Abstract
Objectives
The biomodification of dentin is a biomimetic approach, mediated by bioactive agents, to enhance and reinforce the dentin by locally altering the biochemistry and biomechanical properties. This review provides an overview of key dentin matrix components, targeting effects of biomodification strategies, the chemistry of renewable natural sources, and current research on their potential clinical applications.
Methods
The PubMed database and collected literature were used as a resource for peer-reviewed articles to highlight the topics of dentin hierarchical structure, biomodification agents, and laboratorial investigations of their clinical applications. In addition, new data is presented on laboratorial methods for the standardization of proanthocyanidin-rich preparations as a renewable source of plant-derived biomodification agents.
Results
Biomodification agents can be categorized as physical methods and chemical agents. Synthetic and naturally occurring chemical strategies present distinctive mechanism of interaction with the tissue. Initially thought to be driven only by inter- or intra-molecular collagen induced non-enzymatic collagen cross-linking, multiple interactions with other dentin components are fundamental for the long-term biomechanics and biostability of the tissue. Oligomeric proanthocyanidins show promising bioactivity, and their chemical complexity requires systematic evaluation of the active compounds to produce a fully standardized intervention material from renewable resource, prior to their detailed clinical evaluation.
Significance
Understanding the hierarchical structure of dentin and the targeting effect of the bioactive compounds will establish their use in both dentin-biomaterials interface and caries management.
1. Overview
Dentin is a complex mineralized tissue arranged in an elaborate 3-dimensional framework composed of tubules extending from the pulp to the dentin-enamel junction, intra-tubular and peri-tubular dentin. The mineral portion is composed of carbonate apatites. Fibrillar type I collagen accounts for 90% of the organic matrix while the remaining 10% consists of non-collagenous proteins, such as phosphoproteins and proteoglycans (Fig. 1). The peri-tubular dentin, i.e. dentin surrounding the tubules, is highly mineralized (95 vol% of mineral), while most organic content is localized at the inter-tubular dentin (30 vol% of mineral) [1]. Dentin undergoes modifications by physiological aging and disease processes to produce different forms of dentin [2]. This process affects the biomechanics and biochemistry of the tissue.
Figure 1.
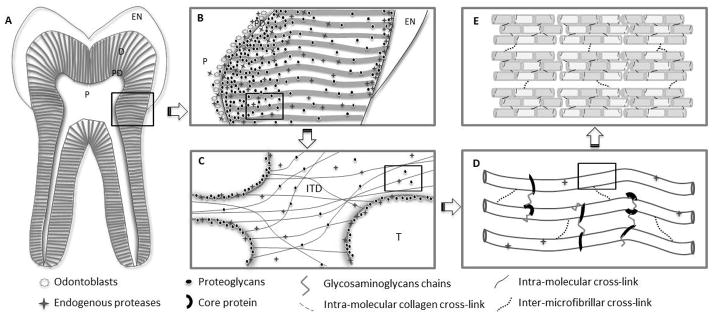
Distribution and hierarchical structure of dentin extracellular matrix components relevant to dentin biomodification. (A) Tooth structure. EN: enamel, D: dentin, PD: predentin, P: pulp. (B) Proteoglycans and endogenous proteases distribution in coronal dentin. Endogenous proteases include MMPs and cysteine-cathepsins with greater presence of both enzymes in deep dentin areas and predentin and the dentin enamel junction [31, 134, 135]. Proteoglycans are also identified in deep dentin, especially predentin region [136]. (C) Dentinal tubule cross-section view at higher magnification representing collagen fibrils, proteoglycans and endogenous proteases. ITD: intertubular dentin; T: dentin tubule. Proteoglycans are bound to collagen and immunolocalized throughout and highly concentrated at the peritubular dentin [136, 137]. Cathepsin B was localized in the odontoblasts and dentinal tubules, especially in peritubular dentin [31]. MMP-2, -9 and -3 are localized along collagen fibrils, mainly at intertubular dentin [28, 138], but no MMPs were identified inside the tubules [134]. (D) Collagen fibril interactions with non-collagenous proteins in dentin organic matrix. The protein core present in proteoglycans binds to collagen and forms aggregates of microfibrils by holding the collagen network together [137]. (E) Enzymatic collagen cross-links provide tensile properties and stability to the collagen fibrils.
Although similar in composition to bone, dentin does not share the same ability to remodel. This limits site regenerative therapies. An advantage of dentin over enamel is the presence of a collagen based scaffold that provides an appropriate cell-free backbone for tissue repair and regeneration. The presence of such a scaffold is a key to advance new concepts in tissue engineering approaches to the treatment of missing hard tissue. Recently, biomodification of dentin has been investigated as a biomimetic strategy therapy to mechanically strengthen the existing collagen network and also control biodegradation rates of extracellular matrix (ECM) components. This review provides an overview of important extracellular matrix components of dentin, as well as mechanisms and application of dentin biomodification, and specifically addresses the broad application of naturally occurring biomodification agents.
2. Extracellular Matrix Components Relevant to Dentin Biomodification
2.1. Type I Collagen
Fibrillar collagen is a strong and elastic biomaterial arranged into highly organized hierarchical structures [3, 4]. Type I collagen is the most abundant of all collagen types and is defined as a coiled-coil trimer molecule, each of which is composed of the repeated sequence of amino acids Gly–X–Y, where X and Y are commonly found to be proline and hydroxyproline, respectively. Type I collagen molecules are biosynthesized from a larger precursor, procollagen, by cleavage at both its C- and N-terminal ends. The collagen fibrils are formed by spontaneous self-assembly of the molecules into a periodic structure with 67–69 nm repeat period overlap between neighboring molecules which is crucial for the development of covalent inter-molecular cross-linking. The inter-molecular cross-linking, the final post-translational modification of collagen, is the basis for the stability, tensile strength and viscoelasticity of the collagen fibrils (Fig. 1). The slope of elastic stress-strain curve for collagen fibers increases with increased degree of cross-linking [5], subtle perturbations to the cross-linking profile have been correlated with the strength of hard tissue [6]. In addition, the biodegradability and thermal stability of the tissue is also controlled by the amount and type of collagen cross-linking.
Endogenous collagen cross-linkings are mediated by enzymatic and non-enzymatic reactions. Enzymatic intra- and inter-molecular cross-links, formed between telopeptides and adjacent triple helical chains through lysine–lysine covalent bonding [6–8], are controlled by a number of factors, such as lysine hydroxylation, glycosylation, turnover rate, molecular packing and external forces [9]. Non-enzymatic collagen cross-linkings are mediated by oxidation and glycation processes [10]. Exogenous collagen cross-linking can be induced by non-enzymatic reaction sources such as chemical agents and physical methods, both of which have distinct mechanisms of interaction with type I collagen (see sub-section 3.1).
2.2. Proteoglycans
Proteoglycans (PGs) are a major group of non-collagenous proteins identified in both pre-dentin and dentin. PGs play a crucial role in dentin mineralization [11, 12] and the structural integrity of collagen fibrils [13]. They are classified into two distinct categories: the large aggregating chondroitin/keratan sulfate family, composed of molecules such as versican and aggregan, and the family of small leucine-rich proteoglycans (SLRPs) [14–16]. PGs found in dentin are mainly small leucine-rich collagen-binding carrying chondroitin- (CS, e.g. biglycan or decorin) with a limited distribution of keratan-sulfate (KS, e.g. fibromodulin, lumican) glycosaminoglycans chains (GAGs) [17]. Although there are similarities in the structure of decorin and biglycan, they differ in the pre-dentin/dentin distribution [18, 19] and in gene-expression during tooth mineralization [20]. In addition to their roles in mineralization, PGs control the tissue hydration and molecule diffusivity [21]. Hence, modified forms of the tissue, such as sclerotic dentin, can affect the distribution of PGs [22].
2.3. Endogenous proteases
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases; able to degrade different components of the ECM [23]. In the oral cavity, MMPs are related to periodontal disease, caries progression, pulp inflammation and cancer [24–26]. In the dentin-pulp complex, several MMPs have been identified such as MMP-2, -3, -8 and -9 [27–29]. Another important family of proteases are the cysteine cathepsins [30, 31]. Cathepsins belong to the papain family and were initially considered as lysosomal proteases, although they can act extracellularly [32]. They become active at acidic pH and most are endopeptidases, with some exceptions like cathepsin B that can also act as a carboxypeptidase [33]. Cathepsins participate in ECM degradation in physiological and pathological processes like bone remodeling, inflammation, rheumatoid arthritis, diabetes, multiple sclerosis and cancer [32, 34]. In addition to their role in caries progression [30], the cathpesins have also been associated with other oral diseases such as periodontitis, bone resorption and oral cancer [35–37]. Like MMPs, cathepsins are active in intact and carious dentin and show decreased activity with age in sound dentin [31].
3. Biomodification of dentin – a bio-inspired strategy
Current therapies to replace missing tissue have primarily relied on synthetic biomaterials to restore function and shape of the tissue. Advances in tissue engineering and regeneration have grown exponentially in the past decades but with limited application in dental hard tissue repair resulting from the limited ability of the tissue to remodel. This site-specific limitation in tissue regeneration of dentin requires unconventional approaches to be used in an attempt to promote tissue repair. Therefore any shift from a restorative to a reparative/regenerative dentistry will likely rely on better understanding on the tissue biochemistry, hierarchical structure (Fig. 1) and implication to the biomechanics of the tooth.
One relatively small but significant step towards dentin tissue repair/regenerations is the development of a biomimetic strategy to enhance the tissue properties by modifying the chemistry of the tissue. The biomodification of existing hard tissue structures, specifically tooth dentin, is a novel approach to improve the biomechanical and biochemical properties of the tissue for preventive or reparative/restorative purposes. The approach was initially thought to be driven by inter- or intra-molecular collagen induced non-enzymatic collagen cross-linking [38]. However the multi-interactions between bioactive agents with various extracellular components of the dentin matrix are likely the determinants of the tissue enhanced biomechanics and biostability. Therefore the term biomodification is more appropriate to define the bioactivity of these highly bioactive chemical mediators.
