Abstract
Rationale: Little is known about outcomes of infection with nontuberculous mycobacteria (NTM) in cystic fibrosis (CF) or about the significance of a positive NTM culture. Determining which patients are at risk for active NTM disease is clinically valuable.
Objectives: To examine the clinical course of subjects with CF with an initial positive NTM culture and identify characteristics associated with active NTM disease.
Methods: We performed a retrospective study of pediatric and adult subjects with CF with at least one positive NTM culture at the Colorado CF Center from 2000 to 2010.
Measurements and Main Results: Mycobacterium avium complex was the first identified NTM in the majority of subjects (73%). The frequency of growing a second NTM species was 26% at 5 years. Clinical characteristics and distribution of NTM species between pediatric and adult subjects were similar except for differences in baseline FEV1 (89% vs. 71%; P < 0.001) and coinfection with Pseudomonas aeruginosa (33% vs. 55%; P = 0.04). Over 60% of subjects had transient or persistent infection but not active NTM disease. Subjects who developed active NTM disease were distinguished from those with transient or persistent infection, respectively, by FEV1 at the time of first positive NTM culture (72% vs. 84 or 86%; P = 0.02) and FEV1 decline in the prior year (−5.8%/yr vs. −0.7%/yr [P = 0.009] or −0.4%/yr [P = 0.001]).
Conclusions: The majority of patients with CF with a first positive NTM culture do not progress to active disease. Lower lung function and accelerated lung function decline appear to be indicators of the significance of an initial positive NTM culture.
Keywords: nontuberculous mycobacteria, Mycobacterium avium complex, Mycobacterium abscessus
Nontuberculous mycobacteria (NTM) are increasingly implicated as a clinically important infection in cystic fibrosis (CF). Positive cultures for NTM have been reported in 6 to 22% of patients with CF from various CF Centers worldwide (1–10). In the largest prospective study within the United States, the most common NTM pathogen isolated was Mycobacterium avium complex (MAC), followed by Mycobacterium abscessus (1). However, more recent studies have reported M. abscessus to be more prevalent in certain regions of the United States and in France (2, 3, 7).
The majority of studies have focused on defining the prevalence of NTM in the CF population and identifying associated risk factors but do little to guide difficult clinical decisions (1–4, 6, 8, 11–16). The initial recovery of NTM in respiratory cultures represents a significant clinical challenge to the clinician and to the patient. Virtually nothing is known regarding the natural history of NTM infection in CF or the clinical significance of an initial positive culture. In the absence of evidence-based criteria for diagnosis and treatment in the CF population, clinicians are often unsure how to counsel patients and families regarding their potential to develop NTM disease, the duration of treatment if needed, the probability of successful clearance, and the lifetime risk of reinfection. Treatment of NTM poses a tremendous burden on patients with CF and providers because therapeutic regimens are often taxing and associated with significant side effects and expense (17, 18). Uncertainty over the significance of a first positive NTM culture may result in a delay in the timely evaluation and decision to treat in some patients, whereas in other cases it may result in early initiation of potentially unnecessary multidrug therapy.
The objectives of this study were to examine the clinical course of patients with CF from the time of a first positive NTM culture and to identify characteristics associated with active NTM disease requiring treatment. We hypothesized that the majority of patients with CF with a first positive NTM culture do not progress to NTM disease. We further hypothesized that, in those who do progress, there are clinical characteristics that can help identify need for treatment beyond the criteria described by the American Thoracic Society and Infectious Diseases Society of America (17). Preliminary results of this study were previously reported in abstract form (19, 20).
Materials and Methods
Study Population and Design
Patients (or guardians for minors) gave informed consent permitting their deidentified records to be used for research purposes from the Colorado CF Center at Children’s Hospital Colorado (CHCO) and National Jewish Health (NJH). The Colorado Multiple Institutional Review Board (10–1558) and the National Jewish Institutional Review Board (HS-2599CO) approved this study. Clinical and research databases were queried for all patients receiving standard care at the CF Center who had at least one positive NTM respiratory culture from January 2000 to December 2010. For subjects identified as having a positive NTM culture, all available records were reviewed to confirm the date of the first positive culture. As part of clinical practice at our CF center, patients are screened for NTM annually by expectorated or induced sputum starting at 10 years of age. In addition, sputum obtained during hospitalizations and all bronchoalveolar lavage samples were tested for NTM. Subjects were classified as pediatric if the first positive NTM culture was documented before 18 years of age. Subjects were excluded if the date of their first positive NTM culture was uncertain or if less than 1 year of follow-up data were available. In addition, subjects diagnosed with CF after referral for NTM lung disease evaluation were excluded to limit the study to patients receiving long-term standard CF care.
Sex, age, CF diagnostic information, and coinfection data were recorded from the date of the first positive culture. Anthropometric data and FEV1 were obtained. FEV1 were expressed as percent of predicted normal using reference equations (21). Information regarding azithromycin use at the time of the first positive NTM culture, NTM drug susceptibility results, treatment regimens prescribed, and results of all NTM cultures was recorded. For comparison purposes, similar data were captured from 2005 (study midpoint) for all pediatric and adult patients with CF followed at the Colorado Center without previously positive NTM cultures. Respiratory cultures for bacterial and fungal CF pathogens were performed at the CHCO and NJH per CF consensus guidelines (22). A detailed description of NTM characterization techniques used is provided in the online supplement.
NTM Outcome–based Groups
Subjects were assigned to three groups based on NTM culture results and clinical outcomes. Although the ATS criteria (17) have not been validated, they provide the generally accepted clinical criteria and were used for diagnosis of NTM disease. The “transient infection” group consisted of those with only a single positive NTM culture. The “persistent infection” group included those with at least two positive NTM cultures who did not meet ATS criteria of NTM disease due to a lack of clinical or radiographic progression not attributable to their underlying CF (17). The third group consisted of subjects with active NTM disease, defined by at least two positive cultures and clinical and radiographic progression of disease not attributable to known coinfections and comorbidities. All subjects classified as having active NTM disease were started on antimycobacterial treatment. As part of clinical practice at our CF center, all patients are treated for their typical CF pathogens and are assessed and treated for comorbidities before starting NTM treatment. If clinical symptoms, pulmonary function decline, and/or progression of disease on chest imaging persist, then a patient is considered to have active disease, and NTM treatment is initiated.
