrifice at 20 weeks postoperatively with a Nicolet Viking IV (Viasys Healthcare, Inc., Conshohocken, PA). Compound muscle action potentials (CMAPs) in the tibial and peroneal nerve-innervated foot muscles of the left limb were recorded. The nerve conduits were then harvested and fixed in TRUMPs fixative (2% paraformaldehyde and 1% glutaraldehyde in PBS). Mid-portion of the nerve conduits were processed and embedded in SPUR as previously described [17]. 1 µm thick sections were obtained and stained with 1% toluidine blue. The number of vessels in each cross-section was counted. The gastrocnemius muscle and tibialis anterior muscle from both hind limbs were collected for wet weight and cross-sectional area. After being weighed on an electronic balance, the muscles were fixed in 10% formalin, processed and embedded in paraffin. 4 µm thick sections were obtained from mid-portion of the muscle belly and stained with Haematoxylin & Eosin. The entire cross-sectional area of the muscle tissue was measured using IH Cscore (Bacus labs,Inc., North Lombard, IL). Muscle weights and cross-sectional areas were expressed as the percentage of the left side to the non-operated right side.
2.10. Statistical Analysis
The data were represented as mean ± SD. One-way analysis of variance (ANOVA) tests were used in this study. Tukey’s post-hoc tests were used to perform multiple comparisons. Statistical significance was defined as p< 0.05. Rank sum test was performed to analyze the tibial and peroneal nerve function combining CMAP amplitude, muscle weight and cross-sectional area using the method of O’Brien and Shampo for combining multiple endpoints [18].
3. RESULTS
3.1. Microsphere Size and Encapsulation Efficiency
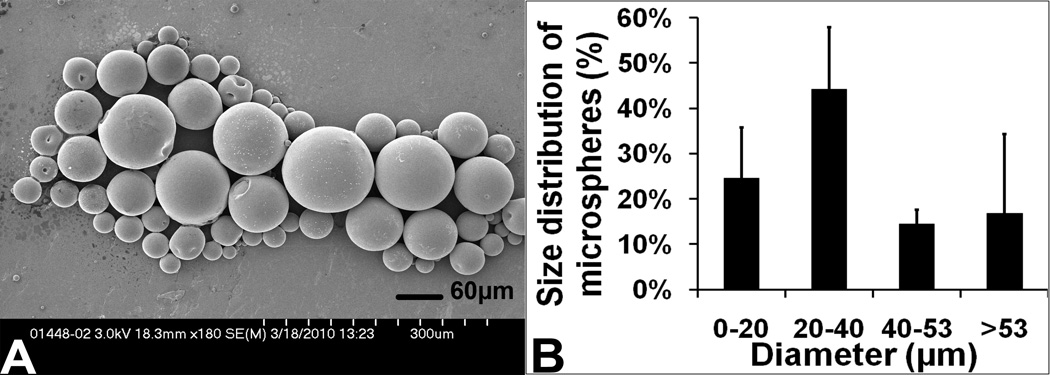
The double emulsion-solvent evaporation technique resulted in VEGF-PLGA microspheres with spherical morphology (Figure 1A). The diameters of the microspheres ranged from 2.1 µm to 205.9 µm (35.3±22.8 µm). About 83.16% of the microspheres had a diameter < 53 µm (Figure 1B). When no extra amount of BSA was added during the process of fabricating VEGF-containing PLGA microspheres, the content of encapsulated VEGF determined by ELISA was 0.94±0.11 µg. This corresponded to an encapsulation efficiency of 93.79±10.64%. When a 2000 fold concentration of BSA was added during the process, the encapsulation efficiency was 76.74% (S1).
Fig 1.
3.2. Microsphere Degradation
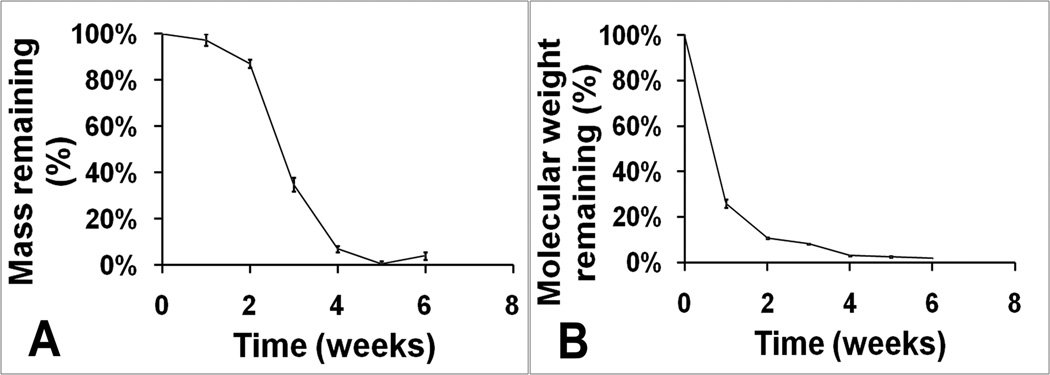
Figure 2A showed the mass loss profile of sieved PLGA microspheres during 6 weeks of degradation in PBS at 37°C. Initial weight loss was slow with ~90% remaining at 2 weeks. After the first 2 weeks, PLGA microsphere weight started to decrease rapidly with only ~35% and ~10% weight remaining after 3 and 4 weeks. At the end of the 5th week, degradation was almost complete.
