Abstract
Rationale: Treatment of pulmonary nontuberculous mycobacteria, especially Mycobacterium abscessus, requires prolonged, multidrug regimens with high toxicity and suboptimal efficacy. Options for refractory disease are limited.
Objectives: We reviewed the efficacy and toxicity of inhaled amikacin in patients with treatment-refractory nontuberculous mycobacterial lung disease.
Methods: Records were queried to identify patients who had inhaled amikacin added to failing regimens. Lower airway microbiology, symptoms, and computed tomography scan changes were assessed together with reported toxicity.
Measurements and Main Results: The majority (80%) of the 20 patients who met entry criteria were women; all had bronchiectasis, two had cystic fibrosis and one had primary ciliary dyskinesia. At initiation of inhaled amikacin, 15 were culture positive for M. abscessus and 5 for Mycobacterium avium complex and had received a median (range) of 60 (6, 190) months of mycobacterial treatment. Patients were followed for a median of 19 (1, 50) months. Eight (40%) patients had at least one negative culture and 5 (25%) had persistently negative cultures. A decrease in smear quantity was noted in 9 of 20 (45%) and in mycobacterial culture growth for 10 of 19 (53%). Symptom scores improved in nine (45%), were unchanged in seven (35%), and worsened in four (20%). Improvement on computed tomography scans was noted in 6 (30%), unchanged in 3 (15%), and worsened in 11 (55%). Seven (35%) stopped amikacin due to: ototoxicity in two (10%), hemoptysis in two (10%), and nephrotoxicity, persistent dysphonia, and vertigo in one each.
Conclusions: In some patients with treatment-refractory pulmonary nontuberculous mycobacterial disease, the addition of inhaled amikacin was associated with microbiologic and/or symptomatic improvement; however, toxicity was common. Prospective evaluation of inhaled amikacin for mycobacterial disease is warranted.
Keywords: amikacin, nontuberculous mycobacteria, therapeutics, antibacterial agents
Nontuberculous mycobacteria are environmentally ubiquitous organisms that cause severe lung disease in certain patient groups (1–3). Current treatment guidelines for mycobacterial lung disease recommend a lengthy multidrug regimen, which for Mycobacterium avium complex includes clarithromycin or azithromycin, rifampin, and ethambutol (4). At best, lasting sputum conversion occurs in only around 65% (5). No consensus recommendations exist for treatment of Mycobacterium abscessus. Some retrospective studies have reported treatment outcomes for M. abscessus similar to those for M. avium complex. A recent study of patients with M. abscessus reported treatment success defined as culture conversion and clinical improvement of 80% using a macrolide and one or more injectable agents that included amikacin over several months (6). A similar retrospective study noted clinical improvement in a majority of patients and sputum conversion lasting at least 12 months in 58% of patients treated with a combination of oral antibiotics and initial intravenous amikacin and cefoxitin (7). A marked difference in response to this regimen was determined by the presence or absence of inducible macrolide resistance (8). The use of amikacin or streptomycin is recommended for patients with rapidly growing mycobacteria or extensive cavitary M. avium complex disease and those who have failed conventional treatments (4). However, the use of systemic aminoglycosides is limited by the associated risks of nephrotoxicity (15%), ototoxicity (37%), and vestibular toxicity (9%). Ototoxicity is greater with the use of kanamycin (42%) or amikacin (55%) than streptomycin (19%) (9). The use of aerosolized amikacin has the potential for reduced toxicity. However, there is minimal literature pertaining to the efficacy of and side effects associated with inhaled amikacin. We evaluated the effectiveness and toxicity of inhaled amikacin in 20 patients with treatment-refractory nontuberculous mycobacterial lung disease.