Assessment of Microbiologic Response
NTM conversion after treatment was defined as having at least three consecutive negative NTM cultures over 1 year, consistent with current guidelines (23–25). Recurrence was defined as two consecutive positive cultures for the same NTM species after sputum conversion. Subjects who continued to have positive cultures after or during treatment were considered to have never converted.
Statistical Analyses
Baseline data are presented as means and standard deviations or medians and interquartile ranges (IQRs) for continuous variables and percentages for categorical variables. Cross-sectional group comparisons were made using ANOVA or Kruskal-Wallis nonparametric tests. Longitudinal comparisons were assessed through mixed effects models accounting for repeated measures on individual subjects. Turkey tests for multiple comparisons were used. Univariate logistic regression models (bionomial or polytomous) were fit to determine the relationship between the culture-positive groups and the independent variables.
Mean FEV1 (% predicted) was calculated using all FEV1 measurements for 1 year around each time point. FEV1 slope estimates were calculated using a standard random coefficients (mixed) model with a knot at the time of the first positive culture, adjusting for age and baseline FEV1. Subgroup analysis of FEV1 slope estimates were calculated using a standard random coefficients (mixed) model with a knot at the time of the first positive culture separated for sex, genotype, and NTM type. Linear contrasts were used to test for differences between groups. A significance level of 0.05 was set for statistical significance. All statistical analyses were performed using SAS software (version 9.3; SAS Institute, Cary, NC).
Results
Characteristics of Pediatric and Adult Subjects with a Positive NTM Culture
One hundred fifty subjects with CF were identified as having at least one positive NTM culture. Of these, 54 (36%) were excluded due to incomplete data, lack of follow-up, or a diagnosis of CF after recovery of NTM from their respiratory culture (see Figure E1 in the online supplement). A total of 96 subjects (48 pediatric and 48 adult) were included for further analysis.
At the time of the first positive NTM culture, adult subjects had a lower average FEV1 and a higher rate of coinfection with Pseudomonas aeruginosa in comparison to the pediatric cohort (Table E1). Adults also had a later median age of CF diagnosis at 0.67 years (IQR, 0.12–10.15 yr) versus 0.09 years (IQR, 0.05–0.59 yr) for pediatric subjects (P = 0.003). The pediatric and adult cohorts were similar in terms of sex distribution, azithromycin use at the time of the first positive NTM culture, and percentage of first NTM isolated resistant to macrolide antibiotics. Differences in CF genotype distribution and coinfection with other CF pathogens did not reach statistical significance. Staphylococcus aureus was the most common coinfection at the time of the first positive NTM culture in both cohorts.
MAC was the most common NTM species isolated in pediatric and adult subjects. Seventy-five percent (36 of 48) of pediatric and 69% (33 of 48) of adult subjects had only MAC isolated from respiratory tract specimens at the time of their first positive culture (Table E1). M. abscessus was cultured at the time of the first positive culture in 10 of 48 (21%) children and in 13 of 48 (27%) adults. A single child grew both MAC and M. abscessus at the time of the first positive culture. In addition, one child grew M. kansasii, one adult grew M. parascrofulaceum, and another adult grew a nonidentifiable NTM.
Subjects analyzed had an average of five positive NTM cultures (range, 1–28) and 10 negative NTM cultures (range, 0–32). Follow-up data were available on average for 4.25 years after the date of the first positive culture. These data demonstrate that, except for age-related differences in baseline FEV1 and coinfection with P. aeruginosa, the clinical characteristics and distribution of NTM species between pediatric subjects and adults were remarkably similar. Therefore, all ages were combined for subsequent comparisons.
Compared with the NTM-negative cohorts from study midpoint, pediatric subjects with NTM had a lower median body mass index percentile, whereas adults with NTM were younger, had a higher baseline FEV1, and were more commonly infected with S. aureus (Table 1). Pediatric and adult NTM-positive subjects had higher rates of coinfection with Stenotrophomonas maltophilia and Aspergillus fumigatus compared with the NTM-negative populations (Table 1).
Table 1.
