Abstract
Purpose
ALT-801 is a bifunctional fusion protein comprising interleukin-2 (IL-2) linked to a soluble, single-chain T cell receptor domain that recognizes a peptide epitope (aa264-272) of the human p53 antigen displayed on cancer cells in the context of HLA-A*0201 (p53+/HLA-A*0201). We evaluated the safety, pharmacokinetics and pharmacodynamics of ALT-801 in p53+/HLA-A*0201 patients with metastatic malignancies.
Experimental Design
p53+/HLA-A*0201 patients were treated with ALT-801 on a schedule of 4 daily 15-minute intravenous infusions, then 10 days rest and 4 more daily infusions. Cohorts of patients were treated at 0.015, 0.040, and 0.080 mg/kg/dose.
Results
Four, sixteen, and six patients were treated at the 0.015, 0.04 and 0.08 mg/kg cohorts, respectively. Two dose limiting toxicities (a grade 4 transient thrombocytopenia and a myocardial infarction) in the 0.08 mg/kg cohort established the maximum tolerated dose (MTD) at 0.04 mg/kg. Patients treated at the MTD experienced toxicities similar to those associated with high-dose IL-2 but of lesser severity. The serum half-life of ALT-801 was 4 hours and ALT-801 serum recovery was as expected based on the dose administered. ALT-801 treatment induced an increase of serum interferon-γ but not tumor necrosis factor-α. Response assessment showed 10 subjects with stable disease at at least 11 weeks, and in one who had melanoma metastasis, there is an ongoing complete absence of identifiable disease after resection of radiographically identified lesions.
Conclusion
This first-in-man study defines an ALT-801 regimen that can be administered safely and is associated with immunological changes of potential antitumor relevance.
Keywords: Immunotherapy, interleukin-2, single-chain T cell receptor, p53, Phase 1 trial
Introduction
While treatment with high dose, bolus recombinant human interleukin-2 (IL-2; aldesleukin) can induce durable complete responses in a small fraction of patients with metastatic melanoma and kidney cancer, treatment is limited by significant toxicities, particularly hypotension, capillary leak syndrome and oliguria (1). One possible approach to improve the therapeutic ratio for IL-2-based treatment is more efficient targeting of lymphocytes with activated IL-2 receptor into the tumor microenvironment. To achieve this, ALT-801 has been developed as a tumor-targeting immunotherapeutic (2). Tumor cell recognition by ALT-801 is mediated by a T cell receptor domain expressed in a recombinant single-chain format (scTCR) that has high-affinity for a tumor associated antigen peptide spanning amino acids 264-272 of the p53 protein, presented on the cell surface by the HLA-A*0201 complex (3). In the ALT-801 protein, this scTCR has been engineered genetically to be linked to an IL-2 molecule. Intermediate and high affinity IL-2 receptors on lymphocytes can be ligated by ALT-801, with subsequent immune activation comparable to that from unmodified IL-2. Theoretically, ALT-801 activated lymphocytes will have the same phenotype as IL-2 activated lymphocytes, but with the additional feature of a protein domain that recognizes a target on tumor cells.
The p53 protein has diverse physiologic function, including regulation of the cell cycle, and particularly G2/M arrest in the context of DNA damage. When p53 is mutated, as it is in many cancers, two key changes occur. Central to the malignant phenotype, the G2/M checkpoint function is impaired and cell cycling without adequate repair is accelerated. Additionally, the stability of the normally labile protein increases, resulting in its accumulation in tumor cell s and elevated presentation of p53-derived peptide antigens on the cell surface s (4, 5). The p53 (aa 264-272)/HLA-A*0201 complex is significantly elevated in a wide range of human tumor tissues (6, 7). The quantitatively differential display of p53 peptide antigens on tumor versus on normal cells defines an opportunity to use p53 positivity to direct TCR-based immunotherapeutics to target tumor cells. In several human xenograft murine models, we have demonstrated that ALT-801 exhibits potent antitumor activity against p53+/HLA-A*0201 tumors but not p53-/HLA-A*0201 tumors regardless of the tissue of origin of the tumors (2, 3, 8). Thus, ALT-801 has therapeutic potential as an immunotherapeutic targeting any tumor which is p53+ in an HLA-A*0201 individual.
In this first-in-man, phase 1 dose escalation study (ClinicalTrials.gov ID: NCT00496860), patients whose tumors bore p53 (aa 264-272)/HLA-A*0201 complexes were treated with ALT-801. The goals of the study were to determine the safety, maximally tolerated dose and the anticancer activity of ALT-801 as monotherapy in humans. We assessed ALT-801 pharmacokinetics and pharmacodynamic effects of ALT-801 on peripheral blood lymphocytes and serum cytokines.
Materials and Methods
Study population
The study protocol and consents were approved by the institutional review boards of each clinical site. Eligible subjects were 18 years of age or older with progressive metastatic malignancy considered surgically and medically incurable who provided written informed consent. Serologic HLA-A2 testing (reactivity to anti-human HLA-A2 monoclonal antibodies (mAbs), BB7.2 and MA2.1) was used to screen subjects and if positive, formalin-fixed paraffin-embedded tumor specimens were tested at a central laboratory (Altor Bioscience Corp.) for immunohistochemically identifiable p53 (aa264-272)/HLA-A*0201. This assessment was scored as 0, 1+, 2+ or 3+, using p53 peptide-specific and control STAR™ multimer reagents as described previously (7). Patients were eligible for enrollment if their tumor cells stained with a ≥1+ score relative to non-tumor cells.
Eligibility criteria included: adequate cardiac function (pre-treatment testing at treatment physician discretion); pulmonary reserve (for smokers, FEV1 ≥ 75% required); liver (aspartate aminotransferase and alkaline phosphatase ≤ 2.5× upper limit of normal [ULN]; PT, aPTT ≤ 1.5× ULN); kidney (creatinine ≤ 1.5× ULN); and marrow function (absolute neutrophil count ≥ 1,500/μL, platelets ≥100,000/μL, hemoglobin ≥ 10g/dL); an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; life expectancy >12 weeks; and no concurrent corticosteroid use. Prior therapies must have been completed more than four weeks before enrollment (> six weeks for nitrosoureas and > eight weeks for monoclonal antibodies).