Fig 2.
The molecular weight loss profile over time for PLGA microspheres is shown in Figure 2B. Unlike mass loss, the molecular weight decreased immediately after placement in PBS. The most significant molecular weight loss was observed for the initial 2 weeks followed by a slower decrease up to the end of week 6.
3.3. Release Kinetics
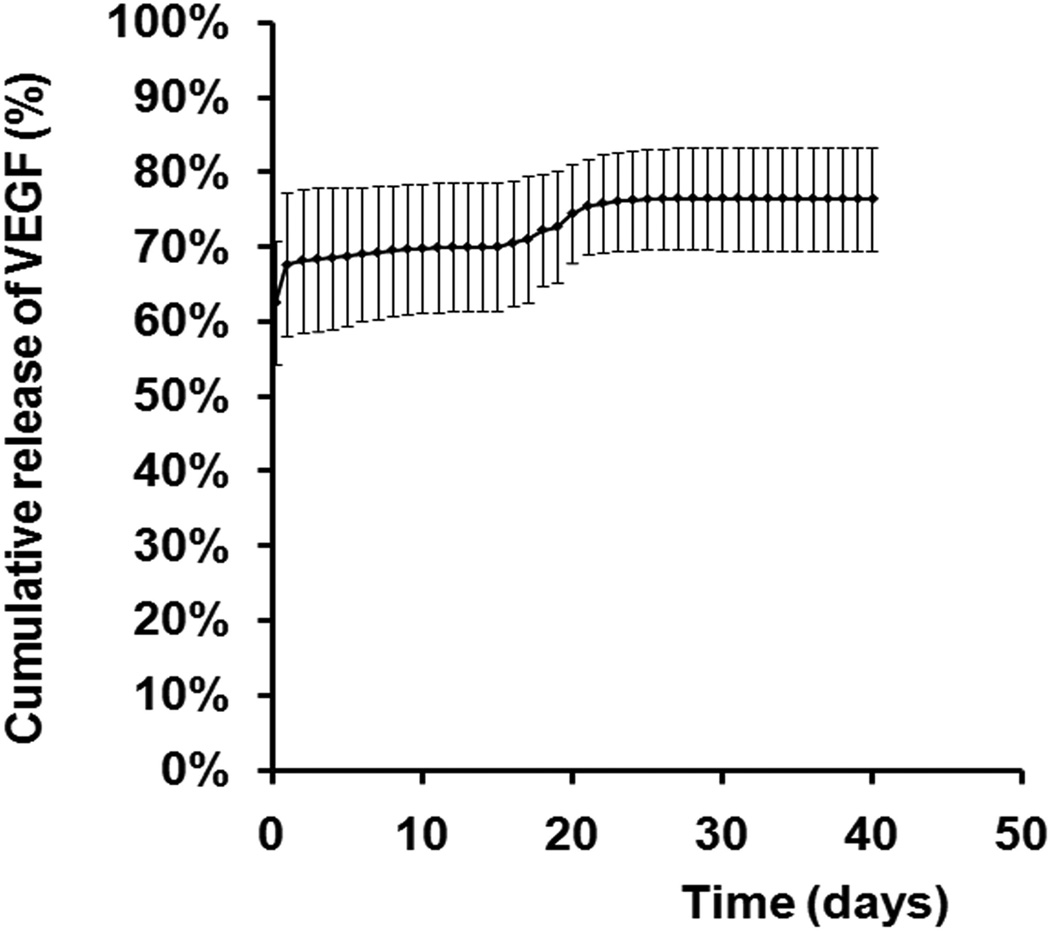
The in vitro release kinetics was quantified by incubating VEGF-containing microspheres in PBS at 37°C for 40 days. The first burst release of VEGF was seen within the first 24 hours which totaled 67.6 ± 8.25% of the entire amount of VEGF encapsulated into the microspheres. This was followed by a phase of sustained release of approximately 0.34% per day for 4 weeks, followed by a second burst release at day 18 to 21 at a rate of approximately 1.11% per day. The cumulative release of VEGF at the end of the assay was 76.36%. (Figure 3)
Fig 3.
