Abstract
We have demonstrated survival of living allogeneic bone without long-term immunosuppression using short-term immunosuppression and simultaneous creation of an autogenous neoagiogenic circulation. In this study bone morphogenic protein-2 (rhBMP-2), and/or vascular endothelial growth factor (VEGF), were used to augment this process. Femoral diaphyseal bone was transplanted heterotopically from 46 Dark Agouti to 46 Lewis rats. Microvascular repair of the allotransplant nutrient pedicle was combined with intra-medullary implantation of an autogenous saphenous arteriovenous (AV) bundle and biodegradable microspheres containing buffer (control), rhBMP-2 or rhBMP-2 + VEGF. FK-506 given daily for 14 days maintained nutrient pedicle flow during angiogenesis. After an 18 weeks survival period, we measured angiogenesis (capillary density) from the AV bundle and cortical bone blood flow. Both measures were greater in the combined (rhBMP-2 + VEGF) group than rhBMP-2 and control groups (p<0.05). Osteoblast counts were also higher in the rhBMP-2 + VEGF group (p<0.05). A trend towards greater bone formation was seen in both rhBMP2 + VGF and rhBMP2 groups as compared to controls (p=0.059). Local administration of VEGF and rhBMP-2 augments angiogenesis, osteoblastic activity and bone blood flow from implanted blood vessels of donor origin in vascularized bone allografts.
Keywords: bone, allotransplantation, microspheres, BMP, VEGF
Introduction
Massive loss of bone, at times including adjacent joints, may occur as the result of tumor resection, trauma, infection, or prosthetic implant failure. Currently available reconstructive methods (vascularized autograft, non-vascularized structural allograft and prosthetic replacement) are problematic in some instances due to nonunion, infection, implant failure and late stress fractures1. A future alternative may be the use of vascularized bone or composite tissue allotransplantation (CTA). Maintaining circulation and tissue bone viability in the face of rejection in these allotransplants currently requires long-term immunosuppression with attendant risks2, 3. A means to maintain circulation in allogeneic bone transplantation without need for long-term immunosuppression has been described4. This is accomplished by placing vascularized recipient tissue in the form of an arteriovenous (AV) bundle or fascial flap within the bone at the time of transplantation. Subsequent capillary proliferation thereby develops a neoangiogenic autogenous vascular bed within the transplanted bone4-6. We have previously demonstrated that short-term immunosuppression (2 weeks) is sufficient for this process to take place in the rat femur. Bone blood flow, bone formation, and osteocyte viability are subsequently maintained long-term with this method without further drug immunosuppression5, 7. The overall goal of the current study was to investigate the possibility of enhancing angiogenesis and bone remodeling using recombinant human bone morphogenic protein 2 (rhBMP-2) and vascular endothelial growth factor (VEGF) in the same experimental model. We administered biodegradable microspheres encapsulating rhBMP2, VEGF, or buffer (control) at the time of surgery within the transplanted femur adjacent to the AV bundle. We measured the effect of the growth factor(s) on angiogenesis, bone blood flow, bone viability and bone formation. We hypothesized that the addition of the rhBMP-2 would improve angiogenesis from the AV bundle as well as increase both bone blood flow and new bone formation in the allotransplanted bone, as compared to no growth factor controls. A synergistic further positive effect on these parameters was expected by the addition of VEGF to the rhBMP-2-encapsulated microspheres.
Methods
Inbred Dark Agouti (DA) rats (n=46) were used as donors in a no survival procedure. Inbred Lewis rats were used as the recipient animals, resulting in transplantation across a major histocompatibility barrier (n=46)8, 9. Immunosuppression was administered in the form of FK-506 (1 mg/kg/day IM X 2 weeks) (Tacrolimus©, Fujisawa Pharmaceutical Co., Osaka, Japan)10. Sterile technique was maintained throughout the procedures. After an 18 weeks survival time the animals were euthanized according to protocol. All experiments were performed according to established National Institutes of Health guidelines and under the direction of our Institutional Animal Care and Use Committee. Animals were allowed to move freely in their cages and fed standard rodent feed and water ad libitum. The group distribution is shown in Table 1.
Table 1.
