Abstract
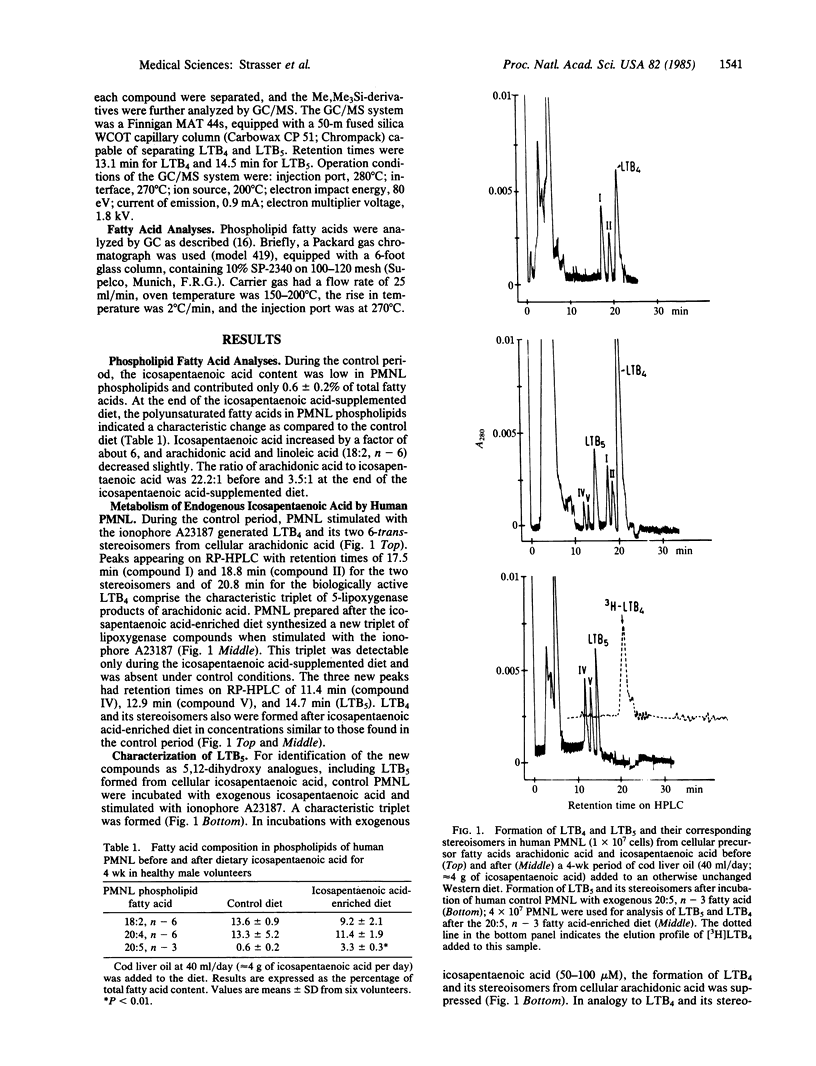
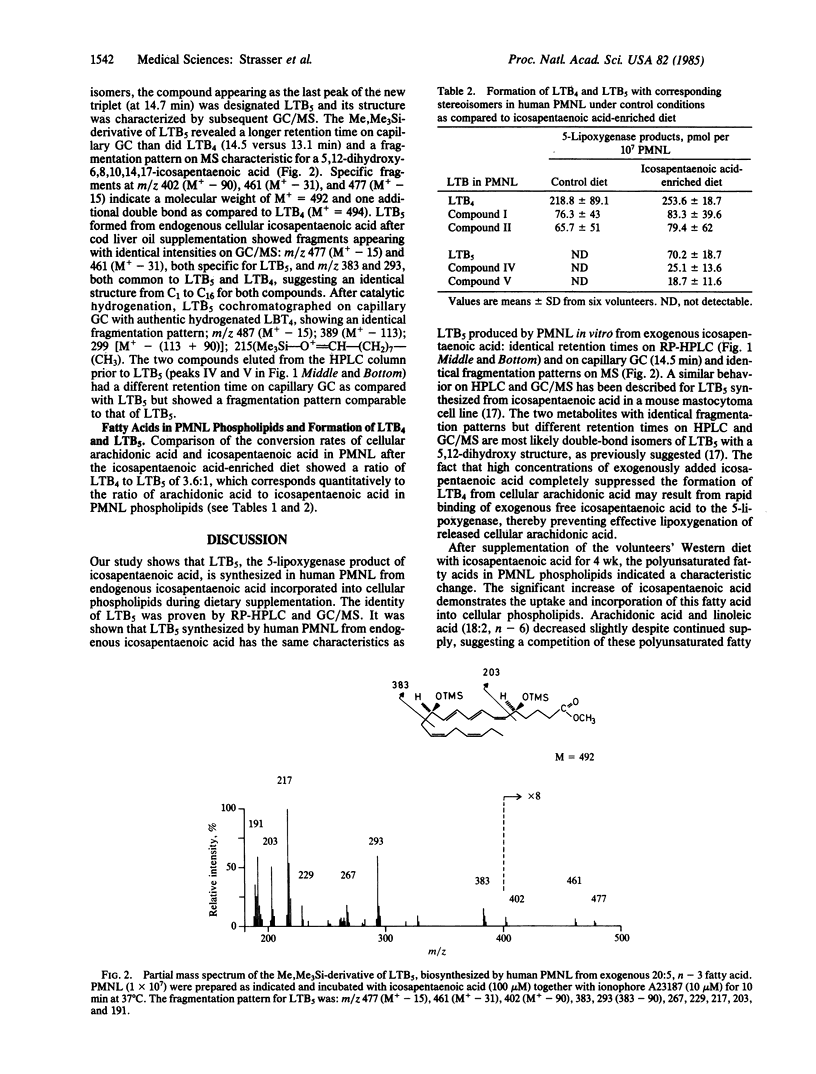
Incorporation and conversion of icosapentaenoic acid (20:5, n - 3) by human polymorphonuclear leukocytes were studied in volunteers (n = 6) ingesting a normal Western diet supplemented with icosapentaenoic acid (approximately equal to 4 g daily). Ingestion of icosapentaenoic acid leads to formation of biologically less active leukotriene B5 (LTB5) from polymorphonuclear leukocytes (PMNL) stimulated with ionophore A23187. LTB5 was identified on HPLC by UV absorption and by GC/MS and showed a behavior identical to that of in vitro synthesized LTB5 produced by incubation of human PMNL with icosapentaenoic acid. The ratio of icosapentaenoic acid/arachidonic acid (20:4, n - 6) in cellular phospholipids increased from 0.045 during control to 0.28 after the supplemented period. LTB5 increased from undetectable values to 70.2 +/- 18.7 pmol of LTB5 per 10(7) PMNL during the experimental period. Synthesis of LTB4 did not change significantly (control, 218.8 +/- 89.1; icosapentaenoic acid-enriched diet, 253.6 +/- 18.7 pmol per 10(7) PMNL). The ratio of LTB4/LTB5 corresponded to the ratio of arachidonic acid/icosapentaenoic acid in PMNL phospholipids. Our findings prove that LTB5, which is 10 to 30 times less potent than LTB4 to cause aggregation, chemotaxis, and degranulation of PMNL, can be formed in vivo in man after dietary icosapentaenoic acid. This may modify the contribution of leukotrienes in processes in which these metabolites are of pathogenetic relevance.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black K. L., Culp B., Madison D., Randall O. S., Lands W. E. The protective effects of dietary fish oil on focal cerebral infarction. Prostaglandins Med. 1979 Nov;3(5):257–268. doi: 10.1016/0161-4630(79)90067-3. [DOI] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: unstable intermediate in formation of dihydroxy acids. