Abstract
Neurite outgrowth is a fundamental step in establishing proper neuronal connections in the developing central nervous system. Dynamic control of outgrowth has been attributed to changes in growth cone Ca2+ levels in response to extracellular cues. Here we have investigated a possible role for Ca2+ permeable kainate (KA) receptors in regulating neurite outgrowth of nociceptive-like dorsal root ganglion (DRG) neurons. To identify KA receptor subunits likely to be involved, we used quantitative RT-PCR on acutely dissociated DRG and dorsal horn neurons. DRG neurons expressed more GluK1, particularly the GluK1b spice variant, than dorsal horn neurons. Conversely, dorsal horn neurons expressed more GluK2, particularly GluK2a, than DRG neurons. Further, an RNA editing assay indicated that the majority of GluK1 and GluK2 mRNA transcripts in DRG were unedited. Imaging Ca2+ transients following application of a KA receptor agonist to DRG and dorsal horn co-cultures revealed increases in intracellular Ca2+ in the growth cones of DRG neurons. In the majority of cases, this increase in Ca2+ was partly or completely blocked by Joro spider toxin (JSTX), an antagonist for Ca2+-permeable AMPA and KA receptors. Treatment of DRG/dorsal horn co-cultures with KA for 18 hours suppressed neurite outgrowth while application of the rapidly desensitizing KA receptor agonist SYM 2081, the competitive AMPA/KA receptor antagonist, CNQX, and JSTX or philanthotoxin enhanced neurite outgrowth and prevented KA effects on neurite outgrowth. Thus, Ca2+ entry through KA receptors at the growth cone of DRG neurons may be an important regulator of neurite outgrowth.
Keywords: Dorsal root ganglion, growth cone, microisland, neurite outgrowth, RNA editing
Introduction
During embryonic and early postnatal development, neurons assemble into functional networks by growing axons and dendrites that become synaptically connected. Outgrowth proceeds due to the dynamic behavior of growth cones that guide the growing neurites through the complex environment of the developing tissue to reach their appropriate targets for selective synaptic contacts (Tessier-Lavigne and Goodman, 1996; Dickson, 2002). The guidance of the growth cone during this process of extension depends heavily on its response to extracellular cues, including specific molecules bound to extracellular matrix and diffusible chemotropic factors such as netrins and growth factors (Dickson, 2002). Additionally, diffusible neurotransmitters, including glutamate, have been implicated in regulation of the rate and direction of neurite outgrowth (Mattson et al., 1988; Tashiro et al., 2003; Schmitz et al., 2009). Previous studies have demonstrated that some extracellular cues regulate growth cone dynamics by causing different spatiotemporal changes in cytosolic Ca2+ level (Gomez and Spitzer, 1999), possibly by shifting the membrane potential of the growth cone (Nishiyama et al., 2008) or by directly mediating Ca2+ influx (Metzger et al., 1998; Catsicas et al., 2001).
Small diameter, high threshold dorsal root ganglion (DRG) neurons, including Aδ and C fiber-associated neurons, contact dorsal horn neurons around the time of birth in rodents (Fitzgerald and Jennings, 1999). These afferent neuron populations are mainly responsible for detecting noxious, temperature and itch stimuli. Many of these peripheral neurons, particularly the C fiber associated neurons, express KA receptors, a subpopulation of glutamate receptors (Huettner, 1990). Over the last few days of embryonic development through the first few postnatal days in rats, these KA receptors change from a predominantly Ca2+-permeable form to a predominantly Ca2+-impermeable form (Lee et al., 2001) This raises the interesting question of what function the Ca2+ permeable KA receptors have during the development and outgrowth of the high threshold primary afferents within the dorsal horn at this critical time.
The molecular structure that determines Ca2+ permeability of KA receptors has been known for some time (Traynelis et al., 2010). Kainate receptors containing GluK1 (formerly GluR5) or GluK2 (formerly GluR6) subunits can be Ca2+-permeable when the subunits are unedited (Egebjerg and Heinemann, 1993; Kohler et al., 1993). These unedited subunits are highly expressed in most brain areas during embryonic development (Paschen et al., 1994; Paschen et al., 1995). Similarly, embryonic and early postnatal nociceptive DRG neurons express unedited GluK1 and the corresponding Ca2+ permeable KA receptors (Lee et al., 2001). It is possible that these receptors may initiate Ca2+ entry to regulate early developmental processes such as neurite outgrowth in DRG neurons. A similar role has been proposed for Ca2+ permeable KA receptors expressed by ciliary ganglion neurons (Olsen et al., 2007). Here we test the hypothesis that KA receptors are expressed on growth cones of putative nociceptive DRG neurons, are highly Ca2+-permeable and are able to influence neurite outgrowth. We have used a model system in which one or two embryonic DRG neurons are grown on a microisland together with one or two dorsal horn neurons. Synapses form between the DRG and dorsal horn neurons within 5 days in this model system (Joseph et al., 2010), allowing us to test whether KA receptors play a role in the events leading up to synapse formation.
Materials and Methods
Preparation of acute and microisland co-cultures
Preparation of collagen microisland co-cultures has been previously described in detail (Albuquerque et al., 2009; Albuquerque et al., 2009; Albuquerque et al., 2009; Albuquerque et al., 2009; Joseph et al., 2010). Briefly, DRG and dorsal horn neurons were isolated from rat Sprague-Dawley rat embryos aged 17 days in utero. Pregnant rats were killed by CO2 asphyxiation followed by cervical dislocation, and the embryos were removed and transferred to ice-cold Leibowitz-15 medium (Invitrogen). Animals were sacrificed according to the guidelines approved by Columbia University Institutional Animal Care and Use Committee. Isolated DRG and dorsal horns were incubated in dishes maintained at 37°C in Ca2+-Mg2+-free Modified Eagle Medium (MEM) containing 0.25% trypsin (Invitrogen) for 20 min. After enzymatic digestion, isolated cells were suspended in MEM plating medium (Invitrogen) containing 4.5 g/l of glucose, MEM vitamins (Invitrogen), and 4% B-27 supplement (Invitrogen) before plating on astrocyte microislands(Albuquerque et al., 2009; Albuquerque et al., 2009). Plating density was usually 6,000–8,000 dorsal horn neurons per dish, and 10,000–15,000 DRG neurons per dish. 2.5S nerve growth factor (10 ng/ml) and glial-derived neurotrophic factors (50ng/ml) were added at the time of plating. 5-fluoro-2′-deoxyuridine (10 μM) was added after two days in culture. For experiments using acutely dissociated DRG neurons, cells were plated directly on glass coverslips pretreated with poly-D-lysine (PDL; 1 mg/ml) and laminin (20 μg/ml). Cultures were kept at 37°C in a 5% CO2-enriched atmosphere. DRG neurons were easily distinguished from dorsal horn neurons by their distinctly larger diameter, phase-bright soma, and finer neurites.
Ratiometric Ca2+ imaging
Acute DRG and dorsal horn neurons were loaded by using 3 μM Fluo-4 AM and 5 μM FuraRed AM Ca2+-sensitive dyes (Molecular Probes) in HEPES buffer containing (in mM) 145 NaCl, 5 KCl, 2 CaCl2, 10 HEPES, 2 MgCl2, and 5.5 glucose, pH 7.3, 325 mOsm for 30-40 min at room temperature and then washed three times in the same buffer to remove surplus dyes. A coverslip of Fluo-4 and Fura Red-loaded DRG/dorsal horn neurons was mounted in a perfusion chamber (400 μl volume) and perfused with HEPES buffer at 2 ml/min. Changes in intracellular Ca2+ were detected with a confocal imaging system (C1; Nikon) mounted on a TE300 inverted microscope (Nikon). Dyes were excited with the 488 nm line of a solid state laser. Fluorescence was detected using 505–550 nm band-pass (Fluo-4 emission) and >635 nm long-pass (Fura Red emission) filters. Images were captured using a 40×, 1.30 NA Plan Fluo oil-immersion objective and at a rate of 3 Hz. Ca2+ transients were recorded in response to domoate application in the presence of strychnine (1 μM), SR 95531 (10 μM), and GYKI 53655 (50 μM). Analysis of the specified subcellular regions of DRG neurons was established by outlining regions of interest whose mean fluorescent intensity in each frame was determined by Nikon confocal software. To assess whether the KA receptors activated during drug application included the Ca2+ permeable subpopulation of KA receptor, domoate was reapplied in the presence of JSTX (3 μM). Each drug was diluted to final concentration in HEPES buffer and applied for 20-40 sec via bath perfusion. The change in Ca2+ signal at the growth cone, the process near the growth cone, and cell body was determined by the percentage change in the ratio (R) of Fluo-4 to Fura red fluorescence (ΔR/R)*100%) in those regions of the DRG neuron, normalized to the baseline ratio measured before drugs application. All experiments were performed at room temperature.
