Abstract
Rationale: The 2007 American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) recommend that patients with pulmonary nontuberculous mycobacterial (PNTM) disease caused by Mycobacterium avium complex (MAC) or M. abscessus be treated with a macrolide-based multidrug antibiotic regimen until sputum culture negative for 1 year. After 6 years, the degree of adherence to recommended guidelines among physicians remains unknown.
Objective: To describe antibiotic treatment practices among physicians treating patients with PNTM in the United States.
Methods: A nationally representative sample of 1,286 U.S. physicians was contacted in December 2011 through January 2012; 582 of the responding physicians were treating patients with PNTM and were eligible to participate. Physicians were asked to extract medical record data on the last four patients they treated in the past year with PNTM disease from either MAC or M. abscessus. Treatment patterns were assessed for all patients by NTM species and physician specialty, and compared with the 2007 recommended ATS/IDSA guidelines.
Main Results: Questionnaires were completed by 349 physicians on 915 patients with PNTM, including 744 (81%) with MAC and 174 (19%) with M. abscessus; 3 patients were positive for both. Physicians treated 76 (44%) patients with M. abscessus and 411 (55%) patients with MAC. Only 13% of antibiotic regimens prescribed to patients with MAC met ATS/IDSA guidelines, 56% did not include a macrolide, and 16% were for macrolide monotherapy. Among patients with M. abscessus, 64% of regimens prescribed did not include a macrolide.
Conclusions: Adherence to the 2007 ATS/IDSA guidelines for treating PNTM disease is poor. Across all physician specialties evaluated, suboptimal or potentially harmful antibiotic regimens were commonly prescribed.
Keywords: Mycobacterium infection, nontuberculous, treatment, guideline adherence
Nontuberculous mycobacteria (NTM) are ubiquitous throughout the environment, and are frequently detected in soil and water samples. Exposure to environmental sources can presumably result in pulmonary infection with NTM in susceptible hosts, with potentially severe outcomes (1, 2). Recently, pulmonary NTM (PNTM) disease prevalence has increased throughout the United States by approximately 8% per year (3). Over 80% of U.S. PNTM infections are caused by Mycobacterium avium complex (MAC), although other species, including M. abscessus, are also isolated (4). To advance the diagnosis and treatment of these chronic lung infections, the American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) jointly published clinical management guidelines in 2007 (6). However, managing PNTM disease is a lengthy, complicated, and time-consuming process (5), and some components of recommended therapy remain controversial.
Although the 2007 ATS/IDSA guidelines specify evidence-based treatment regimens for PNTM disease caused by MAC, no established regimens of proven efficacy exist for M. abscessus, which can cause significant morbidity and mortality; rather, the guidelines suggest only that clarithromycin may be beneficial (6). Even where antibiotic regimens are specified, compliance is thought to be poor due to the complex nature of treatments and frequently reported drug intolerances (7–9). Still, despite recognized limitations in treating PNTM disease, the current guidelines are frequently effective (10). In contrast, deviating from ATS/IDSA guidelines with certain two-drug regimens or with macrolide monotherapy places the patient at increased risk for developing macrolide-resistant infections, which carry a more dismal prognosis (10).
The actual treatment practices and antibiotic regimens prescribed by physicians actively managing the care of patients with PNTM throughout the United States remain largely unknown. Given the implications of inappropriate treatments for NTM lung disease, understanding current treatment approaches and trends is critical for identifying and addressing gaps that may exist in physician-led practices for effectively treating PNTM disease. We conducted a nationwide survey of U.S. physicians treating patients with PNTM disease to provide nationally representative estimates of antibiotic treatment–related practices.
Methods
From December 2011 through January 2012, U.S.-based physicians were randomly sampled from the American Medical Association Physician Masterfile. This file is updated every 3 months, and contains complete contact information for all U.S. physicians. The sample was stratified by specialty to ensure representation of specialties that treat the majority of patients with PNTM. Overall, 95% of the 1,286 physicians selected were in the following specialties: pulmonology, infectious diseases (IDs), internal medicine, or family/general practice. All physicians included in the sample were actively engaged in patient care; the sample selected was geographically representative. Demographics of the final sample were compared with that of the American Medical Association Masterfile to ensure sample validity.
Selected physicians received an electronic invitation to participate in the survey. To be eligible for the study, a physician must have seen one patient or more in the previous 12 months with a PNTM diagnosis, who was being managed for M. abscessus or MAC. For a patient to be eligible for inclusion, he or she must have had: (1) been seen by the physician within the previous 12 months; (2) been diagnosed with M. abscessus– or MAC-associated lung disease; (3) been currently under the study physician’s care; and (4) not been diagnosed with tuberculosis in the previous 12 months. Eligible physicians were asked to extract demographic, laboratory, and treatment data from the records of patients with diagnosed PNTM disease under their care. Questionnaires were completed by the physician either online, by telephone, or via hardcopy. Technical support was made available to all participants to clarify questionnaire items and minimize missing data. To limit any bias in patient selection by the physician, physicians provided information for up to their last four qualified cases.