Methods
Study Population and Data Collection
Records from patients who consented to an institutional review board–approved mycobacterial natural history study at the National Institutes of Health (NIH) Clinical Center were queried from 2003 through 2010 to identify those who met the diagnostic criteria for pulmonary nontuberculous mycobacterial disease as established by the American Thoracic Society (ATS) guidelines (4), were treated with aerosolized amikacin, and had a baseline and at least one follow-up visit. The baseline visit was defined as the initiation date for inhaled amikacin. Treatment regimens before initiation of inhaled amikacin were noted; patients who were not on an ATS guidelines–based treatment regimen with persistently positive mycobacterial cultures were excluded.
Microbiology results from lower respiratory tract specimens (sputum, bronchoalveolar lavage, or lung biopsy) from the inhaled amikacin treatment period were identified. All respiratory specimen results, including source and mycobacterial smear, species, growth quantification, and clarithromycin susceptibility, were recorded. Mycobacterial smear quantification was categorized as negative, few (1–99 organisms per 100 high-power fields), moderate (1–10 organisms per field), and many (>10 organisms per field); culture growth quantification was designated as liquid media only, scant (<20 colonies per media), light (20–100 colonies per media), moderate (101–300 colonies per media), and heavy (>300 colonies per media).
Subspecies level identification of M. abscessus group isolates was performed with a typing scheme using polymerase chain reaction primers designed for four discriminatory locations and the erm(41) gene; for some isolates, multilocus sequence typing based on partial sequencing of secA1, rpoB, and hsp65 was also used (10–12). Minimum inhibitory concentration of clarithromycin was determined in Mueller-Hinton medium by the broth microdilution method using Sensititer RAPMYCO plates (Trek Diagnosis, Cleveland, OH). Plates were subjected to an extended incubation of 14 days at 30°C for final reading to ensure detection of inducible resistance to clarithromycin, and the interpretative breakpoints used were those recommended by the Clinical and Laboratory Standards Institute (13).
Symptoms were scored (1, 0) for the presence or absence of fever, cough, fatigue, hemoptysis, weight loss, night sweats, shortness of breath, and sputum production. Total symptom scores, calculated by summation of symptoms present, were compared between baseline and final visit, defined as the visit coinciding with stopping amikacin or with data abstraction (if amikacin was continuing). Inflammatory markers, erythrocyte sedimentation rate, C-reactive protein, β2 microglobulin, and total white blood cell count were recorded from each visit. Chest computed tomography (CT) images at baseline were scored by a radiologist (L.R.F.) based on the severity (absent = 0, mild = 1, moderate = 2, severe = 3) of cavities, airspace opacities, and nodular bronchiectasis. The radiologist was blinded to the clinical data. Each lung was scored separately, and severity scores for the three abnormality categories from both lungs were combined to achieve a potential severity score range from 0 (none of these abnormalities) to 18 (bilateral severe findings for all three categories). Scores of CT scans within 6 months of first or last culture (n = 19; one patient had no appropriately timed follow-up scan) were compared globally for either no change, improvement, or worsening by two radiologists (L.R.F., J.R.S.) blinded to the order of the scans. Discrepancies were resolved by consensus review. Audiology reports were also reviewed, and hearing loss was identified by two audiologists (C.C.B., C.K.Z.); change from baseline was noted for patients with serial audiograms performed while on inhaled amikacin based on the American Speech Language Hearing Association guidelines (9).
Amikacin Dosing
Commercial amikacin sulfate 250 mg/ml solution was diluted with 3 ml of saline and placed in a jet nebulizer for inhalation. Patients were generally started at 250 mg once daily and told to titrate to twice daily after 2 weeks if no dysphonia was noted. They were then told to titrate toward 500 mg twice daily at 2-week intervals. If dysphonia was encountered, they were instructed to hold dosing until it resolved and then resume at the prior tolerated dose and interval. If 250 mg once daily was not tolerated, the interval was increased or dose reduced. Data were collected until the final amikacin visit if it was stopped, with recorded reasons for discontinuation, or until the date of data abstraction if amikacin was continuing past this date.
Statistical Analysis
Summary statistics for quantitative markers are presented as mean ± SD or, if data were appreciably skewed, as median with the range (minimum, maximum). Average monthly change in inflammatory markers while on amikacin was calculated for each individual.