3.1. Biomodification of dentin – sources and types
Type I collagen plays a major role on the replacement of missing tooth structure as the tooth component that provides micromechanical retention of resin-based materials and also as scaffold for tissue remineralization. Well-known synthetic agents, nature derived agents and also physical methods have been shown to effectively interact with type I collagen [39–41] and will be explored in this section. The sources and types of biomodification agents can be similarly classified as collagen cross-linking agents. Table 1 summarizes currently investigated agents for dental application. A brief overview of specific agents is given below with special emphasis on naturally occurring polyphenols due to their enhanced bioactivity, biocompatibility and applications when compared to other well-known agents.
Table 1.
Summary of current biomodification strategies with reported dentin matrix interaction.
| Types | Mediate non-enzymatic collagen cross-linking | Enzymatic Inhibition | Proteoglycans | Dentin matrix interaction | |||
|---|---|---|---|---|---|---|---|
| Endogenous proteases | Exogenous proteases | Mechanical properties | Enzymatic degradation | ||||
|
Physical Methods
| |||||||
| UVA Radiation/Riboflavin |
+ | + | N/N | N/N | N/N | + | |
|
| |||||||
|
Chemical Agents
| |||||||
| Synthetic | Glutaraldehyde | + | + | + | −− | +++ | + |
| Carbodiimide/NHS | + | + | + | −− | ++ | + | |
|
| |||||||
| Naturally Occurring | Genipin Polyphenols |
+ | − | N/N | N/N | + | + |
| Oligomeric Proanthocyanidins | + | + | + | + | ++++ | + | |
| Others Polyphenols | −/+ | + | + | N/N | −/+++ | + | |
N/N: Not known/not reported, +: positive effect, −−: no effect
3.1.1. Synthetic Resources
Physical Methods
Also called the photo-oxidative method; most synthetic biomodifiers use light exposure especially ultraviolet radiation [42, 43]. The photo-oxidative method requires the presence of singlet oxygen; and riboflavin (vitamin B2) is one of the most potent producers of oxygen radicals [41]. Photo-activation of riboflavin results in singlet-oxygen-inducing chemical covalent bonds, bridging amine groups (N-H) of glycine of one chain with carbonyl groups (C=O) of hydroxyproline and proline in adjacent chains [44, 45]. Riboflavin is non-toxic and collagen cross-linking induced by UVA and radiation/riboflavin is a successful treatment for ophthalmic diseases such as keratoconus [41, 46]. While proven effective in the laboratory as a photo-activation method of riboflavin [47], safety issues regarding the use of UVA and its practicality for dental use need to be considered.
Chemical agents
Encompass the largest selection of potential synthetic and naturally occurring mediators. Their established and postulated mechanisms of action are described below:
Aldehydes
While Glutaraldehyde (GA) is the most widely known agent of this class, similar cross-linking ability is found with other aldehydes such as formaldehyde and glyceraldehyde. GA is a dialdehyde with high affinity for free primary amine groups of amino acids [39]. GA has been associated with a decrease in the rate of collagen degradation [40, 48] and improved dentin collagen properties [49], reacting primarily with the ε-amino groups of peptidyl lysine and hydroxylysine residues of collagen fibrils. A disadvantage of GA is its high cytotoxicity [40], which limits clinical applicability.
Carbodiimide hydrochloride (EDC) is known as a zero-length agent due to its ability to cross-link peptides without introducing additional linkage groups. The cross-linking mechanism is mediated by the activation of carboxylic acid groups of glutamic and aspartic acids to form an O-acylisourea intermediate. The latter reacts with the ω-amino groups of lysine or hydroxylysine to form an amide cross-link, leaving urea as the terminal by-product. The addition of N-hydroxysuccinimide (NHS) to the EDC-containing solution is effective in increasing the number of induced collagen cross-linking and preventing the hydrolysis of activated carboxyl groups [50, 51]. While the cross-linking potential is limited [52], EDC is less toxic than GA [53, 54].
3.1.2 Natural Renewable Resources
Also part of the family of chemical agents, naturally occurring compounds have received great attention in the past decade for their potential use in dentistry. Two of their most attractive characteristics are their very low toxicity when compared to synthetic agents [40] and renewable/sustainable resources. Sources such as Genipin (GE, an aglycone of an iridoid glycoside, one of the major components in the fruit of Gardenia jasminoides) have found limited application due to its slow cross-linking reaction [55]. Inter- and intra-molecular cross-linking is believed to be mediated by reactions with free amino acid (lysine, hydroxylysine, or arginine) to form a nitrogen iridoid that undergoes dehydration to form an aromatic monomer [56–58].
A large variety of bioactivities have been reported for polyphenols from plants. Their biological functions are the foundation for their prominent roles in plant-based dietary supplements and nutritionals, and recent research continues to explore new applications. Of particular interest to dental application are the proanthocyanidins (PACs), belongings to a category known as condensed tannins, highly hydroxylated structures capable of forming an insoluble complex with carbohydrates and proteins [59]. In addition to their dentin activity, PACs increase collagen synthesis, are potent anti-microbial and anti-tumorigenics [60]. The interaction of PACs and collagen results in the formation of complexes that are believed to be stabilized primarily by hydrogen bonding between the protein amide carbonyl and the phenolic hydroxyl [61] and also covalent and hydrophobic bonds. The relatively large stability of PACs-protein complexes suggests structure specificity [62], which although encouraging hydrogen binding also creates hydrophobic pockets [40]. Such microenvironments decrease the dielectric constant enhancing the stability of such H-bonds. Non-specific inhibition of protease activity and interactions with proteoglycans have broadened their dental applications. Similar to other natural occurring agents, the source and preparation from the raw materials are essentials for determining their interactions with the tissue. In an attempt to unravel this complexity, the section below provides comprehensive description of their chemistry, manipulation and characterization.
4. Proanthocyanidins
4.1. Polyphenol Chemistry Underlying Dentin Interactions
Polyphenols represent secondary metabolites which are produced by plants likely as part of their defense mechanism. Different classes of polyphenolic compounds commonly observed in plants are phenolic acids, flavonoids (sub-classes: flavones, isoflavones, flavanols, flavanones, and anthocyanins), stilbenes, and lignans [63]. An important aspect of the flavonoid chemistry is the formation of oligomers/polymers from monomers such as epicatechin or quercetin by mutual condensation, or conjugation with sugars to form O-glycosides (e.g., rutin = quercetin-3-O-rutinoside) or C-glycosides (e.g., epicatechin-8-C-β-D-galactopyranose in Theobroma cacao) [64] and other phenolic acids like gallic acid by condensation reactions forming gallates e.g., epigallocatechin gallate in Camellia sinensis (green tea). These conjugated/condensed products can be easily confused with the monomers in the context of both their structure and nomenclature. For example, hesperidin, a flavanone glycoside, is derived from the aglycone hesperitin, with the only difference between the two compounds being the presence of a disaccharide, rutinose.
Proanthocyanidins (PACs) are divided into different classes based on the hydroxylation patterns in the A and B rings of the flavanoid skeleton. PACs containing (−) epicatechin (majority) or (+) catechin as their building blocks are known as procyanidins. The monomeric building blocks of proanthocyanidins are flavan-3-ol units composed of three rings (Fig. 2A) which based on IUPAC rules are labeled on the basis of their biosynthesis (A – triketide, B –phenylpropanoid, C – pyran ring formed by condensation). Oligomeric proanthocyanidins (OPACs) are most frequently composed of (−) epicatechin (2,3-cis) and (+) catechin (2,3-trans) terminal and extension units. The presence of chiral centers at positions 2 and 3 in the monomers and 2, 3, 4 in the dimers result in 4 and 8 stereoisomers, respectively. This imparts an exceptional amount of structural diversity and complexity to the already complicated biocombinatorial chemistry of the PACs.
Figure 2.
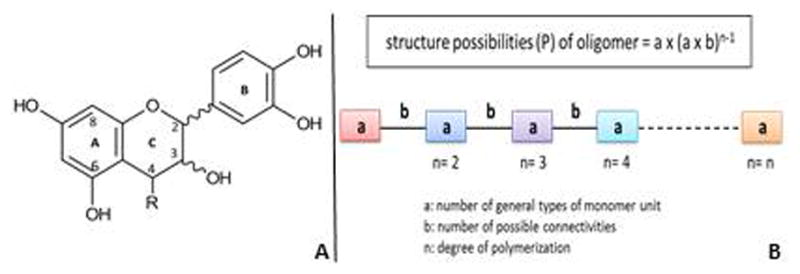
(A) Composition of the monomeric building block of proanthocyanidins. (B) Schematic representation of the permutation of catechin monomers in PACs with various degrees of polymerization.
In PAC oligomers and polymers, the monomers are linked together via A-type (C-4→C-6/C-8 and C-2→oxygen at C-7) and B-type (C-4→C-6/C-8) linkages. The monomers range from (290–306 Da) molecular weight (MW) while the OPACs range from 500 to 3000 Da. All PACs above 3000Da are considered polymers whose MWs can reach up to 20,000 Da or even higher. As the molecular weight of PACs increase, they become increasingly polar due to the presence of additional hydroxyl groups and hence potential for hydrogen bonding. The polymeric PACs, which are frequently called “tannins”, have a tendency to form complex aggregates. They possess significant solubilization difficulties in commonly used polar organic solvents and are also known to precipitate proteins. Tannins have molecular weights in the range of 1000 to 5000 Da. Two distinct and major classes of tannins are the hydrolysable tannins, containing hydrolysable ester bonds between the major building blocks, and the condensed tannins or proanthocyanidins, in which the monomers are linked by C-C or C-O-C bonds.