3.4. Bioactivity of Released VEGF
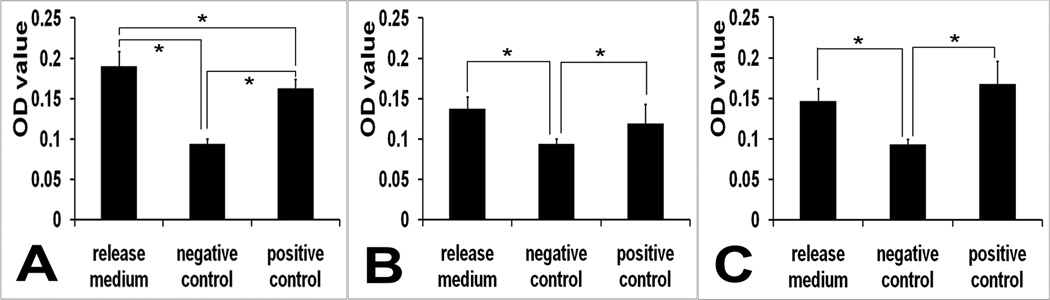
The bioactivity of VEGF released from the microspheres over time was assessed using an in vitro HUVECs proliferation assay. In the pilot study, dose-proliferation response was most obvious when the cell number was 3000/well. The GF- medium curve showed a positive dose response. HUVEC proliferation was more robust when cultured for 1 or 2 days. The 2 day curve seemed to show the most optimal dose-growth response (S2). Therefore GF- medium, 3000 cells/well and culturing for 2 days were determined as the optimal combination for the bioassay. Supernatant collected from the first 24 hours where the first and major burst release occurred was diluted to under 100 ng/mL. Supernatant collected at other time points were within physiological concentrations and were used directly in the assay. VEGF released at all time points was confirmed to be bioactive except day 25 and day 28. HUVEC proliferation was enhanced by the release medium when compared to that of the negative control, and was comparable to that of the positive control. Data from 6 hours, day 12 and day 24 are shown in Figure 4. The 6 hours release medium’s VEGF concentration was 2347.5 ng/mL and diluted to 94 ng/mL, that of day 12 was 2 ng/mL, and that of day 24 was 6.8 ng/mL. VEGF of the same concentration as the release medium for each time point was aliquoted from fresh VEGF stock solution to serve as the positive control. The cells exposed to the release medium showed an increased rate of proliferation as indicated by an increased number of cells per well represented by a higher OD value (p<0.05).
Fig 4.
3.5. Nerve Regeneration across Conduits Supplemented with VEGF-containing Microspheres
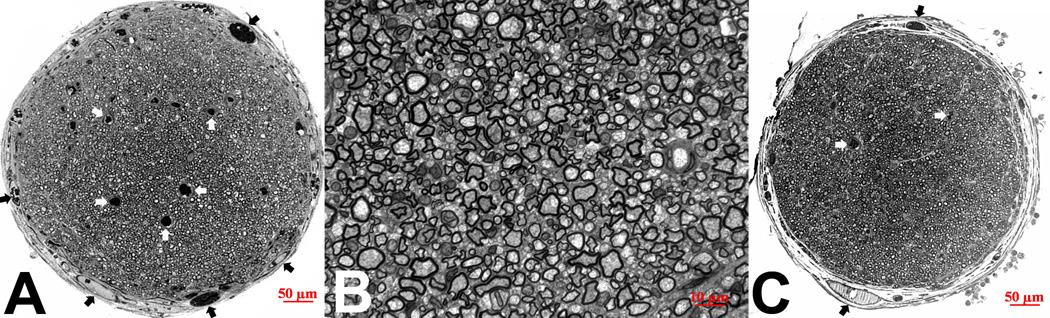
PCLF nerve conduits containing VEGF loaded microspheres suspended in matrigel within the conduit lumen were implanted in the 1cm rat sciatic nerve defect model. Robust nerve regeneration across the segmental defect was observed morphologically. Cross sections at the mid-tube level of the nerve conduits demonstrated a single large and round nerve fascicle surrounded by a thin layer of perineurium (Figure 5A). It contained numerous regenerated nerve fibers and several capillaries. Higher magnification of the cross-sections revealed that these nerve fibers were densely populated and well myelinated (Figure 5B). The average number of vessels in the VEGF treated group (n=8; average 34.8 vessels/per profile) was higher than the controls (n=6, average 24.5 vessels/profile) (Figure 5). Although statistically the difference was not significant, the trend of increase in vascularity was clear (34.8/profile vs 24.5/profile).