Group Distribution
| Group | Growth factor | N |
|---|---|---|
| Group 1 | None | 15 |
| Group 2 | rhBMP-2 | 15 |
| Group 3 | rhBMP-2 + VEGF | 16 |
Femoral Transplantation Model
Microvascular transfer of the rat femur was first described in 1984 based on anatomical dissection and vessel arborization11. The vascular distribution to the bone is provided by proximal and distal nutrient branches arising from the lateral femoral circumflex artery and femoral artery, respectively.
Microsphere Manufacture
Microsphere fabrication was performed under aseptic conditions. Briefly 0.2 ml of a 1.5 mg/ml of rhBMP-2 solution (INFUSE©, Medtronic Sofamor Danek, Memphis TN), 0.17 ml of a 1.8 mg/ml VEGF growth factor solution (Trevigen Inc., Gaithersburg, MD), or buffered saline were encapsulated in biodegradable PLGA microspheres using methods developed by a collaborating laboratory 12, 13. The manufacturing parameters used have been previously determined to provide a zero-order drug release for 28 days after an initial burst, when embedded in a substrate such as a collagen matrix. Each bone transplant was loaded with 15mg of microspheres containing 0.7 μg per mg growth factor (= 10 μg total).
Operative Procedure
In a DA donor, the right femur was dissected together with its nutrient vascular pedicle. The proximal common iliac artery and vein were ligated and cut at their origin, to be used for the microvascular repair. The pedicle was then irrigated with heparinized saline, the femoral head and neck and distal femoral condyles were removed. The medullar canal was reamed with a 2-mm–diameter drill bit. Allografts varied from 18 to 22mm in length. In a Lewis recipient, the right femoral artery and vein were ligated at the saphenous origin to be used for microvascular repair with the graft vessels in an end-to-end fashion (Figure 1). The collateral flow to the limb was sufficient to prevent vascular compromise postoperatively. The use of smaller size donor rats, with a body weight 50–70 g less than that of the recipient allowed an appropriate match of vessel diameters. The graft was wrapped with a reinforced silicone membrane, to prevent angiogenesis from surrounding tissues, and placed in an abdominal skin pocket. The left saphenous artery and vein were harvested, along with a small distal fascial flap, and implanted as an AV bundle into the medullar canal to run the entire length of the allograft. Growth factors (rhBMP2, rhBMP2 + VGF) were delivered as encapsulated poly (D,L-lactide-co-glycolide) (PLGA) microspheres placed on the AV bundle. The medullar canal was then filled with 20 ml of collagen I solution at pH 7.4. Closure of the subcutaneous and skin layers was done using interrupted 4-0 absorbable and nylon sutures and staples. After an 18 weeks survival time the animals were euthanized and data analysis started, following the order detailed below.
Figure 1.
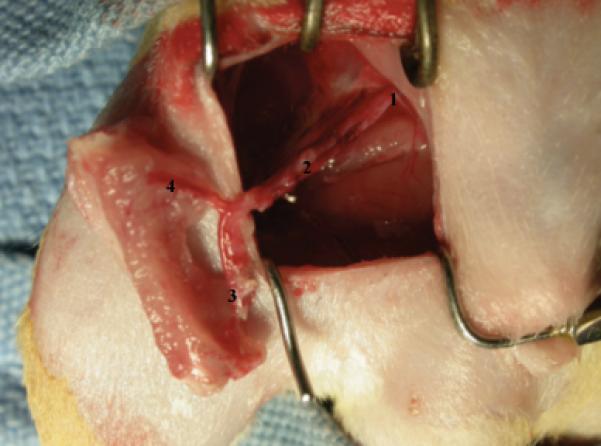
Allograft in place after anastomoses in the recipient rat vessels: (1) Common iliac vessels, (2) Lateral femoral circumflex vessels, (3) Distal nutrient branch to femur, (4) Femoral vessels with proximal nutrient branch.
Assessment
Bone Blood Flow
We have previously described and validated the use of hydrogen washout to measure cortical blood flow in the rat femur14, 15. Measurements were performed on all grafts, immediately prior to euthanasia (18 weeks survival time). We used the established methodology without modification. Briefly, animals were anesthetized, a tracheotomy was placed and a mixture of 30% oxygen and 70% hydrogen begun. A hydrogen sensor (H2-50©, Unisense, Aarhus, Denmark) was inserted into a 0.36mm cortical opening with a micromanipulator and connected to a picoammeter (Picoammeter2000©, Unisense, Aarhus, Denmark). The hydrogen concentration was measured and allowed to reach a state of equilibrium, as recorded using LabVIEWTM© software (National Instruments, Austin, TX; Figure 2). Hydrogen was removed from the mixture, and cortical blood flow calculated based upon the rate of hydrogen washout was measured and cortical bone blood flow calculated, expressed as ml/min per 100 g of tissue16, 17.
