Abstract
Lignans and phenylethanoid glycosides purified from Forsythia suspensa were reported to display various bioactivities in the previous literature, including the antimicrobial activity. Therefore, the present research is aimed to purify and identify the chemical constituents of the methanol extracts of fruits of F. suspensa. The methanol extracts of fruits of F. suspensa were fractionated and further purified with the assistance of column chromatography to afford totally thirty-four compounds. Among these isolates, 3β-acetoxy-20α-hydroxyursan-28-oic acid (1) was reported from the natural sources for the first time. Some of the purified principles were subjected to the antimicrobial activity examinations against Escherichia coli to explore new natural lead compounds.
1. Introduction
Food safety is an important public health issue continuously attracting researchers from various fields. The use of biopreservatives and pathogen antagonists had been completed as a means of protecting the microbiological safety of fresh and processed products [1–4]. Lignans and phenylethanoid glycosides are widely distributed among plant bioresources and those purified from Forsythia suspensa have already been reported to exhibit antimicrobial bioactivities in the previous literature [5–13]. Although these natural compounds did not exhibit better inhibition of the bacterial growth, they were not very toxic while compared with the synthetic antibiotics. F. suspensa (Oleaceae) is an important original plant of the crude drug “rengyo” (Forsythiae Fructus) which has been used for anti-inflammatory, diuretic, drainage, and antimicrobial purposes in Oriental medicine [6, 8]. Previous phytochemical investigations of Forsythia genus afforded a series of steroids, triterpenoids, lignans, and phenylethanoid glycosides [5–16]. In our continuous program aimed to the bioactive principles from natural sources, the fruits of F. suspensa were selected as the target due to their antimicrobial potential in our preliminary bioassay (Table 1). In the present study, we wished to report the structural characterization of one new triterpene, 3β-acetoxy-20α-hydroxyursan-28-oic acid (1), along with thirty-three known compounds, as well as their antimicrobial effects against E. coli. We hoped to explore new lead compounds which could be performed for further investigation of the new antibiotic agents.
Table 1.
The minimum inhibitory concentrations (MICs) of the crude extract and partial purified fractions of F. suspensa against E. coli (BCRC-11634).
| Sample | MIC (mg/mL) |
|---|---|
| FS (crude extracts) | 4.25 |
| FSC (chloroform fraction) | 6.25 |
| FSW (water fraction) | 12.50 |
2. Materials and Methods
2.1. General Procedure
Melting point was determined by a Fisher Scientific melting point measuring apparatus without corrections. The IR spectrum was obtained, as a KBr disc, on a Bruker Tensor 27 FT-IR spectrometer. Optical rotation was measured with an Atago AP-300 automatic polarimeter. 1H- and 13C-NMR, COSY, HMQC, HMBC, and NOESY spectra were recorded on the Varian Unity 400 and Bruker AV 500 NMR spectrometers, using tetramethylsilane (TMS) as the internal standard. Standard pulse sequences and parameters were used for the NMR experiments and all chemical shifts were reported in parts per million (ppm, δ). The low and high-resolution FAB mass spectra were obtained on a JEOL JMS-700 spectrometer operated in the positive-ion mode. All the chemicals were purchased from Merck KGaA (Darmstadt, Germany) unless specifically indicated. Column chromatography was performed on silica gels (Kieselgel 60, 70–230 mesh, Merck KGaA). Thin layer chromatography (TLC) was conducted on precoated Kieselgel 60 F 254 plates (Merck) and the compounds were visualized by UV light or spraying with 10% (v/v) H2SO4 followed by heating at 110°C for 10 min.
2.2. Plant Materials
The fruits of Forsythia suspensa were purchased from the herbal markets in Yunlin, Taiwan, and authenticated by Dr. C. S. Kuoh (Department of Bioscience, National Cheng Kung University, Tainan, Taiwan). A voucher specimen (PCKuo_2007001) was deposited in the herbarium of the Department of Biotechnology, National Formosa University, Yunlin, Taiwan.
2.3. Extraction and Isolation
The fruits of Forsythia suspensa (6.0 Kg) were powdered and refluxed with methanol (20 L × 7), and the combined extracts were concentrated under reduced pressure to give a brown syrup (1.4 Kg). The crude extract was suspended into water and partitioned with chloroform, successively to afford chloroform (450 g) and water soluble fractions (950 g), respectively.