Clinical characteristics of pediatric and adult subjects at the time of the first positive nontuberculous mycobacteria culture with cystic fibrosis center comparisons for patients without positive nontuberculous mycobacteria cultures from 2005
| Subject Characteristics | Pediatric Subjects |
Adult Subjects |
||||
|---|---|---|---|---|---|---|
| Positive NTM (n = 48) | 2005 Pediatric CF Center, Negative NTM (n = 451) | Positive NTM vs. Negative NTM | Positive NTM (n = 48) | 2005 Adult CF Center, Negative NTM (n = 103) | Positive NTM vs. Negative NTM | |
| Age, median yr (IQR) | 12.9 (11.4–14.5) | 11.2 (6.0–17.1) | P = 0.16 | 26.4 (21.0–34.2) | 32.3 (25.6–39.8) | P < 0.01* |
| Female sex, n (%) | 24 (50) | 216 (48) | P = 0.78 | 27 (56) | 61 (59) | P = 0.73 |
| FEV1% predicted, mean (SD) | 89% (19.6) | 88% (23.8) | P = 0.66 | 71% (22.0) | 58% (23.7) | P < 0.01* |
| Pediatric BMI percentile, median (IQR) | 29.5th (12.0–53.0) | 40.9th (21.5–63.4) | P = 0.02* | — | — | |
| Adult BMI, median (IQR) | — | — | 21.3 (19.1–24.1) | 21.0 (19.0–23.8) | P = 0.06 | |
| Azithromycin use, n (%) | 15 (31) | N/A | N/A | 18 (38) | N/A | N/A |
| Macrolide resistant NTM, n (%) | 4 (8) | 7 (15) | ||||
| CF genotype, n (%) | P = 0.24 | P = 0.01* | ||||
| F508del homozygous | 29 (61) | 221 (49) | 20 (42) | 50 (49) | ||
| F508del heterozygous | 15 (31) | 159 (35) | 25 (52) | 43 (42) | ||
| Other | 4 (8) | 45 (10) | 3 (6) | 6 (6) | ||
| Unknown | 0 (0) | 26 (6) | 0 (0) | 4 (4) | ||
| Coinfection, n (%) | ||||||
| Staphylococcus aureus (any) | 35 (73) | 296 (69) | P = 0.30 | 34 (72) | 47 (51) | P < 0.01* |
| Pseudomonas aeruginosa (any) | 16 (33) | 174 (41) | P = 0.47 | 26 (55) | 67 (73) | P = 0.20 |
| Stenotrophomonas maltophilia | 14 (29) | 49 (12) | P < 0.001* | 10 (21) | 8 (9) | P = 0.02* |
| Aspergillus fumigatus | 18 (38) | 72 (17) | P < 0.001* | 14 (30) | 7 (8) | P < 0.001* |
| NTM type isolated, n (%): | ||||||
| MAC | 36 (75) | N/A | N/A | 33 (69) | N/A | N/A |
| M. abscessus | 10 (21) | 13 (27) | ||||
| MAC and M. abscessus | 1 (2) | 0 (0) | ||||
| Other | 1 (2) | 2 (4) | ||||
Definition of abbreviations: BMI = body mass index; IQR = interquartile range; MAC = Mycobacterium avium complex; N/A = not available; NTM = nontuberculous mycobacteria
Statistically significant correlations.
Characteristics of Subjects Infected with MAC versus M. abscessus
Characteristics of subjects with MAC compared with those with M. abscessus were not different in terms of age, sex distribution, baseline FEV1, anthropometric measures, azithromycin use and resistance, CF genotype distribution, or frequencies of coinfection with other CF pathogens (Table 2). These findings demonstrate that subjects with MAC or M. abscessus were similar; therefore, both NTM species were combined for subsequent NTM-outcome based group analyses.
Table 2.
Clinical characteristics of subjects at the time of the first positive nontuberculous mycobacteria culture divided by first nontuberculous mycobacteria type isolated
| Subject Characteristics | MAC (n = 70) | Mycobacterium abscessus (n = 24) | P Value |
|---|---|---|---|
| Age, median years (IQR) | 16.7 (13.0–24.8) | 18.9 (11.6–30.4) | 0.52 |
| Female sex, n (%) | 37 (53) | 12 (50) | 0.81 |
| FEV1% predicted, mean (SD) | 81% (22.2) | 82% (23.4) | 0.60 |
| Pediatric BMI percentile, median (IQR) | 30th (10–53) | 27th (21–50) | 1.00 |
| Adult BMI, median (IQR) | 21 (18.6–13.7) | 23.5 (20.1–23.8) | 0.64 |
| Azithromycin use, n (%) | 22 (31) | 9 (38) | 0.59 |
| Macrolide resistant NTM, n (%) | 5 (7) | 2 (8) | 0.85 |
| CF genotype, n (%) | |||
| F508del homozygous | 35 (50) | 14 (58) | 0.74 |
| F508del heterozygous | 30 (43) | 9 (38) | |
| Other | 5 (7) | 1 (4) | |
| Coinfection, n (%) | |||
| Staphylococcus aureus (any) | 53 (76) | 15 (63) | 0.21 |
| Pseudomonas aeruginosa (any) | 31 (44) | 10 (42) | 0.82 |
| Stenotrophomonas maltophilia | 17 (24) | 7 (29) | 0.64 |
| Aspergillus fumigatus | 25 (36) | 7 (29) | 0.56 |
| NTM group distribution, n (%) | |||
| Transient infection | 14 (20) | 6 (25) | 0.43 |
| Persistent infection | 31 (44) | 7 (29) | |
| Active disease | 25 (36) | 11 (46) |
Definition of abbreviations: BMI = body mass index; CF = cystic fibrosis; IQR = interquartile range; MAC = Mycobacterium avium complex; NTM = nontuberculous mycobacteria.
Clinical Outcome after an Initial Positive NTM Culture
The majority of subjects with a first positive NTM culture had transient (22 of 96 subjects, 23%) or persistent (37 of 96 subjects, 38.5%) NTM infection and did not meet the ATS definition of active NTM disease (59 of 96 subjects [61.5%]). The frequency of active NTM disease was not statistically different in subjects with M. abscessus compared with those with MAC (Table 2). Specifically, 45 out of 70 subjects (64.3%) with MAC and 13 out of 24 subjects (54.2%) with M. abscessus did not receive treatment (P = 0.43).