Figure 2.
Picture showing the LabViewTM Software© measure (National Instruments, Austin, TX) of the Hydrogen Washout Curve and Cortical Bone Blood Flow.
Microangiography
Following euthanasia, the abdominal aorta was filled with blue microangiographic solution (Microfil©, Flow Tech Inc., Carver, MA) under physiologic pressure with an outflow cannula in the vena cava. The compound was allowed to polymerize up to 24 h at 4°C. Grafts were then fixed in 10% formalin for 24 h and a 2-mm transverse section removed for histomorphometric analysis using a diamond-coated circular drill. The grafts were then decalcified in 14% EDTA acid for 7 h in a calibrated laboratory microwave at 750W (Pelco Biowave 3450 Laboratory Microwave©, Ted Pella, Inc., Redding, CA)18. The intraosseous vasculature was visualized by optical bone clearing 19 and the capillary density (D) of each specimen was measured using image analysis software (Scion Image for Windows 4.0.2©): D=Vessel pixels/Total pixels (Vessel Area/ Bone Area). Patency of the AV bundle was documented from the presence of the angiographic polymer after sacrifice (Figure 3 and 4).
Figure 3.

Picture of the bone after bone decalcification and clearing. Patency of the AV bundle is attested by the presence of the angiographic polymer
Figure 4.

Image analysis showing the pixel conversion of the colorized vessel, allowing capillarity density calculation
Histologic Grading of Bone Viability
A 2-mm transverse section was cut for histological analysis from the end of each decalcified bone prior to optical clearing. Hematoxylin-eosin stained specimens were used for this analysis. To measure bone viability, the number of osteocyte-occupied lacunae versus empty lacunae was counted in three randomly selected fields at 400X using a 40 X lens and 10 X eyepiece (Nikon Eclipse 50i©, Nikon Instruments, Melville, NY; Figure 5). Osteoblast and Osteoclast were also counted during this analysis.
Figure 5.
Figure showing a hematoxylin-eosin stained specimen.
Quantitative Histomorphometry for Bone Remodeling
Calcein and tetracycline fluorescent labels (20 mg/kg) were administered ten days apart, at 2 weeks and 4 days prior to sacrifice, respectively. These compounds fluoresce with green and orange colors respectively, and are deposited in areas of active osteoblastic activity. Quantitative histomorphometry of the allotransplanted bone was performed on unstained transverse sections at X 200 with bone image analysis software (Osteomeasure©; Osteometrics, Atlanta, GA; Figure 6)20. Multiple parameters were calculated from each complete cross-section. These included mineral apposition rate (MAR: the distance between the two labels divided by the time period between the labels) and bone formation rate to bone surface ratio (BFR/BS).
Figure 6.
Histomorphometry picture showing a double bone label. Calcein= Green / Tetracycline=Orange
Data Analysis
Continuous data, including capillary density, bone blood flow, and histomorphometry measures, were reported as mean values with standard deviations. Categorical data was described as median values with ranges. Capillary density, bone blood flow, and histomorphometric data were compared across three growth factor treatment groups, using either analysis of variance or the Kruskal–Wallis test as appropriate. If a significant difference between groups was detected, the Wilcoxon rank sum test was used to investigate the difference between the individual groups. Correlation between dependent variables was assessed with Spearman's rank correlation coefficient. All statistical tests were two-sided, and the threshold of statistical significance was set at a 0.05. Analyses were performed with the help of personnel from our institution's Division of Biomedical Statistics and Informatics.
Results
No infections or other problems occurred in the animals listed. All animals with non-patent AV bundle were excluded from the analysis. The individual group results are summarized in Table 2.
Table 2.