The chloroform soluble extracts were purified by silica gel column chromatography (SiO2 CC) eluted with n-hexane and acetone gradients (100 : 1 to 1 : 1) to afford 8 fractions as monitored by TLC. Fractions 4, 5, and 8 display significant spots and therefore were subjected to the further purification. Fraction 4 was purified by SiO2 CC eluted with n-hexane/ethyl acetate (50 : 1) to yield three subfractions (F4.1~4.3). The subfraction F4.2 displayed significant spots and was applied to SiO2 CC, eluted with n-hexane and acetone gradients (100 : 1 to 1 : 1), to afford β-amyrin acetate (2) (10 mg) and taraxasterol acetate (3) (6 mg). The subfraction F4.3 was purified with SiO2 CC eluted with n-hexane and acetone gradients (300 : 1 to 1 : 1) to yield three minor fractions (F4.3.1~4.3.3). The minor fraction F4.3.1 was further applied to SiO2 CC with benzene : ethyl acetate (50 : 1) solvent system to afford 3β-acetyl-20,25-epoxy-dammarane-24α-ol (4) (25 mg). F4.3.2 was repeatedly subjected to SiO2 CC and preparative TLC (pTLC) (eluted with benzene : acetone, 20 : 1) to yield 3β-acetoxy-20α-hydroxyursan-28-oic acid (1) (10 mg). F4.3.3 was recrystallized with acetone to produce acetyl oleanolic acid (5) (20 mg). Fraction 5 was purified by SiO2 CC eluted with n-hexane/ethyl acetate (50 : 1) to yield ten subfractions (F5.1~5.10). Subfractions F5.5, 5.6, 5.8, and 5.10 were major fractions and displayed significant spots by TLC monitoring. F5.5 was further isolated by SiO2 CC with a mixed eluent of benzene and acetone (200 : 1) to afford 3β-acetyl-20,25-epoxy-dammarane-24α-ol (4) (20 mg). F5.6 was also subjected to SiO2 CC with a mixed eluent of benzene and acetone (200 : 1) and further recrystallization of the minor fractions with chloroform/methanol to yield betulinic acid (6) (30 mg) and labda-8(17),13E-dien-15,18-dioic acid 15-methyl ester (7) (5 mg), respectively. F5.8 was recrystallized with chloroform/methanol to produce mixture of β-sitosterol (8) and stigmasterol (9) (630 mg). F5.10 was repeatedly subjected to SiO2 CC and pTLC (eluted with benzene : acetone, 20 : 1) to yield ψ-taraxasterol (10) (8 mg).
Fraction 8 was subjected to SiO2 CC eluted with chloroform/methanol gradients (50 : 1 to 1 : 1) and monitored by TLC to afford five subfractions (F8.1~8.5). Subfraction F8.1 was further recrystallized with chloroform/methanol to yield betulinic acid (6) (2 mg). F8.2 was repeatedly subjected to SiO2 CC and pTLC (eluted with chloroform : methanol, 50 : 1) to afford ψ-taraxasterol (10) (2 mg) and 3β-hydroxyanticopalic acid (11) (12 mg), respectively. The subfraction F8.3 was purified with SiO2 CC eluted with chloroform and methanol gradients (50 : 1 to 1 : 1) to yield three minor fractions (F8.3.1~8.3.3). The minor fraction F8.3.2 was further applied to pTLC eluted with benzene/acetone (10 : 1) to yield agatholic acid (12) (9 mg). F8.3.3 was repeatedly subjected to SiO2 CC (eluted with chloroform/acetone, 50 : 1) and pTLC (eluted with benzene/acetone, 30 : 1) to yield 3,4-dimethoxybenzoic acid (13) (6 mg). Subfraction F8.4 was applied to SiO2 CC eluted with chloroform and methanol gradients (50 : 1 to 1 : 1) to yield four minor fractions (F8.4.1~8.4.4). The minor fractions F8.4.2 and 8.4.3 were major fractions and displayed significant spots by TLC monitoring. F8.4.2 was further repeatedly subjected to SiO2 CC and pTLC (eluted with n-hexane/acetone, 1 : 1) to yield vanillic acid (14) (12 mg) and syringic acid (15) (3 mg). F8.4.3 was further recrystallized with chloroform/methanol to yield phillyrin (16) (30 mg). Subfraction F8.5 was purified by SiO2 CC eluted with chloroform and methanol gradients (50 : 1 to 1 : 1) to yield three minor fractions (F8.5.1~8.5.3). The minor fraction F8.5.1 was further repeatedly subjected to SiO2 CC and pTLC (eluted with chloroform/ethyl acetate, 10 : 1) to afford p-hydroxyphenylacetic acid (17) (10 mg). F8.5.2 was isolated by pTLC eluted with chloroform/acetone (4 : 1) to produce p-hydroxybenzoic acid (18) (15 mg). F8.5.3 was further recrystallized with acetone to yield benzoic acid (19) (16 mg).