A weighting process using a calculated adjustment factor was applied to the data collected to correct for two sources of potential bias: (1) patients of certain specialists may be over- or underrepresented in the sample; and (2) patients of a physician who sees many patients with PNTM would be less likely to be selected for the study than a patient of a physician who sees fewer patients with PNTM. Applying the appropriate weight adjusted the survey data so that each survey patient represented the approximate corresponding proportion of the total PNTM population diagnosed annually. This allowed all findings to be extrapolated to the entire United States population of patients with PNTM.
Patient and physician characteristics were assessed using descriptive statistics. Significant differences (P < 0.05) were evaluated using t tests and Chi-square tests of independence, which were adjusted using the Bonferroni correction for multiple pairwise comparisons. Multivariate logistic regression analysis was used, and adjusted odds ratios (aOR) with 95% confidence intervals (CI) were generated to assess factors associated with when a physician opted to treat versus not treat a patient with antibiotics. Antibiotic treatment patterns were summarized and described by NTM species. In addition, for all patients treated with antibiotics, the specific treatment regimens prescribed to patients over the course of their treatment history were summarized by NTM species and by physician specialty for patients with MAC. All regimens were categorized based on whether or not they were in adherence with the 2007 ATS/IDSA MAC treatment guidelines. Regimens not in adherence with these guidelines were further classified into the following subcategories, as determined by this study’s senior author, who was also a major contributor to the ATS/IDSA guideline development (6): (1) regimens that may increase the risk of developing macrolide resistance; (2) regimens that are of unknown clinical significance; and (3) regimens that do not include macrolides. Antibiotic regimens prescribed for patients with M. abscessus were categorized similarly for comparison, although, according to the 2007 ATS/IDSA guidelines, there are no drug regimens of proven efficacy for the treatment of M. abscessus lung disease (6).
Analyses were conducted using Statistical Analysis System (SAS Institute, Cary, NC) and Statistical Product and Service Solutions (SPSS, Inc., Chicago, IL). All results reflect weighted data that were adjusted to allow estimates to be extrapolated to the national PNTM physician and patient population.
Results
Physician and Patient Characteristics
Of the 1,286 physicians selected, 582 (45%) were eligible to participate. Of those eligible, 349 (60%) completed questionnaires on 915 patients diagnosed with PNTM disease, including 744 (81%) with MAC- and 174 (19%) M. abscessus–associated infections; lower airway cultures from three patients were positive for both (Table 1). Physician specialties represented in this study included pulmonology (46%), ID (23%), internal medicine (21%), and family/general practice (10%). Most patients were treated primarily by ID specialists (39%) or pulmonologists (37%). A total of 19% of physicians practiced at NTM specialty centers, representing 23% of patients. A total of 73% of physicians were practicing for more than 10 years. The median volume of patients with PNTM seen by all study physicians in the previous year was 15 (ranging from 8 among Family/General Practitioners to 20 among ID physicians). No one specialty was more likely to treat patients with M. abscessus or MAC.
Table 1.
Percentage of patients with Mycobacterium avium complex and Mycobacterium abscessus by patient and physician characteristics
| |
All Patients with PNTM (n = 915) |
MAC (n = 744) |
M. abscessus (n = 174) |
|---|---|---|---|
| (%) | (%) | (%) | |
| Physician specialty | |||
| Pulmonology | 37 | 38 | 35 |
| Infectious disease | 39 | 38 | 41 |
| Internal medicine | 17 | 17 | 18 |
| Family/general practitioner | 6 | 6 | 6 |
| Practice setting | |||
| NTM specialty center | 43 | 41 | 51 |
| Physician’s office | 57 | 59 | 48 |
| Region | |||
| Northeast | 20 | 21 | 14 |
| Southeast | 35 | 36 | 35 |
| Midwest | 20 | 20 | 21 |
| West | 25 | 23 | 30 |
| Patient age, yr | |||
| <50 | 29 | 29 | 30 |
| ≥50 | 71 | 71 | 70 |
| Patient sex | |||
| Female | 48 | 48 | 45 |
| Male | 52 | 52 | 55 |
| Patient race/ethnicity | |||
| White | 49 | 48 | 52 |
| Black | 27 | 29 | 18 |
| Hispanic/Latino | 11 | 11 | 12 |
| Asian/Pacific Islander | 9 | 9 | 10 |
| Selected patient comorbidities | |||
| Asthma | 27 | 27 | 28 |
| COPD | 26 | 27 | 24 |
| Bronchiectasis | 19 | 22 | 7 |
| Diabetes mellitus | 17 | 15 | 24 |
| HIV/AIDS infection | 10 | 11 | 7 |
| Rheumatoid arthritis | 5 | 6 | 3 |
Definition of abbreviations: AIDS = acquired immune deficiency syndrome; COPD = chronic obstructive pulmonary disease; HIV = human immunodeficiency virus; MAC = Mycobacterium avium complex; NTM = nontuberculous mycobacteria; PNTM = pulmonary NTM.
Three patients were positive for both MAC and M. abscessus.
Among the patients evaluated, 64% were diagnosed with PNTM within the previous year. Overall, 72% of patients were 50 years of age or older (mean = 57 yr), and 53% were male. Altogether, 49% of patients were white, 27% black, 11% Hispanic, and 9% Asian/Pacific Islander. Female patients were significantly older than male patients, with a median age of 59 versus 55 years (P < 0.05), respectively. Only 19% of black patients and 22% of Hispanic patients were 65 years of age or older compared with 30% of Asian/Pacific Islander patients and 44% of white patients. Significantly more black patients were 46–55 years old compared with white patients (34% vs. 16%, respectively; P < 0.05); significantly more white patients were 75 years of age or older compared with black and Hispanic patients (21% vs. 4% and 6%, respectively; P < 0.05). Patients aged 18–34 years were significantly more likely to be diagnosed with M. abscessus versus MAC (P < 0.05).