The hydrolysable tannins are classified into gallitannins and ellagitannins which are composed of gallic acid and ellagic acid units, respectively. These tannins can be hydrolyzed by acid or enzymes such as tannase. These polyphenols were primarily used by the leather industry for tanning purposes, but are also found in many plants of medicinal interest such as pomegranate rind and bark, bearberry leaves and cloves.
The condensed tannins or PACs are not readily hydrolysable like the gallitannins or ellagitannins, and glycosidation is not commonly observed [65]. Most of the observed medicinal properties of tannins are attributed to the class of condensed tannins.
The third, relatively rarely studied class of tannins called the “complex tannins” consists of conjugations between PACs and hydrolysable tannins via C-C bonds [64].
4.2. Structural Diversity and Chemical Space of Oligomeric Plant Phenols
4.2.1. Structures of Oligomeric Proanthocyanidins (OPACs)
OPACs are one of the two large groups of polyphenols, which consist of condensed polyphenolic moieties. Representing oligomeric or even polymeric condensates, the building blocks are flavan-3-ols which are mainly linked through C-4→C-8 or C-4→C-6 carbon-carbon bonds (B-type proanthocyanidins). Structures with an additional inter-flavanoid ether bond between C-2β→O→C-7 (or C-2β→O→C-5), they are classified as A-type PACs [66]. PACs are divided into different subclasses depending on the specific structure of the monomeric flavan-3-ol units, which include catechin (C), epicatechin (EC), gallocatechin (GC), epigallocatechin (EGC), afzelechin (AZ), epiafzelechin (EAZ), and all their 3-O-gallic acid esters, i.e., catechingallate (CG), epicatechingallate (ECG), gallocatechingallate (GCG), epigallocatechingallate (EGCG), respectively [66, 67]. The most abundant types of PAC are procyanidins containing catechin (C) and epicatechin (EC) subunits (Fig. 3) [68].
Figure 3.
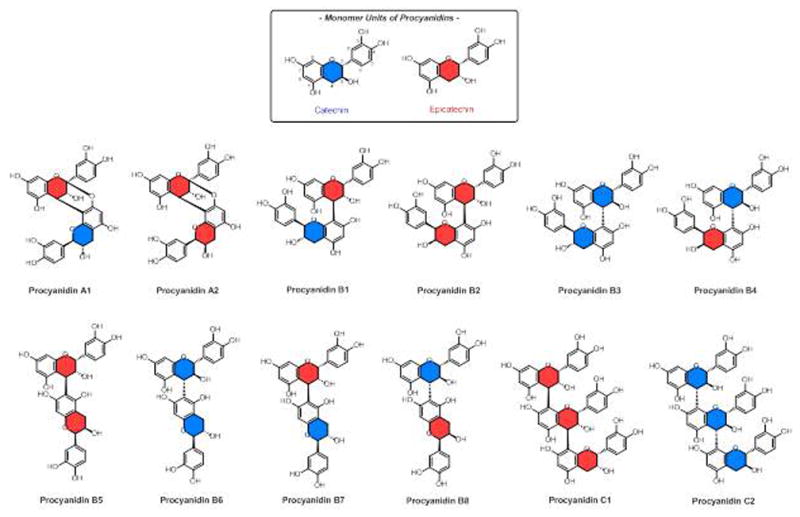
Structures of common procyanidins. The color coding distinguishes the two most abundant monomers, catechin(C) and epicatechin (EC).
From the perspective of 3D structural diversity, OPACs possess a large number of structural variations, which result from the different monomer types, the different inter-flavanoid linkages (bond types and linked positions), and degree of polymerization (Table 3). The main flavan-3-ol units of grape seed PACs are C, EC, CG, and ECG, and these monomers are mainly linked with C-4 (α or β)→C-8 or C-4 (α or β)→C-6 inter-flavanoid bonds [69]. Accordingly, there are 64 theoretical structural possibilities for grape seed PAs dimers, as calculated using the principle of permutation as shown in Fig. 3. Table 3 provides an overview of the theoretical numbers of potential permutations in the OPACs of various plants under investigation in the authors’ laboratory. The calculations take into account the degree of polymerization (DP), the known types of monomers, and the linkages that have been described for each plant. While the assumption of a “combinatorial biosynthesis” approach may be an overestimate of the actual OPACs metabolomes, it still demonstrates the exponential increase in the number of theoretical structural variations of PACs oligomers, with thousands of variations already starting at the level of tetramers (DP = 4). In addition to the rapidly increasing numbers of combinations and linkages, it is important to note that, despite their apparent similarities in 2D drawings, the molecules are in fact very different from a 3D perspective (Fig. 5). Accordingly, the distinctly different shapes, spatial arrangement, and chemical properties of seemingly similar structures need to be considered when interpreting their biological activities.
Table 3.
Theoretical Structural Possibilities of PACs. The calculations are permutations based on the Degree of Polymerization (DP) and the numbers/types of monomers and linkages known to occur in the selected PAC-rich plants.
Figure 5.
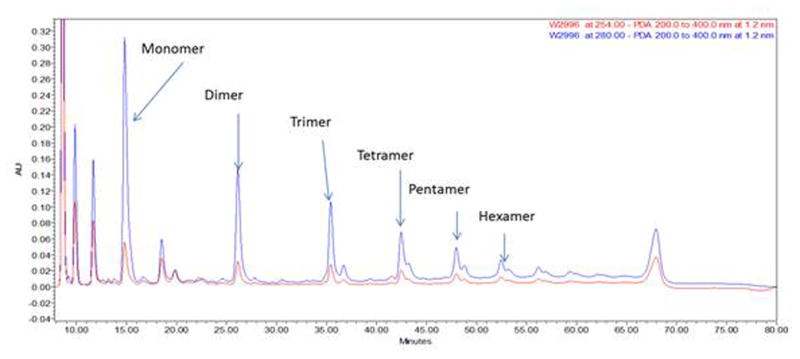
Diol-Phased HPLC UV 280 nm trace of procyanidins of cacao.
4.2.2. Analysis of Oligomeric Polyphenols
Separation and chemical analysis of oligomeric polyphenols have been performed with various chromatographic methods, such as size-exclusion, reverse-phased, and normal-phased chromatographies. However, analysis of oligomeric polyphenols is rather complex as it involves distinction of relatively large numbers of structurally very similar molecules with many types of isomers, high polarity (phenolic OHs), and relatively high molecular weights. Size-exclusion chromatography with Sephadex LH-20 has been commonly used to fractionate PACs by using a gradient solvent system of aqueous acetone and aqueous alcohol [70–72]. This method is commonly used at the pre-separation step for preparative HPLC or before quantitative analysis. Reverse-phase HPLC is commonly used for the separation of flavan-3-ol monomers, dimers, and trimers. However, higher oligomers do not separate well in this system, especially as the number of possible isomers increases geometrically with DP. Moreover, the elution order of the compounds does not strictly follow the DP linearly [73]. Typically, studies involving the analysis or separation of PACs have been performed to gain insights on the distribution of the degree of DP. Normal-phase HPLC can also be used for the separation and quantitative analysis of proanthocyanidins in plants and foods. In fact, normal-phased HPLC has been reported to separate OPACs according to their DP up to decamers [73–75]. However, polymeric compounds are not easily eluted from LC columns, especially from silica gel based stationary phases to which their affinities are too high for elution. This irreversible adsorption behavior can be avoided with the modified normal phase materials. Such an example is the newly developed method which uses a Diol stationary phase to isolate PACs from cacao [76] (Fig. 5). The Diol column can be used with solvents having a wide-range of polarity and it does not adsorb proanthocyanidins.
Nuclear magnetic resonance (NMR) spectroscopy is generally used for structure elucidation of chemical compounds. However, NMR analysis of PACs is challenged by the generally broad signals which results from atropisomerism and the occurrence of distinct conformers at room temperature. Derivatization by, e.g., permethylation or peracetylation to increase the steric hindrance of each unit eliminates or ameliorates these dynamic affects and reduces the spectral complexity, but also increases the molecular weight and produces samples which are no longer suitable for biological evaluation (in place of their underivatized parent compounds) due to loss hydroxyl group. While synthetic methods and NMR analysis at low temperatures can be applied for the elucidation of higher oligomers, there are practical limits for the identification of their exact structures. Accordingly, in the last 20 years, the monomers and dimers are by far the most commonly studied compounds (>200 publications per year). Phytochemical reports about higher oligomers are scarce: 28 publications elucidate trimers (citation), only five reports describe tetrameric PACs (citation), and no reports on pentameric or higher oligomers could be located.
4.2.4. PACs-Dentin Matrix Interactions
Since 2007, the topic of natural cross-linkers of dentin collagen and their enhancement of dentin strength has attracted more and more attention. Studies of PACs or PACs-rich extracts have primarily focused on their ability to enhance the mechanical properties of the tissue as well as to reduce biodegradability of the tissue by host-derived and exogenous proteases [49, 55, 77–91]. Other PACs-rich extracts, such as cocoa seed extract have also been shown to greatly decrease the enzymatic degradation, increase stiffness and decrease the swelling ratio of demineralized dentin [92, 93]. However it is a concentration and time dependent process that also can cause a brownish coloration of dentin. Reported discolorations are likely related to the content of polymeric, high-molecular weight PACs and their oxidation products. Specific information about the chemical species that are colored is unavailable in the literature. Current studies are underway by the authors research group to elucidate the underlying chemical mechanisms and determine methods to produce PAC-rich materials that do not cause discoloration.
The authors’ collaborative research project on the development of a clinical preparation with dentin enhancing properties evolved from preliminary studies of polyphenol-rich plant extracts of grape seeds (Vitis vinifera) and cocoa (Theobroma cacao) [83]. Aimed at the identification of active components from these extracts and standardization of a clinical preparation, we have been using phytochemical methodology and chromatography methods to investigate the components soluble in aqueous alcohol by chromatographic methods. Gel Permeation Chromatography (GPE) with Sephadex LH-20 was applied to fractionate both extracts, and the active PAC-rich fractions were concentrated into several fractions. The preliminary data showed fractions 5–10 were the most active and that the degree of polymerization of the PACs in these fractions was 3–10 based on HPLC analysis (Fig. 6).