Results
| Group 1 (no growth factor) | Group 2 (rhBMP-2) | Group 3 (rhBMP2+VEFG) | |
|---|---|---|---|
| Capilarity density (%) | 13.35 (+/- 6.73) | 18.98 (+/- 9.71) | 32.13 (+/- 13.99) |
| Cortical blood flow (ml/min/100 g) | 0.12 (+/- 0.06) | 0.15 (+/- 0.08) | 0.25 (+/- 0.11) |
| Single label/bone surface (SLS/BS) | 5.56 (+/- 2.87) | 4.98 (+/- 2.61) | 5.23 (+/- 2.94) |
| Double label/bone surface (DLS/BS) | 3.82 (+/- 2.99) | 9.15 (+/- 7.42) | 10.15 (+/- 8.55) |
| Mineralized surface/bone surface (MS/BS;%) | 6.59 (+/- 2.30) | 11.64 (+/- 6.90) | 12.77 (+/- 8.14) |
| Mineral apposition rate (MAR;mm/day) | 6.60 (+/- 2.30) | 11.64 (+/- 6.90) | 12.77 (+/- 8.14) |
| Bone formation rate/bone surface (BFR/BS;mm3/mm2/year) | 24.78 (+/- 19.63) | 73.32 (+/- 75.32) | 93.47 (+/- 91.17) |
| Bone volume/total volume (BV/TV) | 62 (+/- 9.7) | 63 (+/- 9.8) | 65 (+/- 8.5) |
| Bone surface/bone volume (BS/BV; mm/mm2) | 4.73 (+/- 2.51) | 4.95 (+/- 1.79) | 4.44 (+/- 2.36) |
| Osteocyte-filled lacunae (%) | 57.37 (+/- 8.55) | 60.45 (+/- 8.55) | 61.34 (+/- 8.89) |
| Osteoblast count (N cells)* | 22.93 (+/- 8.14) | 26.00 (+/- 9.23) | 41.44 (+/- 19.22) |
| Osteoclast count (N cells)* | 10.73 (+/- 3.41) | 11.8 (+/- 3.45) | 11.8 (+/- 2.64) |
3 fields at 400 × magnification.
Capillary density was greater in the rhBMP-2 + VEGF group (Mean= 32.14%) than rhBMP-2 alone (Mean= 18.98%) and control group (Mean= 13.35%; Figure 7). This difference was statistically significant (p<0.05). The use of rhBMP-2 and VEGF combined increased cortical blood flow more than rhBMP-2 and control group (p<0.05). The mean cortical blood flow was 0.12 ml/min/100 g in Group 1 (control), 0.15 ml/min/100 g in Group 2 (rhBMP-2) and 0.25 ml/min/100 g in Group 3 (rhBMP-2 + VEGF; Figure 8). The percentage of cortical bone lacunae occupied by osteocytes (a measure of osteocyte viability) was 57.37 %, 60.45% and 61.34% for groups 1, 2 and 3 respectively. We observed a trend towards higher rates of bone formation (BFR) in the rhBMP-2 + VGF and rhBMP-2 group than in the control group, but this were not statistically significant (p=0.059). Also, MAR mean values were higher in Group 3 and 2 compared to the control group, with no significant difference between groups. Osteoblast counts were greater in the rhBMP-2 + VEGF group than either the rhBMP-2 or control group, with a significant difference between them (p<0.05) (Figure 9). There was a strong correlation between capillary density and cortical blood flow values (Pearson correlation coefficient= 0.938). Local administration of either VEGF or rhBMP-2 augmented angiogenesis and bone blood flow from implanted blood vessels. This effect is amplified when used together. We found a similar positive effect on numbers of osteoblasts.
Figure 7.
Capillary density (% total bone area)
Figure 8.
Cortical blood flow (ml/min/100 g)
Figure 9.
Osteoblast Count (mean number of cells: 3 random fields at 400X magnification)
Discussion
Growth factors have been of interest to investigators as a means to improve rates of bone healing and enhance new bone formation in difficult clinical circumstances21, 22. Local, sustained delivery of the growth factor may be achieved by implantation of biodegradable PLGA microspheres at the time of surgery23. The slow and regular release achieved, and their ability to deliver the factors in the area of concern may be important to achieve a physiologic effect. Multiple growth factors can be encapsulated together for possible synergistic effect24.
Several types of bone morphogenic proteins have been identified that may significantly accelerate bone regeneration in both human and animal models25, 26. The recombinant human BMP-2 used in this study has been shown to stimulate osteogenic differentiation of both mesenchymal steam cells and osteoblasts in standard tissue culture settings when used alone or in combination with VEGF27, 28,29. Their successful delivery by PLGA microsphere encapsulation has been demonstrate in vivo and in vitro30, 31. We have observed that the rhBMP-2 had the expected action in promoting greater bone growth (greater MAR, BFR and Osteoblast Count values), as cited, but also had angiogenic effects that increased cortical blood flow and the density of newly-formed capillaries when compared with the control group (greater Capillarity Density and Hydrogen Washout values).