The water extracts were applied to a reversed-phase Diaion HP-20 column eluted with water and methanol gradients to afford six fractions as monitored by C-18 TLC; however, no constituents were identified from fractions 1–3. Fraction 4 (wF4) was subjected to SiO2 CC eluted with chloroform/methanol gradients (100 : 1 to 1 : 1) and monitored by TLC to afford five subfractions (wF4.1~4.5). The subfraction wF4.1 was purified with SiO2 CC eluted with chloroform and acetone gradients (100 : 1 to 1 : 1) to yield p-hydroxyphenylacetic acid methyl ester (20) (5 mg). Subfraction wF4.2 was applied to SiO2 CC eluted with chloroform and acetone gradients (200 : 1 to 1 : 1) to yield four minor fractions (wF4.2.1~wF4.2.4). The minor fraction wF4.2.1 was further recrystallized with chloroform/methanol to afford p-tyrosol (21) (10 mg). The minor fractions wF4.2.2 and wF4.2.3 were further repeatedly subjected to SiO2 CC and pTLC (eluted with chloroform/methanol, 30 : 1) to afford p-hydroxybenzoic acid (18) (5 mg) and p-hydroxyphenylacetic acid (17) (4 mg), respectively. The minor fraction wF4.2.4 was subjected to SiO2 CC and further purified by pTLC (eluted with chloroform/methanol, 20 : 1) to yield hydroxytyrosol (22) (3 mg). Subfraction wF4.4 was subjected to SiO2 CC eluted with chloroform and acetone gradients (100 : 1 to 1 : 1) to yield five minor fractions (wF4.4.1~wF4.4.5). The minor fractions wF4.4.2, wF4.4.4, and wF4.4.5 displayed significant spots and were applied to SiO2 CC, eluted with chloroform/methanol (10 : 1) to afford 2-furancarboxylic acid (23) (15 mg), salidroside (24) (18 mg), and (6S,9R)-roseoside (25) (10 mg), respectively. Subfraction wF4.5 was repeatedly subjected to SiO2 CC (eluted with chloroform/methanol, 10 : 1) and further recrystallization of the minor fractions with chloroform/methanol to result in forsythoside D (26) (8 mg), methyl-α-D-glucopyranoside (27) (10 mg), and adoxosidic acid (28) (15 mg), respectively.
Fraction 5 (wF5) was subjected to SiO2 CC eluted with chloroform/methanol gradients (200 : 1 to 1 : 1) and monitored by TLC to afford five subfractions (wF5.1~5.5). Subfractions wF5.1, wF5.3, and wF5.4 displayed significant spots and therefore were subjected to the further purification. Subfraction wF5.1 was repeatedly subjected to SiO2 CC (eluted with chloroform/acetone, 300 : 1 to 1 : 1) and further recrystallized with chloroform/methanol to result in p-hydroxyphenylacetic acid methyl ester (20) (3 mg). Subfraction wF5.3 was applied to SiO2 CC (eluted with chloroform/acetone, 300 : 1 to 1 : 1) and further recrystallized with chloroform/methanol to yield p-hydroxyphenylacetic acid (17) (5 mg) and protocatechualdehyde (29) (5 mg). Subfraction wF5.4 was repeatedly purified by SiO2 CC (eluted with chloroform/acetone, 200 : 1 to 1 : 1) and further recrystallization of the minor fractions with chloroform/methanol to yield esculetin (30) (3 mg) and caffeic acid (31) (12 mg), respectively. Fraction 6 (wF6) was isolated by SiO2 CC eluted with chloroform/methanol gradients (100 : 1 to 1 : 1) and monitored by TLC to result in five subfractions (wF6.1~6.5). Only subfractions wF6.2 and wF6.3 displayed significant spots and therefore were subjected to the further purification. Subfraction wF6.2 was repeatedly purified by SiO2 CC (eluted with chloroform/acetone, 200 : 1 to 1 : 1) and further recrystallization of the minor fractions with acetone to yield trans-coumaric acid (32) (5 mg) and trans-ferulic acid (33) (5 mg). Subfraction wF6.3 was further recrystallized with acetone to result in quercetin (34) (45 mg).
2.3.1. Spectral Data of 1
White powder (CHCl3), mp 238–245°C; [α]D 25−118.0 (c 0.09, CHCl3); IR (Neat) ν max: 3442, 2948, 1760, 1727, 1444, 1375, 1250 cm−1; 1H NMR (CDCl3, 400 MHz) δ 0.83 (15H, m, CH3-23, 24, 25, 27, 29), 0.94 (3H, s, CH3-26), 1.35 (3H, s, CH3-30), 2.05 (3H, s, CH3-32), 2.10 (1H, m, H-15), 2.60 (2H, m, H-16), 4.48 (1H, dd, J = 10.4, 5.6 Hz, H-3α); 13C NMR (CDCl3, 125 MHz) δ 15.5 (C-26), 16.2 (C-23, 24, 25), 16.5 (C-27), 18.1 (C-6), 21.3 (C-32), 21.4 (C-11), 23.7 (C-2), 25.0 (C-12), 25.4 (C-30), 26.8 (C-22), 28.0 (C-29), 29.2 (C-16), 31.2 (C-15, 21), 35.1 (C-7), 37.1 (C-10), 37.9 (C-4), 38.7 (C-1), 40.4 (C-8), 43.2 (C-14, 18), 49.4 (C-13), 50.2 (C-17), 50.5 (C-9), 55.9 (C-5), 80.9 (C-3), 90.1 (C-20), 171.0 (C-31), 176.8 (C-28); FAB-MS m/z (rel. int.) 517 ([M+H]+, 100); HR-FAB-MS m/z 517.3896 [M+H]+ (calcd for C32H53O5, 517.3893).
2.4. Antimicrobial Activity
2.4.1. Microorganisms
The antimicrobial activity was evaluated against Escherichia coli (BCRC-11634). The strains were kept at −70°C in Luria-Bertani agar (LBA), activated by transferring into nutritive agar and incubating at 37 ± 1.0°C for 18 h. The bacterial suspension of each strain was prepared in a sterile tube with glass pearls and turbidity adjusted with distillated water, according to McFarland scale number 1 tube, which corresponds to approximately 3 × 108 CFU/mL [13].