Figure 6.
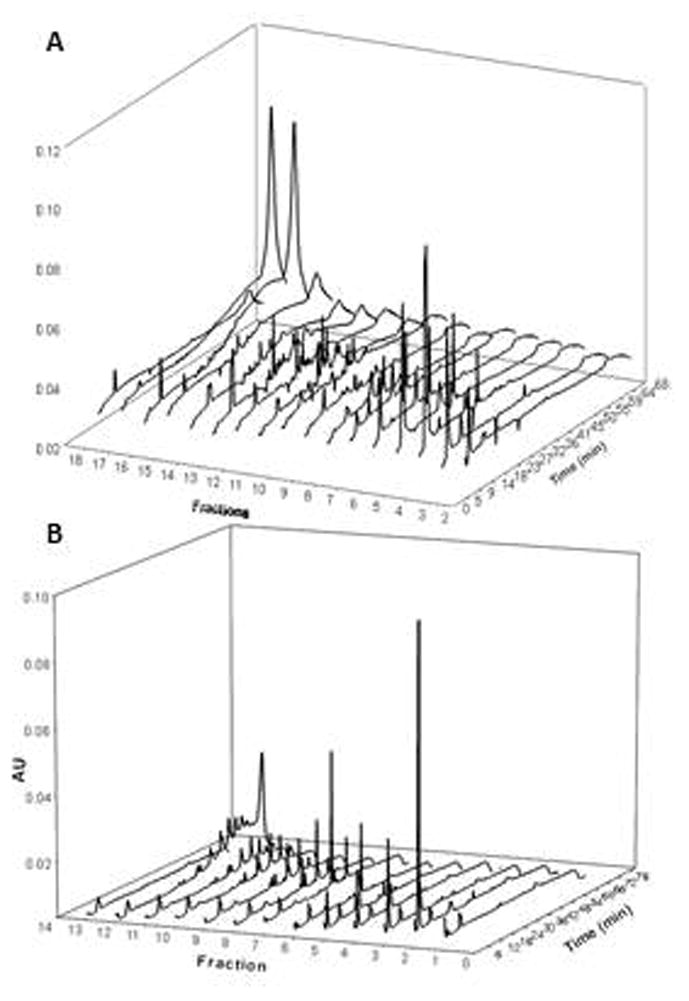
HPLC Profiles of Fractions (UV 280 nm): (A) Grape seed extract sub-fractions and (B) Cocoa seed extract sub-fractions.
The removal of GAGs from dentin has also been observed only following biomodification with high concentrations of PACs [82]. Due to the GAGs hydrophilicity, PGs contribute to dentin-resin bonds during re-wetting procedures by maintenance of the collagen fibril architecture [94, 95]. In contrast, the removal of PGs and/or GAGs by MMP-3 (proteoglycanase) [96] and chondroitinase ABC (to remove CS GAGs) [97] increases the penetrability of the resin monomers into the fully expanded collagen network. The removal of proteoglycans may be partially associated with the mechanical changes to the dentin matrix treated with PAC sources, and perhaps biodegradation rates by changing the tissue hydration and molecule diffusivity through the tissue [21, 98]; consequently reducing biodegradation and increasing resin diffusion through the matrix. It is important to highlight that PGs play an important role in dentin biomechanics [82] and tissue hydration/diffusivity [21], and therefore effects of PACs on PGs raises more questions than answers regarding the hierarchical structure of the tissue and its biomechanical behavior.
Inhibition of proteases by PACs has been extensively investigated for various systemic applications and particularly shown to prevent, reduce or reverse cancer [99] due to their inhibitory MMP activity [99, 100]. Isolated PAC compounds from cranberry have shown inhibitory effects of MMPs associated with reduction of periodontal disease progression [101–103]. The direct effects of PACs on endogenous proteases broaden their application as a multi-targeting agent for reparative and preventive therapies of dental hard tissue.
5. Clinical Applications of Dentin Biomodification
5.1. Tooth-Biomaterials Interfaces
The most common cause of replacement of resin composite restorations is secondary caries [104, 105]. These warrant the need for novel treatment strategies to reinforce the dentin structure. Resin-dentin bonded interfaces may be considered a unique form of tissue engineering in which a collagen-based dentin matrix scaffold is reinforced by resin to produce a hybrid layer that couples adhesives/resin composites to the underlying mineralized dentin [106]. Instead of serving as a stable anchor between the bulk adhesive and subjacent intact dentin, the hybrid layer is rather the weakest link at the adhesive interface bond [107]. These denuded collagen matrices are also filled with water, which serves as a functional media for the degradation of resin matrices and collagen [107, 108]. These pitfalls have been recognized by the research community as a major factor leading to the short service life of adhesive resin composite restorations.
In addition, altered forms of dentin in general have a negative impact on the bonding ability of current adhesive systems. Caries-affected dentin, a common substrate in dental preparations requiring restorative therapies, is well-known to impair the bonding performance of contemporary systems [85, 109, 110]. The development of new therapies has not received the much needed consideration of pathological and physiological variations of the tissue substrate. Strengthening of type I collagen and reduced biodegradation are likely to reinforce the dentin structure and in combination are promising strategies to develop a strong and long lasting tooth-biomaterial interface. Evidence of targeting effects of dentin biomodification in dentin-resin bonded interfaces is summarized below.
Improving tissue biomechanics
Even if degradation of resin-sparse collagen fibrils can be prevented, the mechanical properties of these denuded collagen fibrils are far inferior to those of resin-infiltrated collagen or mineralized collagen [111]. Enhanced properties of dentin matrix have been extensively demonstrated for various types of dentin biomodification strategies [47, 49, 55, 82–84]. In addition, recently, tissue specific action of different agents has been reported in carious teeth [112], with enhanced activity at dentin zones around the carious lesion as opposed to apparently sound sites.
Enhancement of the biomechanical properties of the dentin matrix is directly related to immediate increased dentin-resin bond strength to sound [47, 86, 113, 114] and caries-affected dentin [85]. A closer look at the interface components shows that the mechanical properties of the underlying dentin and hybrid layer are significantly improved and largely attributed to agent-mediated non-enzymatic collagen cross-linking [79, 80]. Changes to the surface hydration and molecule diffusivity may have a considerable effect on the bond strength as well [82]. Long-term stability has also been reported and attributed to irreversible collagen cross-linking and reduced dentin matrix biodegradation [81, 115]. Mediators such as EDC/NHS have a significant long-term effect on the resin-dentin bond strength without affecting the immediate bond strength, likely due to reduced biodegradation rates [116]. It is also noteworthy that the effect on the resin-dentin bonded stability depends on the restorative material [115].
Biodegradation – inhibition of proteases
The low pH environment found during restorative procedures can potentially release and activate MMPs and cathepsins, leading to subsequent cleavage of exposed dentin collagen [108]. Collagenolytic and gelatinolytic activities have been shown to occur in dentin and hybrid layers [29, 117, 118].
In general, most dentin biomodification strategies impair exogenous and endogenous proteolytic activity in a concentration dependent manner. Biomodification of dentin matrices by PACs induces remarkable resistance against degradation either from bacterial collagenase [82, 87, 88, 91, 119] or endogenous proteases such as MMPs [102]. Inhibitory effects of PACs on proteases have been described as non-specific, making the determination of their mechanisms of action challenging. However, the potent inhibitory effects are likely to involve several mechanisms such as down regulation of endogenous proteases expression, protease inactivation/silencing and protection of cleavage sites within collagen; expanding their potency and clinical application.
Recently, potent inhibitory effects of EDC and Riboflavin/UVA on MMPs [114, 116] have highlighted the potential of these agents to reduce dentin matrix breakdown and enhance the long-term stability of dentin-resin interfaces. Aldehyde-based inhibitory activity on proteases has been shown to affect bacterial collagenase [82], while preliminary studies exhibit a dose dependent inhibitory activity of MMP-2 and MMP-9 (unpublished data).
5.2. Dental Caries Prevention and Remineralization
The effects of dentin biomodification on caries prevention or on remineralization have been largely confined to naturally derived agents. Plant extracts have been used as oral hygiene agents for hundreds of years [120]. While several studies have investigated direct effect on dental biofilm and microorganisms, limited studies addressed the role of polyphenol-tooth complex in caries progression and remineralization therapies. The use of polyphenols in particular, as dentin-active agents has appeared in the dental literature mostly in the last decade. The first report in 2001 concerns the effect of miswak extract in the treatment of dentin [121]. In subsequent studies, a comparison of 50% miswak extract and chlorhexidine gluconate, showed that the plant extract had similar benefits as to those of chlorhexidine gluconate. Although the studies did not include phytochemical analyses, general phytochemical research on miswak by others showed that the plant contains tannins, saponins, flavonoids and sterols.
The PACs affinity for proline-rich proteins increases their applicability on root dentin caries. Recently, decreased in vitro caries progression and remineralization of root dentin were shown to be promoted by PAC-rich grape seed extract [78, 122–124]. The beneficial effect is believed to involve two mechanisms: stabilization of dentin matrix for matrix remineralization and formation of calcium-PAC complexes promoting mineral deposition. PACs also demonstrate a potent anti-bacterial effect [125, 126], further contributing to their use for caries prevention.
Polyphenols from green tea are among the most well studied PACs and many bioactivities have been reported, including those affecting dentin. Investigations of the protective function of green tea extract on dentin erosion and abrasion [127–130] found that green tea extract solution significantly reduced dentin wear and this observation was attributed to the polyphenols in the green tea acting as inhibitors of MMP. Epigallocatechin-3-gallate (EGCG) is a major bioactive component in green tea extract, and known to be an inhibitor of proteases [131]. However, the mechanism of action of green tea extract is likely more complex, involving multi-functional activities in biofilm and hard tissue. Two recent published articles have evaluated its function as an agent acting against the degradation of collagen in dental erosion and of dentin matrix loss [132, 133].