The proportion of PNTM infections from MAC was significantly greater in the Northeast (87%) relative to other regions (P < 0.05), where the proportion of infections with MAC ranged from 77% in the West to 82% in the Southeast. The proportion from M. abscessus was significantly greater in the West (23%) relative to other regions (P < 0.05), where the proportion ranged from 14% in the Northeast to 20% in the Midwest. Frequencies of comorbid conditions evaluated were similar between patients with MAC and patients with M. abscessus, with some exceptions: diabetes mellitus was more common among patients with M. abscessus (24%) than patients with MAC (15%), and bronchiectasis was more prevalent among patients with MAC (22%) than patients with M. abscessus (7%) (P < 0.05).
Treatment Practices and Regimens
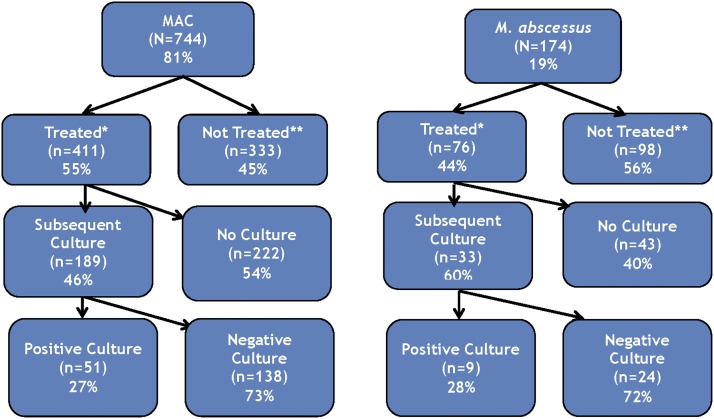
Overall, 55% (n = 411) of patients with MAC and 44% (n = 76) of patients with M. abscessus received some type of antibiotic treatment from their physician (Figure 1). Physicians were significantly more likely to treat patients who reported night sweats (aOR = 2.4; 95% CI = 1.5–3.7) or weight loss (aOR = 2.1; 95% CI = 1.3–3.4), had symptoms perceived as being more severe by the physician at initial presentation (aOR = 2.0; 95% CI = 1.4–2.9), tested positive for NTM via bronchial wash or lavage (aOR = 1.7; 95% CI = 1.1–2.5), were located in the South relative to all other United States regions (aOR = 2.2; 95% CI = 1.5–3.4), or who were treated in an out-patient facility (aOR = 4.0; 95% CI = 1.7–9.6), single-specialty group practice (aOR = 3.4; 95% CI = 2.0–5.7), or multispecialty group practice (aOR = 2.2; 95% CI = 1.4–3.6) relative to a medical school–affiliated research teaching hospital setting.
Figure 1.
Percentage and number of patients with Mycobacterium avium complex (MAC) and M. abscessus lung disease diagnosed, treated with antibiotics, and cultured by their treating physician. *The treated group includes patients who received any antibiotic treatment regimen from their physician. **The nontreated group includes patients who were diagnosed with nontuberculous mycobacterial (NTM) lung disease for over 6 months and did not receive any antibiotic treatment regimen for this condition.
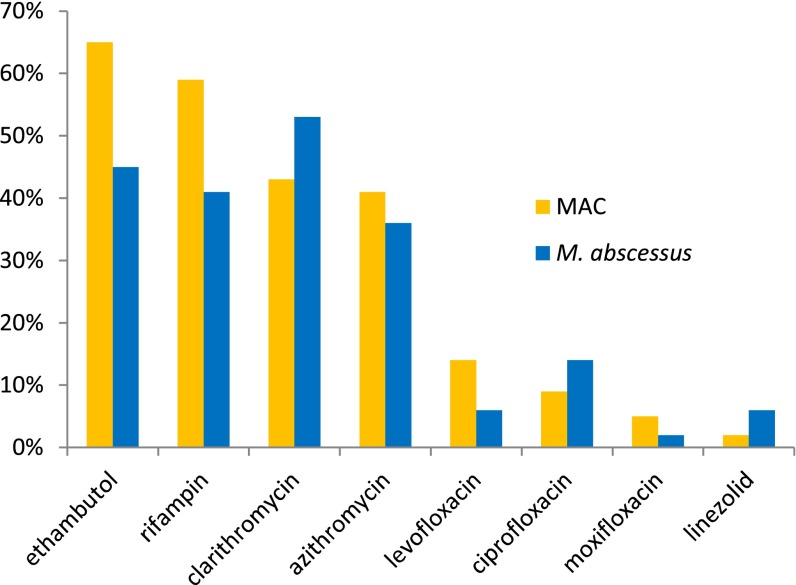
Among treated patients with MAC and those with M. abscessus, 93 and 68% received oral antibiotics, 13 and 64% received intravenous antibiotics, and 1 and 7% received inhaled antibiotics as part of their prescribed treatment, respectively. A total of 39% of physicians indicated that the oral antibiotics they selected met ATS/IDSA criteria, 18% selected regimens to minimize drug-related toxicities, and 12% selected regimens due to concerns over drug resistance. Among those receiving oral antibiotics (Figure 2), patients with MAC were more frequently treated with azithromycin than patients with M. abscessus (41% vs. 36%), whereas patients with M. abscessus were more frequently treated with clarithromycin than patients with MAC (53% vs. 43%). Patients with MAC were also more likely than patients with M. abscessus to receive rifampin (59% vs. 41%) and/or ethambutol (65% vs. 45%). The most frequently prescribed intravenous antibiotics for patients with MAC and patients with M. abscessus included amikacin (31 and 38%), imipenem/cilastatin (8 and 35%), cefoxitin (4 and 17%), tigecycline (8% for both), meropenem (4 and 6%), and linezolid (2% for both), respectively. Among those receiving inhaled antibiotics, all received inhaled amikacin.