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3213–3217. doi: 10.1073/pnas.76.7.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray M. A. The pharmacology and pathophysiology of leukotriene B4. Br Med Bull. 1983 Jul;39(3):249–254. doi: 10.1093/oxfordjournals.bmb.a071828. [DOI] [PubMed] [Google Scholar]
- Culp B. R., Lands W. E., Lucches B. R., Pitt B., Romson J. The effect of dietary supplementation of fish oil on experimental myocardial infarction. Prostaglandins. 1980 Dec;20(6):1021–1031. doi: 10.1016/0090-6980(80)90056-8. [DOI] [PubMed] [Google Scholar]
- Dyerberg J., Jørgensen K. A. Marine oils and thrombogenesis. Prog Lipid Res. 1982;21(4):255–269. doi: 10.1016/0163-7827(82)90011-x. [DOI] [PubMed] [Google Scholar]
- Fischer S., Weber P. C. Prostaglandin I3 is formed in vivo in man after dietary eicosapentaenoic acid. Nature. 1984 Jan 12;307(5947):165–168. doi: 10.1038/307165a0. [DOI] [PubMed] [Google Scholar]
- Fischer S., Weber P. C. Thromboxane A3 (TXA3) is formed in human platelets after dietary eicosapentaenoic acid (C20:5 omega 3). Biochem Biophys Res Commun. 1983 Nov 15;116(3):1091–1099. doi: 10.1016/s0006-291x(83)80254-x. [DOI] [PubMed] [Google Scholar]
- Goldman D. W., Pickett W. C., Goetzl E. J. Human neutrophil chemotactic and degranulating activities of leukotriene B5 (LTB5) derived from eicosapentaenoic acid. Biochem Biophys Res Commun. 1983 Nov 30;117(1):282–288. doi: 10.1016/0006-291x(83)91572-3. [DOI] [PubMed] [Google Scholar]
- Hjorth R., Jonsson A. K., Vretblad P. A rapid method for purification of human granulocytes using percoll. A comparison with dextran sedimentation. J Immunol Methods. 1981;43(1):95–101. doi: 10.1016/0022-1759(81)90040-5. [DOI] [PubMed] [Google Scholar]