6. Conclusions
In summary, dentin biomodification is a bio-inspired strategy to enhance the properties of dentin matrix with major impact on novel preventive and reparative/regenerative dental therapies. It is essential to consider the mechanism and limitations of a wide range of available strategies. Preliminary in vitro studies have provided strong evidence of its application in dentin-resin bonded interfaces and caries development/progression. PACs appear to be most promising due to their biocompatibility, high dentin bioactivity and renewable resources. For their further development and investigation, care must be taken to select appropriate source materials and perform rigorous standardization to develop intervention materials that are suitable for clinical use.
Figure 4.
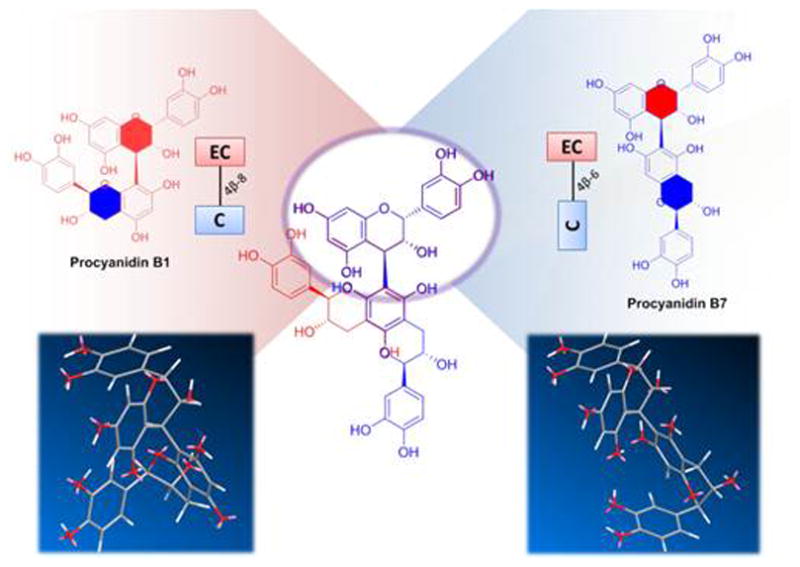
Comparison of the structures of procyanidin B1 and B7 in 2D structure drawings and a 3D-model. The difference in their inter-flavanol linkages leads to an important change in the overall geometry. While the overlay of their 2D structures can demonstrates the effect when arranging the molecules as shown here, a comparison of 3D models is required to assess the actual differences in molecular shape. C- Cathechin; EC -Epicatechin
Table 2.
Examples of Common Oligomeric Proanthocyanidins (OPACs) and Their Simplified Codesa)
| Degree of Oligomerization | Name | Code |
|---|---|---|
| dimer | procyanidin A1 |
|
| procyanidin A2 |
|
|
| procyanidin B1 |
|
|
| procyanidin B2 |
|
|
| procyanidin B3 |
|
|
| procyanidin B4 |
|
|
| procyanidin B5 |
|
|
| procyanidin B6 |
|
|
| procyanidin B7 |
|
|
| procyanidin B8 |
|
|
| prodelphinidin B1 |
|
|
| prodelphinidin B2 |
|
|
| prodelphinidin B3 |
|
|
| prodelphinidin B4 |
|
|
|
| ||
| trimer | procyanidin C1 |
|
| procyanidin C2 |
|
|
|
| ||
| tetramer | cinnamtannin A2 |
|
| cinnamtannin B2 |
|
|
C=catechin, EC=epicatechin, GC=gallocatechin, EGC=epigallocatechin
Acknowledgments
The authors were supported by NIH-NIDCR research grants R01DE021040, DE017740 and DE017740-01S4 for work that generated some of the data reported in this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marshall GW., Jr Dentin: microstructure and characterization. Quintessence Int. 1993;24:606–17. [PubMed] [Google Scholar]
- 2.Marshall GW, Jr, Marshall SJ, Kinney JH, Balooch M. The dentin substrate: structure and properties related to bonding. J Dent. 1997;25:441–58. doi: 10.1016/s0300-5712(96)00065-6. [DOI] [PubMed] [Google Scholar]
- 3.Gelse K, Poschl E, Aigner T. Collagens--structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–46. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Ortiz C, Boyce MC. Materials science. Bioinspired structural materials. Science. 2008;319:1053–4. doi: 10.1126/science.1154295. [DOI] [PubMed] [Google Scholar]
- 5.Silver FH, Horvath I, Foran DJ. Viscoelasticity of the vessel wall: the role of collagen and elastic fibers. Crit Rev Biomed Eng. 2001;29:279–301. doi: 10.1615/critrevbiomedeng.v29.i3.10. [DOI] [PubMed] [Google Scholar]
- 6.Knott L, Bailey AJ. Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance. Bone. 1998;22:181–7. doi: 10.1016/s8756-3282(97)00279-2. [DOI] [PubMed] [Google Scholar]
- 7.Light ND, Bailey AJ. The chemistry of the collagen cross-links. Purification and characterization of cross-linked polymeric peptide material from mature collagen containing unknown amino acids. Biochem J. 1980;185:373–81. doi: 10.1042/bj1850373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiser K, McCormick RJ, Rucker RB. Enzymatic and nonenzymatic cross-linking of collagen and elastin. FASEB J. 1992;6:2439–49. doi: 10.1096/fasebj.6.7.1348714. [DOI] [PubMed] [Google Scholar]
- 9.Yamauchi M. Advances in Tissue Banking. New Jersey, NJ: World Scientific Publishing; 2000. Collagen biochemistry: an overview; pp. 455–500. [Google Scholar]
- 10.Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2010;21:195–214. doi: 10.1007/s00198-009-1066-z. [DOI] [PubMed] [Google Scholar]
- 11.Haruyama N, Sreenath TL, Suzuki S, Yao X, Wang Z, Wang Y, et al. Genetic evidence for key roles of decorin and biglycan in dentin mineralization. Matrix biology: journal of the International Society for Matrix Biology. 2009;28:129–36. doi: 10.1016/j.matbio.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruggeri A, Orsini G, Mazzoni A, Nato F, Papa V, Piccirilli M, et al. Immunohistochemical and biochemical assay of versican in human sound predentine/dentine matrix. Eur J Histochem: EJH. 2009;53:125–33. doi: 10.4081/ejh.2009.e15. [DOI] [PubMed] [Google Scholar]
- 13.Panwar P, Du X, Sharma V, Lamour G, Castro M, Li H, et al. Effects of cysteine proteases on the structural and mechanical properties of collagen fibers. J Biol Chem. 2013;288:5940–50. doi: 10.1074/jbc.M112.419689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waddington RJ, Hall RC, Embery G, Lloyd DM. Changing profiles of proteoglycans in the transition of predentine to dentine. Matrix Biology. 2003;22:153–61. doi: 10.1016/s0945-053x(03)00019-2. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg M, Kulkarni A, Young M, Boskey A. Dentin: structure, composition and mineralization. Front Biosci (Elite Ed) 2011;1:711–35. doi: 10.2741/e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikdin H, Olsson ML, Hultenby K, Sugars RV. Osteoadherin accumulates in the predentin towards the mineralization front in the developing tooth. PloS one. 2012;7:e31525. doi: 10.1371/journal.pone.0031525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg M, Takagi M. Dentine proteoglycans: composition, ultrastructure and functions. Histochem J. 1993;25:781–806. [PubMed] [Google Scholar]
- 18.Yoshiba N, Yoshiba K, Iwaku M, Ozawa H. Immunolocalization of the small proteoglycan decorin in human teeth. Arch Oral Biol. 1996;41:351–7. doi: 10.1016/0003-9969(95)00138-7. [DOI] [PubMed] [Google Scholar]
- 19.Orsini G, Ruggeri A, Jr, Mazzoni A, Papa V, Mazzotti G, Di Lenarda R, et al. Immunohistochemical identification of decorin and biglycan in human dentin: a correlative field emission scanning electron microscopy/transmission electron microscopy study. Calcif Tissue Int. 2007;81:39–45. doi: 10.1007/s00223-007-9027-z. [DOI] [PubMed] [Google Scholar]
- 20.Matsuura T, Duarte W, Cheng H, Uzawa K, Yamauchi M. Differential expression of decorin and biglycan genes during mouse tooth development. Matrix Biol. 2001;20:367–73. doi: 10.1016/s0945-053x(01)00142-1. [DOI] [PubMed] [Google Scholar]
- 21.Torzilli PA, Arduino JM, Gregory JD, Bansal M. Effect of proteoglycan removal on solute mobility in articular cartilage. J Biomech. 1997;30:895–902. doi: 10.1016/s0021-9290(97)00059-6. [DOI] [PubMed] [Google Scholar]
- 22.Suppa P, Ruggeri AJ, Tay F, Prati C, Biasotto M, Falconi M, et al. Reduced Antigenicity of Type I Collagen and Proteoglycans in Sclerotic Dentin. J Dent Res. 2006;85:133–7. doi: 10.1177/154405910608500204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16:558–64. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gusman H, Santana RB, Zehnder M. Matrix metalloproteinase levels and gelatinolytic activity in clinically healthy and inflamed human dental pulps. Eur J Oral Sci. 2002;110:353–7. doi: 10.1034/j.1600-0722.2002.21347.x. [DOI] [PubMed] [Google Scholar]
- 25.Sorsa T, Tjaderhane L, Salo T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 2004;10:311–8. doi: 10.1111/j.1601-0825.2004.01038.x. [DOI] [PubMed] [Google Scholar]
- 26.Tjaderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J Dent Res. 1998;77:1622–9. doi: 10.1177/00220345980770081001. [DOI] [PubMed] [Google Scholar]
- 27.Martin-De Las Heras S, Valenzuela A, Overall CM. The matrix metalloproteinase gelatinase A in human dentine. Arch Oral Biol. 2000;45:757–65. doi: 10.1016/s0003-9969(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 28.Mazzoni A, Papa V, Nato F, Carrilho M, Tjaderhane L, Ruggeri A, Jr, et al. Immunohistochemical and biochemical assay of MMP-3 in human dentine. J Dent. 2011;39:231–7. doi: 10.1016/j.jdent.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjaderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol. 2007;52:121–7. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Nascimento FD, Minciotti CL, Geraldeli S, Carrilho MR, Pashley DH, Tay FR, et al. Cysteine cathepsins in human carious dentin. J Dent Res. 2011;90:506–11. doi: 10.1177/0022034510391906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tersariol IL, Geraldeli S, Minciotti CL, Nascimento FD, Paakkonen V, Martins MT, et al. Cysteine cathepsins in human dentin-pulp complex. J Endod. 2010;36:475–81. doi: 10.1016/j.joen.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 32.Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B, et al. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim Biophys Acta. 2012;1824:68–88. doi: 10.1016/j.bbapap.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turk B, Turk D, Turk V. Lysosomal cysteine proteases: more than scavengers. Biochim Biophys Acta. 2000;1477:98–111. doi: 10.1016/s0167-4838(99)00263-0. [DOI] [PubMed] [Google Scholar]