Treatment plan
Inpatient hospitalization and placement of a central venous catheter were required. Infusions were performed at 4 U.S. clinical sites that had extensive experience in IL-2 (aldesleukin, Proleukin™) high dose bolus therapy. The treatment course was a cycle of four daily 15 minute intravenous infusions, with planned discharge home on the 5th day, then a 10-day interval off treatment, followed by a second similar cycle. This is illustrated in Table 1A. A second course of treatment was offered to patients with stable disease (SD), partial response (PR) or complete response (CR), at 7 weeks or later. Dose omission criteria are below.
Table 1.
A, the 2-cycle treatment course of ALT-801. B, dose escalation scheme and number of patients treated.
| A. | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 1 (Cycle 1) | Week 2 (Rest) | Week3 (Cycle 2) | |||||||||||||||||||
| Day | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| ALT-801 | X | X | X | X | X | X | X | X | |||||||||||||
| B. | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | ALT-801 dose (mg/kg) | Number of patients treated | |||||||||||||||||||
| 1 | 0.015 | 4 | |||||||||||||||||||
| 2 | 0.04 | 6 + MTD expansion | |||||||||||||||||||
| 3 | 0.08 | 6 | |||||||||||||||||||
| MTD expansion | 0.04 | 10 | |||||||||||||||||||
The definition for dose limiting toxicity (DLT) was based on the fact that toxicities of IL-2 are often significant, but rapidly reversible. The DLT events included those which would cause a discontinuation of the treatment during the first treatment course, specifically: grade 2+ cardiac arrhythmia, ischemia or infarction; systolic hypotension below 90 mmHg and longer than 24 hours with vasopressor; grade 4 hypotension; proteinuria of grade 3+ for over 3 days; grade 3+ liver function abnormality (alkaline phosphatase, ALT, AST) not recovering within 3 days or occurring for a second time; platelet count below 50,000 per mm3 for over 3 days; grade 2 allergic reaction with bronchospasm, or grade 3+ allergic or infusion-related reactions; other grade 3 events that were either not controllable or symptomatic and which persisted for more than 3 days (without resolution to grade 1 or 0) or which recurred after retreatment; or grade 4 events (except controllable nausea or vomiting that resolved to grade 1 in less than 72 hours, or lymphopenia, which may be directly attributed to the mechanism of action).
Based on the pharmacokinetic (PK) profile in animals, the plasma concentrations of ALT-801 achieved at relatively low dose levels were expected to be sufficient to fully occupy intermediate-affinity (Kd 10-9M) and high-affinity (Kd 10-11M) IL-2 receptors on the surface of leukocytes (3, 9). In addition, animal toxicity studies suggested the potential side effects of ALT-801 treatment would be qualitatively similar to that of IL-2 therapy. Thus, a dose level of 0.015 mg/kg/dose (equivalent to approximately 50,000 IL-2 IU/kg) was chosen as the first dosing level with a subsequent planned dose escalation up to 0.16 mg/kg/dose in a total of up to five cohorts (Table 1B).
A conventional 3+3 format dose escalation was used, with cohort escalation if 0 of 3 subjects had DLT events or expansion to 6 subjects if 1 of 3 subjects had DLT events. If 0 to 1 of 6 subjects had DLT events, the dose level would be considered tolerated. If 2 or more of initial 3-6 subjects had DLT events, the dose level would be considered to exceed the maximum tolerated dose. Ten additional patients were treated at the maximum tolerated dose (MTD).
Subject evaluations & correlative testing
Subjects had daily blood tests (complete blood count (CBC) and comprehensive metabolic panel) during inpatient treatment, with additional testing as clinically indicated. Clinical response, determined by the Response Evaluation Criteria in Solid Tumors (RECIST) (1.0) (10), was assessed at 7 weeks (corresponding to 4 weeks after end of treatment), at 11 weeks as necessary to confirm a response at 7 weeks, or as otherwise clinically indicated.
Pharmacokinetics (PK)
Blood samples were collected at pre-dosing on the first study and at approximately 15 and 30 min, and 1, 2, 4, 8, 12 and 24 hours post-dosing on the first and fourth days in each of cycles. Serum concentrations of ALT-801 were determined using two different sandwich enzyme-linked immunosorbent assays (ELISAs) in which the sample was captured on anti-TCR Cβ mAb (BF-1)-coated wells and detected with either biotinylated anti-IL-2 mAb (Pierce, Rockford, IL) or biotinylated anti-TCR Cβ mAb (W4F), as described previously (3). The first ELISA format measures intact ALT-801 whereas the second ELISA detects the TCR domain of ALT-801 only.
Immunogenicity
An ELISA method was used to screen for antibodies with specificity for ALT-801. A cell proliferation assay using IL-2-dependent CTLL-2 cells was used to test for neutralizing antibodies to the IL-2 domain of ALT-801 (8).
Pharmacodynamics
Serum interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) levels were measured by ELISA methods according to the manufacturer's instructions (BD Bioscience-Pharmingen, San Diego, CA). To detect immune cell activation in patients during the ALT-801 treatment course, peripheral blood mononuclear cells (PBMCs) were prepared from patient blood samples collected pre-dosing (Cycles 1 & 2) and 2 hours after the drug infusion of doses 3 and 7. Following overnight incubation of PBMCs (2 × 104 cells /well) in medium alone or medium containing ALT-801 (0.2 μg/well), p53-positive A375 tumor cells (1 × 104 cells /well) or ALT-801 (0.2 μg/well) + A375 tumor cells (1 × 104 cells/well), IFN-γ positive cells were measured in anti-human IFN-γ antibody-coated ELISPOT plates (Biolegend, San Diego, CA).
Medication supply
ALT-801 (8) provided by Altor BioScience Corporation in 2 mg/ml solution and reconstituted in D5W. The study drug was produced under cGMP conditions by recombinant CHO.K1 cells in serum-free culture medium and purified by immunoaffinity chromatography using the BF-1 mAb capture followed with ion-exchange chromatography and nanofiltration (8).
All supportive medications were from commercial supply.
Results
Serologic HLA-A2 and target-epitope screening
Between August 2007 and May 2009, 118 patients were consented and screened, of whom 67 were HLA-A2 positive. Of the 67 positive for HLA-A2, 56 had tumor specimens with positive test results for the target p53 (aa 264-272)/HLA-A*0201 complex. Fig. 1A shows representative positive immunohistochemistry (IHC) staining of tumor specimen from patients with melanoma and renal cell carcinoma using the p53 (aa 264-272)/HLA-A*0201-specific and control STAR™ multimer reagents (7). Positive p53 (aa 264-272)/HLA-A*0201 staining of specimens of other tumor types from other subjects is shown in Fig. 1B. Some of the HLA-A2 positive patients with p53+/HLA-A*0201 tumors either withdrew consent or did not meet other inclusion/exclusion criteria (N = 30).