Ca2+ imaging in combination with focal application of domoate
Neurons were loaded with the Ca2+ indicator dyes and imaged as described above, with the following modifications. Cells were perfused by HEPES buffer solution containing the cocktail of drugs described above with the addition of tetrodotoxin (TTX; 500 nM). Domoate (1 μM; prepared in the perfusion solution) was focally applied from a puffer pipette fabricated from borosilicate thin-walled glass capillary tube (1.5 mm O.D., 1.1 mm I.D.) using a P-97 Flaming-Brown micropipette puller (Sutter Instruments). The dimensions of the puffer pipette were similar to those of a pipette typically used for patch clamp experiments. Prior to the growth cone experiments, the optimum size and orientation of the pipette and the duration of the “puff” was ascertained using DIC imaging and a tracer dye. Domoate was ejected from the pipette tip by two pulses of pressurized N2 (20 kPa) of 100 ms duration, 20 ms apart, controlled with a Picospritzer (Parker-Hannifin). The pipette was positioned 30 μm laterally and 10 μm vertically from the center of the growth cone or soma under investigation. The local application of domoate was synchronized to image acquisition using Clampex 9.2 software, a Digidata 1322A A/D converter (both Molecular Devices) and the trigger function of the EZ 3.60 software (Nikon) used to acquire confocal images. For each domoate application, a series of 35 images were acquired at approximately 2 Hz. Images were analyzed using ImageJ software (NIH, http://rsb.info.nih.gov/ij) using the “Bio-Formats” plug-in and custom-written macros.
Preparation of cDNA templates
Total RNA was extracted from separately dissociated DRG and dorsal horn neurons isolated from E17 rats using the RNeasy kit (Qiagen). Reverse transcription was carried out with SuperScript III reverse transcriptase following manufacturer's instructions (Invitrogen). Briefly, RNA (1–2 μg), oligo (dT) or random hexamer as primers (Invitrogen), dNTP mix (New England Biolabs, 20 mmol of each) were mixed on ice. The mixture was heated to 65°C for 5 min to allow for primer annealing and then incubated on ice for at least 1 min. Following incubation on ice, another mixture of 5× first strand buffer, 14 mM DTT, 200 units of SuperScript III reverse transcriptase, and 40 units of RNAseOUT recombinant RNAse inhibitor (Invitrogen) was added to the previous reaction mixture. The total reaction volume was 20 μl. Incubation for 50 min at 50°C was carried out in a thermal cycler, followed by 70°C for 15 min to inactivate the transcriptase. Reverse transcription was performed on each RNA sample with no added SuperScript III reverse transcriptase. PCR carried out on this resultant RT product generated no PCR product, confirming absence of genomic DNA contamination. The cDNA samples were stored at −20°C until required.
Primer design
The oligonucleotide primers used for the editing assay and deaminase expression have previously been described (Belcher and Howe, 1997). Primers used in the quantitative and end point RT-PCR experiments were designed with Frodo software (MIT). To avoid amplification of genomic DNA, primers were designed to span an intron. To ensure that the primers were specific for the gene of interest, primer sequences were searched against the mouse and rat genome using BLAST. Quantitative and end-point RT-PCR GluK1 and GluK2 primers were designed to recognize homologous regions in the respective isoforms (GluK1 or 2 total primers). Isoform specific primers were designed to recognize unique regions of the GluK1 or GluK2 isoforms. Primer sequences are shown in Table 1.
Table 1.
Sequences for the primers used in qualitative (A), editing assay (B), and quantitative real time RT-PCR (C). Isoform specific primers were generated from nucleotide sequences for the different isoforms of GluK1 and GluK2 constructs previously described in (Jaskolski et al., 2004). Constructs and nucleotide sequences of the GluK1 and GluK2 isoforms were kindly provided by Christophe Mulle. The sizes of the resulting products from primer pairs are given in base pairs.
| Gene | PRIMERS | ACCESSION # | SIZE |
|---|---|---|---|
| Qualitative RT-PCR | |||
| GluK1 (total) | 5′-GGTATAACCCCCACCCATGCAACCC-3′ | M83560 | 313bp |
| 5′-GAAGGTCATCGTCGAGCCATCTCTG-3 | |||
| GluK1a | 5′-ATTGAGTATGTGACGGAGAGGA-3′ | 350bp | |
| 5′-TACGCAACTTCAGTAATGCTG-3′ | |||
| GluK1b | 5′-ATTGAGTATGTGACGGAGAGGA-3′ | 455bp | |
| 5′-TCTCTGAGTTCGTCTGGGTGA-3′ | |||
| GluK1c | 5′ ATTGAGTATGTGACGGAGAGGA-3′ | 400bp | |
| 5′-ACCAAGGCTTTCTGTTTTGCT-3′ | |||
| GluK2 (total) | 5′-GGTATAACCCACACCCTTGCAACCC-3′ | Z11548 | 451bp |
| 5′-TGACTCCATTAAGAAAGCATAATCCGA-3′ | |||
| GluK2a | 5′-AAATGTGGGCGTTTATGAGC-3′ | 500bp | |
| 5′-CTGGCACTTCAGGGACATTC-3′ | |||
| GluK2b | 5′-AAATGTGGGCGTTTATGAGC-3′ | 490bp | |
| 5′-CTGGATGGTATGGTGGCACT-3′ | |||
| ADAR1 | 5′-CCCTAGCTATGAGCACAG-3′ | U18942 | 520bp |
| 5′-GCACAAGGCTCCACAAAGG-3′ | |||
| ADAR2 | 5′-CCATGGATATAGAAGACGAAGAG-3′ | U43534 | 550bp |
| 5′-CTTGTCTGGAGTCTCAAAGCC-3′ | |||
|
| |||
| RNA Editing RT-PCR | |||
| GluK1 | 5′-GGTATAACCCACACCCTTGCAACCC-3′ | M83560 | 313bp |
| 5′-GAAGGTCATCGTCGAGCCATCTCTG-3′ | |||
| GluK2 | 5′-GGTATAACCCCCACCCATGCAACCCC-3′ | Z11548 | 451bp |
| 5′-TGACTCCATTAAGAAAGCATAATCCGA-3′ | |||
|
| |||
| Quantitative Real time RT-PCR | ACCESSION # | SIZE | |
| Total GluK1 | 5′-GCAAATATGGAGCACAGAATGA-3′ | 173 | |
| 5′-CTTCCGGTAAAGGATGCTAATG-3′ | |||
| GluK1a | 5′-AGGACTCGTGCTTTCTGTGTTT-3′ | 108 | |
| 5′-GGGTTATCAAGGCATACGACAC-3′ | |||
| GluK1b | 5′-GAAGAACAATGACGTTGAGCAG-3′ | 163 | |
| 5′-TCTCTGAGTTCGTCTCTGGTGA-3′ | |||
| GluK1c | 5′-AAATCACGGAAGAACAATGACA-3′ | 108 | |
| 5′-ACCAAGGCTTTCTGTTTTGCT-3′ | |||
| Total GluK2 | 5′-CATAAAGCTGACCTTGCAGTTG-3′ | 227 | |
| 5′-CTGGCTATGACAAAGAGCACAC-3′ | |||
| GluK2a | 5′-AGCGTCGGCTCAAACATAAG-3′ | 100 | |
| 5′-TTTCTTTACCTGGCAACCTTCT-3′ | |||
| GluK2b | 5′-TTTGTGGCAGTGGGAGAGTT-3′ | 100 | |
| 5′-ACAGTGTCTGGATGGTATGGTG-3′ | |||
| ADAR1 | 5′-CTTAGAAAGGCAAGGCGATG-3′ | U18942 | 156 |
| 5′-GAGGTGCTTTGAGTGGCTTC-3′ | |||
| ADAR2 | 5′-CAAGTACCGCCTGAAGAAGC-3′ | U43534 | 172 |
| 5′-AAAGACCTGCCCGTTTACCT-3′ | |||
| ß-Actin | 5′-TGAGAGGGAAATCGTGCGTGACAT-3′ 5′-ACCGCTCGTTGCCAATAGTGATGA-3′ |
NM007393 | 127 |
End-point RT-PCR
PCR was carried out in a total volume of 50 μl, using HotStart Plus DNA polymerase (Qiagen). A ‘master mix’ of the PCR reaction components was prepared before addition to individual PCR tubes to minimize pipetting errors, and all samples underwent PCR at the same time in the same experiment. Reaction mixture consisted of 1 μg of DRG or dorsal horn complementary DNA (cDNA), 10 pmol of each primer pair, 200 μM deoxynucleoside triphosphates (dNTPs), 50 mM KCl2, 10 mM Tris–HCl (pH 8.3), 1.5 mM MgCl2, and 0.5 μl of HotStart Plus DNA polymerase (Qiagen). Unless otherwise noted, standard PCR conditions consisted of 1 cycle of 94°C for 2 min; 35 cycles of: 94°C for 30 s, annealing temperature for 30 s, 72°C for 30 s; 1 cycle of 72°C for 10 min. The annealing temperatures used for qualitative RT-PCR were as follow: 57°C for GluK6a, 58°C for ADAR2 and GluK1, 60°C for ADAR1 and GluK2, and 50°C for GluK2b and all GluK1 splice forms. . The primers used for PCR amplification, expected PCR product sizes, and their respective annealing temperatures are listed in Table 1. PCR products were run on 2% agarose gels and stained with ethidium bromide. Appropriate negative control PCR reactions were run in parallel.