Fig 5.
Tibial nerve function (Table 1), as evaluated by combining tibial CMAP amplitude, gastrocnemius muscle weight and cross-sectional area, was significantly better in VEGF-containing microsphere group than in the control group (p=0.01). The difference of peroneal nerve function, as evaluated by combining peroneal CMAP amplitude, tibialis anterior muscle weight and cross-sectional area, was not statistically significant between the VEGF-microsphere group and the control group (p=0.51).
Table 1.
Comparison of Tibial Functions between VEGF-microsphere Group and Control Group
| Group | CMAP amplitude (mV) |
Gastrocnemius muscle weight (%) |
Muscle cross- sectional area (%) |
|---|---|---|---|
| VEGF group (n=8) | 3.39±1.07 | 60.5±6.3 | 59.6±8.0 |
| Control group (n=6) | 2.73±2.01 | 56.5±6.0 | 49.2±11.0 |
4. DISCUSSION
Angiogenesis and vascular remodeling play important roles in nerve regeneration. This is especially true in the presence of an ischemic environment such as a long nerve graft in a fibrotic nerve bed or when a non-permeable nerve conduit is used. Penkert et al [19] found that revascularization from the extraneural vessels alone begins on the third postoperative day. Longitudinal revascularization into the graft by way of endoneural vessels developed more slowly, reaching the middle part of the graft at 6 to 15 days and requiring 24 days for complete revascularization. Our goal is to establish a novel system to stimulate vascularization during this critical period of nerve revascularization and regeneration, which can be used to condition nerve conduits when extraneural revascularization is depleted and endoneural revascularization is compromised.
VEGF was chosen as the bioactive factor due to its strong angiogenic effect. VEGF increases microvascular permeability, stimulates the proliferation and migration of endothelial cells, and promotes angiogenesis. VEGF also has direct effects on neurons and glial cells, including inhibition of apoptosis, promotion of survival and stimulation of neurogenesis. Other neuroprotective and neurotrophic activities of VEGF include stimulation of proliferation of astrocytes, Schwann cells, microglia and cortical neurons.
Among the several different VEGF isoforms, VEGF121 is the smallest form. It lacks the residues encoded by exon 6 and exon 7, and can diffuse freely without binding to heparin [20]. Other longer forms bind to heparin with increasing affinity. VEGF165 appears to be the most abundant and potent isoform, followed by VEGF121 [21]. Although VEGF affinity is highest for binding to the VEGFR-1 receptor, VEGFR-2 appears to be the primary mediator of VEGF angiogenic activity [21, 22]. Both VEGF121 and VEGF165 can bind to VEGFR-1 and VEGFR-2, and hence execute their biological effects. Although VEGF121 is the second most potent isoform, it was chosen in our study because of its ability to diffuse rapidly and its heparin-independence. This allows easier tailoring of encapsulation and release in the delivery system that we designed. Since VEGF has a short half-life (<90 min) in the body, a carrier is needed to release VEGF in a controlled fashion in order to have a sustained effect of VEGF.
PLGA is biocompatible and degrades by hydrolytic cleavage into nontoxic molecules (lactic acid and glycolic acid) that are easily eliminated from the body. It is approved for human use by the Food and Drug Administration and has been extensively investigated for sustained and controlled delivery of therapeutic agents aimed at local therapy [23–31] . A previous study [20] employing a similar microsphere fabrication approach showed that NGF can be encapsulated and released consistently both in vitro and in vivo by the PLGA microsphere delivery system. PLGA with different lactic to glycolic acid ratios and different inherent viscosities was used and the results showed encapsulation efficiency was highest (74.6%) for a PLGA ratio of 50:50 1A, with an inherent viscosity of 0.1 dL g−1. 79–98% of all spheres had a diameter of 59 μm or smaller. This microsphere size distribution was comparable to our results. The encapsulation efficiency of our study however was much higher, at 93.8% for PLGA 50:50 4A microspheres comparing to the just over 40% encapsulation efficiency of PLGA microspheres with the same composition. This might be attributed to the lower molecular weight of the VEGF isoform we chose. Also, instead of radiolabelling the therapeutic agent with iodine-125 and quantifying encapsulation and release by measuring the radioactivity using a gamma counter, we employed ELISA which is a more accurate evaluation.