Figure 1.
Detection of p53 (aa264-272)/HLA-A*0201 complexes on formalin-fixed paraffin-embedded tumor specimens. A, IHC staining of tumor specimens from patients with melanoma and renal cell carcinoma (RCC) using p53 (aa 264-272)/HLA-A*0201-specific (264scTCR) and control (CVMscTCR) reagents. B, positive IHC staining of other tumor histologies using the p53 (aa 264-272)/HLA-A*0201-specific reagent. The staining of tumor cells was assessed as 3+ (4006, 1011, 1006, 2004) or 2+ (2001, 2002) versus 1+ for normal cells in these specimens.
Clinical characteristics
Twenty-six patients received at least one dose of ALT-801, with an age range of 27-79 years. The most frequent diagnoses were melanoma and clear cell kidney cancer. The dose levels are shown in Table 1B; subjects' clinical characteristics are summarized in Table 2. Three subjects were withdrawn from the study during treatment due to adverse events (AEs). One subject died during the 28-day follow-up period, due to disease progression.
Table 2.
Patient characteristics by dose level.
| Dose Level | 0.015 mg/kg/dose | 0.040 mg/kg/dose | 0.080 mg/kg/dose | Retreatment |
|---|---|---|---|---|
| Subjects, n | 4 | 16 | 6 | 3 |
| Age, y, Median (Range) | 65 (50 - 79) | 51 (27 - 72) | 58 (53 - 65) | 45 (27 - 47) |
| Gender, M:F, n | 2: 2 | 10: 6 | 5:1 | |
| Race, n (%) | ||||
| - Caucasian | 4 (100%) | 15 (94%) | 6 (100%) | 3 (100%) |
| - Black, African/American | 1 (6%) | |||
| ECOG Performance Status, n (%) | ||||
| 0 | 3 (75%) | 10 (63%) | 5 (83%) | 2 (67%) |
| 1 | 1 (25%) | 6 (38%) | 1 (17%) | 1 (33%) |
| Tumor Type (n) | Melanoma (1), renal cell carcinoma (1), colon cancer (1), neuroendocrine cancer (1) | Melanoma (6), renal cell carcinoma (4), prostate cancer (2), transitional cell carcinoma (1), collecting duct renal cancer (1), Hodgkin's lymphoma (1), head and neck cancer (1) | Melanoma (3), renal cell carcinoma (3) | Melanoma (1), collecting duct renal cancer (1), head and neck cancer (1) |
| Disease Stage, n (%) | ||||
| III | 1 (25%) | 2 (13%) | 1 (17%) | 1 (33%) |
| IV | 3 (75%) | 14 (88%) | 5 (83%) | 2 (67%) |
Adverse events
The range of observed AEs was similar across the three tested dose levels, with a high frequency of IL-2-associated side effects. As shown in Table 3, the most frequent constitutional AEs observed in the dose escalation and MTD expansion phase of the study were fever (100% of subjects), chills/rigors (85%) and fatigue (42%). Common laboratory AEs were asymptomatic electrolyte imbalances (hypophosphatemia, hypocalcemia, hyponatremia) (81% of subjects) and hypoalbuminemia (96%). Hematologic changes reported as AEs included thrombocytopenia (69% of subjects), anemia (65%) and lymphopenia (35%). Frequent cardiac-related AEs were hypotension (62% of subjects) and transient asymptomatic arrhythmias (42%).
Table 3.
Profile of adverse event, any attribution, of severity grade 3+ or occurring in more than 30% of patients.
| Number of Patients with AEs by ALT-801 Dose Level | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organ-System | 0.015 mg/kg (n of 4) | 0.040 mg/kg (n of 16) | 0.080 mg/kg (n of 6) | All AEs | ||||||||||
|
|
|
|
|
|||||||||||
| - CTCAE Term Grade | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | n | (%) |
| Constitutional | ||||||||||||||
| - Fever | 1 | 3 | 0 | 0 | 3 | 9 | 4 | 0 | 0 | 5 | 1 | 0 | 26 | (100%) |
| - Rigors/chills | 0 | 2 | 0 | 0 | 2 | 12 | 0 | 0 | 1 | 4 | 1 | 0 | 22 | (85%) |
| - Fatigue | 1 | 1 | 0 | 0 | 4 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 11 | (42%) |
| Gastrointestinal | ||||||||||||||
| - Nausea | 1 | 0 | 0 | 0 | 7 | 3 | 0 | 0 | 3 | 0 | 0 | 0 | 14 | (54%) |
| - Vomiting | 0 | 1 | 0 | 0 | 9 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 12 | (46%) |
| - Diarrhea | 0 | 0 | 0 | 0 | 5 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 8 | (31%) |
| Blood | ||||||||||||||
| - Thormbocytopenia | 3 | 0 | 0 | 0 | 4 | 5 | 0 | 1 | 0 | 4 | 0 | 1 | 18 | (69%) |
| - Anemia | 1 | 1 | 0 | 0 | 4 | 6 | 2 | 0 | 0 | 3 | 0 | 0 | 17 | (65%) |
| - Lymphopenia | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 6 | 0 | 0 | 1 | 1 | 9 | (35%) |
| Cardiac/Vascular | ||||||||||||||
| - Hypotension | 1 | 1 | 0 | 0 | 1 | 7 | 1 | 0 | 1 | 2 | 2 | 0 | 16 | (62%) |
| - Supraventricular/sinus tachycardia | 0 | 0 | 0 | 0 | 7 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 11 | (42%) |
| - Cardiac ischemia/infarction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | (8%) |
| - Thrombosis/embolism | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | (8%) |
| - Elevated c-troponin I | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | (4%) |
| Hepatic | ||||||||||||||
| - Elevated alkaline phosphatase | 1 | 0 | 0 | 0 | 5 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 10 | (38%) |
| - Elevated bilirubin | 0 | 1 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 2 | 0 | 0 | 7 | (27%) |
| Pulmonary | ||||||||||||||
| - Hypoxia | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 3 | (12%) |
| - Pleural effusion | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | (4%) |
| - Pneumonitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | (4%) |
| Renal | ||||||||||||||
| - Hypoalbuminemia | 1 | 2 | 0 | 0 | 2 | 11 | 3 | 0 | 0 | 4 | 2 | 0 | 25 | (96%) |
| - Decreased urine output | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 3 | (12%) |
| Skin | ||||||||||||||
| - Pruritus | 0 | 0 | 0 | 0 | 3 | 6 | 0 | 0 | 1 | 3 | 0 | 0 | 13 | (50%) |
| - Rash/desquamation | 0 | 2 | 0 | 0 | 3 | 4 | 1 | 0 | 1 | 1 | 0 | 0 | 12 | (46%) |
| Pain | ||||||||||||||
| - Musculoskeletal | 0 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 8 | (31%) |
| - Pulmonary | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | (8%) |
| - Chest | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | (4%) |
| - General | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | (4%) |
| Metabolic | ||||||||||||||
| - Hypophosphatemia | 0 | 0 | 2 | 0 | 1 | 5 | 8 | 1 | 0 | 0 | 4 | 0 | 21 | (81%) |
| - Hypocalcemia | 0 | 3 | 0 | 0 | 3 | 8 | 1 | 0 | 2 | 2 | 1 | 0 | 20 | (77%) |
| - Hyponatremia | 0 | 0 | 2 | 0 | 11 | 0 | 2 | 0 | 4 | 0 | 0 | 0 | 19 | (73%) |
| - Hypokalemia | 1 | 1 | 0 | 0 | 7 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 11 | (42%) |
| - Hypomagnesemia | 1 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 11 | (42%) |
| - Hyperglycemia | 0 | 0 | 0 | 0 | 4 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 6 | (23%) |
| - Hyperkalemia | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | (12%) |
Dose escalation experience
The first subject (age 79, metastatic renal cancer) on the first dose level (0.015 mg/kg/dose) had moderate hypotension, was treated with dopamine, and resolved within 24 hours. The protocol initially designated this as an AE causing discontinuation of treatment (which would be a DLT event); the protocol was revised. The patient was offered further treatment but declined and was replaced. No DLT events occurred in the next 3 subjects in cohort 1 and enrollment to the second cohort commenced.