Results
Twenty-three patients who met the ATS criteria for pulmonary mycobacterial disease were treated with inhaled amikacin. Two patients who did not have at least one follow-up visit recorded were excluded. One patient was excluded because the inhaled amikacin was started as part of an initial treatment regimen for M. abscessus and thus was not previously on a failing ATS guidelines–based treatment regimen. The majority of patients (80%) were women, and the mean age at initiation of inhaled amikacin treatment was 56 ± 16 years. All 20 patients had a history of bronchiectasis, two had cystic fibrosis, an additional three were single cystic fibrosis transmembrane conductance regulator mutation carriers, and one had primary ciliary dyskinesia.
Patients had a median (range) of 8 (0, 39) positive cultures obtained at NIH (some had multiple positive cultures obtained elsewhere before referral to NIH; one patient was referred to NIH shortly after starting inhaled amikacin, and therefore all prior positive cultures were done elsewhere) before starting inhaled amikacin. Patients had received an average of 60 (6, 190) months of treatment for mycobacteria before starting inhaled amikacin. Positive mycobacterial smears were noted in 18 (90%) patients at the initial visit. All 20 patients were culture positive at the initial visit; 15 (75%) were identified with persistent M. abscessus and 5 (25%) with persistent M. avium complex infections (Table 1). Of the M. abscessus group isolates, 10 were identified as M. abscessus sensu stricto and 5 were M. abscessus subspecies massiliense. All were resistant to macrolides. Four of the M. avium complex isolates were M. intracellulare (two susceptible, one intermediate, one not tested), and one was M. avium (susceptible) (Table 2).
Table 1.
Patient characteristics at initiation of inhaled amikacin (N = 20)
| Sex, female, n (%) | 16 (80) |
| Age, mean (SD), yr | 56 (16) |
| Comorbidities, n (%) | |
| CFTR carriers | 3 (15) |
| Cystic fibrosis | 2 (10) |
| Chronic obstructive pulmonary disease | 2 (10) |
| Asthma | 1 (5) |
| Primary ciliary dyskinesia | 1 (5) |
| CT findings | |
| Initial score, mean (SD) | 6 (3) |
| Cavitary disease, n (%) | 9 (45) |
| Mycobacterium species, n (%) | |
| Mycobacterium abscessus group | 15 (75) |
| Mycobacterium avium complex | 5 (25) |
| Mo on mycobacterial treatment (before amikacin), median (range) | 60 (6, 190) |
Definition of abbreviations: CFTR = cystic fibrosis transmembrane conductance regulator; CT = computed tomography.
Table 2.
Mycobacterial status and treatment regimens
| ID # | Species | Macrolide Susceptibility | Final Mycobacterial Status | Prior Treatment | Regimen at Initiation of Amikacin | Drugs Started with Amikacin | Drugs Started during Amikacin Treatment Period |
|---|---|---|---|---|---|---|---|
| 1 | Mint | I | Negative | az, cla, et, rib, rim, | az, et, rim | ||
| 2 | Mabs | R (inducible) | Negative | li, am, cla, ti | mo, cla | ||
| 3 | Mmas | R (inducible) | Relapse | az, et, rif | az | li, ti | mo |
| 4 | Mmas | R | Positive | ce, am, cla, az, mo, im | ce, am | cla, mo, li | me |
| 5 | Mint | S | Relapse | rim, et, az, le, cla, clo | rim, az | ||
| 6 | Mabs | R | Positive | am, er, az, cla, et, rib, me, ti | me, li | ||