Figure 2.
Percentage of patients with Mycobacterium avium complex (MAC) and M. abscessus prescribed oral antibiotics by type of antibiotic.
Treatment Regimens for MAC
Table 2 summarizes the specific antibiotic regimens that treated patients with MAC received. Only 13% of regimens prescribed to patients with MAC met the 2007 ATS/IDSA guidelines, which includes a macrolide, ethambutol, and rifamycin. The majority of regimens prescribed to patients with MAC (57%) did not include a macrolide at all. In addition, 30% of regimens prescribed are associated with an increased risk of developing macrolide resistance, including macrolide monotherapy (16%), macrolide and rifampin only (13%), and/or macrolide plus fluoroquinolone (1%); 1% of patients was treated with an antibiotic regimen of currently unknown clinical significance.
Table 2.
Summary of antibiotic regimens prescribed to patients treated for pulmonary disease associated with Mycobacterium avium complex (n = 411) and Mycobacterium abscessus (n = 76)
| |
Regimens for MAC (n = 579) |
Regimens for M. abscessus* (n = 101) |
|---|---|---|
| Treatment Regimen | n (% of All Regimens) | n (% of All Regimens) |
| Treatment regimens meeting ATS/IDSA guidelines for MAC* | 77 (13) | 7 (7) |
| Macrolide, ethambutol, and rifamycin | 75 (13) | 7 (7) |
| Macrolide, ethambutol, rifamycin, and parenteral aminoglycoside | 2 (0.3) | — |
| Treatment regimens not meeting ATS/IDSA guidelines for MAC* | 502 (87) | 94 (93) |
| Regimens that may increase macrolide resistance | 174 (30) | 18 (18) |
| Macrolide monotherapy | 93 (16) | 10 (10) |
| Macrolide plus fluoroquinolone | 7 (1) | 1 (1) |
| Macrolide plus rifampin | 74 (13) | 7 (7) |
| Regiments of unknown clinical significance | 3 (0.5) | 11 (11) |
| Macrolide plus inhaled amikacin | 1 (0.2) | 6 (6) |
| Macrolide plus linezolid | 2 (0.3) | 4 (4) |
| Macrolide plus parenteral aminoglycoside without ethambutol and with or without other drugs | — | 1 (1) |
| Regimens that do not include macrolides | 325 (56) | 65 (64) |
| Ethambutol plus rifamycin | 117 (20) | 8 (8) |
| Fluoroquinolone based regimen | 94 (16) | 15 (15) |
| Parenteral aminoglycoside based regimen | 15 (3) | 15 (15) |
| Linezolid based regimen | 1 (0.2) | 1 (1) |
| Any nonmacrolide antibiotic monotherapy regimen | 98 (17) | 26 (26) |
Definition of abbreviations: ATS = American Thoracic Society; IDSA = Infectious Diseases Society of America; MAC = Mycobacterium avium complex.
Macrolides include clarithromycin and azithromycin; rifamycins include rifampin and rifabutin; parenteral aminoglycosides include streptomycin and amikacin; and fluorquinolones include ofloxacin, ciprofloxacin, levofloxacin, and moxifloxacin. Data presented in this table refer to the total number of regimens prescribed; patients may have received more than one regimen.
There are currently no drug regimens of proven efficacy for the treatment of M. abscessus lung disease according to the 2007 ATS/IDSA guidelines (6).
Although more regimens prescribed to patients with MAC by pulmonologists met ATS/IDSA guidelines (18%) than those prescribed by ID (10%) or family/general practice/internal medicine (9%) physicians, pulmonologists also more frequently prescribed regimens associated with an increased risk for developing macrolide resistance (40%) than ID (23%) or family/general practice/internal medicine (23%) physicians (Table 3). Pulmonologists were less likely to prescribe regimens without macrolides than other physician specialties, although regimens without macrolides still constituted 42% of all those prescribed; nearly 70% of regimens prescribed by other physician specialties did not include a macrolide (Table 3).
Table 3.