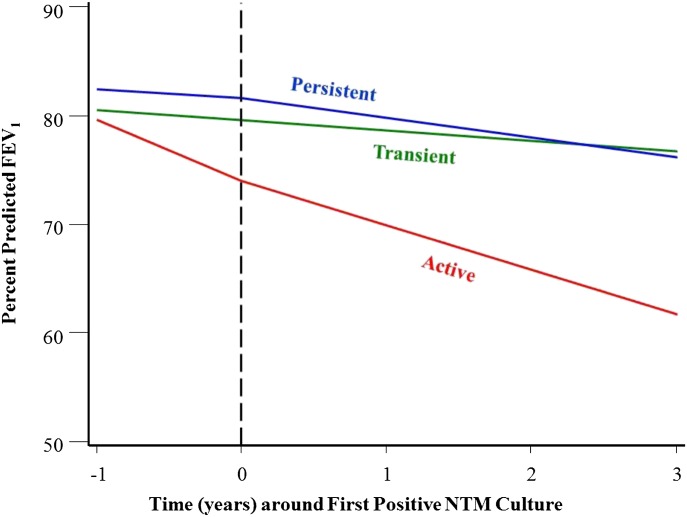
Differences in lung function are apparent when comparing subjects with active NTM disease versus those with transient or persistent infection (Figure 1). Subjects with active NTM disease had a lower baseline FEV1 at the time of the first positive culture compared with those with transient or persistent infection (P = 0.02) (Table E2). Subjects with active disease had an increased rate of decline in FEV1 in the year preceding the first positive culture (−5.8% predicted/yr) compared with those with transient (−0.7% predicted/yr; P = 0.009) or persistent infection (−0.4% predicted/yr; P = 0.001). In the 3 years after the first positive culture, subjects with active disease had a persistently increased rate of decline in FEV1 (−4.1% predicted/yr) compared with those with transient (−1.6% predicted/yr; P = 0.002) or persistent (−2.1% predicted/yr; P = 0.001) infection, as may be expected on the basis of our set criteria of having a presence or absence of clinical progression. There was no significant difference in decline in FEV1 between subjects with transient or persistent infection before (P = 0.89) or after (P = 0.61) the first positive NTM culture. No other clinical characteristics, including sex, anthropometric data, azithromycin use, azithromycin resistance, CF genotype, or coinfection with other CF pathogens, distinguished these three groups (Table E2). Subjects with NTM on azithromycin were not more likely to develop active disease than those not on azithromycin (relative risk, 0.98; 95% confidence interval, 0.55–1.73); nor were they less likely to clear their NTM with treatment (relative risk, 1.51; 95% confidence interval, 0.71–3.22).
Figure 1.
Change in percent predicted FEV1 over time from 1 year before to 3 years after the date of the first positive culture in the three nontuberculous mycobacteria (NTM) groups. Subjects are divided into those with transient infection, those with persistent infection, and those with active disease. The vertical dashed line (at time 0) represents the time of first positive NTM culture. Subjects with active NTM disease have a lower baseline FEV1 at time of first positive culture, an increased rate of decline in FEV1 over the 1 year preceding the first positive culture, and an accelerated rate of decline in FEV1 after the first positive culture when compared with the other groups. There were no differences in baseline FEV1 or decline in FEV1 for subjects with transient or persistent infection before or after the first positive NTM culture.
Long-Term Acquisition of a Second NTM Infection
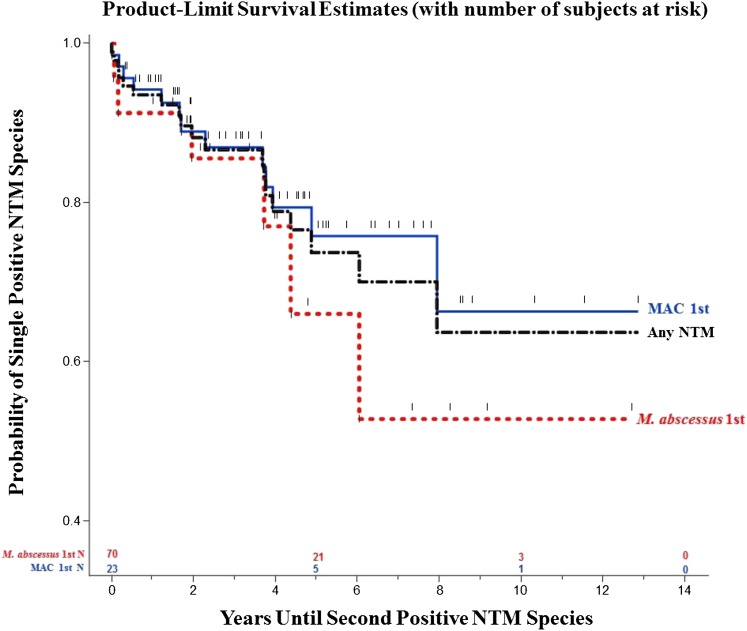
During the follow-up period, 20 subjects had a second NTM species isolated from respiratory tract specimens (Figure 2). Using Kaplan-Meier survival analyses, 24% of subjects who grew MAC initially went on to grow a second NTM species (primarily M. abscessus) during 5 years of follow-up. For subjects growing M. abscessus first, 34% grew a second NTM species (primarily MAC) at 5 years. Overall, 26% of subjects grew a second NTM species at 5 years, and 36% grew a second NTM species at 10 years.
Figure 2.
Probability of detecting a second nontuberculous mycobacteria (NTM) species after culturing a first NTM by Kaplan-Meier analysis, separated by initial positive NTM culture species. Twenty-four percent of subjects with Mycobacterium avium complex (MAC) initially grew a second NTM species during 5 years of follow-up, whereas 34% of subjects with M. abscessus first grew a second NTM species at 5 years. Overall, 26% of subjects grew a second NTM species at 5 years, and 36% grew a second NTM species at 10 years.
Response to Treatment
Thirty-seven subjects analyzed in this study were started on treatment for active NTM disease. On average, treatment started 18 months after the first positive NTM culture. Follow-up data were available for an average of 48 months after the initiation of treatment. Subjects were treated with a variety of regimens, including oral, intravenous, and inhaled antibiotics, because there are no standardized NTM treatment regimens for patients with CF. No subjects had surgical resection as part of their treatment plan. Overall, 43% of subjects did not clear their first NTM species cultured. The majority (60%) of the 25 subjects treated for MAC converted their cultures to negative (Figure E2). Thirty-two percent of subjects did not convert their cultures to negative, whereas in 8%, MAC recurred after an average of 1.75 years of negative cultures. The majority (55%) of subjects with M. abscessus did not convert their cultures to negative (Figure E2). There was no significant difference in culture conversion rates between MAC and M. abscessus (P = 0.42). Among those who did not convert their cultures, a single adult subject died of respiratory failure deemed related to M. abscessus 8 years after bilateral lung transplant.