BbvI restriction fragment length polymorphism analysis of editing at the Q/R site
The extent of RNA editing at the Q/R sites of GluK1 and GluK2 were determined using a protocol based on differential digestion of RT-PCR products with the restriction enzyme BbvI (Belcher and Howe, 1997; Bernard et al., 1999). DRG and dorsal horn neuron RT-PCR products were amplified using GluK1 or GluK2-specific primers under conditions described above. Resulting PCR products (10 μl of a 50 μl reaction) were completely digested for 4–6 h at 37°C with 4-6 units of BbvI (New England Biolabs). The assay, as reported by previous studies (Belcher and Howe, 1997; Bernard et al., 1999), contains an additional endogenous BbvI restriction site that provides an internal positive control for the completeness of digested PCR products. Only those samples that were completely digested were analyzed. Following digestion, samples were washed and collected using a PCR clean-up system kit (Promega). Samples were then fractionated on 5% (GluK2 or GluR6) or 6% (GluK1 or GluR5) polyacrylamide gels in TBE buffer. Gels were stained with ethidium bromide for 5 min and destained for 10–15 min prior to being photographed using an Alpha imager 2200 system (Alpha Innotech Corporation).
Quantitative real-time RT-PCR
Quantitative real-time RT-PCR (qPCR) was performed using SYBR Green Master Mix following the manufacturer's instructions (Stratagene). Each qPCR reaction comprised 12.5 μl 2× SYBR Green PCR Master Mix, forward and reverse primers at an optimized concentration of 800 nM, 10 ng/μl complementary DRG/dorsal horn co-cultured DNA template and sterile water up to a final volume of 25 μl. Primer pairs were designed with the aid of Frodo (MIT), the melting temperature was set at 60°C, and the length of the amplicon was kept between 100 and 240 bases. Sequences of qPCR primers are listed in Table 1. All reactions were conducted in duplicate using a Stratagene Mx3000 qPCR Detection System. Negative control was performed with sterile purified deionized water. Unless otherwise noted, standard PCR conditions consisted of three segments. The initial segment was 10 min at 95°C and the second segment or thermal profile consisted of 35 cycles of 15 sec at 95°C, 30 sec at 56°C, and 10 sec at 72°C. The third segment consisted of 60 sec at 95°C, 30 sec at 55°C, and 30 sec at 95°C. Melting-curve analysis showed that all PCR amplifications led to a single and specific product. The threshold cycle (Ct) values, the cycle where the fluorescent signal starts to rise above the background signal, were automatically measured by Stratagene Mx3000 qPCR Detection System software. GluK subunit or splice variant expression in DRG neurons relative to expression in DH neurons was quantified using the 2 –ΔΔCT method (Livak and Schmittgen, 2001). ΔCT values were calculated using β-actin expression as an internal control. ΔΔCT values were calculated by subtracting DRG neuron ΔCT values from dorsal horn neuron ΔCT values.
Immunocytochemistry
DRG dorsal horn co-cultures prepared as described above were washed with PBS two to three times before and after each of the following steps. Cultures were fixed with 4% paraformaldehyde for 20 min, permeabilized with 0.1% Triton X-100 for 1hr and incubated overnight with a GAP-43-specific primary antibody (1:1000; Chemicon). The cultures were then incubated with an Alexa 488-conjugated secondary antibody (1:400; Invitrogen) then mounted on coverglass with Fluoromount-G (SouthernBiotech). Phase-contrast or DIC and fluorescence images were captured with an Axiocam CCD camera mounted on a Zeiss Axioplan 2 microscope with a 20× 1.4 NA objective (Zeiss) using Openlab imaging software (Improvision). For clarity, adjustments to the brightness and contrast of the images were made using Photoshop 7.2 software (Adobe Systems).
Image analysis
For morphological analysis, digital images from 30–35 microislands were acquired blind and randomly from 10 dishes over 5 different cultures. The number and length of primary neurites and growth cone area were quantified using Metamorph software (Molecular Devices). Measurements represent length of longest process neurites/cell and does not include branches. Additional analysis was performed using Excel (Microsoft) and Prism (GraphPad). Statistical comparisons of immunofluorescence were made using One-way ANOVA. All data are reported as mean ± SEM.
Drugs
Strychnine, Gramicidin D, philanthotoxin (PhTX) and JSTX were purchased from Sigma. Kainate, CNQX, GYKI 53655, SR 95535 and SYM 2081 were purchased from Tocris Cookson. NGF was from Boehringer Mannheim and GDNF from Promega. All of the drugs were freshly diluted in bath solution to their final concentrations before experiments.
Results
GluK1 and GluK2 mRNA expression
To test the hypothesis that KA receptors are expressed on DRG neuron growth cones and influence neurite outgrowth under conditions in which synapses can form, we used a culture system that included both embryonic day 17 (E17) DRG neurons and their dorsal horn synaptic target neurons grown together on microisland cultures. Based on prior observations, we knew that GluK1 subunits were expressed by DRG neurons at E17 in primarily an unedited form (Lee et al., 2001). However, several splice variants have been described for GluK1 and GluK2 (Pinheiro and Mulle, 2006), and no information was available about these splice variants in embryonic DRG or dorsal horn neurons. Furthermore, no antibodies were available to clearly distinguish subunit and splice variant distribution in these two populations of neurons. Therefore, we generated specific primers to detect the expression of GluK1 and GluK2 splice variants in acutely dissociated, E17 DRG and dorsal horn cells (Table 1). Our results show that GluK1 and GluK2 mRNA transcripts and their splice variants are expressed in both DRG and dorsal horn neurons [Fig. 1(A)].
Figure 1.