We have previously reported the value of the angiogenic growth factor VEGF to promote angiogenesis from AV bundles implanted into necrotic autogenous bone. Either direct delivery or endothelial cell viral transfection will promote new capillary formation32, 33. VEGF has been reported to work synergistically with fibroblast growth factor 2 to stimulate angiogenesis in vitro34 and in vivo5, 35. It will similarly induce neovascularization from vessels implanted in cryopreserved allograft bone36. When used in combination, rhBMP-2 and VEGF have a synergic effect in bone formation24, 37, 39, 40. Our data supports these previous reports, demonstrating combined delivery to increase neovascularization (capillarity density), bone blood flow (hydrogen washout) and bone formation (histomorphometric analysis and osteoblast count), when compared to rhBMP-2 or buffered saline alone. It is likely that VEGF-stimulated neoangiogenesis improves the local environment to enhance the function of rhBMP-2-stimulated osteoblasts5. Bone viability was similar in all groups, as demonstrated by the percentage of osteocyte-occupied cortical bone lacunae. This demonstrates the effectiveness of our allotransplantation method, which maintains allotransplant viability short-term using Tacrolimus immunosuppression, and long-term viability due to angiogenesis from the implanted autologous vessels. No long-term drug therapy or effort to induce a state of tolerance is needed, as in conventional organ or composite tissue allotransplantation. This study demonstrates that growth factors may have a role to play to improve the rate and extent of vessel proliferation and osteoblast stimulation in allotransplanted bone. In the future, a large animal study with orthotopic reconstruction of a segmental defect may better emulate clinical use and serve as a bridge to clinical bone allotransplantation. It is possible that future clinical use may also include local delivery of VEGF and rhBMP-2.
Acknowledgements
This study was funded by the National Institutes of Health (NIH) (grant number RO1 AR49718).
The authors thank Fujisawa Pharmaceutical Co., Ltd., Osaka, Japan, for the generous donation of FK-506.
The authors acknowledge the Mayo Statistics Department for this article Statistical analysis, especially Christina M. Wood-Went for her work in this research.
Footnotes
The authors declare that there are no further conflicts of interested related to this research.
References
- 1.Bishop AT, Pelzer M. Vascularized bone allotransplantation: current state and implications for future reconstructive surgery. Orthop Clin North Am. 2007;38(1):109–22. vii. doi: 10.1016/j.ocl.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Doi K, Kawai S, Shigetomi M. Congenital tibial pseudoarthrosis treated with vascularised bone allograft. Lancet. 1996;347(9006):970–1. doi: 10.1016/s0140-6736(96)91458-0. [DOI] [PubMed] [Google Scholar]
- 3.Hofmann GO, Kirschner MH. Clinical experience in allogeneic vascularized bone and joint allografting. Microsurgery. 2000;20(8):375–83. doi: 10.1002/1098-2752(2000)20:8<375::aid-micr6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Ohno T, Pelzer M, Larsen M, Friedrich PF, Bishop AT. Host-derived angiogenesis maintains bone blood flow after withdrawal of immunosuppression. Microsurgery. 2007;27(8):657–63. doi: 10.1002/micr.20427. [DOI] [PubMed] [Google Scholar]
- 5.Giessler GA, Zobitz M, Friedrich PF, Bishop AT. Host-derived neoangiogenesis with short-term immunosuppression allows incorporation and remodeling of vascularized diaphyseal allogeneic rabbit femur transplants. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2009;27(6):763–70. doi: 10.1002/jor.20764. [DOI] [PubMed] [Google Scholar]