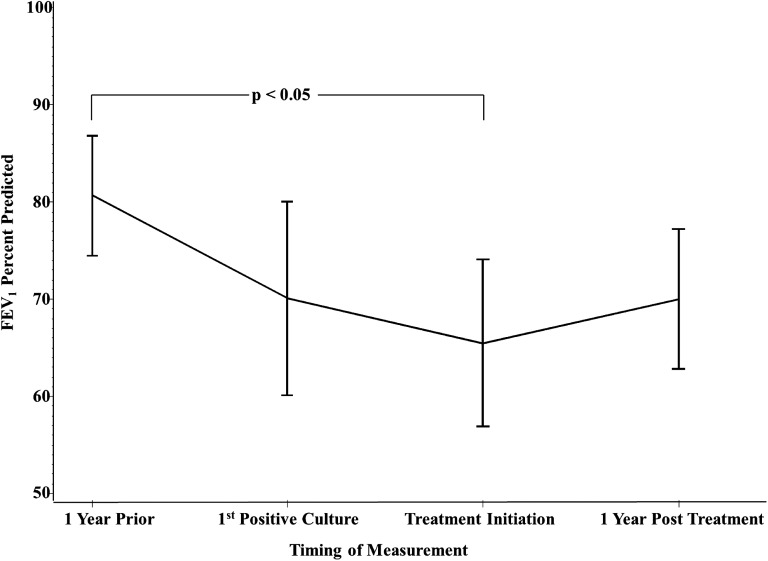
In subjects treated for active NTM disease, there was a significant difference between the mean FEV1 1 year before the first positive culture and the mean FEV1 at the time of treatment initiation (81% ± 3.1 SEM vs. 65% ± 4.3 SEM; P < 0.05) (Figure 3). There were no other statistically significant differences, although FEV1 appears to stabilize and improve with initiation of NTM treatment.
Figure 3.
Change in FEV1 percent predicted (mean, SD) in subjects treated for active nontuberculous mycobacteria (NTM) disease from the year before the date of the first positive culture, time of first NTM culture, time of treatment initiation, and 1 year after treatment initiation. The mean FEV1 at the time of treatment initiation was significantly lower than the mean FEV1 1 year before the first positive NTM culture.
Discussion
NTM is widely recognized as a significant complication of CF (1–10). Although much has been learned concerning the epidemiology of NTM in CF, previous studies examining the prevalence of NTM and potential risk factors for acquisition were not designed to guide clinical decision-making. Several additional reports have prospectively or retrospectively analyzed the impact of NTM on progression of CF lung disease (7, 8, 13, 16, 26–28). However, even in multicenter trials, cases are relatively rare, and distinguishing between patients with a single positive culture, patients with apparent indolent infection, and patients with NTM disease is difficult from reviewing databases in the absence of a detailed history and extended clinical follow-up (28); thus, data from these different populations may be combined (8, 16, 27). Due to these limitations, it is difficult to make conclusions concerning clinical outcomes of patients with CF with NTM from the available literature.
Herein, we report the clinical outcomes of the largest CF cohort described to date from the time of subjects’ first positive NTM culture in which NTM culture data were combined with longitudinal clinical data. In this population, MAC was most often the first positive NTM recovered. Contrary to previous reports (3, 4, 29), we did not observe less MAC in the pediatric population. In fact, except for the expected age-related differences in FEV1 and coinfection rate with P. aeruginosa, we did not see a significant difference in any clinical characteristic tested or distribution of NTM species between pediatric and adult subjects at the time of first positive culture.
Although MAC was typically the first identified NTM, a subsequent positive culture for M. abscessus was common, whereas subjects who first cultured M. abscessus also had a high rate of secondary positive cultures for MAC. Together, 26% of subjects were identified with a second NTM species at 5 years, and 36% were identified with a second NTM species at 10 years. Because routine identification of various species within the M. avium and M. abscessus complexes was not performed during the study, these findings almost certainly underrepresent the true frequency of recovering a second NTM. Many previous trials have anecdotally noted the presence of individuals with more than one species of NTM recovered from their sputum (1–4, 11, 14, 29–31). With longitudinal analysis starting at the time of initial detection of NTM, our data demonstrate that the presence of a second species of NTM is more than an occasional occurrence; rather, it is a predictable development in patients with CF after an initial positive culture. These findings support the need for lifelong strategies for NTM surveillance and management in patients with CF who present with a positive NTM culture.
Within our study group, about one quarter of subjects apparently cleared their sputum spontaneously after a single positive culture. With NTM widely present in the environment, these organisms are likely transiently present in the CF airway at any point in time, with no clinical significance. Of greater interest, we demonstrated the presence of a large cohort of patients with CF with recurrent positive cultures for NTM but no clinical evidence of disease. The potential for patients with CF to have “indolent infection” by NTM, including M. abscessus, has previously been described in case series but is not well defined or universally accepted (8, 12, 14, 31, 32). Over the period of analysis, spanning 1 year before and 3 years after the initial positive culture, we were unable to demonstrate a difference in FEV1 decline between subjects with transient or persistent infection. These findings support the conclusion that the majority of subjects with CF will not progress to require treatment for their NTM after a first positive culture. A larger sample size may have increased our power to detect a difference between these groups. In addition, the possibility exists that over decades of follow-up, patients with apparently indolent infection will eventually demonstrate accelerated lung decline. It is also possible that, over time, the NTM will acquire additional virulence factors, such as a “rough” morphotype (33, 34), which could potentially alter the host response and result in active disease in an individual after years of indolent infection (31, 35). A long-term prospective trial is needed to conclusively determine if the benefit of not treating patients with apparently indolent infection outweighs the long-term risk of developing NTM disease.