Antibiotic regimens prescribed to patients treated for Mycobacterium avium complex by physician specialty
| Regimens Prescribed to Patients with MAC by Treating Physician Specialty |
|||
|---|---|---|---|
| |
Infectious Disease |
Pulmonology |
Family/General Practice and Internal Medicine |
| Treatment Regimen | n (%) | n (%) | n (%) |
| Total number of regimens prescribed (mean # regimens per patient) | 194 (1.5) | 237 (2.0) | 88 (1.5) |
| Treatment regimens meeting ATS/IDSA guidelines | 20 (10) | 42 (18) | 8 (9) |
| Macrolide, ethambutol, and rifamycin | 19 (10) | 41 (17) | 8 (9) |
| Macrolide, ethambutol, rifamycin, and parenteral aminoglycoside | 1 (0.5) | 1 (0.4) | — |
| Treatment regimens not meeting ATS/IDSA guidelines for MAC* | 174 (90) | 195 (82) | 80 (91) |
| Treatment regimens that may increase macrolide resistance | 45 (23) | 95 (40) | 20 (23) |
| Macrolide monotherapy | 23 (12) | 51 (22) | 10 (11) |
| Macrolide plus fluoroquinolone | 4 (2) | 3 (1) | 1 (1) |
| Macrolide plus rifampin | 18 (9) | 41 (17) | 9 (10) |
| Treatment regiments that are of unknown clinical significance | 3 (2) | — | — |
| Macrolide plus inhaled amikacin | 1 (0.5) | — | — |
| Macrolide plus linezolid | 2 (1) | — | — |
| Treatment regimens that do not include macrolides | 126 (65) | 100 (42) | 60 (68) |
| Ethambutol plus rifamycin | 35 (18) | 53 (22) | 17 (19) |
| Fluoroquinolone based regimen | 44 (23) | 25 (11) | 13 (15) |
| Parenteral aminoglycoside based regimen | 1 (0.5) | 1 (0.4) | 10 (11) |
| Linezolid based regimen | 1 (0.5) | — | — |
| Any nonmacrolide antibiotic monotherapy regimen | 45 (23) | 21 (9) | 20 (23) |
Definition of abbreviations: ATS = American Thoracic Society; IDSA = Infectious Diseases Society of America; MAC = Mycobacterium avium complex.
Patients with treatment regimens overseen by more than one physician specialty type or by physician specialties other than those listed were excluded from this table (n = 44). Macrolides include clarithromycin and azithromycin; rifamycins include rifampin and rifabutin; parenteral aminoglycosides include streptomycin and amikacin; and fluorquinolones include ofloxacin, ciprofloxacin, levofloxacin, and moxifloxacin.
There are currently no drug regimens of proven efficacy for the treatment of M. abscessus lung disease according to the 2007 ATS/IDSA guidelines (6).
Treatment Regimens for M. abscessus
A total of 64% of regimens prescribed to patients with M. abscessus during the course of their treatment did not include a macrolide (Table 2). Other regimens prescribed to patients with M. abscessus included those that are recommended by the ATS/IDSA for patients with MAC (7%), regimens associated with an increased risk of macrolide resistance (18%), and regimens including a macrolide together with other antibiotics, such as parenteral aminoglycosides (1%), inhaled amikacin (6%), and linezolid (4%).
Discussion
By using a sample of physicians who manage the care of patients with MAC- and M. abscessus–associated lung disease, and applying robust weighting procedures to correct for the under- and/or oversampling of certain patient and physician segments, this study aimed to provide nationally representative estimates of antibiotic treatment–related practices for PNTM disease in the United States. We found that fewer than 15% of antibiotic regimens prescribed to patients with PNTM who were actively treated by their physicians adhered to evidence-based guidelines. In addition, a concerning proportion of regimens were prescribed that are associated with the emergence of macrolide-resistant infections, which are more difficult to treat and are associated with high mortality rates (10, 11). This study is the first to identify major gaps that exist in physician-led treatment practices for PNTM disease throughout the United States, and highlights several disconcerting trends that place patients at greater risk for more severe and potentially life-threatening PNTM infections.
The 2007 ATS/IDSA guidelines specify that treatment for MAC-associated lung disease should include a daily or three-times-weekly drug regimen with a macrolide, ethambutol, and rifamycin until culture negative while on therapy for 1 year (6). Yet, in our study population, only 13% of patients with MAC were prescribed this regimen for any duration of time. Even among patients of pulmonologists, only 18% of regimens prescribed met these criteria. Physicians may be hesitant to prescribe recommended multidrug regimens due to concerns over suboptimal cure rates and frequently reported drug-related side effects (7–9), which can range from mild, including nausea or diarrhea, to quite severe, with hepatitis or peripheral neuropathy (11). However, when recommended regimens are well monitored and closely adhered to, MAC-associated lung disease eradication rates can reach 60–90% (8, 12–14). Although treatment is imperfect (8, 12–14), a macrolide-based regimen with ethambutol and rifamycin still represents the most effective treatment currently recognized (6, 15). Furthermore, several studies suggest that success is more likely during the initial treatment attempt, with decreased chances of success on subsequent treatment attempts (12, 13, 16). Although the reasons for this remain incompletely understood, and may be related to the presence of more advanced PNTM disease by the time adequate treatment is administered (13), it underlines the importance of initial treatment of newly diagnosed patients with an appropriate antibiotic regimen.