Studies have shown that by modifying formulation parameters and fabrication methods, the release of the therapeutic agent from PLGA devices can be altered [23, 32]. PLGA 50:50 4A degrades between 3 and 4 weeks. This rate of degradation meets the requirement of sustained delivery of VEGF for 4 weeks, especially for the initial 2 weeks that is critical for revascularization in nerve regeneration. Several groups [29–31, 33–36] have reported loading VEGF into PLGA microspheres with various levels of success.
The most commonly reported method for manufacturing microspheres is the double-emulsion technique. This technique yields microspheres of various sizes. The average diameters ranged from 0.4 µm to 48 µm [26, 29–31, 33, 34]. It has been shown that vortex speed affects the size of the microspheres and encapsulation efficiency [37]. We tried different vortex speeds and found that speeds higher than 3100 rpm led to smaller PLGA microspheres with 93.8% encapsulation efficiency. This encapsulation efficiency is higher than the 5.3% to 83.8% encapsulation efficiency reported in the literature [29–31, 33]. The diameters of the microspheres yielded from our fabrication ranged from 2.1 µm to 205.9 µm (35.3±22.8 µm). About 83.16% of the microspheres had a diameter < 53 µm. In order to obtain only the smaller microspheres while still maintaining reasonable encapsulation efficiency, we used a 53µm grid to sieve the microspheres. This also eliminated clumps of microspheres that could potentially be a physical obstacle when placed in a nerve conduit. Sieved, smaller microspheres would have faster degradation due to the larger overall surface area of a given weight.
Another important consideration during the microsphere fabrication process is to maintain the bioactivity of VEGF. King [38] and Iwata [39] observed inactivation of protein using the double-emulsion technique. In this process, the protein dissolved in solution would emulsify in an organic solvent containing the dissolved PLGA. This can cause the protein to be denatured by the organic solvent. They therefore added 2,000 and 10,000 fold amounts of BSA to the target protein in the fabrication process to protect the agent. In a pilot study, we measured the bioactivity of released VEGF with and without a 2000 fold amount of BSA added during microsphere fabrication and found that VEGF retained its bioactivity without the addition of large amount of BSA. We attribute this to BSA that was already contained as a carrier protein for VEGF that is provided by the manufacturer and to BSA that was added to the VEGF stock solution (60 fold the VEGF amount). We also noticed that the addition of a large quantity of BSA lowered the encapsulation efficiency.
The degradation of PLGA involves chain scissions of ester bond linkages in the polymer backbone by hydrolysis [40]. At the initial 2 weeks of the degradation process, most of the long chains have been hydrolyzed into smaller molecular lengths. After that, the oligomers are hydrolyzed into lactic and glycolic acid. This explains the observation that molecular weight loss happened earlier than mass loss that became obvious after 2 weeks. Our results also showed that only 7% mass and 3% molecular weight were retained at the end of 4 weeks, which concurred with the degradation profile of PLGA 50:50 4A.
Release kinetics of any given agent is related to three important processes: degradation of the polymer, diffusion through the polymer and the dissolution of the dispersed agent [24]. In the early phases, the release of the agent takes place mainly through its diffusion while in the later phases, the release is mediated through both diffusion of the agent and degradation of the polymer. This is compatible with our findings from the release kinetics study. The first burst release occurred at the initial 24 hours due to the diffusion of VEGF molecules that were loosely attached to the surface of the microspheres. The second burst release occurred around 3 weeks because of the final degradation of the polymer, which coincided with the degradation timeline.
Our results also demonstrated that VEGF encapsulated in PLGA microspheres was released in a controlled manner with preserved bioactivity up to 4 weeks. The HUVEC proliferation assay has been used in many studies to assess the bioactivity of VEGF [34, 38, 41, 42] . At the end of HUVECs culture, MTS colorimetric assay was applied. MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) is bioreduced by cells into a formazan product that is soluble in tissue culture medium. The conversion of MTS into aqueous, soluble formazan is accomplished by dehydrogenase enzymes found in metabolically active cells. The quantity of formazan product as measured by the amount of 490nm absorbance is directly proportional to the number of living cells in culture and thus reflects cell proliferation. Although MTS could be a marker of increased cell size as well, we chose MTS assay because in the relevant literature this is a very common and well-accepted assay to test the bioactivity of VEGF exerted on HUVECs. It is critical to use appropriate controls for results to be valid. Prior to the experiment, we did a pilot study to investigate the sensitivity and validity of this assay. This screening identified the combination of 3000 cells/well, base medium plus serum and 2 day assay duration to be the optimal conditions for the assay. We carefully controlled and calibrated the assay which then confirmed that the released VEGF was bioactive and functional in that it enhanced HUVECs proliferation equivalent to fresh VEGF of the same concentration.