The capacity of NTM to accelerate progression of CF lung disease has been uncertain, and in some reports no clinical impact was detected (27). In longitudinal analysis of patients identified with NTM from the prospective US multicenter prevalence study, only 22 cases of NTM disease were identified, and the trend toward worsening lung function did not reach significance over the 15 months of follow-up (28). In a large retrospective review with a mean of 6 years of follow-up, 38 cases of chronic NTM infection defined by at least three cultures demonstrated greater decline in FEV1 than a cohort without NTM, including a subset of 22 cases of chronic M. abscessus infection, but not in the subset without M. abscessus (7). Within our study, in which clinical status was analyzed relative to the first positive NTM culture, we demonstrated that by clearly distinguishing patients who meet ATS criteria for NTM disease, the contribution of NTM to progression of CF lung disease is apparent within 3 years after the initial culture irrespective of the type of NTM.
At the time of the first positive culture, patients with CF who eventually met ATS criteria for active NTM disease appeared distinct from those with transient or persistent infection. These subjects had a lower baseline FEV1 and an increased rate of decline in FEV1 over the year before the date of the first positive culture. Previously, it has been reported that cultures positive for NTM occur more frequently in patients with milder lung disease (1). However, other researchers have found greater NTM prevalence in subjects with lower FEV1 (2) or were unable to detect a difference (6, 8, 14, 15). Our results demonstrate that although adults with NTM-positive cultures had milder lung disease compared with adults without NTM, subjects who developed active NTM infections had lower FEV1. The accelerated decline in lung function in patients with NTM disease before the time of a first positive culture likely reflects the presence of NTM before initial detection. Our data indicate that an unexpected decrease in FEV1 in patients receiving standard CF care is a potentially useful indicator of the significance of a first positive NTM culture.
There is general consensus that, in CF and non-CF populations, M. abscessus is a more virulent bacteria than MAC (13, 17, 28, 29, 36), that it may be associated with a greater rate of FEV1 decline (7, 30), and that it is more difficult to treat (17, 26, 37). However, our results demonstrate that both MAC and M. abscessus can be cleared spontaneously in a subset of patients with CF or can result in an apparently indolent infection. In patients who progressed to NTM disease, the prevalence of M. abscessus was not different from MAC, and both appear to predispose patients to infection with the other organism. Our results support the conclusion that, at the time of a first positive culture, both M. abscessus and MAC have the potential to accelerate FEV1 decline and require treatment but that successful treatment of MAC is more common.
Treatment success of NTM pulmonary disease at our CF center is similar to rates reported in patients without CF (23–25, 37, 38). Forty percent of subjects with MAC never converted their sputum cultures to negative or achieved temporary clearance with recurrence, and 55% of subjects with M. abscessus never converted their sputum cultures during the observation period. In those who were treated, lung function decline stabilized in the year after therapy initiation even though the organism was often not eradicated. Azithromycin use has recently been shown to block autophagy, suggesting that it has the potential to predispose patients with CF to mycobacterial infection (39), although this effect has not been seen in case-control studies (29, 40). In addition, a recent nested case-control study by Binder and colleagues showed that patients with CF with incident NTM infections were less likely to have had chronic azithromycin treatment in the last year (41). Azithromycin was used in about one third of our subjects equally across the three outcome groups and was not associated with worse clinical outcomes. Subjects on azithromycin or with an initial NTM isolate resistant to azithromycin were not more likely to develop active disease requiring treatment; nor were they less likely to clear their NTM with treatment.
The limitations of this study include its retrospective, single-center design. However, data were collected from the largest CF center in the United States, and the number of subjects studied exceeded previous multicenter trials. NJH is a national referral center for the treatment of NTM disease, and this clinical focus has contributed to frequent surveillance for NTM in our CF population. Determination of NTM disease was made by experienced CF Center physicians, typically in consultation with the physicians at the NTM Center of Excellence at NJH. Unfortunately, during the study period, organisms identified as MAC or M. abscessus were not further subspeciated; nor was colony morphotype routinely reported as “smooth” or “rough.” There have been recent data to suggest differing pathogenic potential between M. avium and M. intracellulare (42) and improved treatment response rates with M. bolletii (previously M. massilliense) infection compared with M. abscessus based on the presence of a nonfunctioning erythromycin ribosomal methylase gene (25), which may be a confounding factor that could be contributing to disease progression in our patients.
In summary, over the past decade, the majority of patients with CF with a first positive NTM culture did not require treatment, and one fourth of subjects cleared their sputum spontaneously. Among these subjects was a group of patients who persistently grew NTM from their sputum but did not progress to active NTM disease. Baseline FEV1 and rate of decline in the year before culturing NTM may help distinguish which patients progress to active NTM disease requiring treatment. These findings and the identification of other predictive biomarkers need to be evaluated in a natural history prospective study of NTM infection in CF to determine who will require treatment early in the course of disease.
Acknowledgments
Acknowledgment
The authors thank Elinor Towler and Christine Barboa for their outstanding assistance in data collection for this work and Dr. Frank Accurso and Dr. Jeffrey Wagener for their mentorship, invaluable feedback, and critical review of this manuscript.
Footnotes
This work was supported by the National Institutes of Health grant T32 HL007670–21.
Author Contributions: Conception and design: S.L.M., C.L.D., J.A.N., S.D.S. Analysis and interpretation: M.K.S., S.L.M., J.A.N., S.D.S. Drafting the manuscript for important intellectual content: S.L.M., M.K.S., C.L.D., J.A.N., S.D.S.