Another concerning trend identified involves the surprising percentage of regimens prescribed that have been associated with the development of macrolide resistance, which, at 30%, exceeded that of regimens meeting ATS/IDSA guidelines. Over 16% of regimens prescribed to patients with MAC—and 22% of those prescribed by pulmonologists—were for macrolide monotherapy. The 2007 ATS/IDSA guidelines clearly state that macrolides should never be used as monotherapy treatment for MAC lung disease (6). In a study by Griffith and colleagues (10), 20% of 59 patients on macrolide monotherapy for 4 months before incorporating ethambutol and rifamycin developed macrolide resistance over the subsequent months of therapy, whereas only 4% of 141 patients started immediately on a three-drug, macrolide-based regimen developed macrolide resistance. Several other alarming two-drug combinations of antibiotics were also noted, including macrolides alone with rifampin (13%) and with fluoroquinolone (1%). Not only is there no evidence to support the use of fluoroquinolones for treatment of MAC-associated lung disease, but, when used in conjunction with a macrolide, this regimen increases the risk of developing macrolide-resistant strains (10). Macrolides are the only antibiotics used for the treatment of MAC-associated lung disease for which in vitro susceptibility correlates with clinical response (6, 13, 16). Patients with macrolide-resistant infections no longer respond favorably to standard macrolide-based regimens, leaving them susceptible to reinfections and poor clinical outcomes, often succumbing to respiratory failure (10).
More than half of the regimens prescribed to patients with MAC and patients with M. abscessus did not include a macrolide. These patients were placed on regimens that included ethambutol plus rifamycin, were fluoroquinolone based, or involved monotherapy with a nonmacrolide antibiotic agent. On the basis of existing evidence, ATS/IDSA guidelines assert that macrolides are the most critical component of any multidrug treatment regimen for MAC lung disease (6), with their introduction over 20 years ago serving as the major therapeutic advance for the treatment of pulmonary MAC infections.
Several challenges associated with macrolide therapy have since been recognized (11, 17). In particular, the inclusion of a macrolide in a multidrug regimen is inappropriate if resistance is detected (10, 11). In this study, 12% of physicians indicated that they selected treatment based on concerns over drug resistance. However, given the data collected, we are unable to determine how many patients had macrolide-resistant infections. Current recommendations advise that such patients be placed on regimens that include ethambutol, rifabutin, and a parenteral agent (6). A total of 18% of all regimens prescribed were for ethambutol plus rifamycin without a macrolide, and only 4% were a parenteral aminoglycoside–based regimen. Alternatively, concerns about in vitro susceptibility or resistance may have been pertinent to other agents, such as ethambutol and rifampin, which is a concern not supported on the basis of numerous studies, and may reflect an inappropriate reliance on in vitro susceptibility tests for antimicrobial choices other than macrolides (6).
Physicians may be frustrated with MAC treatment regimens, even those recommended by ATS/IDSA guidelines, due to frequent medication toxicity and all-too-often disappointing outcomes. A recent study suggests that suboptimal pharmacodynamics and pharmacokinetics are the rule for medications in ATS/IDSA–recommended MAC treatment regimens, and are probably important contributing factors for poor overall MAC treatment outcomes (7). Although there are limitations to this study, principally the lack of a clear correlation between favorable pharmacodynamic indices and treatment success (5), the analysis at least offers hope that, with aggressive MAC medication manipulations, such as drug-level determinations, drug-dosage changes based on the drug levels obtained, or drug substitutions, clinicians might improve MAC treatment outcomes. Our study further suggests that an important first step—and one that is far easier to accomplish—would be for clinicians to simply follow current ATS/IDSA guidelines for MAC therapy.
For M. abscessus–associated pulmonary infections, no antibiotic regimens of proven efficacy are currently recognized, making it difficult to assess the quality of treatments prescribed. In a study evaluating outcomes among 69 patients treated for M. abscessus pulmonary disease, 29% remained culture positive, despite extended multidrug antibiotic therapy, and 23% of patients with culture conversion later experienced relapse (18). ATS/IDSA guidelines currently recommend use of a long-term, macrolide-based multidrug regimen to treat M. abscessus lung disease (6). In this study, just over half of treated patients with M. abscessus received clarithromycin at some point in time, though two-thirds of all regimens prescribed to these patients over the full course of their treatment history did not include a macrolide.
In a recent study, Choi and colleagues (19) demonstrated emergence of inducible macrolide resistance in all of 14 M. abscessus isolates tested after incubation for 14 days in the presence of clarithromycin or azithromycin, with significantly greater levels of resistance in isolates exposed to clarithromycin than azithromycin. Their findings reinforce the importance of performing macrolide sensitivity testing that includes evaluation of both mutational and inducible macrolide resistance before prescribing a treatment regimen for M. abscessus. Because M. abscessus has inducible macrolide resistance, which accounts for the vast majority of macrolide-resistant M. abscessus isolates, it is unlikely that macrolides will continue to be recommended as first-line M. abscessus therapy. For the purposes of this analysis, however, it is also very unlikely that our survey respondents did not include macrolide in M. abscessus regimens because of awareness of this inducible resistance concern, as essentially no mycobacterial laboratories in the United States were reporting the inducible resistance at the time of the survey; only mutational resistance, which accounts for a small percentage of macrolide-resistant M. abscessus, has routinely been reported. Clearly, given the challenges that exist in successfully treating M. abscessus lung disease, and the poor outcomes associated with treatment failure, it is imperative that additional drug treatment trials be performed to allow the development of an evidence base for improved guidelines.