2.4.2. Determination of the In Vitro Antimicrobial Activity
The antimicrobial activities against E. coli of different concentrations of tested samples were determined by the microtiter plate method described by the United States Pharmacopeia [17]. A twofold microdilution broth method was used to determinate the minimum inhibitory concentrations (MIC) value for each test substance [18–21]. Each well contained 106 CFU/mL of test bacteria and LB medium (100 μL). 100 μL of MeOH solutions of tested samples (5 mg/mL for pure compounds and 20 mg/mL for the fractions) was added to wells of the first row. Dilutions were used to dispense 100 μL into the other sterile 96 wells of an ELISA plate using a multichannel micropipette, resulting in eight concentrations to be tested for each compound. A negative control containing inoculated growth medium and methanol was prepared. Each experiment was performed in triplicate.
2.4.3. Minimum Inhibitory Concentration (MIC) Determination
The MIC value is a measure to define the antibacterial activity of a compound and is defined as the lowest concentration of drug that inhibits visible growth. The amount of growth in the wells containing test samples was compared with the amount of growth in the control wells when determining the growth end points. When a single skipped well occurred, the highest MIC was read.
3. Results and Discussion
3.1. Isolation and Characterization of Compounds
Dried and powdered fruits of F. suspensa were extracted with methanol, and the combined extracts were concentrated under reduced pressure to give deep brown syrup. The crude extract was suspended into water and partitioned with chloroform to afford chloroform and water soluble fractions, respectively. Purification of the chloroform fraction of the methanol extracts of fruits of F. suspensa by a combination of chromatographic techniques yielded one new triterpene, 3β-acetoxy-20α-hydroxyursan-28-oic acid (1) (Figure 1), β-amyrin acetate (2) [22], taraxasterol acetate (3) [23], 3β-acetyl-20,25-epoxy-dammarane-24α-ol (4) [24], acetyl oleanolic acid (5) [25], betulinic acid (6) [26], labda-8(17),13E-dien-15,18-dioic acid 15-methyl ester (7) [27], mixture of β-sitosterol (8) and stigmasterol (9) [28], ψ-taraxasterol (10) [29], 3β-hydroxyanticopalic acid (11) [30], agatholic acid (12) [31], 3,4-dimethoxybenzoic acid (13) [32], vanillic acid (14) [33], syringic acid (15) [33], phillyrin (16) [15], p-hydroxyphenylacetic acid (17) [34], p-hydroxybenzoic acid (18) [33], and benzoic acid (19) [35], respectively. The water fraction was subjected to the reversed-phase Diaion HP-20 column chromatography and successive isolation to afford p-hydroxyphenylacetic acid (17), p-hydroxybenzoic acid (18), p-hydroxyphenylacetic acid methyl ester (20) [36], p-tyrosol (21) [37], hydroxytyrosol (22) [38], 2-furancarboxylic acid (23) [39], salidroside (24) [40], (6S,9R)-roseoside (25) [41], forsythoside D (26) [8], methyl-α-D-glucopyranoside (27) [42], adoxosidic acid (28) [43], protocatechualdehyde (29) [44], esculetin (30) [45], caffeic acid (31) [46], trans-coumaric acid (32) [47], trans-ferulic acid (33) [48], and quercetin (34) [49], respectively. The chemical structures of known compounds 2–34 were identified by comparison of their physical and spectroscopic data with those reported in the literature. Among the isolates, compounds 2, 4, 6, 8, 14, 16, 17, 24, 26, 28, 31, and 34 had been identified from the titled plant. Other compounds were reported from F. suspensa for the first time. Compound 1 was a new compound and its structure was established by the spectral analysis.
Figure 1.
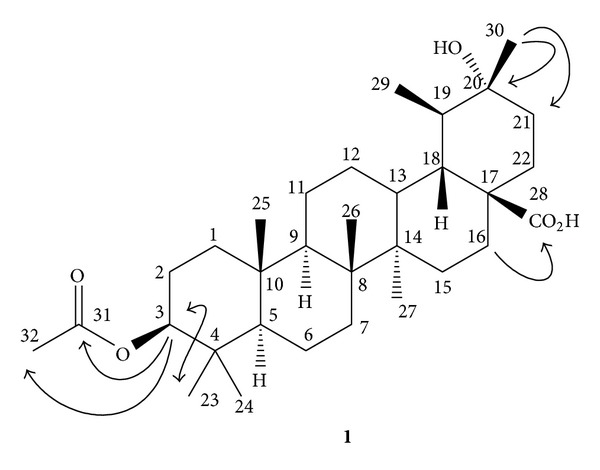
Chemical structure, significant HMBC (→) and NOESY (↔) correlations of compound 1.