This article has an online data supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Olivier KN, Weber DJ, Wallace RJ, Jr, Faiz AR, Lee JH, Zhang Y, Brown-Elliot BA, Handler A, Wilson RW, Schechter MS, et al. Nontuberculous Mycobacteria in Cystic Fibrosis Study Group. Nontuberculous mycobacteria. I: multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med. 2003;167:828–834. doi: 10.1164/rccm.200207-678OC. [DOI] [PubMed] [Google Scholar]
- 2.Levy I, Grisaru-Soen G, Lerner-Geva L, Kerem E, Blau H, Bentur L, Aviram M, Rivlin J, Picard E, Lavy A, et al. Multicenter cross-sectional study of nontuberculous mycobacterial infections among cystic fibrosis patients, Israel. Emerg Infect Dis. 2008;14:378–384. doi: 10.3201/eid1403.061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roux AL, Catherinot E, Ripoll F, Soismier N, Macheras E, Ravilly S, Bellis G, Vibet MA, Le Roux E, Lemonnier L, et al. Jean-Louis Herrmann for the OMA Group. Multicenter study of prevalence of nontuberculous mycobacteria in patients with cystic fibrosis in france. J Clin Microbiol. 2009;47:4124–4128. doi: 10.1128/JCM.01257-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pierre-Audigier C, Ferroni A, Sermet-Gaudelus I, Le Bourgeois M, Offredo C, Vu-Thien H, Fauroux B, Mariani P, Munck A, Bingen E, et al. Age-related prevalence and distribution of nontuberculous mycobacterial species among patients with cystic fibrosis. J Clin Microbiol. 2005;43:3467–3470. doi: 10.1128/JCM.43.7.3467-3470.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sermet-Gaudelus I, Le Bourgeois M, Pierre-Audigier C, Offredo C, Guillemot D, Halley S, Akoua-Koffi C, Vincent V, Sivadon-Tardy V, Ferroni A, et al. Mycobacterium abscessus and children with cystic fibrosis. Emerg Infect Dis. 2003;9:1587–1591. doi: 10.3201/eid0912.020774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radhakrishnan DK, Yau Y, Corey M, Richardson S, Chedore P, Jamieson F, Dell SD. Non-tuberculous mycobacteria in children with cystic fibrosis: isolation, prevalence, and predictors. Pediatr Pulmonol. 2009;44:1100–1106. doi: 10.1002/ppul.21106. [DOI] [PubMed] [Google Scholar]
- 7.Esther CR, Jr., Esserman DA, Gilligan P, Kerr A, Noone PG. Chronic mycobacterium abscessus infection and lung function decline in cystic fibrosis. J Cyst Fibros. 2010;9:117–123. doi: 10.1016/j.jcf.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aitken ML, Burke W, McDonald G, Wallis C, Ramsey B, Nolan C. Nontuberculous mycobacterial disease in adult cystic fibrosis patients. Chest. 1993;103:1096–1099. doi: 10.1378/chest.103.4.1096. [DOI] [PubMed] [Google Scholar]
- 9.Valenza G, Tappe D, Turnwald D, Frosch M, Konig C, Hebestreit H, Abele-Horn M. Prevalence and antimicrobial susceptibility of microorganisms isolated from sputa of patients with cystic fibrosis. J Cyst Fibros. 2008;7:123–127. doi: 10.1016/j.jcf.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Nick JA. Nontuberculous mycobacteria in cystic fibrosis. Semin Respir Crit Care Med. 2003;24:693–702. doi: 10.1055/s-2004-815665. [DOI] [PubMed] [Google Scholar]
- 11.Bange FC, Brown BA, Smaczny C, Wallace RJ, Jr, Böttger EC. Lack of transmission of mycobacterium abscessus among patients with cystic fibrosis attending a single clinic. Clin Infect Dis. 2001;32:1648–1650. doi: 10.1086/320525. [DOI] [PubMed] [Google Scholar]
- 12.Oliver A, Maiz L, Cantón R, Escobar H, Baquero F, Gómez-Mampaso E. Nontuberculous mycobacteria in patients with cystic fibrosis. Clin Infect Dis. 2001;32:1298–1303. doi: 10.1086/319987. [DOI] [PubMed] [Google Scholar]
- 13.Fauroux B, Delaisi B, Clément A, Saizou C, Moissenet D, Truffot-Pernot C, Tournier G, Vu Thien H. Mycobacterial lung disease in cystic fibrosis: a prospective study. Pediatr Infect Dis J. 1997;16:354–358. doi: 10.1097/00006454-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Kilby JM, Gilligan PH, Yankaskas JR, Highsmith WE, Jr, Edwards LJ, Knowles MR. Nontuberculous mycobacteria in adult patients with cystic fibrosis. Chest. 1992;102:70–75. doi: 10.1378/chest.102.1.70. [DOI] [PubMed] [Google Scholar]
- 15.Girón RM, Máiz L, Barrio I, Martínez MT, Salcedo A, Prados C. [Nontuberculous mycobacterial infection in patients with cystic fibrosis: a multicenter prevalence study] Arch Bronconeumol. 2008;44:679–684. [PubMed] [Google Scholar]
- 16.Hjelte L, Petrini B, Källenius G, Strandvik B. Prospective study of mycobacterial infections in patients with cystic fibrosis. Thorax. 1990;45:397–400. doi: 10.1136/thx.45.5.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, et al. ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 18.Ballarino GJ, Olivier KN, Claypool RJ, Holland SM, Prevots DR. Pulmonary nontuberculous mycobacterial infections: antibiotic treatment and associated costs. Respir Med. 2009;103:1448–1455. doi: 10.1016/j.rmed.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martiniano SL, Sontag MK, Nick JA, Sagel SD. Nontuberculous mycobacterial infection in cystic fibrosis patients at a large CF care center [abstract] Am J Respir Crit Care Med. 2011;183:A2398. [Google Scholar]
- 20.Martiniano SL, Sontag MK, Nick JA, Sagel SD. Nontuberculous mycobacterial infection in cystic fibrosis patients at the Colorado CF care center [abstract] Pediatr Pulmonol Suppl. 2011;34:A255. [Google Scholar]
- 21.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 22.Burns JL, Emerson J, Stapp JR, Yim DL, Krzewinski J, Louden L, Ramsey BW, Clausen CR. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis. 1998;27:158–163. doi: 10.1086/514631. [DOI] [PubMed] [Google Scholar]