In the 0.04 mg/kg/dose cohort, the third subject (age 30, refractory Hodgkin's lymphoma) experienced a grade 4 pleural effusion, designated as a DLT. The subject was intubated and required extensive supportive hospitalization, but recovered and was discharged from hospital. There were no further DLT events in cohort 2 (0.04 mg/kg/dose, 6 subjects) and the third cohort commenced.
In the 0.08 mg/kg/dose cohort, a subject (age 65, metastatic melanoma) had a DLT event of grade 4 thrombocytopenia that was asymptomatic. The event lasted 6 days and resolved without intervention. This event occurred after the full course of study drug infusion had been given. The treating investigator-designated attribution was not to the study drug; fluid administration and the disease site may have contributed. A second subject (age 64, nodular melanoma) had an acute myocardial infarction (grade 4 toxicity) as a DLT event after the sixth dose of ALT-801. This was considered probably related to the study drug; however, previously unsuspected high-grade atherosclerotic coronary obstruction was subsequently identified. Following angioplasty, the event resolved and the subject was discharged from the hospital and had no significant cardiac symptoms after discharge. A third serious AE in cohort 3 was grade 3 pneumonitis after the 5th dose, requiring prolonged hospitalization. The event subsequently resolved without additional treatment or intervention. A fourth event was an uncomplicated catheter-associated thrombosis, which was likely not drug- or dose-related, but was still considered directly related to the study procedures. The two DLTs of the 0.08 mg/kg cohort established the MTD at 0.04 mg/kg.
Three patients received a second course of treatment. For one of these patients (age 27, collecting duct/spindle cell cancer of kidney), abrupt visual loss occurred that was related to previously unappreciated bulky metastasis within the sinus causing impingement on the optic chiasm. This toxicity represented disease progression and was not attributed to ALT-801 treatment; however, ALT-801 treatment was discontinued.
In coordination with the pharmacodynamic testing (see below), the original treatment plan and discussion with the members of the Data Safety Monitoring Board, the 0.04 mg/kg/dose level was designated as the MTD, completing the dose escalation phase of the study. Ten additional patients were treated at this dose level, as an expansion phase of the study. In the expansion cohort, the toxicities were qualitatively similar to those from the escalation phase, dominated by low-grade constitutional symptoms, gastrointestinal symptoms and hypotension (Table 2).
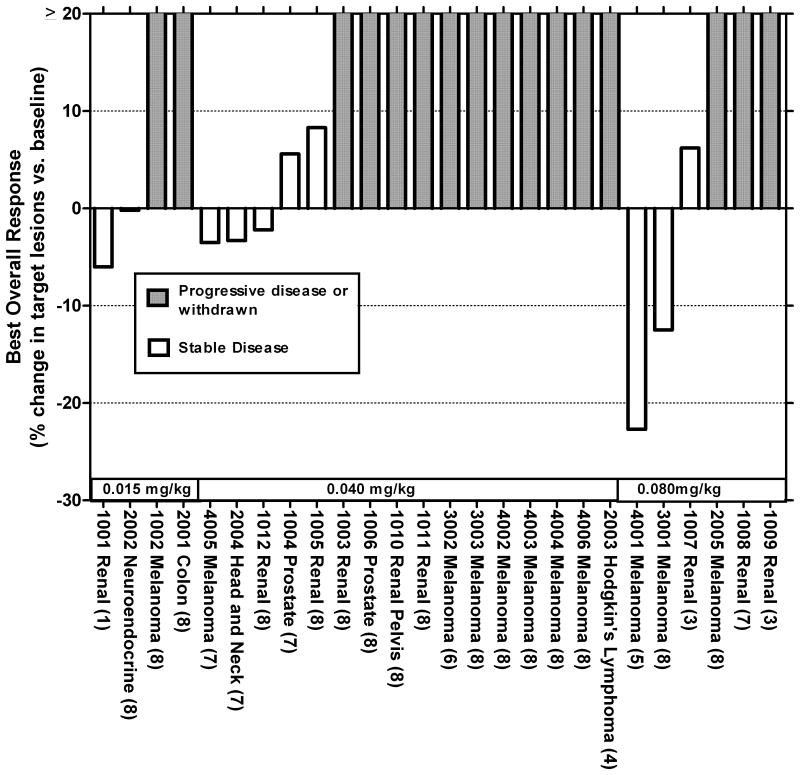
Tumor response
Fig. 2 shows the best response, by diagnosis and by cohort. Melanoma was the most frequent diagnosis, and the one for which there was the most identifiable stabilization or regression. One subject (age 47, malignant melanoma) died within 28 days of last treatment (day +26); the treating physician attributed the death to disease progression.