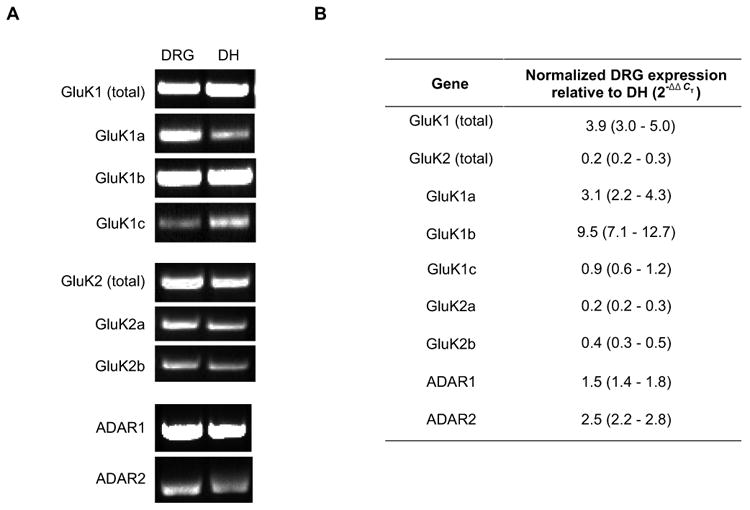
Expression of GluK1, GluK2, ADAR1, and ADAR2 mRNA in acutely prepared DRG and dorsal horn neurons. (A) total mRNA were isolated from tissues, reverse transcribed and the transcripts of interest then amplified using PCR. The PCR products were run on a gel and visualized using ethidium bromide staining. (B) Table showing expression of GluK and ADAR transcripts in DRG neurons relative to dorsal horn neurons. Quantification was carried out using qPCR and the ΔΔCT method. Data are displayed as fold expression in DRG neurons relative to dorsal horn neurons. GluK1 is more prevalent in DRG neurons and GluK2 in dorsal horn neurons. GluK1b splice form more prevalent in DRG neurons, whereas GluK2a is most prevalent in dorsal horn neurons. Both types of ADAR and ADAR are more prevalent in dorsal horns neurons.
We examined the relative abundance of GluK1 and GluK2 transcripts and their known splice variants by qPCR in both DRG and dorsal horn neurons. Using primers specific for all GluK1 splice variants (GluK1 total), we found that GluK1 mRNA transcript was approximately 4 fold higher in DRG than in dorsal horn neurons [Fig. 1(B)]. In contrast, total GluK2 mRNA transcript was 5 fold higher in dorsal horn than in DRG neurons [Fig. 1(B)]. These results are consistent with the prevalence of GluK1 mRNA transcripts in DRG neurons shown previously (Partin et al., 1993; Sato et al., 1993; Tolle et al., 1993).
The abundance of GluK subunit splice variants in DRG neurons relative to dorsal horn neurons was determined using qPCR with primers specific for known splice variants. Of the GluK1 splice variants assayed, the GluK1b mRNA transcripts had the largest difference in expression level between the two neuron populations, having approximately 10 fold greater expression in DRG neurons than in dorsal horn neurons [Fig. 1(B)]. Conversely, GluK2a mRNA transcripts were 5 fold more prevalent in dorsal horn neurons compared to DRG neurons [Fig. 1(B)].
Editing of the Q/R site in GluR5 and GluR6
In addition to splice variants, the diversity of KA receptors is enhanced by RNA editing. As in the case of the Q/R editing of AMPA receptors, subunit editing results in KA receptors with low permeability to Ca2+ and low single-channel conductance (Lerma et al., 2001). To determine the extent of editing of GluK1 and GluK2 subunits, a restriction enzyme based assay was used (Belcher and Howe, 1997). GluK1 and GluK2 express a codon for glutamine (Q) at a critical site in the pore loop, M2 domain that can be edited to a codon for arginine (R) by a dsRNA adenosine deaminase (Dingledine et al., 1999; Seeburg and Hartner, 2003). Editing of KA receptor subunits at the Q/R site results in blocking of receptor Ca2+ permeability when the edited subunit is included in the receptor tetramer of subunits. This Q (CAG) to R (CGG) substitution in GluK1 and GluK2 also destroys one of the two BbvI recognition sequences (GCAGC) from unedited RT-PCR products. As a result, digestion of GluK1 and GluK2 RT-PCR products with the restriction enzyme BbvI produces restriction fragments of unique lengths for edited and unedited cDNAs (Paschen et al., 1994; Belcher and Howe, 1997; Bernard et al., 1999).
GluK1 and GluK2 RT-PCR products were generated from acutely dissociated E17 DRG and dorsal horn neurons using specific primer sets (Table 1). The resulting products were digested to completion with BbvI and separated by polyacrylamide gel electrophoresis [Fig. 2(A)]. The molar ratios of the edited and unedited digestion products were determined by quantitative comparison of the fluorescent intensities of specific restriction fragments (Belcher and Howe, 1997). As shown in Figure 2(B), GluK2 pre-mRNA transcripts were edited to a greater extent than GluK1 in both DRG and dorsal horn neurons. Further, the extent of GluK2 editing was more pronounced in dorsal horn neurons than in DRG neurons [Fig. 2(B)]. In DRG neurons, the majority of mRNA for both subunits was unedited.
Figure 2.
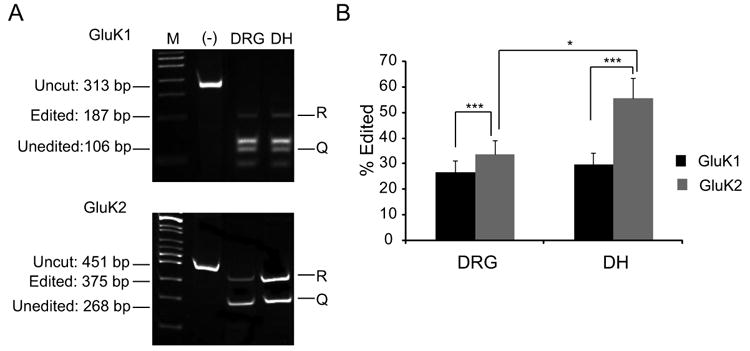
Editing of GluK1 and GluK2 KA receptor subunits in DRG and dorsal horn neurons. (A) GluK1 and GluK2 mRNA were amplified from acutely prepared DRG and dorsal horn neurons by RT-PCR. The products were cut with Bbv1 to reveal the extent of RNA editing. (B) The extent of editing was calculated by the molar ratio of R (Edited) band over that of the uncut band (Q). Notice that editing activity is more prevalent for GluK2 over GluK1. All values are expressed as mean ± SEM, *P < 0.05, **P < 0.005, ***P < 0.0005 by the Student's t-test, and n ≥ 25 for each condition.
Expression of adenosine deaminases in acutely dissociated DRG and dorsal horn cells
RNA editing of GluK receptor subunits has been attributed to two structurally related adenosine deaminases: ADAR1 (dsRNA-specific adenosine deaminase, dsRAD or DRADA) and ADAR2 (dsRNA-specific editase 1, RED1) (Schmauss and Howe, 2002). To determine which of these adenosine deaminases are expressed in acutely dissociated embryonic DRG or dorsal horn cells, end point RT-PCR was used to detect ADAR1 or ADAR2 mRNA. Both ADAR1 and ADAR2 mRNA transcripts were detected in both DRG and dorsal horn cells [Fig. 1(A)]. The relative abundance of these transcripts was tested by qPCR. Both ADAR1 and ADAR2 are more abundantly expressed in dorsal horn neurons than in DRG neurons [Fig. 1(B)]. These results show that ADAR1 and 2 are present in neurons in DRG and dorsal horn.
Activation of Ca2+-permeable KA receptors in growth cones of developing DRG neurons
We found that GluK1b is expressed by embryonic day 17 DRG neurons and that the GluK1 mRNA is mostly unedited, consistent with expression of a receptor that is Ca2+ permeable. The key question then became whether KA receptors are expressed at the level of the growth cone where they could best influence neurite outgrowth and if so, are they Ca2+ permeable there. We used Ca2+ imaging to address these questions because it provides a way to record activity at the subcellular sites of interest.
Kainate receptor activation may lead to intracellular Ca2+ elevation that is not directly caused by direct influx of Ca2+ through the KA receptor. For example, all KA receptors are permeable to monovalent cations, and influx of these cations following receptor activation causes membrane depolarization. If the depolarization is sufficient, voltage-gated Ca2+ channels will be activated, providing an additional source of Ca2+ entry. In addition, ryanodine receptor-mediated Ca2+-induced Ca2+ release from intracellular Ca2+ stores may also contribute to Ca2+ transients. However, in all of these cases, the Ca2+ response is initiated by KA receptor activation and thus Ca2+ provides a good indicator of the presence of KA receptors, regardless of whether or not the Ca2+ transient is directly caused by influx of Ca2+through the receptor. .