| 7 | Mabs | R (inducible) | Negative | cla, az, et, le | li, az | mo | |
| 8 | Mmas | R (inducible) | Positive | ci, cla, am, le, me, IFN-γ, IL12, mo, ce | az, | ti, me, mo | |
| 9 | Mabs | R (inducible) | Positive | cla, mo, az, ce, am, im | az, mo | ti, me | |
| 10 | Mabs | R (inducible) | Positive | az, mo, li | az, mo, li | ||
| 11 | Mabs | R (inducible) | Positive | ci, az, mo, cla, li | az, mo | ti | |
| 12 | Mint | S | Positive | cla, et, rim, am, mo | az, mo, rim | ||
| 13 | Mmas | R | Relapse | az, li, ti, ce, | az, ti | ce, me | |
| 14 | Mabs | R (inducible) | Positive | ce, cla, am, az, mo, | mo, li | ||
| 15 | Mmas | R | Positive | mo, li, cla, et, rim, | mo, li | ||
| 16 | Mint | NT | Positive | az, et, rim, rib, cla | cla, et, rib | mo | |
| 17 | Mabs | R (inducible) | Negative | cla, et, rim, im, az | az, et | ||
| 18 | Mavi | S | Positive | cla, et, rim, le | cla, et, rim | clo | |
| 19 | Mabs | R (inducible) | Positive | cla, rim, et, me, im, ce, li, mo, ti | cla, et, mo, rim | IFN-γ | |
| 20 | Mabs | R (inducible) | Negative | im, cla, az | az | li |
Definition of abbreviations: am = intravenous amikacin; az = azithromycin; ce = cefoxitin; ci = ciprofloxacin; cla = clarithromycin; clo = clofazimine; er = ertapenem; et = ethambutol; I = intermediate; im = imipenem; IFN-γ = interferon gamma; IL12 = interleukin 12; le = levofloxacin; li = linezolid; Mabs = Mycobacterium abscessus sensu stricto; Mavi = Mycobacterium avium; me = meropenem; Mint = Mycobacterium intracellulare; Mmas = Mycobacterium abscessus subspecies massiliense; mo = moxifloxacin; R = resistant; rib = rifabutin; rim = rifampin; S = susceptible; ti = tigecycline.
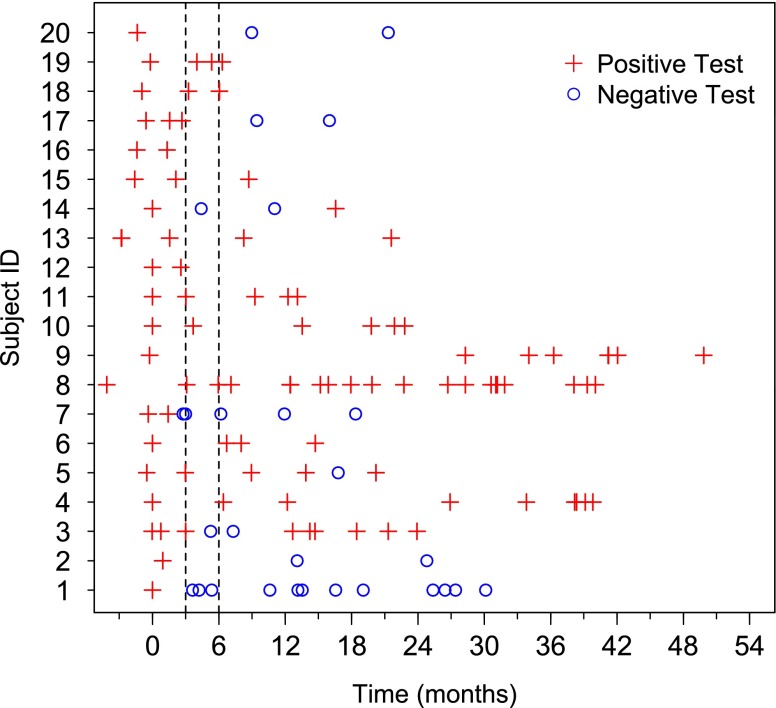
Patients were followed for a median of 19 (1, 50) months, and the median time to the first follow-up visit was 3 (1, 28) months (Figure 1). Six (30%) patients were continued on inhaled amikacin past the date of abstraction (May 2011). Eight patients (40%) had at least one negative culture and smear during the course of follow-up. All had at least one follow-up specimen beyond their first negative culture; three (38%) were observed to have at least one subsequent positive culture, and five (62%) were persistently negative (Figure 1). Of the five patients who remained culture negative, four (80%) had M. abscessus and one (20%) had M. avium complex. For simplicity, those with subsequent positive cultures after an initial negative result are referred to as “relapses.” The relapses occurred while still on inhaled amikacin at 3, 7, and 12 months after the initial negative culture. The latter two relapses had two consecutive negative cultures at least 2 months apart, whereas the relapse at 3 months lacked a confirmatory negative culture. Among those with persistently negative cultures, they were observed to be negative on treatment for at least 7 to 27 months post conversion. Overall, limited numbers of cultures were obtained during the first 6 months of treatment. Of nine patients with a culture obtained within the first 3 months, 1 (11%) had a negative culture by 3 months; 4 of 16 (25%) patients with a culture in the first 6 months had at least one negative culture. Compared with the baseline visit, a decrease in smear quantitation was noted in 9 of 20 (45%) patients. Culture growth quantity (e.g., heavy to light growth) of mycobacteria decreased in 10 of 19 (53%) (one patient did not have growth quantity available at initial culture).