- 34.Turk D, Guncar G. Lysosomal cysteine proteases (cathepsins): promising drug targets. Acta Crystallogr D Biol Crystallogr. 2003;59:203–13. doi: 10.1107/s0907444902021479. [DOI] [PubMed] [Google Scholar]
- 35.Dickinson DP. Cysteine peptidases of mammals: their biological roles and potential effects in the oral cavity and other tissues in health and disease. Crit Rev Oral Biol Med. 2002;13:238–75. doi: 10.1177/154411130201300304. [DOI] [PubMed] [Google Scholar]
- 36.Mogi M, Otogoto J. Expression of cathepsin-K in gingival crevicular fluid of patients with periodontitis. Arch Oral Biol. 2007;52:894–8. doi: 10.1016/j.archoralbio.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Tsuji Y, Yamaza T, Kido MA, Goto T, Nakata S, Akamine A, et al. Expression of cathepsin K mRNA and protein in odontoclasts after experimental tooth movement in the mouse maxilla by in situ hybridization and immunoelectron microscopy. Cell Tissue Res. 2001;303:359–69. doi: 10.1007/s004410000327. [DOI] [PubMed] [Google Scholar]
- 38.Stenzel KH, Miyata T, Rubin AL. Collagen as a biomaterial. Annu Rev Biophys Bioeng. 1974;3:231–53. doi: 10.1146/annurev.bb.03.060174.001311. [DOI] [PubMed] [Google Scholar]
- 39.Cheung DT, Perelman N, Ko EC, Nimni ME. Mechanism of crosslinking of proteins by glutaraldehyde III. Reaction with collagen in tissues. Connect Tissue Res. 1985;13:109–15. doi: 10.3109/03008208509152389. [DOI] [PubMed] [Google Scholar]
- 40.Han B, Jaurequi J, Tang BW, Nimni ME. Proanthocyanidin: a natural crosslinking reagent for stabilizing collagen matrices. J Biomed Mater Res Part A. 2003;65:118–24. doi: 10.1002/jbm.a.10460. [DOI] [PubMed] [Google Scholar]
- 41.Snibson GR. Collagen cross-linking: a new treatment paradigm in corneal disease - a review. Clin Experiment Ophthalmol. 2010;38:141–53. doi: 10.1111/j.1442-9071.2010.02228.x. [DOI] [PubMed] [Google Scholar]
- 42.Foote CS. Mechanisms of photosensitized oxidation. There are several different types of photosensitized oxidation which may be important in biological systems. Science. 1968;162:963–70. doi: 10.1126/science.162.3857.963. [DOI] [PubMed] [Google Scholar]
- 43.Barnard K, Light ND, Sims TJ, Bailey AJ. Chemistry of the collagen cross-links. Origin and partial characterization of a putative mature cross-link of collagen. Biochem J. 1987;244:303–9. doi: 10.1042/bj2440303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fraser RD, MacRae TP, Suzuki E. Chain conformation in the collagen molecule. J Mol Biol. 1979;129:463–81. doi: 10.1016/0022-2836(79)90507-2. [DOI] [PubMed] [Google Scholar]
- 45.McCall AS, Kraft S, Edelhauser HF, Kidder GW, Lundquist RR, Bradshaw HE, et al. Mechanisms of corneal tissue cross-linking in response to treatment with topical riboflavin and long-wavelength ultraviolet radiation (UVA) Invest Ophthalmol Vis Sci. 2010;51:129–38. doi: 10.1167/iovs.09-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–7. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 47.Fawzy A, Nitisusanta L, Iqbal K, Daood U, Beng LT, Neo J. Characterization of riboflavin-modified dentin collagen matrix. J Dent Res. 2012;91:1049–54. doi: 10.1177/0022034512459053. [DOI] [PubMed] [Google Scholar]
- 48.Cheung DT, Tong D, Perelman N, Ertl D, Nimni ME. Mechanism of crosslinking of proteins by glutaraldehyde. IV: In vitro and in vivo stability of a crosslinked collagen matrix. Connect Tissue Res. 1990;25:27–34. doi: 10.3109/03008209009009810. [DOI] [PubMed] [Google Scholar]
- 49.Bedran-Russo AK, Pashley DH, Agee K, Drummond JL, Miescke KJ. Changes in stiffness of demineralized dentin following application of collagen crosslinkers. J Biomed Mater Res Part B, Appl Biomater. 2008;86:330–4. doi: 10.1002/jbm.b.31022. [DOI] [PubMed] [Google Scholar]
- 50.Olde Damink LH, Dijkstra PJ, van Luyn MJ, van Wachem PB, Nieuwenhuis P, Feijen J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials. 1996;17:765–73. doi: 10.1016/0142-9612(96)81413-x. [DOI] [PubMed] [Google Scholar]
- 51.Staros JV, Wright RW, Swingle DM. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal Biochem. 1986;156:220–2. doi: 10.1016/0003-2697(86)90176-4. [DOI] [PubMed] [Google Scholar]
- 52.Bedran-Russo AK, Vidal CM, Dos Santos PH, Castellan CS. Long-term effect of carbodiimide on dentin matrix and resin-dentin bonds. J Biomed Mater Res Part B, Appl Biomater. 2010;94:250–5. doi: 10.1002/jbm.b.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang YP, Kimura M, Tawada K. Covalent crosslinking of myosin subfragment-1 and heavy meromyosin to actin at various molar ratios: different correlations between ATPase activity and crosslinking extent. J Muscle Res Cell Motil. 1990;11:313–22. doi: 10.1007/BF01766669. [DOI] [PubMed] [Google Scholar]
- 54.Petite H, Duval JL, Frei V, Abdul-Malak N, Sigot-Luizard MF, Herbage D. Cytocompatibility of calf pericardium treated by glutaraldehyde and by the acyl azide methods in an organotypic culture model. Biomaterials. 1995;16:1003–8. doi: 10.1016/0142-9612(95)94908-4. [DOI] [PubMed] [Google Scholar]
- 55.Bedran-Russo AK, Pereira PN, Duarte WR, Drummond JL, Yamauchi M. Application of crosslinkers to dentin collagen enhances the ultimate tensile strength. J Biomed Mater Res Part B, Appl Biomater. 2007;80:268–72. doi: 10.1002/jbm.b.30593. [DOI] [PubMed] [Google Scholar]
- 56.Frujikawa S, Yokota T, Konga K, JK The continuous hydrolysis of geniposide to genipin using immobilized beta-glucosidase on calcium alginate gel. J Biotechnol Lett. 1987;9:697–702. [Google Scholar]
- 57.Sung HW, Chang WH, Ma CY, Lee MH. Crosslinking of biological tissues using genipin and/or carbodiimide. J Biomed Mater Res Part A. 2003;64:427–38. doi: 10.1002/jbm.a.10346. [DOI] [PubMed] [Google Scholar]
- 58.Sung HW, Chang Y, Chiu CT, Chen CN, Liang HC. Crosslinking characteristics and mechanical properties of a bovine pericardium fixed with a naturally occurring crosslinking agent. J Biomed Mater Res. 1999;47:116–26. doi: 10.1002/(sici)1097-4636(199911)47:2<116::aid-jbm2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 59.Cao N, Fu Y, He J. Mechanical properties of gelatin films cross-linked, respectively, by ferulic acid and tannin acid. Food Hydrocolloids. 2007;21:575–84. [Google Scholar]
- 60.Trouet AU, Pirson P, Steiger R, Masquelier M, Baurain R, Gillet J. Development of new derivatives of primaquine by association with lysosomotropic carriers. Bull World Health Organ. 1981;59:449–58. [PMC free article] [PubMed] [Google Scholar]
- 61.Hagerman AE, Klucher K. Tannin-protein interactions. Prog Clin Biol Res. 1986;213:67–76. [PubMed] [Google Scholar]
- 62.Hagerman AE, Butler LG. The specificity of proanthocyanidin-protein interactions. J Biol Chem. 1981;256:4494–7. [PubMed] [Google Scholar]
- 63.Pandey Kanti B, Rizvi Syed I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–8. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferreira D, Slade D, Marais JPJ. Flavans and proanthocyanidins. Flavonoids. 2006:553–616. [Google Scholar]
- 65.Evans WC. Trease and Evans Pharmacognosy. Elsevier; 2009. [Google Scholar]
- 66.Huemmer W, Schreier P. Analysis of proanthocyanidins. Molecular Nutrition & Food Research. 2008;52:1381–98. doi: 10.1002/mnfr.200700463. [DOI] [PubMed] [Google Scholar]
- 67.Gu L. Analysis methods of proanthocyanidins. Analysis of Antioxidant-Rich Phytochemicals. 2012:247–74. [Google Scholar]
- 68.Ferreira D, Li X-C. Oligomeric proanthocyanidins: naturally occurring O-heterocycles (January 1996 to December 1998) Natural Product Reports. 2000;17:193–212. doi: 10.1039/a705728h. [DOI] [PubMed] [Google Scholar]
- 69.Wu Q, Wang M, Simon JE. Determination of proanthocyanidins in fresh grapes and grape products using liquid chromatography with mass spectrometric detection. Rapid Communications in Mass Spectrometry. 2005;19:2062–8. doi: 10.1002/rcm.2029. [DOI] [PubMed] [Google Scholar]
- 70.Jones WT, Broadhurst RB, Lyttleton JW. The condensed tannins of pasture legume species. Phytochemistry (Elsevier) 1976;15:1407–9. [Google Scholar]
- 71.Ossipova S, Ossipov V, Haukioja E, Loponen J, Pihlaja K. Proanthocyanidins of mountain birch leaves: quantification and properties. Phytochem Anal. 2001;12:128–33. doi: 10.1002/pca.568. [DOI] [PubMed] [Google Scholar]