Due to the high encapsulation efficiency and steady release profile, only 1 mg of PLGA microspheres were needed in the nerve conduit lumen of 1.6 mm inner diameter and 10 mm length to achieve the 5–10 ng/mL VEGF biological concentration. Based on the density of PLGA (1.25g/mL), this amount of PLGA microspheres will translate into 0.8 µL of volume. This is about 1/25 of the volume of nerve conduit lumen (20 µL). In addition, these microspheres were sieved to get rid of clumps and facilitate even distribution/suspension in Matrigel. These allowed regenerating nerve fibers to grow across the gap without obstacles. Our preliminary in vivo data showed supplementing nerve conduits with VEGF microspheres enhanced angiogenesis and subsequently tibial nerve functional recovery, a component of the sciatic nerve whose regeneration usually precedes that of the peroneal component.
5. CONCLUSIONS
In this study, we established PLGA microspheres as a novel system for controlled release of VEGF. This system had high encapsulation efficiency. Sustained release of VEGF for 4 weeks could be achieved with this carrier. VEGF retained its bioactivity through the 4-week time period. Importantly, when the VEGF-containing microspheres were placed in a synthetic (PCLF) nerve scaffold, robust angiogenesis and nerve regeneration was observed. Our future experiments will focus on in vivo studies of comparing delivering VEGF using this system and other methods and their effects on peripheral nerve regeneration.
Supplementary Material
Acknowledgments
This research was sponsored by the Armed Forces Institute of Regenerative Medicine award number W81XWH-08-2-0034. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. The content of the manuscript does not necessarily reflect the position or the policy of the Government, and no official endorsement should be inferred.
Jing Rui, MD, is supported by the Sundt Fellowship fund, Department of Neurological Surgery, Mayo Clinic.
M. Brett Runge is supported by NIH training grant 1T32AR056950-01 and the NIH Loan Repayment Program.
The authors thank Zvonimir S Katusic, M.D., Ph.D. for providing HUVECs, Jewel Podratz, Dr. Bret Ball and Shuya Zhang for technical assistance in ELISA, cell culture and muscle histology, respectively.
References
- 1.Nilsson A, Dahlin L, Lundborg G, Kanje M. Graft repair of a peripheral nerve without the sacrifice of a healthy donor nerve by the use of acutely dissociated autologous Schwann cells. Scand J Plast Reconstr Surg Hand Surg. 2005;39:1–6. doi: 10.1080/02844310410017979. [DOI] [PubMed] [Google Scholar]
- 2.Yla-Herttuala S, Rissanen TT, Vajanto I, Hartikainen J. Vascular endothelial growth factors: biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol. 2007;49:1015–1026. doi: 10.1016/j.jacc.2006.09.053. [DOI] [PubMed] [Google Scholar]
- 3.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 4.Ng YS, D'Amore PA. Therapeutic angiogenesis for cardiovascular disease. Curr Control Trials Cardiovasc Med. 2001;2:278–285. doi: 10.1186/cvm-2-6-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendel RC, Henry TD, Rocha-Singh K, Isner JM, Kereiakes DJ, Giordano FJ, et al. Effect of intracoronary recombinant human vascular endothelial growth factor on myocardial perfusion: evidence for a dose-dependent effect. Circulation. 2000;101:118–121. doi: 10.1161/01.cir.101.2.118. [DOI] [PubMed] [Google Scholar]
- 6.Hedman M, Hartikainen J, Syvanne M, Stjernvall J, Hedman A, Kivela A, et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT) Circulation. 2003;107:2677–2683. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- 7.Makinen K, Manninen H, Hedman M, Matsi P, Mussalo H, Alhava E, et al. Increased vascularity detected by digital subtraction angiography after VEGF gene transfer to human lower limb artery: a randomized, placebo-controlled, double-blinded phase II study. Mol Ther. 2002;6:127–133. doi: 10.1006/mthe.2002.0638. [DOI] [PubMed] [Google Scholar]
- 8.Rissanen TT, Markkanen JE, Arve K, Rutanen J, Kettunen MI, Vajanto I, et al. Fibroblast growth factor 4 induces vascular permeability, angiogenesis and arteriogenesis in a rabbit hindlimb ischemia model. FASEB J. 2003;17:100–102. doi: 10.1096/fj.02-0377fje. [DOI] [PubMed] [Google Scholar]