Figure 1.
Culture test results over time by subject. Subject ID numbers on Y-axis correspond to ID numbers in Table 2. Inhaled amikacin was started at time 0. Vertical dashed lines mark Months 3 and 6. Eight patients achieved negative (o) cultures; five of these had persistently negative cultures. The remaining 12 patients had persistently positive (+) cultures.
Most patients (n = 16, 80%) were on a multidrug regimen at the time amikacin was added. For M. abscessus, the most commonly used drugs were azithromycin, linezolid, and moxifloxacin. For M. avium complex, the most common concomitant medications were azithromycin, ethambutol, and rifampin (Table 2). The most common concomitant pathogens identified at baseline were Aspergillus species (n = 6, 30%), Penicillium species (n = 5, 25%), and Pseudomonas species (n = 2, 10%). In general, the presence of concomitant pathogens decreased during the course of treatment with inhaled amikacin (Table 3).
Table 3.
Baseline and final characteristics (N = 20)
| Baseline | Final | |
|---|---|---|
| Concomitant organisms | ||
| Aspergillus species | 6 (30) | 4 (20) |
| Penicillium species | 5 (25) | 4 (20) |
| Pseudomonas species | 2 (10) | 2 (10) |
| Fusarium species | 1 (5) | 0 (0) |
| Klebsiella oxytoca | 1 (5) | 0 (0) |
| Paecilomyces species | 1 (5) | 1 (5) |
| Symptoms | ||
| Cough | 20 (100) | 17 (85) |
| Sputum | 18 (90) | 16 (80) |
| Shortness of breath | 13 (65) | 12 (60) |
| Fever | 2 (10) | 3 (15) |
| Night sweats | 7 (35) | 4 (20) |
| Fatigue | 4 (20) | 5 (25) |
| Weight loss | 5 (25) | 4 (20) |
Data are presented as n (%). Organisms isolated from sputum specimens at initiation of inhaled amikacin (baseline) and at the time of stopping inhaled amikacin or data abstraction in those patients continuing drug (final). Symptoms noted to be present at baseline or final visits.
The following symptoms were present at baseline: cough 100%, sputum production 90%, shortness of breath 65%, night sweats 35%, weight loss 25%, fatigue 20%, and fever 10% (Table 3). None reported hemoptysis. Total symptom scores improved in nine patients (45%), were unchanged in seven (35%), and worsened in four (20%) (data not shown). There was no significant change in white blood cell count, erythrocyte sedimentation rate, or β2-microglobulin markers. The median C-reactive protein at baseline was 0.53 (0.40, 27) mg/L, and C-reactive protein had a slight mean (95% confidence interval) increase over the follow-up period of 0.108 (0.008, 0.269) mg/L per month (data not shown). Six (30%) patients showed improvement on serial CT scans, 3 (15%) were unchanged, and 11 (55%) worsened while on inhaled amikacin. There was no correlation between sputum conversion and radiographic changes. Of the five patients with sustained negative cultures, 2 had improved CT scans and 3 had unchanged or worse CT scans; out of the 12 patients with persistent positive cultures, 4 had improved and 8 had worsened CTs.
