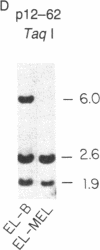
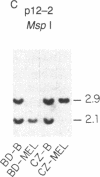
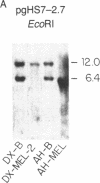
Abstract
Loss of genetic material at certain chromosomal sites is implicated in the etiology of retinoblastoma and Wilms tumor. Whether specific chromosomal deletions are associated with other types of human cancer needs to be explored. We have examined 24 melanoma cell lines, derived from 21 patients with nonfamilial malignant melanoma, for evidence of somatically induced hemizygosity or homozygosity. Twelve DNA probes, recognizing single-copy restriction fragment length polymorphisms (RFLP) determined by loci on 11 different chromosomes, were used to screen autologous combinations of melanoma cells and either B cells or fibroblasts. Loss of heterozygosity in melanoma cells was identified at 27 of 100 informative loci. These losses occurred at loci on 8 different chromosomes, and the frequency of loss at individual loci varied between 8% and 67%. We conclude that somatic mutations resulting in homozygosity or hemizygosity are common in melanoma and evidently not restricted to specific chromosomes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albino A. P., Le Strange R., Oliff A. I., Furth M. E., Old L. J. Transforming ras genes from human melanoma: a manifestation of tumour heterogeneity? Nature. 1984 Mar 1;308(5954):69–72. doi: 10.1038/308069a0. [DOI] [PubMed] [Google Scholar]
- Albino A. P., Lloyd K. O., Houghton A. N., Oettgen H. F., Old L. J. Heterogeneity in surface antigen and glycoprotein expression of cell lines derived from different melanoma metastases of the same patient. Implications for the study of tumor antigens. J Exp Med. 1981 Dec 1;154(6):1764–1778. doi: 10.1084/jem.154.6.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. E. Clinical characteristics of the genetic variety of cutaneous melanoma in man. Cancer. 1971 Sep;28(3):721–725. doi: 10.1002/1097-0142(197109)28:3<721::aid-cncr2820280330>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Balaban G., Herlyn M., Guerry D., 4th, Bartolo R., Koprowski H., Clark W. H., Nowell P. C. Cytogenetics of human malignant melanoma and premalignant lesions. Cancer Genet Cytogenet. 1984 Apr;11(4):429–439. doi: 10.1016/0165-4608(84)90024-4. [DOI] [PubMed] [Google Scholar]
- Barker D., Schafer M., White R. Restriction sites containing CpG show a higher frequency of polymorphism in human DNA. Cell. 1984 Jan;36(1):131–138. doi: 10.1016/0092-8674(84)90081-3. [DOI] [PubMed] [Google Scholar]
- Carey T. E., Takahashi T., Resnick L. A., Oettgen H. F., Old L. J. Cell surface antigens of human malignant melanoma: mixed hemadsorption assays for humoral immunity to cultured autologous melanoma cells. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3278–3282. doi: 10.1073/pnas.73.9.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Cavenee W., Leach R., Mohandas T., Pearson P., White R. Isolation and regional localization of DNA segments revealing polymorphic loci from human chromosome 13. Am J Hum Genet. 1984 Jan;36(1):10–24. [PMC free article] [PubMed] [Google Scholar]
- Dracopoli N. C., Fogh J. Loss of heterozygosity in cultured human tumor cell lines. J Natl Cancer Inst. 1983 Jan;70(1):83–87. [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B., Feinberg A. P. Somatic deletion and duplication of genes on chromosome 11 in Wilms' tumours. Nature. 1984 May 10;309(5964):176–178. doi: 10.1038/309176a0. [DOI] [PubMed] [Google Scholar]
- Greene M. H., Goldin L. R., Clark W. H., Jr, Lovrien E., Kraemer K. H., Tucker M. A., Elder D. E., Fraser M. C., Rowe S. Familial cutaneous malignant melanoma: autosomal dominant trait possibly linked to the Rh locus. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6071–6075. doi: 10.1073/pnas.80.19.6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries P., Barton D., McKay A. M., Humphries M. M., Carritt B. Isolation of a polymorphic DNA segment unique to human chromosome 7 by molecular cloning of hybrid cell DNA. Mol Gen Genet. 1983;190(1):143–149. doi: 10.1007/BF00330337. [DOI] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Lampkin B. C., Workman M. L., Copeland N. G., Jenkins N. A., Cavenee W. K. Loss of alleles at loci on human chromosome 11 during genesis of Wilms' tumour. Nature. 1984 May 10;309(5964):170–172. doi: 10.1038/309170a0. [DOI] [PubMed] [Google Scholar]
- Lowy D. R. Infectious murine leukemia virus from DNA of virus-negative AKR mouse embryo cells. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5539–5543. doi: 10.1073/pnas.75.11.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor S. L., Sakaguchi A. Y., Barker D., White R., Shows T. B. DNA polymorphic loci mapped to human chromosomes 3, 5, 9, 11, 17, 18, and 22. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2447–2451. doi: 10.1073/pnas.81.8.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor S. L., Sakaguchi A. Y., Shen L. P., Bell G. I., Rutter W. J., Shows T. B. Polymorphic human somatostatin gene is located on chromosome 3. Proc Natl Acad Sci U S A. 1983 May;80(9):2686–2689. doi: 10.1073/pnas.80.9.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Goldman D. S., Sallan S. E. Development of homozygosity for chromosome 11p markers in Wilms' tumour. Nature. 1984 May 10;309(5964):172–174. doi: 10.1038/309172a0. [DOI] [PubMed] [Google Scholar]
- Pathak S., Drwinga H. L., Hsu T. C. Involvement of chromosome 6 in rearrangements in human malignant melanoma cell lines. Cytogenet Cell Genet. 1983;36(3):573–579. doi: 10.1159/000131975. [DOI] [PubMed] [Google Scholar]
- Reeve A. E., Housiaux P. J., Gardner R. J., Chewings W. E., Grindley R. M., Millow L. J. Loss of a Harvey ras allele in sporadic Wilms' tumour. Nature. 1984 May 10;309(5964):174–176. doi: 10.1038/309174a0. [DOI] [PubMed] [Google Scholar]
- Skolnick M. H., Willard H. F., Menlove L. A. Report of the Committee on Human Gene Mapping by Recombinant DNA Techniques. Cytogenet Cell Genet. 1984;37(1-4):210–273. doi: 10.1159/000132011. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]