3.2. Structural Elucidation of Compound 1
The purified white powder 1 was visualized by spraying with 1% (w/v) Ce(SO4)2 in 10% (v/v) aqueous H2SO4 followed by heating at 120°C and displayed purplish black spots on TLC plate. It also displayed positive responses against the Lieberman-Burchard test. These results suggested compound 1 to be a triterpenoid [50]. The molecular formula of 1 was established as C32H52O5 by the pseudomolecular [M+H]+ ion peak at m/z 517.3896 in HR-FAB-MS analysis and was further supported by its 13C-NMR spectrum which showed signals for all the 32 carbons of the molecule including one set of acetyl group (δ C 171.0, 21.3), one carboxylic acid group (δ C 176.8), one oxygenated quaternary carbon (δ C 90.1), and one acetoxygenated carbon (δ C 80.9), respectively. In the 1H-NMR spectrum of 1, there were proton signals for seven methyl groups at δ 0.83 (15H, m, and CH3-23, -24, -25, -27, and -29), 0.94 (3H, s, and CH3-26), and 1.35 (3H, s, and CH3-30), and one acetyl methyl group at δ 2.05 (3H, s, and CH3-32), respectively. The spectroscopic data indicated compound 1 to possess oleanane type basic skeleton. In the downfield region, one oxygenated proton at δ 4.48 (1H, dd, J = 10.4, 5.6 Hz, H-3α) was located at C-3 which was further established by the NOESY correlations between CH3-23 and H-3. The 2 J, 3 J-HMBC correlations from δ 4.48 (H-3) to δ C 21.3 (C-32) and 171.0 (C-31) also evidenced the presence of acetoxy group at C-3. The substitution of tertiary alcohol at C-20 was also determined with the HMBC analysis of correlations from CH3-30 to C-21 (δ C 31.2) and C-20 (δ C 90.1). The 2 J, 3 J-HMBC correlation peak between δ 2.60 (m, H-16) and δ C 176.8 (C-28) supported the carboxylic acid group to be attached at C-17. The complete assignments of 1H and 13C NMR signals of 1 were furnished from the NOESY and HMBC spectra. Therefore the chemical structure of 1 was established as 3β-acetoxy-20α-hydroxyursan-28-oic acid and shown in Figure 1.
3.3. The Antimicrobial Effects of Isolated Compounds against Escherichia coli
The crude extracts, partially purified fractions, and some of the purified principles (Figure 2) were subjected to the examinations for the inhibitory effects against E. coli [17–21]. The MIC data of the fractions were presented in Table 1. The MIC value of crude extracts (FS) was 4.25 mg/mL and demonstrated inhibition of the bacterial growth. Comparatively, the chloroform fraction (FSC) displayed more significant inhibitory effects against E. coli (BCRC-11634) than the water fraction (FSW) with MIC values of 6.25 and 12.50 mg/mL, respectively. When studying the influence of the concentration of compounds on the antimicrobial activities against E. coli, twofold microdilution broth method was used for the purified principles from the chloroform fraction (FSC), including triterpenoids 1, 2, 6, and 10; diterpenoids 11and 12; and lignan 16. It was observed that as the concentration increased, the inhibition of the bacterial growth was also increased. All of the tested samples demonstrated the inhibitory effects in a concentration-dependent manner. The MIC data of the examined compounds were presented in Table 2. The MIC values were in the range between 1.20 and 5.00 mg/mL against E. coli (BCRC-11634). Among the tested compounds, triterpenoids betulinic acid (6) and ψ-taraxasterol (10) exhibited the most significant inhibition against E. coli with MIC values of 1.20 mg/mL. These principles should be responsible for the bioactivity of the chloroform fraction (FSC). The results exhibited that the triterpenoids from the methanol extracts of fruits of F. suspensa possessed antibacterial activities against the common bacteria. It also provided evidence for the traditional uses of the fruits of F. suspensa as herbal medicines in the treatment of bacterial diseases. Although these purified compounds did not display better inhibition of the bacterial growth compared with the reported synthetic antibiotics, the extracts and principles from the natural sources usually possessed lower toxicity. Further structural modification could be performed to improve the activity and maintain the safety of these compounds. Therefore, it would be potentially useful in developing new antimicrobial therapeutic agents.
Figure 2.