The surprisingly low percentage of physicians, including pulmonary and ID specialists, who treat NTM lung disease according to published guidelines is not readily explained. Multidrug macrolide-based therapy for MAC and M. abscessus lung diseases was first recommended by the ATS and IDSA, the largest pulmonary and ID subspecialty societies in the United States, in 1997, and was further reinforced by the 2007 statement/guidelines on NTM diseases (6), which are freely accessible online. The NTM guidelines frequently cite expert opinion/consensus to justify specific recommendations, and, perhaps for that reason, some practitioners lack confidence in them and choose to rely on personal experience to guide therapy. Another possible explanation might be the difficulty shepherding patients through the prolonged, expensive, and frequently toxic NTM medication regimens recommended by the guidelines. Successful therapy results from a partnership between the physician and patient, whereby patients have realistic expectations of treatment demands and receive support to overcome inevitable hurdles that arise during NTM therapy. Physicians must communicate clearly to patients that there are a limited number of available effective agents to treat NTM, so that a drug discarded because of side effects may not be easily replaced, or may require replacement by an even more toxic drug. Second, eliminating some drugs from the treatment regimen may have detrimental consequences for long-term management of the NTM disease, such as the development of macrolide resistance.
There are several important points and limitations that impact the findings of this study. The demographics represented in our study population differ from what is typically reported. Whereas over 70% of our patients with NTM are over 50 years old, 30% are between the ages of 50 and 65 years, which is a slightly younger population than what is typically reported (20, 21). This difference in age distribution likely reflects the fact that our patient sample included greater proportions of men (52%) (20, 21) and of non-white/non-Hispanic racial/ethnic groups (51%) than what is typically reported (3). However, because we adjusted for the probability of each physician and patient being included in our study sample, these demographics better reflect the true population of patients with PNTM in the United States than studies that do not apply these added measures to deal with sampling biases.
Treatment-related data were categorized by regimen rather than by patient. Therefore, it is unknown how many unique patients received each type of antibiotic regimen (such as if one individual stopped and restarted the same regimen multiple times). In addition, data on the duration of treatment with each antibiotic regimen prescribed were not readily available, limiting our ability to interpret whether a regimen was the intended final treatment, as may be indicated by long-term use, or rather one that was transient while being “worked up” to a more complicated regimen. However, it is important to note that this would still be out of compliance with guidelines, which state that patients with MAC should be started on the full, specified regimen once diagnosed, assuming macrolide resistance is not detected (6).
Another limitation is the lack of macrolide susceptibility testing data, which would provide greater insight into why certain regimens were prescribed. Still, despite these limitations, our data show that the vast majority of patients with PNTM are being prescribed antibiotic regimens that do not meet ATS/IDSA guidelines, with nearly a third receiving regimens that place them for macrolide-resistant infections. It is critical that further studies are done to understand why these regimens are being prescribed, and that better education on proper PNTM treatment practices for physicians caring for these patients is achieved. Finally, it is always possible that physicians who responded to this survey differ from those who did not in ways that could not be accounted for, but may impact our findings, although the weighting procedures applied helped control for this bias.
The future of medical practice in the United States will almost certainly include more dependence on published treatment guidelines, preferably evidence based, to improve patient outcomes and lower healthcare costs. If this study is representative of physician adherence with published treatment guidelines, there is clearly a great deal of work that must be done to accomplish that goal.
Acknowledgments
Acknowledgments
The authors thank Nick Gurreri for his extensive technical contributions in the conception, design, and analysis of this research.
Footnotes
Supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, and by a contract from INSMED Inc. to Clarity Pharma Research.