- 9.Simons M, Annex BH, Laham RJ, Kleiman N, Henry T, Dauerman H, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation. 2002;105:788–793. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- 10.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 11.Rajagopalan S, Mohler ER, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, et al. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108:1933–1938. doi: 10.1161/01.CIR.0000093398.16124.29. [DOI] [PubMed] [Google Scholar]
- 12.Kastrup J, Jorgensen E, Ruck A, Tagil K, Glogar D, Ruzyllo W, et al. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris A randomized double-blind placebo-controlled study: the Euroinject One trial. J Am Coll Cardiol. 2005;45:982–988. doi: 10.1016/j.jacc.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 13.Tria MA, Fusco M, Vantini G, Mariot R. Pharmacokinetics of nerve growth factor (NGF) following different routes of administration to adult rats. Exp Neurol. 1994;127:178–183. doi: 10.1006/exnr.1994.1093. [DOI] [PubMed] [Google Scholar]
- 14.Pfister LA, Papaloizos M, Merkle HP, Gander B. Nerve conduits and growth factor delivery in peripheral nerve repair. J Peripher Nerv Syst. 2007;12:65–82. doi: 10.1111/j.1529-8027.2007.00125.x. [DOI] [PubMed] [Google Scholar]
- 15.Quaglia F. Bioinspired tissue engineering: the great promise of protein delivery technologies. Int J Pharm. 2008;364:281–297. doi: 10.1016/j.ijpharm.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 16.Kempen DH, Lu L, Zhu X, Kim C, Jabbari E, Dhert WJ, et al. Development of biodegradable poly(propylene fumarate)/poly(lactic-co-glycolic acid) blend microspheres. II. Controlled drug release and microsphere degradation. J Biomed Mater Res A. 2004;70:293–302. doi: 10.1002/jbm.a.30080. [DOI] [PubMed] [Google Scholar]
- 17.Wang S, Yaszemski MJ, Knight AM, Gruetzmacher JA, Windebank AJ, Lu L. Photo-crosslinked poly(epsilon-caprolactone fumarate) networks for guided peripheral nerve regeneration: material properties and preliminary biological evaluations. Acta Biomater. 2009;5:1531–1542. doi: 10.1016/j.actbio.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Brien PC, Shampo MA. Statistical considerations for performing multiple tests in a single experiment. 5. Comparing two therapies with respect to several endpoints. Mayo Clin Proc. 1988;63:1140–1143. doi: 10.1016/s0025-6196(12)65511-6. [DOI] [PubMed] [Google Scholar]
- 19.Penkert G, Bini W, Samii M. Revascularization of nerve grafts: an experimental study. J Reconstr Microsurg. 1988;4:319–325. doi: 10.1055/s-2007-1006938. [DOI] [PubMed] [Google Scholar]
- 20.Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992;267:26031–26037. [PubMed] [Google Scholar]
- 21.Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001;114:853–865. doi: 10.1242/jcs.114.5.853. [DOI] [PubMed] [Google Scholar]
- 22.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- 23.de Boer R, Knight AM, Spinner RJ, Malessy MJ, Yaszemski MJ, Windebank AJ. In vitro and in vivo release of nerve growth factor from biodegradable poly-lactic-co-glycolic-acid microspheres. J Biomed Mater Res A. 2010;95:1067–1073. doi: 10.1002/jbm.a.32900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JM, Seo KS, Jeong YK, Hai BL, Kim YS, Khang G. Co-effect of aqueous solubility of drugs and glycolide monomer on in vitro release rates from poly(D,L-lactide-co-glycolide) discs and polymer degradation. J Biomater Sci Polym Ed. 2005;16:991–1007. doi: 10.1163/1568562054414676. [DOI] [PubMed] [Google Scholar]
- 25.Birnbaum DT, Brannon-Peppas L. Molecular weight distribution changes during degradation and release of PLGA nanoparticles containing epirubicin HCl. J Biomater Sci Polym Ed. 2003;14:87–102. doi: 10.1163/15685620360511155. [DOI] [PubMed] [Google Scholar]
- 26.Ayalasomayajula SP, Kompella UB. Subconjunctivally administered celecoxib-PLGA microparticles sustain retinal drug levels and alleviate diabetes-induced oxidative stress in a rat model. Eur J Pharmacol. 2005;511:191–198. doi: 10.1016/j.ejphar.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Elcin AE, Elcin YM. Localized angiogenesis induced by human vascular endothelial growth factor-activated PLGA sponge. Tissue Eng. 2006;12:959–968. doi: 10.1089/ten.2006.12.959. [DOI] [PubMed] [Google Scholar]