The most common maximum tolerated dosing was 250 mg daily (50% of patients) followed by 250 mg twice daily (20%) and 250 mg thrice weekly (15%). Seven (35%) patients stopped amikacin due to toxicity, including ototoxicity in two (10%), hemoptysis in two (10%), and nephrotoxicity (reversible increase in creatinine), persistent dysphonia, and vertigo in one each (Table 4). Ototoxicity was self-reported in two patients and resolved in one patient after returning to daily dosing instead of twice daily. Baseline audiometry at the time of inhaled amikacin initiation was available in 16 (80%) patients: four patients had normal hearing sensitivity; four had mild high frequency sensorineural hearing loss; seven had high frequency sloping sensorineural hearing loss ranging from mild to moderate in three, mild to moderately severe in three, and mild to profound in one; and one had a mild to profound sensorineural hearing loss across all test frequencies. In addition to a high-frequency sensorineural hearing loss in one ear, a single patient also had moderately severe conductive hearing loss in the other ear. Six (38%) patients had previously been treated with systemic amikacin. Thirteen of the 16 patients with baseline audiology visits had at least one follow-up visit over an average of 19 (±10) months, and two met American Speech Language Hearing Association criteria for hearing loss progression (9) while on inhaled amikacin.
Table 4.
Inhaled amikacin dosing
| Mo on inhaled amikacin, median (range) | 19 (1, 50) |
| Final inhaled amikacin dose, n (%) | |
| 250 mg daily | 10 (50) |
| 250 mg twice daily | 4 (20) |
| 250 mg thrice weekly | 3 (15) |
| 250 mg every other d | 1 (5) |
| 500 mg daily | 1 (5) |
| 125 mg twice daily | 1 (5) |
| Reasons for stopping inhaled amikacin | n (%) |
| Ototoxicity | 2 (10) |
| Hemoptysis | 2 (10) |
| Nephrotoxicity* | 1 (5) |
| Persistent dysphonia | 1 (5) |
| Vertigo | 1 (5) |
Exceeded normal creatinine levels (1.2 mg/dl) at two visits (1.43, 1.42 mg/dl).
Discussion
Treatment of nontuberculous mycobacterial lung disease requires lengthy, and often toxic, antibiotic regimens, which may be unsuccessful. This is especially true for rapidly growing mycobacteria pulmonary infections. The ATS guidelines describe the goal of 12 months of negative sputum cultures on treatment to be unrealistic for M. abscessus. Instead, a more reasonable objective is to achieve short periods of negative sputum with more emphasis being placed on symptomatic and radiographic improvement (4). The addition of inhaled amikacin to standard drug treatment regimens may lead to a greater rate of successfully treated mycobacterial lung disease. In a previous study, four of six people who had failed standard treatment for M. avium complex lung disease were culture negative after 6 months of inhaled amikacin without toxic side effects (14). The dose of inhaled amikacin used in this study (average of 750–1,000 mg/d) was considerably higher than in our study and may have contributed to a higher frequency of sputum conversion. Colin and colleagues reported the use of inhaled amikacin initially at 500 mg twice daily then 250 mg twice daily together with clarithromycin in a patient with cystic fibrosis with recurrent acute flares of pulmonary disease associated with M. abscessus (15). The patient had developed significant nephrotoxicity and mild hearing loss from prior intravenous treatment but was able to be maintained on this regimen for a prolonged period without significant progression of toxicity or recurrence of M. abscessus (15). The high frequency of macrolide resistance and the extended period of failed response to complex multidrug regimens in our study set a very high bar for detectable response from addition of inhaled amikacin. In these patients with refractory M. abscessus or M. avium complex, inhaled amikacin in combination with their continued antibiotic regimen led to clearance in 25% (5 of 20) patients, 4 of whom had M. abscessus. The majority of patients showed improvement in sputum culture quantitation, and 30% had improvement in serial chest CT scans. However, there was no correlation between culture results and CT changes. This may be due to the advanced destructive nature of the bronchiectasis and fibrosis associated with these organisms, such that it is difficult to detect small changes that may correlate with symptomatic or microbiologic improvement. It is also likely that radiographic changes progressed in some patients due to inadequate treatment of mycobacteria, contribution of other bacteria or fungi, or progression of underlying bronchiectasis.