Figure 2.
Tumor assessment by RECIST of subjects following a single course of ALT-801 treatment. For each subject, the number of ALT-801 doses actually administered of the planned 8 doses is shown in parentheses. Subjects with new lesions on assessment or who withdrew from the study prior to assessment were labeled as progressors.
Of the 26 subjects enrolled 3 (Patients 1010, 4002 and 4003) had low level positive staining for p53 (aa 264-272)/HLA-A*0201 complex on their tumor specimens. None of these 3 subjects showed tumor response post treatment whereas stable disease was seen in 10 of 23 patients with tumor specimens staining at “2+” or greater for p53 (aa 264-272)/HLA-A*0201 complex.
In the 0.04 mg/kg cohort, one subject with head and neck cancer, had a target lesion of 30 mm in the nasopharyngeal sinus, and had experienced progression of disease after two courses of cisplatin and docetaxel, prior to enrollment into the ALT-801 study. The patient had stable disease after the first course of ALT-801 treatment, and then received the second course of ALT-801 treatment five months after the completion of the first. The stable disease continued to last for another 3.5 months with no other anticancer therapy.
Of the subjects receiving 0.08 mg/kg ALT-801, one who was diagnosed with stage IIIc had melanoma originating from a primary lesion on his left lower leg with subsequent development of multiple in-transit metastases, as well as enlarged inguinal lymph nodes Prior therapies included isolated limb perfusion but with subsequent progression clinically and by FDG-PET. He was then enrolled on a clinical study of dacarbazine (DTIC) ± the experimental drug PI-88 (Progen Pharmaceuticals, Queensland, Australia, NCT00130442). He was randomized to DTIC alone and received 15 cycles (11 months) of treatment. At that point, the disease status was stable disease. He elected to crossover to PI-88 and received 10 days of treatment but developed an anti-heparin antibody response requiring termination from the study. Two months later, at the point of entry on the treatment reported in the present trial, sites of disease were multiple in-transit metastases of the left leg with an 8 mm target lesion and smaller lesions distally in the left groin. After one 8-dose course at the 0.08 mg/kg level, there was confirmed clinically and radiologically stable disease at week 11 (Fig. 2; Patient 3001). At 7 months after the first dose of ALT-801, restaging CT and FDG-PET scans and a series of biopsies (outside the planned treatment assessment of the present trial) were collected and showed no new lesions and no evidence of residual melanocytic tumor in the specimens examined. There were no pre-ALT-801 PET scans or biopsies for comparisons. The patient continues to exhibit no evidence of disease at 2+ years post ALT-801 treatment, without any additional anticancer therapy.
Two other subjects in the 0.08 mg/kg/dose cohort also had SD; this was despite several missed doses during the course of ALT-801 treatment.
Altogether, ten patients (38%) of 26 with various metastatic malignancies met SD criteria as best response. Available post-study data for these SD patients with various diagnoses indicated a median progression free survival of 7.3 months and a median overall survival of 21 months (Table 4). At least eight of the 10 SD patients survived greater than one year from the initiation of ALT-801 treatment.
Table 4.
Post-study treatment experience of patients with stable disease.
| Subject ID | Disease | ALT-801 dose level (mg/kg) | Time to progression (months)a | Survival (months)a |
|---|---|---|---|---|
| 1001 | RCC | 0.015 | 7.7+ b | 46.9+ c |
| 2002 | Neuroendocrine | 0.015 | 3.9 | 12.4 |
| 1004 | Prostate | 0.04 | 2.3+ d | 19.9+ d |
| 1005 | RCC | 0.04 | 1.4+ d | 1.4+ d |
| 1012 | RCC | 0.04 | 2.8 | 11.6 |
| 2004 | Head and Neck | 0.04 | 10.4 | 21.0 |
| 4005 | Melanoma | 0.04 | 5.0 | 12.4 |
| 1007 | RCC | 0.08 | 3.0 | 16.1 |
| 3001 | Melanoma | 0.08 | 29.0+ e | 29.0+ c |
| 4001 | Melanoma | 0.08 | 9.5 | 35.4+ c |
Contribution of post-study therapy, if any, not assessed
No recent tumor assessment available
Alive as of last report
Lost to follow-up
No evidence of disease as of last report
Pharmacokinetics of ALT-801
Maximum concentration (Cmax) of 405, 875 and 2335 ng/mL occurred 15 to 30 minutes after the start of the first dose of ALT-801 at the 0.015, 0.04, 0.08 mg/kg/dose groups, respectively. The Cmax observed in subsequent dosing was comparable to the values for the initial dosing (data not shown). Assuming an average plasma volume of ∼40 ml/kg and uniform plasma distribution of the study drug, the calculated mean percent recovery of ALT-801 was 108%, 88%, 117% for the 0.015, 0.04 and 0.08 mg/kg/dose levels, respectively. Additionally, the ALT-801 molecule appeared intact throughout the study, based on the results of two ELISAs (data not shown).
Measurement of serum half-life on the initial dose showed comparable values across the dose range (4.4, 4.2, and 4.6 hours for the 0.015, 0.04, 0.08 mg/kg/dose cohorts, respectively). On subsequent doses of ALT-801, there was more variation, with the half-life range from 2.0 to 4.2 hours, depending on dose level and dose number. In subjects administered a second course of ALT-801 at 0.04 mg/kg, a Cmax of about 783 ng/mL and a serum half-life of 3.7 hours were observed after the first dose of the second course, comparable to the first-course treatment parameters.
In summary, the Cmax was proportional to dose and the serum initial half-life of ALT-801 was approximately four hours.
Immunogenicity
Immunogenicity testing by ELISA indicated that at 4 weeks after initial treatment, most treated patients developed anti-ALT-801 antibodies as indicated by an increase in serum antibody titers compared to predose levels. For the 16 subjects receiving 0.04 mg/kg ALT-801, there was considerable variability in anti-ALT-801 antibody levels at week 4 with one subject showing no detectable titer and two subjects with titers greater than 20,000. Overall, the relative levels of anti-ALT-801 antibody decreased at the week 7 and 11 follow-ups (Fig. 3). No IL-2 neutralizing antibodies were detectable in any sample, including samples with high anti-ALT-801 titers. Additionally, none of the three subjects administered a second course of ALT-801 treatment experienced any allergic reaction following dosing.
Figure 3.
Immunogenicity analysis. Serum anti-ALT-801 Ab levels were determined in subjects at 4, 7 and 11 weeks after the initiation of treatment with 0.015 (closed squares), 0.04 (open triangles) or 0.08 mg/kg (closed triangles) ALT-801. Values expressed as median ± interquartile range.