- 28.Lim TY, Poh CK, Wang W. Poly (lactic-co-glycolic acid) as a controlled release delivery device. J Mater Sci Mater Med. 2009;20:1669–1675. doi: 10.1007/s10856-009-3727-z. [DOI] [PubMed] [Google Scholar]
- 29.Formiga FR, Pelacho B, Garbayo E, Abizanda G, Gavira JJ, Simon-Yarza T, et al. Sustained release of VEGF through PLGA microparticles improves vasculogenesis and tissue remodeling in an acute myocardial ischemia-reperfusion model. J Control Release. 2010;147:30–37. doi: 10.1016/j.jconrel.2010.07.097. [DOI] [PubMed] [Google Scholar]
- 30.Golub JS, Kim YT, Duvall CL, Bellamkonda RV, Gupta D, Lin AS, et al. Sustained VEGF delivery via PLGA nanoparticles promotes vascular growth. Am J Physiol Heart Circ Physiol. 2010;298:H1959–H1965. doi: 10.1152/ajpheart.00199.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borselli C, Ungaro F, Oliviero O, d'Angelo I, Quaglia F, La Rotonda MI, et al. Bioactivation of collagen matrices through sustained VEGF release from PLGA microspheres. J Biomed Mater Res A. 2010;92:94–102. doi: 10.1002/jbm.a.32332. [DOI] [PubMed] [Google Scholar]
- 32.Cao X, Schoichet MS. Delivering neuroactive molecules from biodegradable microspheres for application in central nervous system disorders. Biomaterials. 1999;20:329–339. doi: 10.1016/s0142-9612(98)00172-0. [DOI] [PubMed] [Google Scholar]
- 33.Norton LW, Tegnell E, Toporek SS, Reichert WM. In vitro characterization of vascular endothelial growth factor and dexamethasone releasing hydrogels for implantable probe coatings. Biomaterials. 2005;26:3285–3297. doi: 10.1016/j.biomaterials.2004.07.069. [DOI] [PubMed] [Google Scholar]
- 34.Faranesh AZ, Nastley MT, Perez de la Cruz C, Haller MF, Laquerriere P, Leong KW, et al. In vitro release of vascular endothelial growth factor from gadolinium-doped biodegradable microspheres. Magn Reson Med. 2004;51:1265–1271. doi: 10.1002/mrm.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J, Bhang SH, Park H, Kim BS, Lee KY. Active blood vessel formation in the ischemic hindlimb mouse model using a microsphere/hydrogel combination system. Pharm Res. 2010;27:767–774. doi: 10.1007/s11095-010-0067-0. [DOI] [PubMed] [Google Scholar]
- 36.Lee M, Chen TT, Iruela-Arispe ML, Wu BM, Dunn JC. Modulation of protein delivery from modular polymer scaffolds. Biomaterials. 2007;28:1862–1870. doi: 10.1016/j.biomaterials.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Kempen DH, Lu L, Zhu X, Kim C, Jabbari E, Dhert WJ, et al. Development of biodegradable poly(propylene fumarate)/poly(lactic-co-glycolic acid) blend microspheres. I. Preparation and characterization. J Biomed Mater Res A. 2004;70:283–292. doi: 10.1002/jbm.a.30079. [DOI] [PubMed] [Google Scholar]
- 38.King TW, Patrick CW., Jr Development and in vitro characterization of vascular endothelial growth factor (VEGF)-loaded poly(DL-lactic-co-glycolic acid)/poly(ethylene glycol) microspheres using a solid encapsulation/single emulsion/solvent extraction technique. J Biomed Mater Res. 2000;51:383–390. doi: 10.1002/1097-4636(20000905)51:3<383::aid-jbm12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 39.Iwata M, Nakamura Y, McGinity JW. Particle size and loading efficiency of poly(D,L-lactic-co-glycolic acid) multiphase microspheres containing water soluble substances prepared by the hydrous and anhydrous solvent evaporation methods. J Microencapsul. 1999;16:49–58. doi: 10.1080/026520499289301. [DOI] [PubMed] [Google Scholar]
- 40.Gopferich A. Mechanisms of polymer degradation and erosion. Biomaterials. 1996;17:103–114. doi: 10.1016/0142-9612(96)85755-3. [DOI] [PubMed] [Google Scholar]
- 41.Lee J, Lee KY. Local and sustained vascular endothelial growth factor delivery for angiogenesis using an injectable system. Pharm Res. 2009;26:1739–1744. doi: 10.1007/s11095-009-9884-4. [DOI] [PubMed] [Google Scholar]
- 42.Franklin SL, Ferry RJ, Jr, Cohen P. Rapid insulin-like growth factor (IGF)-independent effects of IGF binding protein-3 on endothelial cell survival. J Clin Endocrinol Metab. 2003;88:900–907. doi: 10.1210/jc.2002-020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.