- 6.Larsen M, Willems WF, Pelzer M, Friedrich PF, Yaszemski MJ, Bishop AT. Augmentation of surgical angiogenesis in vascularized bone allotransplants with host-derived a/v bundle implantation, fibroblast growth factor-2, and vascular endothelial growth factor administration. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 28(8):1015–21. doi: 10.1002/jor.21098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pelzer M, Larsen M, Chung YG, Ohno T, Platt JL, Friedrich PF, et al. Short-term immunosuppression and surgical neoangiogenesis with host vessels maintains long-term viability of vascularized bone allografts. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2007;25(3):370–7. doi: 10.1002/jor.20313. [DOI] [PubMed] [Google Scholar]
- 8.Muramatsu K, Bishop AT, Sunagawa T, Valenzuela RG. Fate of donor cells in vascularized bone grafts: identification of systemic chimerism by the polymerase chain reaction. Plastic and reconstructive surgery. 2003;111(2):763–72. doi: 10.1097/01.PRS.0000041532.11604.B5. [DOI] [PubMed] [Google Scholar]
- 9.Muramatsu K, Kurokawa Y, Kuriyama R, Taguchi T, Bishop AT. Gradual graft-cell repopulation with recipient cells following vascularized bone and limb allotransplantation. Microsurgery. 2005;25(8):599–605. doi: 10.1002/micr.20173. [DOI] [PubMed] [Google Scholar]
- 10.Arai K, Hotokebuchi T, Miyahara H, Arita C, Mohtai M, Sugioka Y, et al. Limb allografts in rats immunosuppressed with FK506. I. Reversal of rejection and indefinite survival. Transplantation. 1989;48(5):782–6. doi: 10.1097/00007890-198911000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Schoofs M, Cariou JL, Amarante J, Panconi B, Bovet JL, Baudet J. Microvascular free bone transfer: experimental technique on rat's femur. Microsurgery. 1984;5(1):19–23. doi: 10.1002/micr.1920050105. [DOI] [PubMed] [Google Scholar]
- 12.Kempen DH, Lu L, Zhu X, Kim C, Jabbari E, Dhert WJ, et al. Development of biodegradable poly(propylene fumarate)/poly(lactic-co-glycolic acid) blend microspheres. II. Controlled drug release and microsphere degradation. J Biomed Mater Res A. 2004;70(2):293–302. doi: 10.1002/jbm.a.30080. [DOI] [PubMed] [Google Scholar]
- 13.Kempen DH, Lu L, Kim C, Zhu X, Dhert WJ, Currier BL, et al. Controlled drug release from a novel injectable biodegradable microsphere/scaffold composite based on poly(propylene fumarate). J Biomed Mater Res A. 2006;77(1):103–11. doi: 10.1002/jbm.a.30336. [DOI] [PubMed] [Google Scholar]
- 14.Larsen M, Pelzer M, Friedrich PF, Bishop AT. Measurement of bone blood flow using the hydrogen washout technique-part II: Validation by comparison to microsphere entrapment. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2008;26(6):746–52. doi: 10.1002/jor.20561. [DOI] [PubMed] [Google Scholar]
- 15.Pelzer M, Larsen M, Friedrich PF, Bishop AT. Measurement of bone blood flow using the hydrogen washout Technique-Part I: quantitative evaluation of tissue perfusion in the laboratory rat. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2008;26(6):741–5. doi: 10.1002/jor.20562. [DOI] [PubMed] [Google Scholar]
- 16.Aukland K, Bower BF, Berliner RW. Measurement of Local Blood Flow with Hydrogen Gas. Circulation research. 1964;14:164–87. doi: 10.1161/01.res.14.2.164. [DOI] [PubMed] [Google Scholar]
- 17.Whiteside LA, Lesker PA, Simmons DJ. Measurement of regional bone and bone marrow blood flow in the rabbit using the hydrogen washout technique. Clin Orthop. 1977;122(122):340–6. [PubMed] [Google Scholar]
- 18.Cunningham CD, Schulte BA, Bianchi LM, Weber PC, Schmiedt BN. Microwave decalcification of human temporal bones. Laryngoscope. 2001;111(2):278–82. doi: 10.1097/00005537-200102000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Spalteholz W. Nebst Anhang: Über Knochenfärbung. S. Hirzel; Leipzig: 1914. Über das Durchsichtigmachen von menschlichen und tierischen Präparaten und seine theoretischen Bedingungen. [Google Scholar]
- 20.Taira H, Moreno J, Ripalda P, Forriol F. Radiological and histological analysis of cortical allografts: an experimental study in sheep femora. Arch Orthop Trauma Surg. 2004;124(5):320–5. doi: 10.1007/s00402-004-0653-x. [DOI] [PubMed] [Google Scholar]
- 21.Ennett AB, Kaigler D, Mooney DJ. Temporally regulated delivery of VEGF in vitro and in vivo. J Biomed Mater Res A. 2006;79(1):176–84. doi: 10.1002/jbm.a.30771. [DOI] [PubMed] [Google Scholar]