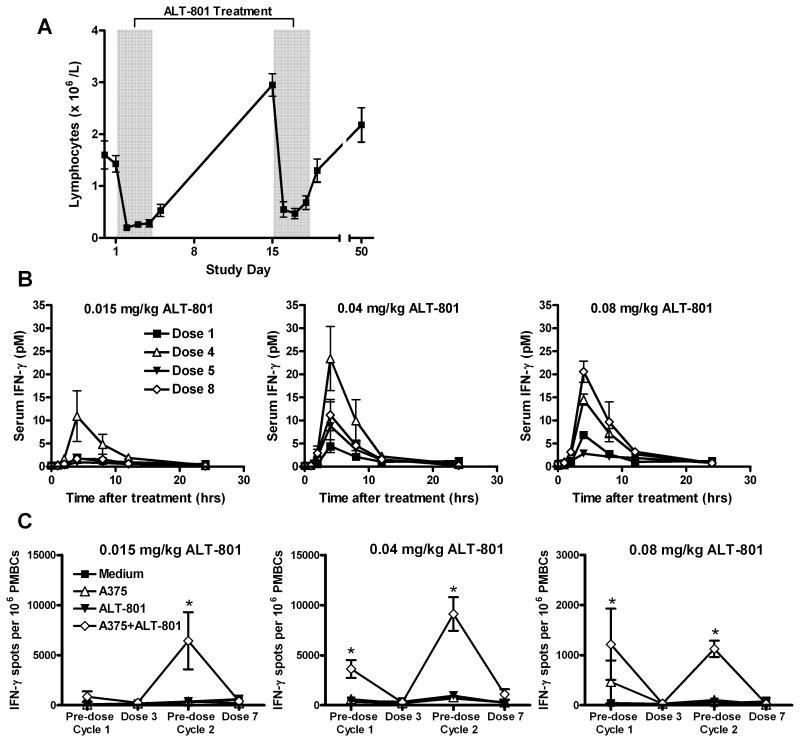
Lymphocyte count and functional testing
An anticipated basic mechanistic feature triggered by ALT-801 treatment is the migration of IL-2 receptor activated lymphocytes from the circulation into the peripheral tissues and p53-positive tumor (3). This mechanism is consistent with the observed induced (total) lymphopenia, as evident in the daily blood cells analysis obtained during the treatment course (Fig. 4A), which may be migration into the tumor, the periphery or another location. Pharmacodynamic measurements included serum IFN-γ and TNF-α levels and ELISPOT titer of IFN-γ producing lymphocytes when tested with A375 human melanoma tumor cells (which bear the p53 (aa264-272)/HLA-A*0201 complex). Following ALT-801 administration, serum IFN-γ levels increased and peaked 4 hours post-treatment before returning to baseline levels at 24 hours (Fig. 4B). These changes were more pronounced in the later doses of the 4-day treatment cycles. In contrast, there was no change in serum TNF-α levels following ALT-801 administration. A detectable increase of numbers of lymphocytes with IFN-γ production when exposed to ALT-801 and A375 cells was observed in PBMC specimens obtained just prior to the second course (Fig. 4C). This was seen at about the same level in the 0.04 mg/kg/dose cohort as in the 0.08 mg/kg/dose cohort, an observation that suggested that the higher dose was not definitely associated with a higher detectable immune response, so that continuation with the development of the 0.04 mg/kg/dose was supported by this portion of the correlative testing. Further studies are required to evaluate leukocyte types and antigen specificities responsible for these cell-mediated responses.
Figure 4.
Effect of ALT-801 on patients immune cell distribution and response. A, blood lymphocyte levels during treatment course in patients receiving 8 doses of 0.04 mg/kg ALT-801. B, serum IFN-γ levels following dose 1 (closed squares), 4 (open triangles), 5 (closed triangles) and 8 (open diamonds) of ALT-801 given at 0.015 (left), 0.04 (top) or 0.08 (right) mg/kg. C, cellular immune responses determined by IFN-γ ELISPOT analysis of patients following treatment with ALT-801 given at 0.015 (left), 0.04 (top) or 0.08 (right) mg/kg. Induction of IFN-γ positive cells in human PBMCs was determined after overnight with medium alone (closed squares) or medium containing ALT-801 (open triangles), p53-positive A375 tumor cells (closed triangles) or ALT-801 + A375 tumor cells (open diamonds). Values expressed as mean ± standard error of the mean. * P < 0.05 compared to medium control by analysis of variance.
Discussion
The primary objective of the study was to define the toxicities and tolerability of and establish a safe dose for further clinical study of ALT-801. This study established the maximum tolerated dose of ALT-801 as 0.04 mg/kg/dose at a schedule of 8-doses over 2 cycles, given days 1-4 and days 15-18. ALT-801 was tolerated at the MTD with acceptable toxicity. Common adverse events were transient, readily managed with supportive care, within the expected range of experience with aldesleukin, and resolved after completion of ALT-801 treatment. Subjects administered ALT-801 at 0.04 mg/kg appeared to have a lower frequency of liver (elevated serum bilirubin) and kidney (oliguria, elevated serum creatinine) abnormalities and grade 3/4 hypotension events than was reported in phase II studies of high-dose IL-2 therapy in treatment of metastatic melanoma or renal cell carcinoma (Table 3). However, direct statistical comparison with other studies was not a trial objective and could be confounded by patient population differences.
A second course of ALT-801 treatment at the MTD was well tolerated in three patients. Anti-ALT-801 antibodies were detectable in the serum of patients 4 weeks after treatment and decreased over time post treatment. No IL-2 neutralizing activity was observed and any effects of anti-ALT-801 antibodies on ALT-801 pharmacokinetics, toxicity and anti-tumor responses remain to be determined. Similar non-neutralizing immunogenicity has been observed in subjects following aldesleukin treatment (aldesleukin prescribing information: www.proleukin.com) and, thus, may be associated with this class of immunotherapeutic protein drugs.