Because of the limited amount of microbiologic sampling within the first 6 months of treatment, it is difficult to estimate precisely the interval for microbiologic clearance or improvement among those who appeared to do so. Of the five patients who had persistently negative sputum cultures, only three had follow-up cultures within the initial 6 months of treatment; two of these converted from positive to negative within the first 4 months of treatment (Figure 1).
Aerosolized amikacin was associated with fewer toxic side effects as compared with previous reports of systemic amikacin (9). Only two patients (10%) discontinued treatment due to hearing loss, and one of these was able to restart at a lower dose without additional ototoxicity. Dysphonia was relatively common but was able to be managed by dose adjustment in all but one patient. The relatively limited side effects seen with inhaled amikacin and its potential capacity to help clear nontuberculous mycobacterial pulmonary infections suggest it may be a useful adjunct in treatment refractory disease.
Further research on the benefits and risks associated with inhaled amikacin is needed. This study is limited by the low number of patients who were both treated with inhaled amikacin and had sufficient follow-up information. An inherent limitation in this type of retrospective analysis of our natural history cohort was the inability to control for number and timing of follow-up visits. In addition, to speak to the true efficacy of amikacin and its ability to help permanently clear nontuberculous mycobacteria, longer follow-up after sputum clearance is necessary. However, given these limitations, it is recognized that there are limited treatment options for patients who have failed or are intolerant of initial treatment attempts. We and others continue to use inhaled amikacin as part of salvage regimens despite the lack of strong supportive evidence. The current multisite, randomized, placebo-controlled trial of liposomal amikacin for inhalation targeting treatment refractory patients with M. avium complex or M. abscessus may shed some light on the efficacy, dosing, and toxicity of this treatment approach.
Footnotes
Supported by the Intramural Research Programs of the National Institute of Allergy and Infectious Diseases, the National Institute of Deafness and Other Communication Disorders, the National Heart, Lung, and Blood Institute, and the Clinical Center of the National Institutes of Health.