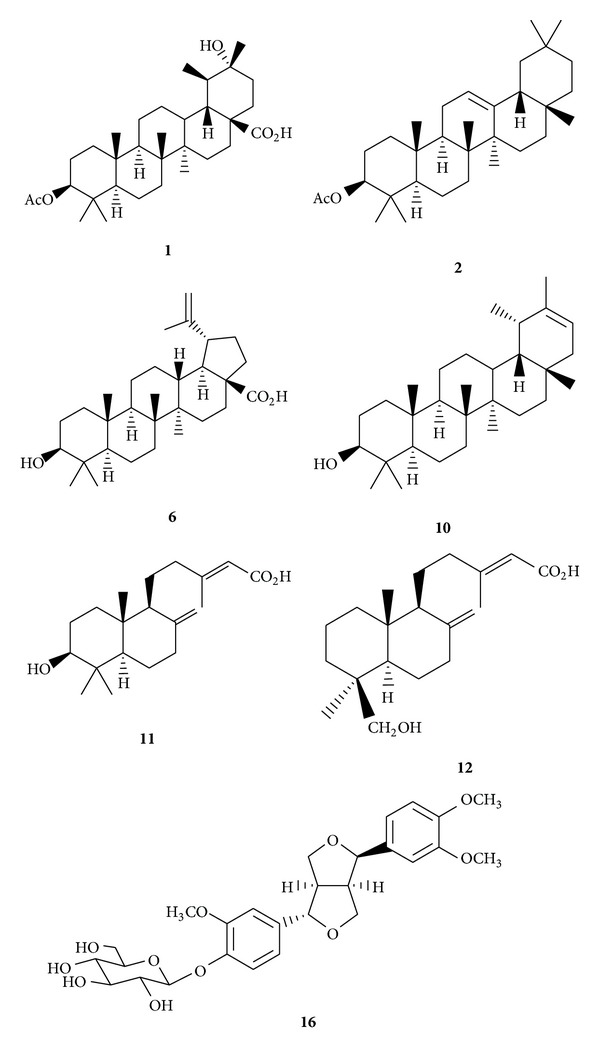
Structures of the isolated compounds subjected to the antimicrobial assay.
Table 2.
The minimum inhibitory concentrations (MICs) of the purified samples from F. suspensa against E. coli (BCRC-11634).
| Compound | MIC (mg/mL) |
|---|---|
| 1 | 4.55 |
| 2 | 5.00 |
| 6 | 1.20 |
| 10 | 1.20 |
| 11 | 3.42 |
| 12 | 2.62 |
| 16 | 3.94 |
Supplementary Material
The 1D and 2D NMR spectra of the new compound (1) were provided.
Acknowledgment
The authors are grateful to the National Science Council, Taiwan, for the financial support of this research.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Abuladze T, Li M, Menetrez MY, Dean T, Senecal A, Sulakvelidze A. Bacteriophages reduce experimental contamination of hard surfaces, tomato, spinach, broccoli, and ground beef by Escherichia coli O157:H7. Applied and Environmental Microbiology. 2008;74(20):6230–6238. doi: 10.1128/AEM.01465-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allende A, Martínez B, Selma V, Gil MI, Suárez JE, Rodríguez A. Growth and bacteriocin production by lactic acid bacteria in vegetable broth and their effectiveness at reducing Listeria monocytogenes in vitro and in fresh-cut lettuce. Food Microbiology. 2007;24(7-8):759–766. doi: 10.1016/j.fm.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Brown AL, Brooks JC, Karunasena E, Echeverry A, Laury A, Brashears MM. Inhibition of Escherichia coli O157:H7 and Clostridium sporogenesin spinach packaged in modified atmospheres after treatment combined with chlorine and lactic acid bacteria. Journal of Food Science. 2011;76(6):M427–M432. doi: 10.1111/j.1750-3841.2011.02260.x. [DOI] [PubMed] [Google Scholar]
- 4.Leverentz B, Conway WS, Janisiewicz W, Abadias M, Kurtzman CP, Camp MJ. Biocontrol of the food-borne pathogens Listeria monocytogenes and Salmonella enterica serovar poona on fresh-cut apples with naturally occurring bacterial and yeast antagonists. Applied and Environmental Microbiology. 2006;72(2):1135–1140. doi: 10.1128/AEM.72.2.1135-1140.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yui CL. A study of the bacteriostatic principle isolated from Forsythia suspensa Vahl. (Lien-chiao) Yaoxue Xuebao. 1960;8(6):241–244. [PubMed] [Google Scholar]
- 6.Endo K, Takahashi K, Abe T, Hikino H. Structure of forsythoside A, an antibacterial principle of Forsythia suspensa leaves. Heterocycles. 1981;16(8):1311–1314. [Google Scholar]
- 7.Nishibe S, Okabe K, Tsukamoto H. Studies on the Chinese crude drug “Forsythiae fructus”. VI: the structure and antibacterial activity of suspensaside isolated from Forsythia suspensa . Chemical and Pharmaceutical Bulletin. 1982;30(12):4548–4553. doi: 10.1248/cpb.30.4548. [DOI] [PubMed] [Google Scholar]
- 8.Endo K, Hikino H. Structures of forsythoside C and D, antibacterial principles of Forsythia suspensa fruits. Heterocycles. 1982;19(11):2033–2036. [Google Scholar]
- 9.Kitagawa S, Nishibe S, Baba H. Studies on the Chinese crude drug “Forsythiae fructus”. VIII: on isolation of phenylpropanoid glycosides from fruits of Forsythia koreana and their antibacterial activity. Yakugaku Zasshi. 1987;107(4):274–278. doi: 10.1248/yakushi1947.107.4_274. [DOI] [PubMed] [Google Scholar]
- 10.Nishibe S, Kitagawa S, Hisada S, et al. Phenolic compounds from Forsythiae fructus and their biological activities. Journal of Pharmacobio-Dynamics. 1987;10(3, s-48) [Google Scholar]
- 11.Kuang HX, Zhang N, Lu ZB. Antibacterial constituents of the unripe fruit of Forsythia suspensa (Thunb.) Vahl. Zhongyao Tongbao. 1988;13(7):32–63. [PubMed] [Google Scholar]
- 12.Qu H, Zhang Y, Wang Y, Li B, Sun W. Antioxidant and antibacterial activity of two compounds (forsythiaside and forsythin) isolated from Forsythia suspensa . Journal of Pharmacy and Pharmacology. 2008;60(2):261–266. doi: 10.1211/jpp.60.2.0016. [DOI] [PubMed] [Google Scholar]