Author Contributions: study concept and design—J.A., D.R.P., J.G., K.H., D.G.; acquisition of data—J.G., K.H.; analysis and interpretation of data—J.A., D.R.P., J.G., K.H., D.G., R.G.; drafting of the manuscript—J.A., D.R.P., J.G., K.H., D.G.; critical revision of the manuscript for important intellectual content—J.A., D.R.P., J.G., K.H., D.G., R.G.; statistical analysis—J.G., K.H.; study supervision—J.A., D.R.P., D.G., R.G.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Falkinham JO. Impact of human activities on the ecology of nontuberculous mycobacteria. Future Microbiol. 2010;5:951–960. doi: 10.2217/fmb.10.53. [DOI] [PubMed] [Google Scholar]
- 2.Tortoli E. Clinical manifestations of nontuberculous mycobacteria infections. Clin Microbiol Infect. 2009;15:906–910. doi: 10.1111/j.1469-0691.2009.03014.x. [DOI] [PubMed] [Google Scholar]
- 3.Adjemian J, Olivier KN, Seitz A, Holland S, Prevots R. Prevalence of pulmonary nontuberculous mycobacterial infections among U.S. Medicare beneficiaries, 1997–2007. Am J Respir Crit Care Med. 2012;185:881–886. doi: 10.1164/rccm.201111-2016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, Montes de Oca R, Shea YR, Seitz AE, Holland SM, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182:970–976. doi: 10.1164/rccm.201002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffith DE, Winthrop KL. Mycobacterium avium complex lung disease therapy. Am J Respir Crit Care Med. 2012;186:477–479. doi: 10.1164/rccm.201207-1321ED. [DOI] [PubMed] [Google Scholar]
- 6.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, et al. ATS Mycobacterial Diseases Subcommittee American Thoracic Society Infectious Disease Society of America An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases Am J Respir Crit Care Med 2007175367–416.[Published erratum appears in Am J Respir Crit Care Med 2007;175:744–745.Dosage error in article text] [DOI] [PubMed] [Google Scholar]
- 7.van Ingen J, Egelund EF, Levin A, Totten SE, Boeree MJ, Mouton JW, Aarnoutse RE, Heifets LB, Peloquin CA, Daley CL. The pharmacokinetics and pharmacodynamics of pulmonary Mycobacterium avium complex disease treatment. Am J Respir Crit Care Med. 2012;186:559–565. doi: 10.1164/rccm.201204-0682OC. [DOI] [PubMed] [Google Scholar]
- 8.Field SK, Fisher D, Cowie RL. Mycobacterium avium complex pulmonary disease in patients without HIV infection. Chest. 2004;126:566–581. doi: 10.1378/chest.126.2.566. [DOI] [PubMed] [Google Scholar]
- 9.Huang JH, Kao PN, Adi V, Ruoss SJ. Mycobacterium avium–intracellulare pulmonary infection in HIV-negative patients without preexisting lung disease: diagnostic and management limitations. Chest. 1999;115:1033–1040. doi: 10.1378/chest.115.4.1033. [DOI] [PubMed] [Google Scholar]
- 10.Griffith DE, Brown-Elliott BA, Langsjoen B, Zhang Y, Pan X, Girard W, Nelson K, Caccitolo J, Alvarez J, Shepherd S, et al. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006;174:928–934. doi: 10.1164/rccm.200603-450OC. [DOI] [PubMed] [Google Scholar]
- 11.Griffith DE, Aksamit TR. Therapy of refractory nontuberculous mycobacterial lung disease. Curr Opin Infect Dis. 2012;25:218–227. doi: 10.1097/QCO.0b013e3283511a64. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka E, Kimoto T, Tsuyuguchi K, Watanabe I, Matsumoto H, Niimi A, Suzuki K, Murayama T, Amitani R, Kuze F. Effect of clarithromycin regimen for Mycobacterium avium complex pulmonary disease. Am J Respir Crit Care Med. 1999;160:866–872. doi: 10.1164/ajrccm.160.3.9811086. [DOI] [PubMed] [Google Scholar]
- 13.Kobashi Y, Matsushima T. The microbiological and clinical effects of combined therapy according to guidelines on the treatment of pulmonary Mycobacterium avium complex disease in Japan—including a follow-up study. Respiration. 2007;74:394–400. doi: 10.1159/000095674. [DOI] [PubMed] [Google Scholar]
- 14.Griffith DE, Brown BA, Cegielski P, Murphy DT, Wallace RJ., Jr Early results (at 6 months) with intermittent clarithromycin-including regimens for lung disease due to Mycobacterium avium complex. Clin Infect Dis. 2000;30:288–292. doi: 10.1086/313644. [DOI] [PubMed] [Google Scholar]
- 15.Daley CL, Griffith DE. Pulmonary non-tuberculous mycobacterial infections. Int J Tuberc Lung Dis. 2010;14:665–671. [PubMed] [Google Scholar]
- 16.Wallace RJ, Jr, Brown BA, Griffith DE, Girard WM, Murphy DT. Clarithromycin regimens for pulmonary Mycobacterium avium complex: the first 50 patients. Am J Respir Crit Care Med. 1996;153:1766–1772. doi: 10.1164/ajrccm.153.6.8665032. [DOI] [PubMed] [Google Scholar]
- 17.Esteban J, García-Pedrazuela M, Muñoz-Egea MC, Alcaide F. Current treatment of nontuberculous mycobacteriosis: an update. Expert Opin Pharmacother. 2012;13:967–986. doi: 10.1517/14656566.2012.677824. [DOI] [PubMed] [Google Scholar]
- 18.Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis. 2011;52:565–571. doi: 10.1093/cid/ciq237. [DOI] [PubMed] [Google Scholar]
- 19.Choi GE, Shin SJ, Won CJ, Min KN, Oh T, Hahn MY, Lee K, Lee SH, Daley CL, Kim S, et al. Macrolide treatment for Mycobacterium abscessus and Mycobacterium massiliense infection and inducible resistance. Am J Respir Crit Care Med. 2012;186:917–925. doi: 10.1164/rccm.201111-2005OC. [DOI] [PubMed] [Google Scholar]
- 20.Winthrop KL, McNelley E, Kendall B, Marshall-Olson A, Morris C, Cassidy M, Saulson A, Hedberg K. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182:977–982. doi: 10.1164/rccm.201003-0503OC. [DOI] [PubMed] [Google Scholar]
- 21.Billinger ME, Olivier KN, Viboud C, de Oca RM, Steiner C, Holland SM, Prevots DR. Nontuberculous mycobacteria-associated lung disease in hospitalized persons, United States, 1998–2005. Emerg Infect Dis. 2009;15:1562–1569. doi: 10.3201/eid1510.090196. [DOI] [PMC free article] [PubMed] [Google Scholar]