- 22.Solheim E. Growth factors in bone. Int Orthop. 1998;22(6):410–6. doi: 10.1007/s002640050290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hiraoka Y, Yamashiro H, Yasuda K, Kimura Y, Inamoto T, Tabata Y. In situ regeneration of adipose tissue in rat fat pad by combining a collagen scaffold with gelatin microspheres containing basic fibroblast growth factor. Tissue Eng. 2006;12(6):1475–87. doi: 10.1089/ten.2006.12.1475. [DOI] [PubMed] [Google Scholar]
- 24.Huang YC, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res. 2005;20(5):848–57. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 25.Geibel PT, Boyd DL, Slabisak V. The use of recombinant human bone morphogenic protein in posterior interbody fusions of the lumbar spine: a clinical series. Journal of spinal disorders & techniques. 2009;22(5):315–20. doi: 10.1097/BSD.0b013e31817d8161. [DOI] [PubMed] [Google Scholar]
- 26.Vaibhav B, Nilesh P, Vikram S, Anshul C. Bone morphogenic protein and its application in trauma cases: a current concept update. Injury. 2007;38(11):1227–35. doi: 10.1016/j.injury.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Chen G, Deng C, Li YP. TGF-beta and BMP Signaling in Osteoblast Differentiation and Bone Formation. International journal of biological sciences. 2012;8(2):272–88. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanczler JM, Ginty PJ, White L, Clarke NM, Howdle SM, Shakesheff KM, et al. The effect of the delivery of vascular endothelial growth factor and bone morphogenic protein-2 to osteoprogenitor cell populations on bone formation. Biomaterials. 2010;31(6):1242–50. doi: 10.1016/j.biomaterials.2009.10.059. [DOI] [PubMed] [Google Scholar]
- 29.Lane JM. Bone morphogenic protein science and studies. J Orthop Trauma. 2005;19(10 Suppl):S17–22. doi: 10.1097/00005131-200511101-00006. [DOI] [PubMed] [Google Scholar]
- 30.Isobe M, Yamazaki Y, Mori M, Amagasa T. Bone regeneration produced in rat femur defects by polymer capsules containing recombinant human bone morphogenetic protein-2. J Oral Maxillofac Surg. 1999;57(6):695–8. doi: 10.1016/s0278-2391(99)90435-4. discussion 9. [DOI] [PubMed] [Google Scholar]
- 31.Zhu L, Chuanchang D, Wei L, Yilin C, Jiasheng D. Enhanced healing of goat femur-defect using BMP7 gene-modified BMSCs and load-bearing tissue-engineered bone. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2010;28(3):412–8. doi: 10.1002/jor.20973. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki O, Bishop AT, Sunagawa T, Katsube K, Friedrich PF. VEGF-promoted surgical angiogenesis in necrotic bone. Microsurgery. 2004;24(1):85–91. doi: 10.1002/micr.10190. [DOI] [PubMed] [Google Scholar]
- 33.Katsube K, Bishop AT, Simari RD, Yla-Herttuala S, Friedrich PF. Vascular endothelial growth factor (VEGF) gene transfer enhances surgical revascularization of necrotic bone. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2005;23(2):469–74. doi: 10.1016/j.orthres.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Pepper MS, Ferrara N, Orci L, Montesano R. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun. 1992;189(2):824–31. doi: 10.1016/0006-291x(92)92277-5. [DOI] [PubMed] [Google Scholar]
- 35.Asahara T, Bauters C, Zheng LP, Takeshita S, Bunting S, Ferrara N, et al. Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo. Circulation. 1995;92(9 Suppl):II365–71. doi: 10.1161/01.cir.92.9.365. [DOI] [PubMed] [Google Scholar]
- 36.Willems WF, Larsen M, Giusti G, Friedrich PF, Bishop AT. Revascularization and bone remodeling of frozen allografts stimulated by intramedullary sustained delivery of FGF-2 and VEGF. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2011;29(9):1431–6. doi: 10.1002/jor.21338. [DOI] [PubMed] [Google Scholar]
- 37.Kempen DH, Lu L, Heijink A, et al. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30:2816–2825. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 38.Young S, Patel ZS, Kretlow JD, et al. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng A. 2009;15:2347–2362. doi: 10.1089/ten.tea.2008.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel ZS, Young S, Tabata Y, et al. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]