- 72.Fu C, Loo AEK, Chia FPP, Huang D. Oligomeric Proanthocyanidins from Mangosteen Pericarps. J Agric Food Chem. 2007;55:7689–94. doi: 10.1021/jf071166n. [DOI] [PubMed] [Google Scholar]
- 73.Karonen M, Loponen J, Ossipov V, Pihlaja K. Analysis of procyanidins in pine bark with reversed-phase and normal-phase high-performance liquid chromatography-electrospray ionization mass spectrometry. Analytica Chimica Acta. 2004;522:105–12. [Google Scholar]
- 74.Sudjaroen Y, Haubner R, Wuertele G, Hull WE, Erben G, Spiegelhalder B, et al. Isolation and structure elucidation of phenolic antioxidants from Tamarind (Tamarindus indica L) seeds and pericarp. Food Chem Toxicol. 2005;43:1673–82. doi: 10.1016/j.fct.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 75.Gu L, Kelm M, Hammerstone JF, Beecher G, Cunningham D, Vannozzi S, et al. Fractionation of polymeric procyanidins from lowbush blueberry and quantification of procyanidins in selected foods with an optimized normal-phase HPLC-MS fluorescent detection method. J Agric Food Chem. 2002;50:4852–60. doi: 10.1021/jf020214v. [DOI] [PubMed] [Google Scholar]
- 76.Kelm MA, Johnson JC, Robbins RJ, Hammerstone JF, Schmitz HH. High-Performance Liquid Chromatography Separation and Purification of Cacao (Theobroma cacao L) Procyanidins According to Degree of Polymerization Using a Diol Stationary Phase. J Agric Food Chem. 2006;54:1571–6. doi: 10.1021/jf0525941. [DOI] [PubMed] [Google Scholar]
- 77.Broyles AC, Pavan S, Bedran-Russo AK. Effect of dentin surface modification on the microtensile bond strength of self-adhesive resin cements. J Prosthodont. 2013;22:59–62. doi: 10.1111/j.1532-849X.2012.00890.x. [DOI] [PubMed] [Google Scholar]
- 78.Pavan S, Xie Q, Hara AT, Bedran-Russo AK. Biomimetic approach for root caries prevention using a proanthocyanidin-rich agent. Caries Res. 2011;45:443–7. doi: 10.1159/000330599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.dos Santos PH, Karol S, Bedran-Russo AK. Nanomechanical properties of biochemically modified dentin bonded interfaces. J Oral Rehab. 2011;38:541–6. doi: 10.1111/j.1365-2842.2010.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dos Santos PH, Karol S, Bedran-Russo AK. Long-term nano-mechanical properties of biomodified dentin-resin interface components. J Biomech. 2011;44:1691–4. doi: 10.1016/j.jbiomech.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Castellan CS, Bedran-Russo AK, Karol S, Pereira PN. Long-term stability of dentin matrix following treatment with various natural collagen cross-linkers. J Mech Behav Biomed Mater. 2011;4:1343–50. doi: 10.1016/j.jmbbm.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bedran-Russo AK, Castellan CS, Shinohara MS, Hassan L, Antunes A. Characterization of biomodified dentin matrices for potential preventive and reparative therapies. Acta Biomater. 2011;7:1735–41. doi: 10.1016/j.actbio.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Castellan CS, Pereira PN, Viana G, Chen SN, Pauli GF, Bedran-Russo AK. Solubility study of phytochemical cross-linking agents on dentin stiffness. J Dent. 2010;38:431–6. doi: 10.1016/j.jdent.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Castellan CS, Pereira PN, Grande RH, Bedran-Russo AK. Mechanical characterization of proanthocyanidin-dentin matrix interaction. Dent Mater. 2010;26:968–73. doi: 10.1016/j.dental.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Macedo GV, Yamauchi M, Bedran-Russo AK. Effects of chemical cross-linkers on caries-affected dentin bonding. J Dent Res. 2009;88:1096–100. doi: 10.1177/0022034509351001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Al-Ammar A, Drummond JL, Bedran-Russo AK. The use of collagen cross-linking agents to enhance dentin bond strength. J Biomed Mater Res Part B, Appl Biomater. 2009;91:419–24. doi: 10.1002/jbm.b.31417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu Y, Wang Y. Proanthocyanidins’ efficacy in stabilizing dentin collagen against enzymatic degradation: MALDI-TOF and FTIR analyses. J Dent. 2013;41:535–42. doi: 10.1016/j.jdent.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Y, Chen M, Yao X, Xu C, Zhang Y, Wang Y. Enhancement in dentin collagen’s biological stability after proanthocyanidins treatment in clinically relevant time periods. Dent Mater. 2013;29:485–92. doi: 10.1016/j.dental.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hechler B, Yao X, Wang Y. Proanthocyanidins alter adhesive/dentin bonding strengths when included in a bonding system. Amer J Dent. 2012;25:276–80. [PMC free article] [PubMed] [Google Scholar]
- 90.Fang M, Liu R, Xiao Y, Li F, Wang D, Hou R, et al. Biomodification to dentin by a natural crosslinker improved the resin-dentin bonds. J Dent. 2012;40:458–66. doi: 10.1016/j.jdent.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 91.Green B, Yao X, Ganguly A, Xu C, Dusevich V, Walker MP, et al. Grape seed proanthocyanidins increase collagen biodegradation resistance in the dentin/adhesive interface when included in an adhesive. J Dent. 2010;38:908–15. doi: 10.1016/j.jdent.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Castellan CS, Pereira PN, Grande RH, Bedran-Russo AK. Mechanical characterization of proanthocyanidin-dentin matrix interaction. Dent Mater. 2010;26:968–73. doi: 10.1016/j.dental.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Castellan CS, Pereira PN, Viana G, Chen SN, Pauli GF, Bedran-Russo AK. Solubility study of phytochemical cross-linking agents on dentin stiffness. J Dent. 2010;38:431–6. doi: 10.1016/j.jdent.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pereira PN, Bedran-de-Castro AK, Duarte WR, Yamauchi M. Removal of noncollagenous components affects dentin bonding. J Biomed Mater Res B Appl Biomater. 2007;80:86–91. doi: 10.1002/jbm.b.30572. [DOI] [PubMed] [Google Scholar]
- 95.Bedran-Russo AK, Pereira PN, Duarte WR, Okuyama K, Yamauchi M. Removal of dentin matrix proteoglycans by trypsin digestion and its effect on dentin bonding. J Biomed Mater Res B, Appl Biomater. 2008;85:261–6. doi: 10.1002/jbm.b.30944. [DOI] [PubMed] [Google Scholar]
- 96.Boukpessi T, Menashi S, Camoin L, Tencate JM, Goldberg M, Chaussain-Miller C. The effect of stromelysin-1 (MMP-3) on non-collagenous extracellular matrix proteins of demineralized dentin and the adhesive properties of restorative resins. Biomaterials. 2008;29:4367–73. doi: 10.1016/j.biomaterials.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 97.Mazzoni A, Pashley DH, Ruggeri A, Jr, Vita F, Falconi M, Di Lenarda R, et al. Adhesion to chondroitinase ABC treated dentin. J Biomed Mater Res B Appl Biomater. 2008;86:228–36. doi: 10.1002/jbm.b.31010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scott JE. Proteoglycan histochemistry--a valuable tool for connective tissue biochemists. Coll Relat Res. 1985;5:541–75. doi: 10.1016/s0174-173x(85)80008-x. [DOI] [PubMed] [Google Scholar]
- 99.Katiyar SK. Matrix metalloproteinases in cancer metastasis: molecular targets for prostate cancer prevention by green tea polyphenols and grape seed proanthocyanidins. Endocr Metab Immune Disord Drug Targets. 2006;6:17–24. doi: 10.2174/187153006776056648. [DOI] [PubMed] [Google Scholar]
- 100.Scoditti E, Calabriso N, Massaro M, Pellegrino M, Storelli C, Martines G, et al. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: a potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch Biochem Biophys. 2012;527:81–9. doi: 10.1016/j.abb.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 101.Bodet C, Chandad F, Grenier D. Inhibition of host extracellular matrix destructive enzyme production and activity by a high-molecular-weight cranberry fraction. J Periodontal Res. 2007;42:159–68. doi: 10.1111/j.1600-0765.2006.00929.x. [DOI] [PubMed] [Google Scholar]
- 102.La VD, Howell AB, Grenier D. Cranberry proanthocyanidins inhibit MMP production and activity. J Dent Res. 2009;88:627–32. doi: 10.1177/0022034509339487. [DOI] [PubMed] [Google Scholar]
- 103.Tipton DA, Babu JP, Dabbous MK. Effects of cranberry components on human aggressive periodontitis gingival fibroblasts. J Periodontal Res. 2012;48:433–42. doi: 10.1111/jre.12023. [DOI] [PubMed] [Google Scholar]
- 104.Kopperud SE, Tveit AB, Gaarden T, Sandvik L, Espelid I. Longevity of posterior dental restorations and reasons for failure. Eur J Oral Sci. 2012;120:539–48. doi: 10.1111/eos.12004. [DOI] [PubMed] [Google Scholar]