The serum half-life of ALT-801 ranged between 2.0 to 4.6 hours depending on the dosing time and level. These results indicate that the half-life of this fusion protein is considerably longer than the reported 13 min distribution and 85 min elimination half-lives reported for aldesleukin (11). Further, the maximum serum ALT-801 concentrations observed were proportional with the ALT-801 dose, in contrast to the 30% serum recovery previously reported for subjects receiving aldesleukin therapy (prescribing information, www.proleukin.com). The Cmax at every dose level was greater than 7 nM and, therefore, was sufficient theoretically to saturate intermediate- or high-affinity IL-2 receptors (KD of 10-9M and 10-11M, respectively) displayed on the surface of lymphocytes (9). Together with the observation of the ability of a single dose of ALT-801 to induce lymphocyte migration for several days and increase serum IFN-γ levels 4-8 hrs post dosing, these data support administration of ALT-801 as a once-daily or every-other day infusion rather than the every 8 hour infusion as used for high-dose IL-2 treatment.
A secondary objective of the study was to evaluate ALT-801 biological effects. An increase in mean serum IFN-γ concentrations was seen 4-8 hours after dosing. Additionally, subjects treated with 0.04 mg/kg or 0.08 mg/kg ALT-801 showed levels of serum IFN-γ induction that were higher than those observed at the 0.015 mg/kg dose level. In contrast, little to no serum TNF-α was detected in any of the subjects' blood samples, in contrast to subjects treated with IL-2 therapy where TNF-α is known to be induced after treatment (12). The induction of TNF-α may be implicated in the etiology of IL-2 toxicity (13), but the relationship between ALT-801 treatment effects and TNF-α induction remains to be determined.
Another secondary objective was to assess the anticancer activity of ALT-801, although clearly the heterogeneity of the treated patients makes it hard to draw any conclusions. Of particular interest, many of the subjects had melanoma or clear cell kidney cancer, and several patients with melanoma had SD with minor tumor shrinkage based on scans at 7 weeks and confirmed at 11 weeks. One patient who received the study drug at the 0.08 mg/kg dose had clinical and radiologic SD on ALT-801 treatment. Later, residual abnormalities were resected surgically and no disease was observed in the specimens, nor is there any detectable melanoma in the patient at 2+ years post treatment. Whether the disease would have been in this complete remission for this duration if the patient had had just the pre-ALT-801 treatments and subsequent resections, as described above, or whether ALT-801 induced the pathologic response and the ongoing absence of any detectable disease is a question that is outside the scope of the available data. However, it is consistent to postulate that ALT-801 may have anti-tumor activity in patients with those diagnoses.
Additionally, our observation in this study that ALT-801 induced stable disease in patients with progressive head and neck tumor, prostate cancer and neuroendocrine cancer is consistent with studies in animal models in which ALT-801 exerted antitumor effects against p53-postive tumors regardless of their histology (8). These findings suggest that the clinical utility of IL-2 immunotherapy could be broadened beyond melanoma and kidney cancer. Clinical trials are currently either underway or being planned to assess this possibility. The anti-tumor effects of ALT-801 also appear to be potentially durable, as evidenced by the long progression-free time or survival (12+ months) achieved in a number of the patients with advanced disease described here (see patients 1001, 2001, 1004, 1007, 2004, 3001, 4001 and 4005 in Table 4). Durability of response is one of the hallmarks of successful immunotherapy, including high-dose IL-2 and anti-CTLA-4 antibodies (14, 15).
There appeared to be a relationship between dose of ALT-801 and clinical response, as the highest dose level (0.08 mg/kg/dose) of ALT-801 was associated with better clinical benefit in some patients including those who did not receive all 8 planned doses (Fig. 2). However, the higher dose level was also associated with higher frequency and severity of adverse events. Although the 4 days in-a-row schedule was developed working from the standard aldesleukin schedule and animal efficacy studies (3, 8), other schedules can be considered. In animal studies, we have found recently that the toxicities associated with the current every-day-dosing regimen could be significantly lessened without sacrificing the efficacy of ALT-801 by employing an every-other-day dosing schedule (Wong, HC, unpublished data). These findings suggest that dosing of ALT-801 at 0.08 mg/kg/dose or higher may still be feasible and pharmacodynamically adequate if ALT-801 doses are spaced more than 24 hours apart. Comparing the measurable pharmacodynamic impact of the 0.04 versus 0.08 mg/kg/doses in the current study, the peak dose was approximately proportional, but either dose was sufficient to meet theoretical concentrations required for IL-2 receptor occupancy and subsequent lymphocyte activation (Fig. 4). The relative real-world clinical relevance or sufficiency of these endpoints, however, is limited in comparison to the key issue of clinical responses. Thus, additional clinical studies and correlative testing will be key for defining a dose and schedule that are biologically optimal.
In a separate preclinical study we have demonstrated that the anticancer effect of ALT-801 could be significantly improved by increasing the density of the p53 (aa264-272)/HLA-A*0201 complex on the tumor cell surface using chemotherapy, such as cisplatin which may induce increased p53 levels (Wong, HC, unpublished data). Thus, further development of ALT-801 clinical application is anticipated in Phase 1 clinical trials (ClinicalTrials.gov ID: NCT01029873 and NCT01326871) testing cisplatin followed by a lower frequency, every-other-day schedule of ALT-801. The relationship between HLA-A2/p53 peptide complex expression on tumors and biological response to ALT-801 will also be further evaluated. While these preliminary results in the current study support the trend that high level tumor expression of p53 (aa 264-272)/HLA-A*0201 complex is important for ALT-801 antitumor responses, such an assessment was limited due to the small number of patients and the heterogeneity in tumor types examined. Thus, we believe that additional studies with more patients in a defined tumor type are needed before concluding on any relationship between p53 (aa 264-272)/HLA-A*0201 tumor staining and patient responses. Although the ALT-801 + cisplatin studies are currently limited to melanoma and urothelial cancer patients, the concept of chemotherapy-mediated p53 induction followed by targeted immunotherapy could be generalized to other cancers and to other p53 inducing treatments.
In summary, this prospective solid tumor malignancy phase 1 dose escalation study provides first-in-human experience of ALT-801. At the MTD of 0.04 mg/kg/dose for a 4-days-in-a-row, multi-cycle dosing regimen, ALT-801 is adequately tolerated for practical use, with demonstrated biological effect (IFN-γ induction) and encouraging clinical activity. The proposed mechanism (i.e., overexpression of p53) spans multiple tumor types. We also observed changes in blood lymphocyte counts and in vitro immune reactivity of lymphocytes against target complex bearing tumor cells in patients received ALT-801. These data are consistent with the proposed mechanism of action of the fusion protein for IL-2-mediated immune cell activation followed by intratumoral accumulation of the activated immune cells via the p53 (aa264-272)/HLA-A*0201-specific targeting domain of ALT-801 (3). Further clinical development of ALT-801 is warranted to establish its clinical utility and to coordinate administration with p53 inducing chemotherapy.