- 23.Kobashi Y, Matsushima T, Oka M. A double-blind randomized study of aminoglycoside infusion with combined therapy for pulmonary Mycobacterium avium complex disease. Respir Med. 2007;101:130–138. doi: 10.1016/j.rmed.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Jeon K, Kwon OJ, Lee NY, Kim BJ, Kook YH, Lee SH, Park YK, Kim CK, Koh WJ. Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am J Respir Crit Care Med. 2009;180:896–902. doi: 10.1164/rccm.200905-0704OC. [DOI] [PubMed] [Google Scholar]
- 25.Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, Park YK, Kim CK, Shin SJ, Huitt GA, et al. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med. 2011;183:405–410. doi: 10.1164/rccm.201003-0395OC. [DOI] [PubMed] [Google Scholar]
- 26.Esther CR, Jr, Henry MM, Molina PL, Leigh MW. Nontuberculous mycobacterial infection in young children with cystic fibrosis. Pediatr Pulmonol. 2005;40:39–44. doi: 10.1002/ppul.20222. [DOI] [PubMed] [Google Scholar]
- 27.Torrens JK, Dawkins P, Conway SP, Moya E. Non-tuberculous mycobacteria in cystic fibrosis. Thorax. 1998;53:182–185. doi: 10.1136/thx.53.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olivier KN, Weber DJ, Lee JH, Handler A, Tudor G, Molina PL, Tomashefski J, Knowles MR Nontuberculous Mycobacteria in Cystic Fibrosis Study Group. Nontuberculous mycobacteria: II. Nested-cohort study of impact on cystic fibrosis lung disease. Am J Respir Crit Care Med. 2003;167:835–840. doi: 10.1164/rccm.200207-679OC. [DOI] [PubMed] [Google Scholar]
- 29.Catherinot E, Roux AL, Vibet MA, Bellis G, Ravilly S, Lemonnier L, Le Roux E, Bernède-Bauduin C, Le Bourgeois M, Herrmann JL, et al. OMA group. Mycobacterium avium and Mycobacterium abscessus complex target distinct cystic fibrosis patient subpopulations. J Cyst Fibros. 2013;12:74–80. doi: 10.1016/j.jcf.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Esther CR, Jr, Esserman DA, Gilligan P, Kerr A, Noone PG. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J Cyst Fibros. 2010;9:117–123. doi: 10.1016/j.jcf.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jönsson BE, Gilljam M, Lindblad A, Ridell M, Wold AE, Welinder-Olsson C. Molecular epidemiology of Mycobacterium abscessus, with focus on cystic fibrosis. J Clin Microbiol. 2007;45:1497–1504. doi: 10.1128/JCM.02592-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cullen AR, Cannon CL, Mark EJ, Colin AA. Mycobacterium abscessus infection in cystic fibrosis: colonization or infection? Am J Respir Crit Care Med. 2000;161:641–645. doi: 10.1164/ajrccm.161.2.9903062. [DOI] [PubMed] [Google Scholar]
- 33.Greendyke R, Byrd TF. Differential antibiotic susceptibility of Mycobacterium abscessus variants in biofilms and macrophages compared to that of planktonic bacteria. Antimicrob Agents Chemother. 2008;52:2019–2026. doi: 10.1128/AAC.00986-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catherinot E, Clarissou J, Etienne G, Ripoll F, Emile JF, Daffé M, Perronne C, Soudais C, Gaillard JL, Rottman M. Hypervirulence of a rough variant of the Mycobacterium abscessus type strain. Infect Immun. 2007;75:1055–1058. doi: 10.1128/IAI.00835-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Catherinot E, Roux AL, Macheras E, Hubert D, Matmar M, Dannhoffer L, Chinet T, Morand P, Poyart C, Heym B, et al. Acute respiratory failure involving an R variant of Mycobacterium abscessus. J Clin Microbiol. 2009;47:271–274. doi: 10.1128/JCM.01478-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mussaffi H, Rivlin J, Shalit I, Ephros M, Blau H. Nontuberculous mycobacteria in cystic fibrosis associated with allergic bronchopulmonary aspergillosis and steroid therapy. Eur Respir J. 2005;25:324–328. doi: 10.1183/09031936.05.00058604. [DOI] [PubMed] [Google Scholar]
- 37.Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis. 2011;52:565–571. doi: 10.1093/cid/ciq237. [DOI] [PubMed] [Google Scholar]
- 38.Lam PK, Griffith DE, Aksamit TR, Ruoss SJ, Garay SM, Daley CL, Catanzaro A. Factors related to response to intermittent treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006;173:1283–1289. doi: 10.1164/rccm.200509-1531OC. [DOI] [PubMed] [Google Scholar]
- 39.Renna M, Schaffner C, Brown K, Shang S, Tamayo MH, Hegyi K, Grimsey NJ, Cusens D, Coulter S, Cooper J, et al. Azithromycin blocks autophagy and may predispose cystic fibrosis patients to mycobacterial infection. J Clin Invest. 2011;121:3554–3563. doi: 10.1172/JCI46095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Catherinot E, Roux AL, Vibet MA, Bellis G, Lemonnier L, Le Roux E, Bernede-Bauduin C, Le Bourgeois M, Herrmann JL, Guillemot D, et al. Inhaled therapies, azithromycin and mycobacterium abscessus in cystic fibrosis patients. Eur Respir J. 2013;41:1101–1106. doi: 10.1183/09031936.00065612. [DOI] [PubMed] [Google Scholar]
- 41.Binder AM, Adjemian J, Olivier KN, Prevots DR. Epidemiology of nontuberculous mycobacterial infections and associated chronic macrolide use among persons with cystic fibrosis. Am J Respir Crit Care Med. 2013;188:807–812. doi: 10.1164/rccm.201307-1200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koh WJ, Jeong BH, Jeon K, Lee NY, Lee KS, Woo SY, Shin SJ, Kwon OJ. Clinical significance of the differentiation between Mycobacterium avium and Mycobacterium intracellulare in M avium complex lung disease. Chest. 2012;142:1482–1488. doi: 10.1378/chest.12-0494. [DOI] [PubMed] [Google Scholar]