Author Contributions: Concept and design: K.N.O., D.R.P. Acquisition of data: T.S.G., D.B., M.F., C.C.B., C.K.Z., S.S., I.K.P. Interpretation of data: K.N.O., P.A.S., C.C.B., C.K.Z., L.R.F., J.R.S., E.P.S., A.M.Z., D.R.P. Drafting manuscript: K.N.O., P.A.S., T.S.G., A.M.Z. Critical review and revision: K.N.O., P.A.S., C.C.B., S.M.H., A.M.Z., D.R.P. Final approval: K.N.O.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Griffith DE. Nontuberculous mycobacterial lung disease. Curr Opin Infect Dis. 2010;23:185–190. doi: 10.1097/QCO.0b013e328336ead6. [DOI] [PubMed] [Google Scholar]
- 2.O’Connell ML, Birkenkamp KE, Kleiner DE, Folio LR, Holland SM, Olivier KN. Lung manifestations in an autopsy-based series of pulmonary or disseminated nontuberculous mycobacterial disease. Chest. 2012;141:1203–1209. doi: 10.1378/chest.11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung JM, Olivier KN. Nontuberculous mycobacteria in patients with cystic fibrosis. Semin Respir Crit Care Med. 2013;34:124–134. doi: 10.1055/s-0033-1333574. [DOI] [PubMed] [Google Scholar]
- 4.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, et al. ATS Mycobacterial Diseases Subcommittee American Thoracic Society Infectious Disease Society of America An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases Am J Respir Crit Care Med 2007175367–416.Published erratum appears in Am J Respir Crit Care Med 2007;175:744–745 [DOI] [PubMed] [Google Scholar]
- 5.Kasperbauer SH, Daley CL. Diagnosis and treatment of infections due to Mycobacterium avium complex. Semin Respir Crit Care Med. 2008;29:569–576. doi: 10.1055/s-0028-1085708. [DOI] [PubMed] [Google Scholar]
- 6.Lyu J, Jang HJ, Song JW, Choi CM, Oh YM, Lee SD, Kim WS, Kim DS, Shim TS. Outcomes in patients with Mycobacterium abscessus pulmonary disease treated with long-term injectable drugs. Respir Med. 2011;105:781–787. doi: 10.1016/j.rmed.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Jeon K, Kwon OJ, Lee NY, Kim BJ, Kook YH, Lee SH, Park YK, Kim CK, Koh WJ. Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am J Respir Crit Care Med. 2009;180:896–902. doi: 10.1164/rccm.200905-0704OC. [DOI] [PubMed] [Google Scholar]
- 8.Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, Park YK, Kim CK, Shin SJ, Huitt GA, et al. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med. 2011;183:405–410. doi: 10.1164/rccm.201003-0395OC. [DOI] [PubMed] [Google Scholar]
- 9.Peloquin CA, Berning SE, Nitta AT, Simone PM, Goble M, Huitt GA, Iseman MD, Cook JL, Curran-Everett D. Aminoglycoside toxicity: daily versus thrice-weekly dosing for treatment of mycobacterial diseases. Clin Infect Dis. 2004;38:1538–1544. doi: 10.1086/420742. [DOI] [PubMed] [Google Scholar]
- 10.Zelazny AM, Root JM, Shea YR, Colombo RE, Shamputa IC, Stock F, Conlan S, McNulty S, Brown-Elliott BA, Wallace RJ, Jr, et al. Cohort study of molecular identification and typing of Mycobacterium abscessus, Mycobacterium massiliense, and Mycobacterium bolletii. J Clin Microbiol. 2009;47:1985–1995. doi: 10.1128/JCM.01688-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shallom SJ, Gardina PJ, Myers TG, Sebastian Y, Conville P, Calhoun LB, Tettelin H, Olivier KN, Uzel G, Sampaio EP, et al. New rapid scheme for distinguishing the subspecies of the Mycobacterium abscessus group and identifying Mycobacterium massiliense isolates with inducible clarithromycin resistance. J Clin Microbiol. 2013;51:2943–2949. doi: 10.1128/JCM.01132-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bastian S, Veziris N, Roux AL, Brossier F, Gaillard JL, Jarlier V, Cambau E. Assessment of clarithromycin susceptibility in strains belonging to the Mycobacterium abscessus group by erm(41) and rrl sequencing. Antimicrob Agents Chemother. 2011;55:775–781. doi: 10.1128/AAC.00861-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes. CLSI document M24–A2. Wayne, PA: Clinical and Laboratory Standards Institute; 2011. p. 14. [PubMed] [Google Scholar]
- 14.Davis KK, Kao PN, Jacobs SS, Ruoss SJ. Aerosolized amikacin for treatment of pulmonary Mycobacterium avium infections: an observational case series. BMC Pulm Med. 2007;7:2. doi: 10.1186/1471-2466-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colin AA. Eradication of Mycobacterium abscessus in a chronically infected patient with cystic fibrosis. Pediatr Pulmonol. 2000;30:267–268. doi: 10.1002/1099-0496(200009)30:3<267::aid-ppul13>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]