Translational Relevance.
Interleukin-2 (IL-2) therapy is effective at providing durable antitumor responses in some patients but its use is limited by severe systemic toxicities. In this article, we report the results of a phase 1 study of ALT-801, a fusion between IL-2 and a soluble T cell receptor domain designed to target IL-2 activity against tumors that overexpress p53. When administered at 0.04 mg/kg/dose intravenously for up to 8 doses, ALT-801 was well tolerated by patients with various metastatic malignancies. The spectrum of adverse events was similar to those associated with bolus IL-2 treatment but less intense. ALT-801 exhibited a longer serum half-life and higher recovery compared to IL-2. ALT-801 treatment also induced cell immune stimulation and showed evidence of dose-dependent clinical benefit. Approximately 40% of 26 treated patients exhibited stable disease and one melanoma patient subsequently developed complete remission, suggesting further clinical evaluation of ALT-801 is warranted.
Acknowledgments
We thank Pierre-Andre Chavillaz, Christian A. Romero, Jack O. Egan, Bai Liu, Lin Kong, Xiaoyun Zhu, and Lijing You of Altor BioScience Corp. for clinical assay support. We thank Drs. Kim Margolin and David Green for serving on the Drug Safety Monitoring Board of this study.
Grant Support: FDA-Orphan Drug Product grant FD-R 03452-01 (M. Fishman, L. Diez), NIH grant CA097550 (B. Y. Huang, S. Tang, P. R. Rhode and H. C. Wong), commercial research support from Altor BioScience Corp.
Footnotes
Disclosure of Potential Conflicts of Interest: B. Y. Huang, S. Tang, P. R. Rhode and H. C. Wong are employees of and equity owners in Altor BioScience Corp.; J. Weber is a paid consultant of Altor Bioscience Corp.
Note: Some preliminary results of this study were previously presented at the following meetings: Fishman M, Pennock G, Diez L, Edwards J, Tang S, Huang B, et al. Immune pharmacodynamics of TCR-IL2 Fusion Protein ALT-801 on cytokines and lymphocytes in initial human experience. Translational Cancer Medicine 2008: Bridging the Lab and the Clinic in Cancer Medicine. Jerusalem, Israel, Nov. 2008, Abstr. 8082; Fishman MN, Pennock GK, Tang S, Huang BY, Chavaillaz PA, Egan JO, et al. Interim Analysis of a Phase I Clinical Trial of TCR-IL2 Fusion Protein ALT-801 in Patients with Metastatic Malignancies. Cancer Immunology and Immunotherapy: Realizing the Promise. (NCI) Bethesda, MD, Sep. 2008; Pennock G, Fishman M, Gonzalez R, Thompson J, Huang B, Tang S, et al. Phase I clinical experience of a targeted TCR-IL2 fusion protein in patients with metastatic malignancies. ASCO Annual Meeting Proceedings: J Clin Oncol 27:15s, 2009: Abstr. 3040; Fishman MN, Pennock G, Gonzalez R, Thompson JA, Huang B, Tang S, et al. Phase I Clinical Experience of a Targeted TCR-IL2 Fusion Protein in Patients with Metastatic Malignancies. Keystone Symposia on Molecular and Cellular Biology: Target Cancer Therapies. Whistler, BC, Canada, Mar. 2009, Abstr. 325.
References
- 1.Rosenberg SA, Lotze MT, Yang JC, Aebersold PM, Linehan WM, Seipp CA, et al. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210:474–484. doi: 10.1097/00000658-198910000-00008. discussion 484-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Card KF, Price-Schiavi SA, Liu B, Thomson E, Nieves E, Belmont H, et al. A soluble single-chain T-cell receptor IL-2 fusion protein retains MHC-restricted peptide specificity and IL-2 bioactivity. Cancer Immunol Immunother. 2004;53:345–357. doi: 10.1007/s00262-003-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wen J, Zhu X, Liu B, You L, Kong L, Lee HI, et al. Targeting activity of a TCR/IL-2 fusion protein against established tumors. Cancer Immunol Immunother. 2008;57:1781–1794. doi: 10.1007/s00262-008-0504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351:453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- 5.Vousden KH, Prives C. P53 and prognosis: new insights and further complexity. Cell. 2005;120:7–10. doi: 10.1016/j.cell.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann TK, Nakano K, Elder EM, Dworacki G, Finkelstein SD, Appella E, et al. Generation of T cells specific for the wild-type sequence p53(264-272) peptide in cancer patients: Implications for immunoselection of epitope loss variants. J Immunol. 2000;165:5938–5944. doi: 10.4049/jimmunol.165.10.5938. [DOI] [PubMed] [Google Scholar]
- 7.Zhu X, Belmont HJ, Price-Schiavi S, Liu B, Lee HI, Fernandez M, et al. Visualization of p53 (264-272)/HLA-A*0201 complexes naturally presented on tumor cell surface by a multimeric soluble single-chain T cell receptor. J Immunol. 2006;176:3223–3232. doi: 10.4049/jimmunol.176.5.3223. [DOI] [PubMed] [Google Scholar]
- 8.Belmont HJ, Price-Schiavi S, Liu B, Card KF, Lee HI, Han KP, et al. Potent antitumor activity of a tumor-specific soluble TCR/IL-2 fusion protein. Clin Immunol. 2006;121:29–39. doi: 10.1016/j.clim.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Minami Y, Kono T, Miyazaki T, Taniguchi T. The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–268. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- 10.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 11.Konrad MW, Hemstreet G, Hersh EM, Mansell PW, Mertelsmann R, Kolitz JE, et al. Pharmacokinetics of recombinant interleukin 2 in humans. Cancer Res. 1990;50:2009–2017. [PubMed] [Google Scholar]
- 12.Atkins MB, Redman B, Mier J, Gollob J, Weber J, Sosman J, et al. A phase I study of CNI-1493, an inhibitor of cytokine release, in combination with high-dose interleukin-2 in patients with renal cancer and melanoma. Clin Cancer Res. 2001;7:486–492. [PubMed] [Google Scholar]
- 13.Mier JW, Vachino G, Klempner MS, Aronson FR, Noring R, Smith S, et al. Inhibition of interleukin-2-induced tumor necrosis factor release by dexamethasone: prevention of an acquired neutrophil chemotaxis defect and differential suppression of interleukin-2-associated side effects. Blood. 1990;76:1933–1940. [PubMed] [Google Scholar]
- 14.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 15.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]