To demonstrate the presence of KA receptors on DRG neuron growth cones, neurons were loaded with Ca2+ indicators and data recorded from 3 subcellular compartments [Fig. 3(A)]. Application of the KA receptor agonist, domoate (3 μM), by perfusion across the entire surface of DRG neurons, produced robust increases in intracellular Ca2+ in the soma, growth cone, and neurite near the growth cone in many neurons. Ca2+ responses were prompt in onset and peaked during the 60 s domoate application in the presence of pre-applied cocktail consisting of GYKI 53655 (50 μM), strychnine (1 μM), SR95535 (10 μM) and lidocaine (0.5 mM) to block AMPA, glycine, GABAA receptors and all Na+ channels, respectively [Fig. 3(B,C,D)]. This concentration of lidocaine is not expected to block the high threshold voltage gated Ca2+ channels (Sugiyama and Muteki, 1994). Seventy five percent (30/40 from 6 different experiments) of the acutely dissociated DRG neurons responded to domoate application with a measurable increase in intracellular Ca2+ in all three subcellular compartments [Fig. 3]. To determine whether Ca2+ permeable KA receptors were involved in the Ca2+ response, domoate-evoked Ca2+ responses were tested in the presence of 3 μM JSTX, a selective antagonist for Ca2+-permeable AMPA and KA receptors. As seen in Figures 3 (B,C,D), JSTX significantly reduced the Ca2+ transients in all three compartments indicating an essential contribution of Ca2+ permeable KA receptors to activation of the Ca2+ transients.
Figure 3.
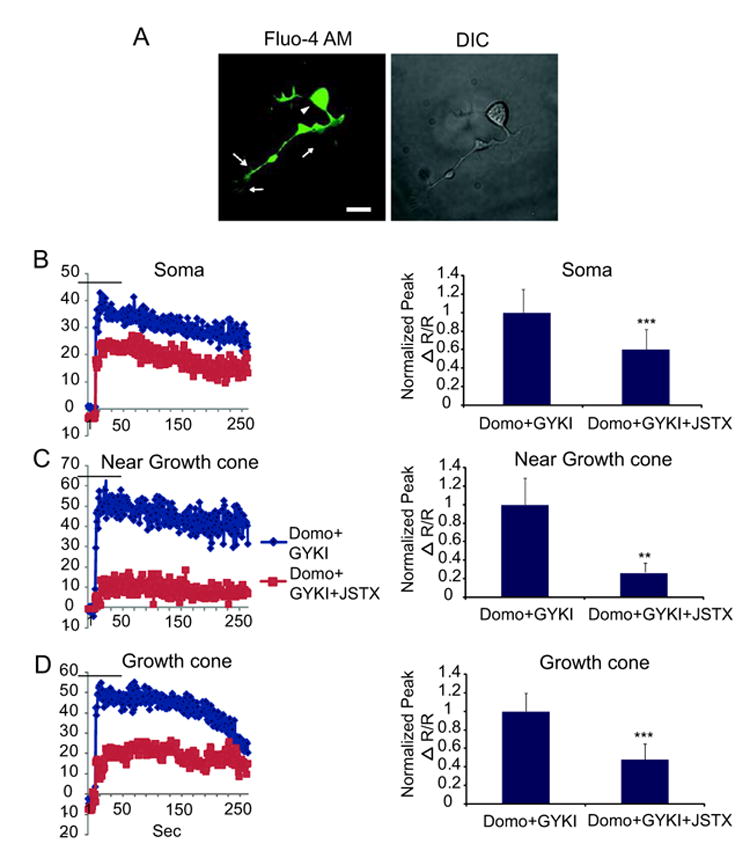
Ca2+-permeable KA receptors in somata, neurites and growth cones of DRG neurons. (A) Representative image of a typical cell showing the subcellular compartments (arrowhead points to the soma, arrows to the growth cone, and broken arrow to the process near the growth cone) in which changes in intracellular Ca2+ were measured. (B) Application of 3 μM domoate in the presence of GYKI 53655 to 40 DRG neurons caused a rapid increase in intracellular Ca2+ in the soma; (C) neurite near growth cone; and (D) in the growth cone - of 30 /40 DRG neurons. Intracellular Ca2+ increases mediated by domoate were reduced by JSTX, a Ca2+-permeable KA or AMPA receptor blocker. Bar graphs to the right of each response trace represent peak Ca2+ responses normalized to baseline of the same cell in each respective compartment. Values are expressed as mean ± SEM and **P < 0.005, ***P < 0.0005 by the Student's t-test. Scale bar is 20 μm.
Bath application of domoate in the presence of lidocaine allowed us to establish that the Ca2+ transients we recorded in the growth cone were likely to be due to activation of KA receptors expressed near DRG growth cones because lidocaine blocked possible action potential activity. However, there remained the possibility that domoate-induced Ca2+ elevation in the growth cone was due to intracellular diffusion of Ca2+ into the growth cone following Ca2+ elevation in the neurites. Therefore, we activated KA receptors on DRG growth cones by focal application of domoate (1 μM) via a puffer pipette. Figures 4(A,B) show the experimental paradigm. In the presence of TTX to block TTX-sensitive Na+ channels, domoate was briefly puffed (approximately 200 msec, see Methods) onto a DRG growth cone, producing an immediate Ca2+ elevation that peaked and quickly returned to baseline as the kainate diffused away [Fig. 4(Aii top panels,iii). When a similar application of domoate was made to the growth cone while imaging the soma, there was no detectable response [Fig. 4(Aii bottom panels,iii)], indicating the absence of back propagating action potentials. The converse experiment in which domoate was focally applied to the soma was also performed [Fig. 4(B)]. In these experiments, a small Ca2+ response was detected at the soma [Fig. 4(Bii top panels,iii)]. Figure 4 (Bii bottom panels,iii) shows that when domoate was applied to the soma, there was no response in the growth cone 1.2 s later when the peak response normally occurs. Interestingly, an elevation of intracellular Ca2+ was observed at the growth cone but it began to rise with a substantial, ∼1.5 s delay, which is after the peak growth cone response when it was activated directly [Fig. 4(A)]. The summary of these data are shown in Figure 4(C). Altogether, these experiments indicate that focal application of domoate onto growth cones elevates intracellular Ca2+ due to activation of local KA receptors on the growth cones.
Figure 4.
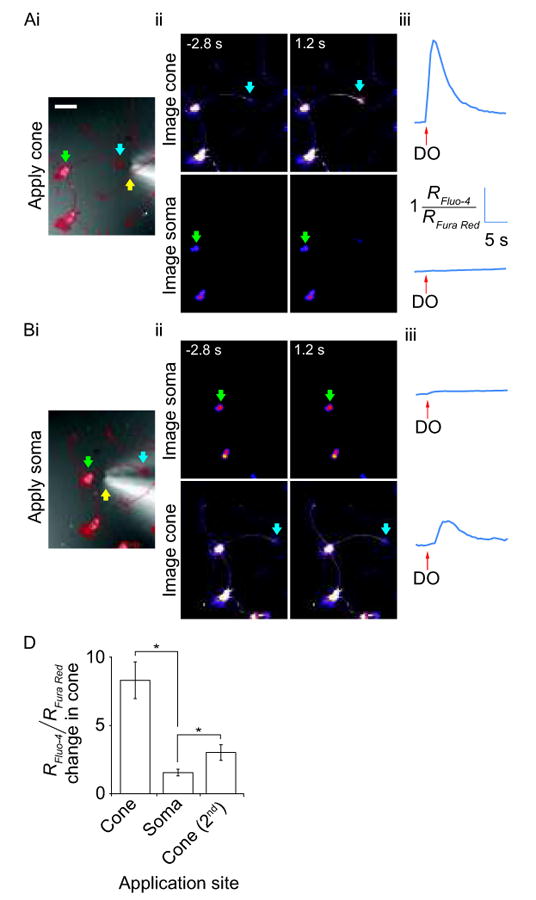
Ca2+ imaging in growth cones and somata in combination with local application of domoate. Domoate (1 μM) was applied from a puffer pipette positioned adjacent to the growth cone (or soma) of the DRG neuron of interest. A) Local domoate application at the growth cone. (i) DIC image with an overlay of a thresholded fluorescence image (in red) showing position of puffer pipette tip (yellow arrow) close to the DRG neuron growth cone (blue arrow). The soma is indicated by a green arrow. The same symbols are used throughout the figure. Scale bar = 50 μm. (ii) Ca2+ dynamics at the growth cone (top) and soma (bottom) 2.8 s before and 1.2 s after domoate application at the growth cone. Confocal images are from Fluo-4 channel alone and warmer colors indicate higher fluorescence intensities. (iii) Trace showing fluorescence changes in the indicated growth cone (top) and soma (bottom) over time. B) Local domoate application at the soma. (i) DIC image of the same cell as in A with an overlay of a thresholded fluorescence image showing the position of puffer pipette tip moved close to the DRG neuron soma. (ii) Images showing Ca2+ dynamics at the soma (top) and growth cone (bottom) 2.8 s before and 1.2 s after domoate application at the soma. (iii) Trace showing fluorescence changes in the soma (top) and growth cone (bottom) following domoate application at the soma. C) Bar chart showing the mean fold-change in Ca2+ dependent fluorescence from resting levels in the growth cone under three conditions; local domoate application to the growth cone, then to the soma and finally, a second application at the growth cone. In each instance, the change in fluorescence was measured at the time-point when the maximum change in fluorescence was observed with the first local application of domoate at the growth cone (error bars show the SEM and * represents a P < 0.05, Student's t-test, n = 5).