- Jakschik B. A., Kuo C. G. Characterization of leukotriene A4 and B4 biosynthesis. Prostaglandins. 1983 Jun;25(6):767–782. doi: 10.1016/0090-6980(83)90002-3. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Menica-Huerta J. M., Shih C., Corey E. J., Lewis R. A., Austen K. F. Characterization and biologic properties of 5,12-dihydroxy derivatives of eicosapentaenoic acid, including leukotriene B5 and the double lipoxygenase product. J Biol Chem. 1984 Feb 25;259(4):2383–2389. [PubMed] [Google Scholar]
- Leitch A. G., Lee T. H., Ringel E. W., Prickett J. D., Robinson D. R., Pyne S. G., Corey E. J., Drazen J. M., Austen K. F., Lewis R. A. Immunologically induced generation of tetraene and pentaene leukotrienes in the peritoneal cavities of menhaden-fed rats. J Immunol. 1984 May;132(5):2559–2565. [PubMed] [Google Scholar]
- Murphy R. C., Pickett W. C., Culp B. R., Lands W. E. Tetraene and pentaene leukotrienes: selective production from murine mastocytoma cells after dietary manipulation. Prostaglandins. 1981 Oct;22(4):613–622. doi: 10.1016/0090-6980(81)90070-8. [DOI] [PubMed] [Google Scholar]
- Needleman P., Raz A., Minkes M. S., Ferrendelli J. A., Sprecher H. Triene prostaglandins: prostacyclin and thromboxane biosynthesis and unique biological properties. Proc Natl Acad Sci U S A. 1979 Feb;76(2):944–948. doi: 10.1073/pnas.76.2.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper P. J. Pharmacology of leukotrienes. Br Med Bull. 1983 Jul;39(3):255–259. doi: 10.1093/oxfordjournals.bmb.a071829. [DOI] [PubMed] [Google Scholar]
- Prickett J. D., Robinson D. R., Steinberg A. D. Dietary enrichment with the polyunsaturated fatty acid eicosapentaenoic acid prevents proteinuria and prolongs survival in NZB x NZW F1 mice. J Clin Invest. 1981 Aug;68(2):556–559. doi: 10.1172/JCI110288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess W., Roth P., Scherer B., Kurzmann I., Böhlig B., Weber P. C. Platelet-membrane fatty acids, platelet aggregation, and thromboxane formation during a mackerel diet. Lancet. 1980 Mar 1;1(8166):441–444. doi: 10.1016/s0140-6736(80)90995-2. [DOI] [PubMed] [Google Scholar]