- 13.Qu H, Zhang Y, Chai X, Sun W. Isoforsythiaside, an antioxidant and antibacterial phenylethanoid glycoside isolated from Forsythia suspensa . Bioorganic Chemistry. 2012;40(1):87–91. doi: 10.1016/j.bioorg.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Kitagawa S, Nishibe S, Benecke R, Thieme H. Phenolic compounds from Forsythia leaves. II. Chemical & Pharmaceutical Bulletin. 1988;36(9):3667–3670. [Google Scholar]
- 15.Rahman MMA, Dewick PM, Jackson DE, Lucas JA. Lignans of Forsythia intermedia . Phytochemistry. 1990;29(6):1971–1980. [Google Scholar]
- 16.Rouf ASS, Ozaki Y, Rashid MA, Rui J. Dammarane derivatives from the dried fruits of Forsythia suspensa . Phytochemistry. 2001;56(8):815–818. doi: 10.1016/s0031-9422(01)00028-0. [DOI] [PubMed] [Google Scholar]
- 17.The United States Pharmacopeia. USP29-NF24 2006. [Google Scholar]
- 18.Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. International Journal of Food Microbiology. 2004;94(3):223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Friedman M, Henika PR, Mandrell RE. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica . Journal of Food Protection. 2002;65(10):1545–1560. doi: 10.4315/0362-028x-65.10.1545. [DOI] [PubMed] [Google Scholar]
- 20.Ooi LSM, Li Y, Kam SL, Wang H, Wong EYL, Ooi VEC. Antimicrobial activities of Cinnamon oil and cinnamaldehyde from the Chinese medicinal herb Cinnamomum cassia Blume. American Journal of Chinese Medicine. 2006;34(3):511–522. doi: 10.1142/S0192415X06004041. [DOI] [PubMed] [Google Scholar]
- 21.Shan B, Cai YZ, Brooks JD, Corke H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. International Journal of Food Microbiology. 2007;117(1):112–119. doi: 10.1016/j.ijfoodmicro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Matsunaga S, Tanaka R, Akagi M. Triterpenoids from Euphorbia maculata . Phytochemistry. 1988;27(2):535–537. [Google Scholar]
- 23.Bhutani KK, Gupta DK, Kapil RS. Occurrence of D/E trans stereochemistry isomeric to ursane (cis) series in a new pentacyclic triterpene from Calotropis procera . Tetrahedron Letters. 1992;33(49):7593–7596. [Google Scholar]
- 24.Jing H, Lee JS, Cao W, Fang CF, Lu P. Determination of forsythoside A from Forsythia suspensa by HPLC. Xibei Yaoxue Zazhi. 2003;18(4):156–157. [Google Scholar]
- 25.Umehara K, Takagi R, Kuroyanagi M, Ueno A, Taki T, Chen YJ. Studies on differentiation-inducing activities of triterpenes. Chemical and Pharmaceutical Bulletin. 1992;40(2):401–405. doi: 10.1248/cpb.40.401. [DOI] [PubMed] [Google Scholar]
- 26.Nick A, Wright AD, Rali T, Sticher O. Antibacterial triterpenoids from Dillenia papuana and their structure-activity relationships. Phytochemistry. 1995;40(6):1691–1695. doi: 10.1016/0031-9422(95)00491-o. [DOI] [PubMed] [Google Scholar]
- 27.Zdero C, Bohlmann F, Niemeyer HM. Friedolabdanes and other constituents from chilean Haplopappus species. Phytochemistry. 1991;30(11):3669–3677. [Google Scholar]
- 28.Kuo YH, Li YC. Constituents of the Bark of Ficus microcarpa L.f. Journal of the Chinese Chemical Society. 1997;44(3):321–325. [Google Scholar]
- 29.Ding HY. Phytochemical and pharmacological studies on Chinese changzhu. Journal of the Chinese Chemical Society. 2000;47(3):561–566. [Google Scholar]
- 30.Braun S, Breitenbach H. Strukturaufklärung einer neuen diterpensäure aus Metasequoia glyptostroboides mit hilfe der 13C-NMR-spektroskopie. Tetrahedron. 1977;33(1):145–150. [Google Scholar]
- 31.de Paiva Campello J, Fonseca SF. Diterpenes from Araucaria angustifolia . Phytochemistry. 1975;14(10):2299–2300. [Google Scholar]
- 32.Trincado M, Grützmacher H, Vizza F, Bianchini C. Domino rhodium/palladium-catalyzed dehydrogenation reactions of alcohols to acids by hydrogen transfer to inactivated alkenes. Chemistry. 2010;16(9):2751–2757. doi: 10.1002/chem.200903069. [DOI] [PubMed] [Google Scholar]
- 33.Chen CY, Chang FR, Teng CM, Wu YC. Cheritamine, a new N-fatty acyl tryptamine and other constituents from the stems of Annona cherimola . Journal of the Chinese Chemical Society. 1999;46(1):77–86. [Google Scholar]