Finally, to further test if the KA receptors present on DRG growth cones are Ca2+ permeable, (as suggested by Figure 3), the effect of 3 μM JSTX on the Ca2+ transient induced by locally-applied domoate was determined. As shown in Figure 5, JSTX depressed or blocked the amplitude of Ca2+ transients induced by local domoate application in the majority of growth cones tested. On average, Ca2+ elevation evoked by domoate was significantly reduced to 49 ± 17% (P < 0.05, n = 7) of the amplitude of control. In the experiments where the JSTX was washed out for 20 minutes and a further domoate application was performed (n = 3/7), the amplitude of the domoate-induced Ca2+ transient showed partial recovery to 59 ± 9% of the control Ca2+ transient. These results indicate that a substantial portion of the KA receptors on DRG growth cones were Ca2+ permeable.
Figure 5.
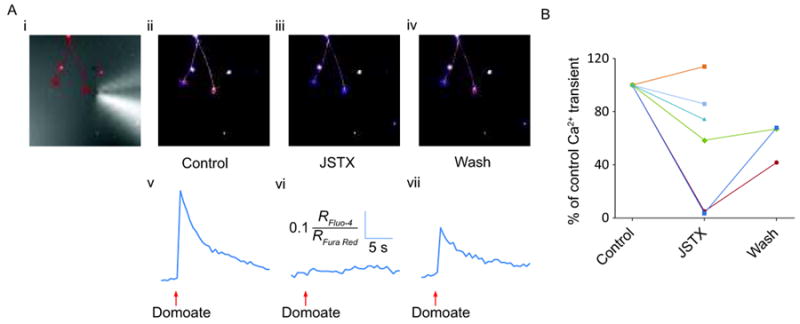
Local domoate application and imaging at the growth cone in the absence and presence of JSTX. (i) DIC image of growth cone with an overlay of a thresholded fluorescence image showing the position of the puffer pipette tip close to the right-hand growth cone. Confocal images of the same cell obtained 1.2 s following domoate puff application in control (ii), after applying JSTX for 20 minutes (iii), and following a 20-minute wash after JSTX removal (iv). (v-vii) Corresponding traces showing fluorescence changes following domoate application in the conditions described in (i-iii). B) Graph showing the effect of JSTX on the amplitude of the domoate-induced fluorescence changes in 6 neurons. Fluorescence changes were normalized to the amplitude of fluorescence changes induced by the control application of domoate.
Effects on neuronal morphology
We tested whether Ca2+ permeable KA receptors influence neurite outgrowth and growth cone morphology under conditions in which synapse formation will occur (Joseph et al., 2010). For these experiments, different concentrations of KA, KA receptor antagonists or KA in the presence of KA receptor antagonists were added to the cells in culture. Eighteen hours after drug addition, cells were fixed and subsequently immunostained for the growth-associated protein, GAP43. This labeling enabled detailed DRG morphology to be clearly identified and any KA receptor-mediated morphological changes to be determined. For each treatment condition, four quantitative morphological measurements were made from DRG neurons stained for GAP43. DRG neurons not in contact with neighboring cells were imaged and the following neuronal morphometrics were measured as illustrated in Figure 6: length of longest primary neurite, number of primary neurites, number of branches off the primary neurite and growth cone area.
Figure 6.
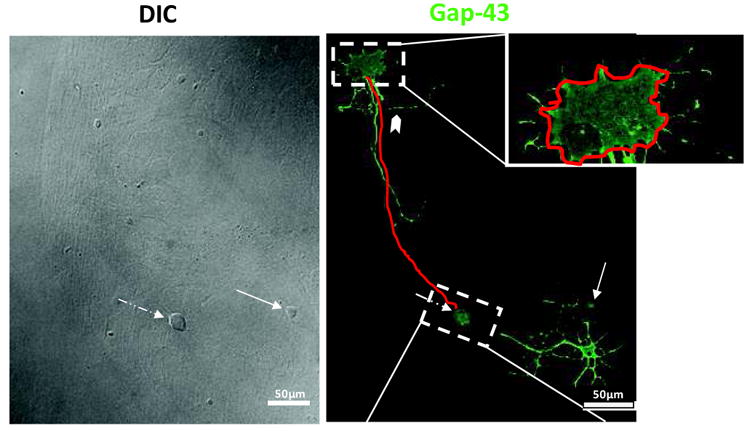
Microisland assay system illustrating the morphological indices measured. E17 rat DRG and dorsal horn neurons were cultured for 18 hours and processed for immunocytochemistry against GAP-43. Left panel (DIC) shows that very few neurons are on the astrocyte microisland. The DRG neuron (broken arrow) is easily distinguished from the dorsal horn neuron (arrow)by its distinct size and round shape. Right panel shows GAP-43 staining of the same microisland. Red line along the process emanating from the body represents the scheme for our neurite length measurements and the red line in the top inset shows our approach for growth cone area measurements. Notched arrowhead points to a typical branch point emanating from the measured longest process that was quantified.
There were no significant differences among growth cone area measurements for vehicle- and KA-treated cultures at the KA concentrations tested (250 nM, 10 μM or 100 μM) or with application of KA receptor antagonists. For the antagonists, we used 3 μM SYM 2081, a rapidly desensitizing KA receptor agonist that has been shown to act as an effective and selective KA receptor antagonist (Wilding and Huettner, 1997) and has been shown to block activation of both Ca2+ permeable and impermeable KA receptors expressed by DRG neurons (Lee et al., 2001). In addition, we used 25 μM CNQX, a nonselective AMPA/ KA receptor antagonist. These antagonists were used alone or in combination with KA. Growth cone area showed no significant differences from controls when treated in this way (Table 2). Furthermore, application of the Ca2+-permeable KA and AMPA receptor antagonists, JSTX and PhTX, alone or in combination with KA had no effect on growth cone area (Table 2).
Table 2.