- 105.Gordan VV, Riley JL, 3rd, Geraldeli S, Rindal DB, Qvist V, Fellows JL, et al. Repair or replacement of defective restorations by dentists in The Dental Practice-Based Research Network. J Amer Dent Assoc. 2012;143:593–601. doi: 10.14219/jada.archive.2012.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu Y, Tjaderhane L, Breschi L, Mazzoni A, Li N, Mao J, et al. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res. 2011;90:953–68. doi: 10.1177/0022034510391799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: aging and stability of the bonded interface. Dent Mater. 2008;24:90–101. doi: 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 108.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216–21. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 109.Yoshiyama M, Urayama A, Kimochi T, Matsuo T, Pashley DH. Comparison of conventional vs self-etching adhesive bonds to caries-affected dentin. Oper Dent. 2000;25:163–9. [PubMed] [Google Scholar]
- 110.Nakajima M, Sano H, Burrow MF, Tagami J, Yoshiyama M, Ebisu S, et al. Tensile bond strength and SEM evaluation of caries-affected dentin using dentin adhesives. J Dent Res. 1995;74:1679–88. doi: 10.1177/00220345950740100901. [DOI] [PubMed] [Google Scholar]
- 111.Sano H, Takatsu T, Ciucchi B, Russell CM, Pashley DH. Tensile properties of resin-infiltrated demineralized human dentin. J Dent Res. 1995;74:1093–102. doi: 10.1177/00220345950740041001. [DOI] [PubMed] [Google Scholar]
- 112.Bedran-Russo AK, Castellan KS, Pashley D, Viana G. Site-specific properties of carious dentin matrices biomodified with collagen cross-linkers. Amer J Dent. 2013 [PMC free article] [PubMed] [Google Scholar]
- 113.Fawzy AS, Nitisusanta LI, Iqbal K, Daood U, Beng LT, Neo J. Chitosan/Riboflavin-modified demineralized dentin as a potential substrate for bonding. J Mech Behav Biomed Mater. 2012;17:278–89. doi: 10.1016/j.jmbbm.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 114.Cova A, Breschi L, Nato F, Ruggeri A, Jr, Carrilho M, Tjaderhane L, et al. Effect of UVA-activated riboflavin on dentin bonding. J Dent Res. 2011;90:1439–45. doi: 10.1177/0022034511423397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bedran-Russo AK, Vidal CM, Dos Santos PH, Castellan CS. Long-term effect of carbodiimide on dentin matrix and resin-dentin bonds. J Biomed Mater Res B, Appl Biomater. 2010;94:250–5. doi: 10.1002/jbm.b.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tezvergil-Mutluay A, Mutluay MM, Agee KA, Seseogullari-Dirihan R, Hoshika T, Cadenaro M, et al. Carbodiimide cross-linking inactivates soluble and matrix-bound MMPs, in vitro. J Dent Res. 2012;91:192–6. doi: 10.1177/0022034511427705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mazzoni A, Mannello F, Tay FR, Tonti GA, Papa S, Mazzotti G, et al. Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. J Dent Res. 2007;86:436–40. doi: 10.1177/154405910708600509. [DOI] [PubMed] [Google Scholar]
- 118.Mazzoni A, Nascimento FD, Carrilho M, Tersariol I, Papa V, Tjaderhane L, et al. MMP activity in the hybrid layer detected with in situ zymography. J Dent Res. 2012;91:467–72. doi: 10.1177/0022034512439210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bedran-Russo AK, Yoo KJ, Ema KC, Pashley DH. Mechanical properties of tannic-acid-treated dentin matrix. J Dent Res. 2009;88:807–11. doi: 10.1177/0022034509342556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Berrin J-G, McLauchlan WR, Needs P, Williamson G, Puigserver A, Kroon PA, et al. Functional expression of human liver cytosolic b-glucosidase in Pichia pastoris. Insights into its role in the metabolism of dietary glucosides. Eur J Biochem. 2002;269:249–58. doi: 10.1046/j.0014-2956.2001.02641.x. [DOI] [PubMed] [Google Scholar]
- 121.Almas K. The effects of extracts of chewing sticks (Salvadora persica) on healthy and periodontally involved human dentine: a SEM study. Indian J Dent Res. 2001;12:127–32. [PubMed] [Google Scholar]
- 122.Xie Q, Bedran-Russo AK, Wu CD. In vitro remineralization effects of grape seed extract on artificial root caries. J Dent. 2008;36:900–6. doi: 10.1016/j.jdent.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guo B, Que KH, Jing Y, Wang B, Liang QQ, Xie HH. Effect of Galla chinensis on the remineralization of two bovine root lesions morphous in vitro. Int J Oral Sci. 2012;4:152–6. doi: 10.1038/ijos.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Que KH, Guo B, Wang B, Liang QQ, Xie HH. Re-mineralization ability of Galla chinensis extracts on root carious lesions with or without non-collagen proteins. Sichuan da xue xue bao Yi xue ban = Journal of Sichuan University Medical science edition. 2012;43:358–61. [PubMed] [Google Scholar]
- 125.Koo H, Duarte S, Murata RM, Scott-Anne K, Gregoire S, Watson GE, et al. Influence of cranberry proanthocyanidins on formation of biofilms by Streptococcus mutans on saliva-coated apatitic surface and on dental caries development in vivo. Caries Res. 2010;44:116–26. doi: 10.1159/000296306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Feng G, Klein MI, Gregoire S, Singh AP, Vorsa N, Koo H. The specific degree-of-polymerization of A-type proanthocyanidin oligomers impacts Streptococcus mutans glucan-mediated adhesion and transcriptome responses within biofilms. Biofouling. 2013 doi: 10.1080/08927014.2013.794456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Buzalaf MA, Kato MT, Hannas AR. The role of matrix metalloproteinases in dental erosion. Adv Dent Res. 2012;24:72–6. doi: 10.1177/0022034512455029. [DOI] [PubMed] [Google Scholar]
- 128.Barbosa CS, Kato MT, Buzalaf MA. Effect of supplementation of soft drinks with green tea extract on their erosive potential against dentine. Aust Dent J. 2011;56:317–21. doi: 10.1111/j.1834-7819.2011.01338.x. [DOI] [PubMed] [Google Scholar]
- 129.Magalhaes AC, Wiegand A, Rios D, Hannas A, Attin T, Buzalaf MA. Chlorhexidine and green tea extract reduce dentin erosion and abrasion in situ. J Dent. 2009;37:994–8. doi: 10.1016/j.jdent.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 130.Kato MT, Magalhaes AC, Rios D, Hannas AR, Attin T, Buzalaf MA. Protective effect of green tea on dentin erosion and abrasion. J Appl Oral Sci. 2009;17:560–4. doi: 10.1590/S1678-77572009000600004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Demeule M, Brossard M, Page M, Gingras D, Beliveau R. Matrix metalloproteinase inhibition by green tea catechins. Biochim Biophys Acta. 2000;1478:51–60. doi: 10.1016/s0167-4838(00)00009-1. [DOI] [PubMed] [Google Scholar]
- 132.Kato MT, Leite AL, Hannas AR, Calabria MP, Magalhaes AC, Pereira JC, et al. Impact of protease inhibitors on dentin matrix degradation by collagenase. J Dent Res. 2012;91:1119–23. doi: 10.1177/0022034512455801. [DOI] [PubMed] [Google Scholar]
- 133.Chen H, Huang B. Pilot study of the effect of green tea extractive epigallocatechin-3-gallate on degradation of collagen in dental erosion. Hua xi kou qiang yi xue za zhi = Huaxi kouqiang yixue zazhi = West China journal of stomatology. 2012;30:549–51. [PubMed] [Google Scholar]
- 134.Boushell LW, Kaku M, Mochida Y, Bagnell R, Yamauchi M. Immunohistochemical localization of matrixmetalloproteinase-2 in human coronal dentin. Arch Oral Biol. 2008;53:109–16. doi: 10.1016/j.archoralbio.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Niu LN, Zhang L, Jiao K, Li F, Ding YX, Wang DY, et al. Localization of MMP-2, MMP-9, TIMP-1, and TIMP-2 in human coronal dentine. J Dent. 2011;39:536–42. doi: 10.1016/j.jdent.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 136.Orsini G, Ruggeri A, Jr, Mazzoni A, Papa V, Piccirilli M, Falconi M, et al. Immunohistochemical identification of type I and type III collagen and chondroitin sulphate in human pre-dentine: a correlative FEI-SEM/TEM study. Int Endod J. 2007;40:669–78. doi: 10.1111/j.1365-2591.2007.01263.x. [DOI] [PubMed] [Google Scholar]
- 137.Bertassoni LE, Stankoska K, Swain MV. Insights into the structure and composition of the peritubular dentin organic matrix and the lamina limitans. Micron. 2012;43:229–36. doi: 10.1016/j.micron.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 138.Mazzoni A, Pashley DH, Tay FR, Gobbi P, Orsini G, Ruggeri A, Jr, et al. Immunohistochemical identification of MMP-2 and MMP-9 in human dentin: correlative FEI-SEM/TEM analysis. J Biomed Mater Res Part A. 2009;88:697–703. doi: 10.1002/jbm.a.31920. [DOI] [PubMed] [Google Scholar]
- 139.Jerez M, Tourino S, Sineiro J, Torres JL, Nunez MJ. Procyanidins from pine bark: Relationships between structure, composition and antiradical activity. Food Chem. 2007;104:518–27. [Google Scholar]
- 140.Sivakumaran S, Meagher LP, Foo LY, Lane GA, Fraser K, Rumball W. Floral procyanidins of the forage legume red clover (Trifolium pratense L) J Agric Food Chem. 2004;52:1581–5. doi: 10.1021/jf035379y. [DOI] [PubMed] [Google Scholar]