- 34.Zhang K, Corrie JET, Munasinghe VRN, Wan P. Mechanism of photosolvolytic rearrangement of p-hydroxyphenacyl esters: evidence for excited-state intramolecular proton transfer as the primary photochemical step. Journal of the American Chemical Society. 1999;121(24):5625–5632. [Google Scholar]
- 35.Laurent P, Lebrun B, Braekman JC, Daloze D, Pasteels JM. Biosynthetic studies on adaline and adalinine, two alkaloids from ladybird beetles (Coleoptera: Coccinellidae) Tetrahedron. 2001;57(16):3403–3412. [Google Scholar]
- 36.Bose DS, Narsaiah AV. An efficient asymmetric synthesis of (S)-atenolol: using hydrolytic kinetic resolution. Bioorganic and Medicinal Chemistry. 2005;13(3):627–630. doi: 10.1016/j.bmc.2004.10.057. [DOI] [PubMed] [Google Scholar]
- 37.Takaya Y, Furukawa T, Miura S, et al. Antioxidant constituents in distillation residue of Awamori spirits. Journal of Agricultural and Food Chemistry. 2007;55(1):75–79. doi: 10.1021/jf062029d. [DOI] [PubMed] [Google Scholar]
- 38.Pouységu L, Sylla T, Garnier T, et al. Hypervalent iodine-mediated oxygenative phenol dearomatization reactions. Tetrahedron. 2010;66(31):5908–5917. [Google Scholar]
- 39.Preobrazhenskaya MN, Rozhkov II, Lazhko EI, Yudina LN, Korolev AM. Reaction of vanilmandelic acid and 4-Hydroxybenzyl alcohol derivatives with L-ascorbic acid. Tetrahedron. 1997;53(20):6971–6976. [Google Scholar]
- 40.Kuwajima H, Takai Y, Takaishi K, Inoue K. Synthesis of 13C-labeled possible intermediates in the biosynthesis of phenylethanoid derivatives, cornoside and rengyosides. Chemical and Pharmaceutical Bulletin. 1998;46(4):581–586. [Google Scholar]
- 41.Yamano Y, Ito M. Synthesis of optically active vomifoliol and roseoside stereoisomers. Chemical and Pharmaceutical Bulletin. 2005;53(5):541–546. doi: 10.1248/cpb.53.541. [DOI] [PubMed] [Google Scholar]
- 42.Albertin L, Stenzel M, Barner-Kowollik C, Foster LJR, Davis TP. Well-defined glycopolymers from RAFT polymerization: poly(methyl 6-O-methacryloyl-α-D-glucoside) and its block copolymer with 2-hydroxyethyl methacrylate. Macromolecules. 2004;37(20):7530–7537. [Google Scholar]
- 43.Suciati S, Lambert LK, Ross BP, Deseo MA, Garson MJ. Phytochemical study of Fagraea spp. Uncovers a new terpene alkaloid with anti-inflammatory properties. Australian Journal of Chemistry. 2011;64(4):489–494. [Google Scholar]
- 44.Chiji H, Tanaka S, Izawa M. Phenolic germination inhibitors in the seed balls of red beet (Beta vulgaris L. var. rubra) Agricultural and Biological Chemistry. 1980;44(1):205–207. [Google Scholar]
- 45.Masamoto Y, Ando H, Murata Y, Shimoishi Y, Tada M, Takahata K. Mushroom tyrosinase inhibitory activity of esculetin isolated from seeds of Euphorbia lathyris L. Bioscience, Biotechnology and Biochemistry. 2003;67(3):631–634. doi: 10.1271/bbb.67.631. [DOI] [PubMed] [Google Scholar]
- 46.Bolzani VDS, Trevisan LMV, Young MCM. Caffeic acid esters and triterpenes of Alibertia macrophylla . Phytochemistry. 1991;30(6):2089–2091. [Google Scholar]
- 47.Chiang YM, Liu HK, Lo JM, et al. Cytotoxic constituents of the leaves of Calocedrus formosana . Journal of the Chinese Chemical Society. 2003;50(1):161–166. [Google Scholar]
- 48.Prachayasittikul S, Suphapong S, Worachartcheewan A, Lawung R, Ruchirawat S, Prachayasittikul V. Bioactive metabolites from Spilanthes acmella Murr. Molecules. 2009;14(2):850–867. doi: 10.3390/molecules14020850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hua Y, Wang HQ. Chemical components of Anaphalis sinica hance. Journal of the Chinese Chemical Society. 2004;51(2):409–415. [Google Scholar]
- 50.Srivastava A, Shukla YN. Aryl esters and a coumarin from Aygyreia speciosa . Indian Journal of Chemistry B. 1998;37(2):192–194. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The 1D and 2D NMR spectra of the new compound (1) were provided.