Summary of analysis for growth cone area, primary neurites emanating from DRG neurons, and the number of branches from primary neurites under various pharmacological conditions. Note that analysis of neurite length was the most reliable analysis possible for dorsal horn neurons. The data show that those morphological indices are unchanged by KA receptor activation or blockade except for the mean number of branches on the DRG primary neurite in Sym2081. All values are expressed as mean ± SEM and P-values were calculated by one way ANOVA followed by Dunnett's multiple comparison post hoc tests.
| DRG neurons | |||||
|---|---|---|---|---|---|
| Agonist | 250 nM KA | 10 μM KA | 100 μM KA | Control | |
| Growth cone area, μm2 | 35.7±3.2 | 33.3±4.3 | 31±3 | 38±3.4 | |
| p>0.05 by ANOVA | n=36 | n=47 | n=41 | n=72 | |
| And Dunnett's test | |||||
| Mean number of branches on the primary neurite | 3±0.4 | 3.8±0.33 | 3.4±0.42 | 4.2±0.4 | |
| p>0.05 by ANOVA | n=30 | n=44 | n=38 | n=61 | |
| And Dunnett's test | |||||
| Number of primary neurites | 2.05±0.18 | 2.52±0.17 | 2.18±0.12 | 2.5±0.18 | |
| p>0.05 by ANOVA | n=37 | n=54 | n=45 | n=48 | |
| And Dunnett's test | |||||
| Antagonists | Sym2081 | CNQX | JSTX | PhTX | Control |
| Growth cone area, μm2 | 46±3.4 | 40±4.8 | 48±4.7 | 48.5±5 | 38±3.4 |
| p>0.05 by ANOVA | n=74 | n=26 | n=54 | n=41 | n=72 |
| And Dunnett's test | |||||
| Mean number of branches on the primary neurite | 6±0.4 | 3. ±0.4 | 5±0.45 | 5±0.5 | 4.2±0.42 |
| P<0.05 by ANOVA | n=77 | n=43 | n=67 | n=51 | n=61 |
| And Dunnett's test | *p<0.05 | p>0.05 | p>0.05 | p>0.05 | |
| Number of primary neurites | 2.2±0.11 | 2.3±0.14 | 2.4±0.14 | 2.2±0.16 | 2.2±0.13 |
| p>0.05 by ANOVA | n=88 | n=42 | n=69 | n=52 | n=58 |
| And Dunnett's test |
| Dorsal horn neurons | ||||||
|---|---|---|---|---|---|---|
| 10 μM KA | Sym2081 | CNQX | JSTX | PhTX | Control | |
| Neurite length μm | 165±24 | 175±18 | 167±24 | 173±16 | 175±18 | 166±18 |
| p>0.05 by ANOVA | n=22 | n=39 | n=27 | n=49 | n=33 | n=69 |
| And Dunnett's test |
As with the growth cone area measurements, the number of branches off primary neurites and the number of primary neurites also showed no differences across most of the drug treatments (Table 2). The one exception was that growing DRG neurons in Sym2081 caused a significant increase in the mean number of branches on the primary neurite (Table 2).
Neurite extension
Neurite extension was defined as a change in length of the longest process growing from DRG neuronal cell bodies. Although application of 250 nM KA had no effect on neurite outgrowth, 10 and 100 μM KA depressed neurite length [Fig. 7(A,B)]. On the other hand, application of SYM 2081 alone enhanced neurite length as did application of 25 μM CNQX, [Fig. 7(C)]. Also, application of 3 μM JSTX or 10 μM PhTX resulted in longer neurites demonstrating the involvement of Ca2+ permeable KA receptors. Co-application of 10 μM KA with 3 μM SYM 2081 not only prevented KA effects on suppression of neurite outgrowth but resulted in enhancement of neurite outgrowth similar to antagonist alone. Similarly, co-application of KA with 3 μM JSTX or 10 μM PhTX enhanced neurite outgrowth [Fig. 7(D)]. These results are consistent with a role for Ca2+-permeable KA receptors in modulating neurite extension during development of DRG neurons. Application of the glycine receptor antagonist, strychnine (3 μM), had no effect on neurite length. None of the drug treatments induced significant differences in neurite length of dorsal horn neurons as shown in Table 2.
Figure 7.
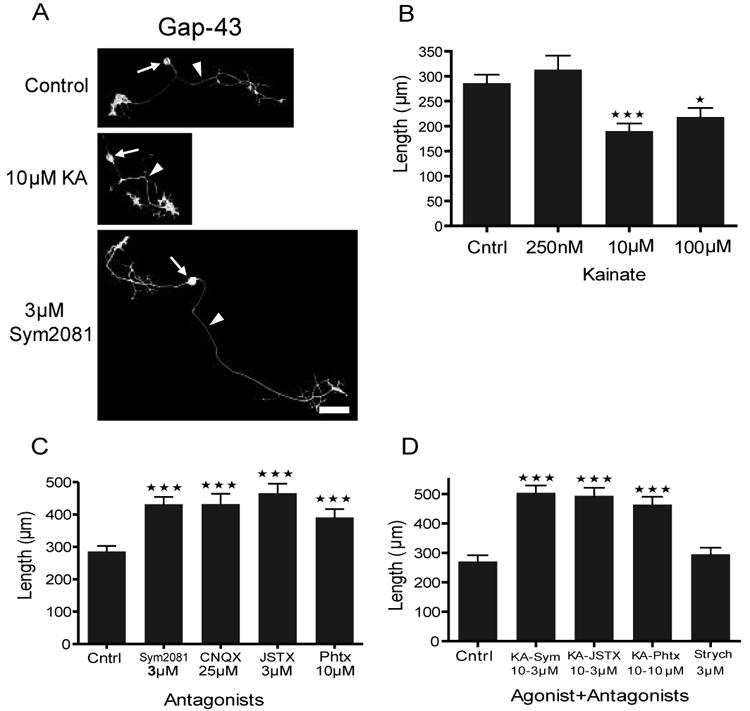
Quantitative analysis of neurite length when cultures are grown in KA receptor agonists and antagonists. (A) Representative images of GAP-43+ DRG neurons (arrows) under control, KA receptor activating, and blocking conditions. Arrowheads point to a typical longest neurite measured. (B) Kainate (10 and 100 μM) depressed neurite length (n = 98, 41, 54, and 41; with mean length of 286 ± 17, 314 ± 28, 190 ± 16, 218 ± 18 μm for control, 250 nM, 10 μM, and 100 μM KA, respectively). (C) Blockade of all KA receptors with SYM 2081 and CNQX and the Ca2+-permeable receptors with JSTX and PhTX enhanced neurite length (n = 98, 88, 42, 67, and 58; with mean length of 286 ± 17, 432 ± 23, 432 ± 32, 466 ± 29, 391 ± 26 μm for control, SYM 2081, CNQX, JSTX, and PhTX, respectively). (D) Simultaneous activation and blockade of these receptors reproduced similar results to antagonists alone (n = 53, 53, 60, 47, and 67; with mean length of 271 ± 21, 504 ± 23, 494 ± 27, 464 ± 27, 295 ± 22 μm for control, KA + SYM 2081, KA + JSTX, and KA + PhTX, and strychnine, respectively. All values are expressed as mean ± SEM and *P < 0.05, **P < 0.005, ***P < 0.0005 by one way ANOVA followed by Dunnett's multiple comparison post hoc tests. Scale bar is 50 μm.
Discussion
KA receptors are known to undergo phenotypic changes in Ca2+-permeability at a time when nociceptive DRG neurons are forming synapses in the spinal cord dorsal horn (Lee et al., 2001). Previously, though, it was not known whether Ca2+ permeable KA receptors are functionally expressed on growing processes of DRG neurons and if so, what impact they have on neurite outgrowth. Hence, we tested the hypothesis that Ca2+ permeable KA receptors are expressed on growth cones of late embryonic DRG neurons and that activation of Ca2+-permable KA receptors influences neurite outgrowth. Toward this end, we have used several complementary approaches to identify the subtype of KA receptors expressed by embryonic day 17 DRG neurons and determine the consequences of their activation during neurite outgrowth in a microisland culture system. Using qPCR, we demonstrated that acutely dissociated DRG neurons primarily express GluK1b and dorsal horn neurons primarily express GluK2a subunits. Consistent with Ca2+ imaging experiments, the majority of GluK1 and GluK2 mRNA transcripts in DRG appeared to be unedited, suggesting that at least some of the channels formed are permeable to Ca2+. This was confirmed by block of domoate-induced Ca2+ transients using JSTX. Finally, both endogenous and exogenous activation of KA receptors resulted in a reduction of neurite length in growing DRG neurons. Our data support the view that Ca2+ permeable KA receptors are important transducers of some of the multiple signals influencing neurite outgrowth during development of DRG neurons.
KA receptor expression in developing DRG and dorsal horn neurons
Pharmacological and knock out studies have suggested that native KA receptors in DRG neurons are constructed of mostly GluK1 subunits while in dorsal horn neurons they are constructed of mostly GluK2 subunits. However, KA responses from DRG neurons in GluK2–/– mice show differences in desensitization kinetics compared to wild-type mice, suggesting a possible contribution of GluK2 subunits to the agonist activated KA receptors (Kerchner et al., 2002). In addition, GluK1 selective antagonists LY382884 and LY293558 produced a substantial block in all of the wild-type and GluK2−/− dorsal horn neurons tested but had virtually no effect on GluK1−/− neurons, suggesting a substantial contribution of GluK1 subunit to KA receptor mediated current in dorsal horn neurons (Kerchner et al., 2002). These experiments demonstrated somewhat selective expression of GluK1 and 2 in DRG and dorsal horn neurons, respectively, but did not determine the splice variants involved.
Because there are no antibodies available that provide subunit or splice variant selective labeling of GluK1 and GluK2, we used qPCR to perform a more extensive investigation of subunit and splice variant expression. Our results showed that all splice variants of GluK1 and GluK2 mRNAs are expressed by E17 acutely dissociated DRG and dorsal horn neurons. However, in DRG neurons, the GluK1b splice variant was the most abundant form of GluK1 compared to dorsal horn neurons. In dorsal horn neurons, the GluK2a splice variant was the most abundant form of GluK2 compared to DRG. Different splice variants affect receptor trafficking differently, with GluK2a having the strongest impact on surface expression. When expressed as a heteromer, GluK2a is able to enhance surface expression of other splice variants (Jaskolski et al., 2004). This raises the possibility that while GluK1b subunits may have a dominant effect on receptor function as shown by antagonist pharmacology (Kerchner et al., 2002), GluK2a may strongly affect trafficking to the neurites and growth cones. These possibilities will need to be tested.
Editing of GluK1 and GluK2 at the Q/R site
We assessed the extent of Q/R editing of GluK1 and GluK2 subunits expressed by acutely dissociated DRG and dorsal horn neurons using differential digestion of RT-PCR products by the restriction enzyme BbvI (Belcher and Howe, 1997). The majority of GluK1 and GluK2 transcripts were unedited in the E17 DRG neurons (75% and 68%, respectively) whereas GluK2 was about 45% unedited in dorsal horn neurons. As was the case for DRG neurons, GluK1 was mostly unedited in dorsal horn neurons (72%). The more extensive editing of GluK2 in dorsal horn neurons paralleled the higher expression of mRNA encoding ADAR1 and ADAR2 in dorsal horn compared to DRG. On the other hand, the overall relatively low level of mRNA editing of the other GluK subunits is consistent with the levels of editing activity previously reported for GluK1 and GluK2 transcripts in embryonic tissues from various brain regions (Paschen et al., 1995; Belcher and Howe, 1997; Bernard et al., 1999; Lee et al., 2001). Furthermore, the relatively minimal editing at the Q/R site in GluK1 and GluK2 transcripts expressed by E17 DRG neurons is consistent with the previously demonstrated expression of Ca2+ permeable KA receptors by embryonic DRG neurons (Lee et al., 2001).
Detection of Ca2+-permeable KA receptors in subcellular compartments of DRG neurons
One of the primary goals of these experiments was to test the hypothesis that KA receptors are expressed at the growth cones of developing DRG neurons and that these KA receptors are Ca2+ permeable. Using Ca2+-imaging, we found evidence for the presence of functional Ca2+-permeable KA receptors at growth cones of acutely dissociated DRG neurons. Both bath application and focal puff application of the KA agonist, domoate, raised intracellular Ca2+ concentration at the soma and growth cones. Attenuation of domoate-evoked Ca2+ transients by antagonist for Ca2+-permeable KA receptors, JSTX, indicated that Ca2+-permeable KA receptors were present and contributed to initiation of the Ca2+ transient. Voltage gated Ca2+ channels and intracellular Ca2+ stores may also contribute to the Ca2+ signal following activation of KA receptors in growth cones, regardless of whether the KA receptors are Ca2+ permeable, Ca2+ impermeable or a mixture of both. Indeed Ca2+ channels and Ca2+ activated Ca2+ release from intracellular stores are likely to be critical sources of Ca2+ elevation in those instances in which the Ca2+ transient was not fully blocked by JSTX. In these cases, domoate would activate the remaining Ca2+ impermeable KA receptors, depolarizing the membrane potential and opening voltage gated Ca2+ channels.
Control mechanisms influencing neurite outgrowth and growth cone dynamics have been frequently attributed to spatiotemporal changes in cytosolic Ca2+ (Gomez and Spitzer, 1999; Henley and Poo, 2004; Gomez and Zheng, 2006). Accordingly, intracellular Ca2+ changes due to glutamate receptor activation have been implicated in regulation of the rate and direction of neurite outgrowth (Mattson et al., 1988; Zheng et al., 1996; Chang and De Camilli, 2001). In most cases, the glutamate receptor-induced changes in intracellular Ca2+ concentration occurs following activation of voltage-gated channels, but a few studies suggest that the Ca2+ changes may be due to Ca2+ flow directly through the glutamate receptors (Metzger et al., 1998; Catsicas et al., 2001). We show here that functional Ca2+-permeable KA receptors are expressed at or near the growth cones of DRG neurons, raising the possibility that they may contribute to Ca2+ signaling that regulates neuronal morphology during development.
Morphological changes
The effects of KA receptor agonists and antagonists were tested on morphological changes in DRG neurites and growth cones. Our results show that activation of Ca2+-permeable KA receptors plays no role in determining growth cone area, number of primary neurites and number of branches off the primary neurite [Table 2]. However, the results show significant impact on neurite outgrowth.
Activation of KA receptors resulted in shorter neurites emanating from DRG neurons, whereas blockade of these receptors with SYM 2081 and CNQX resulted in longer neurites. More importantly, selective blockade of the Ca2+-permeable KA receptors with JSTX and PhTX also resulted in longer neurites, similar to the actions of SYM 2081 and CNQX. There were no changes in dorsal horn neurite length between controls and experimental cultures [Table 2], suggesting that regulation of neurite outgrowth by activation of Ca2+-permeable KA receptors was specific to DRG neurons. These results establish a direct functional role for Ca2+-permeable KA receptors in neurite outgrowth of DRG neurons similar to studies showing involvement of Ca2+-permeable AMPA receptors in neurite outgrowth in rat motor neurons (Metzger et al., 1998) and chick embryonic retinal neurons (Catsicas et al., 2001).
Our analysis was restricted to the length of the longest neurite, which may not be an ideal approach to measuring total neurite outgrowth. This is because the number of primary neurites is an important factor influencing measurement of total neurite outgrowth. In other words, neuronal outgrowth measured in neurons with few processes that are very long and in neurons with many processes that are short, can both result in the same amount of total neurite outgrowth. However, we observed a similar number of primary neurites across treatment groups compared to controls. Branching per neuron can also affect total neurite outgrowth. Neurons with longer neurites and fewer branches can have the same total neuron outgrowth as neurons with shorter neurites, but more branches. However, there were no measurable difference in the average number of branch points for experimental and control cultures.
Thus, the results of our study show that activation of Ca2+-permeable KA receptors may have a profound impact in modulating morphological development of DRG neurons through regulation of outgrowth of neurites in culture, possibly providing a stop signal for DRG neurons during synaptic contact. This scenario is consistent with antagonists of KA receptors blocking the effects of KA on neurite outgrowth. Indeed, such a stabilizing effect of KA receptor activation has been shown for hippocampal neurons (Tashiro et al., 2003; Ibarretxe et al., 2007).
Acknowledgments
This work was supported by NIH grants NS49964 to Donald J. Joseph and NS053575 to Amy B. MacDermott. We thank Asya Haney, Jess Kuhn, Katerina Mancevska, and Papiya Choudhury for invaluable technical support. We are grateful to Christophe Mulle for constructs and nucleotide sequences of the GluK1 and GluK2 isoforms used in this study.
References
- Albuquerque C, Joseph DJ, Choudhury P, MacDermott AB. Dissection, plating, and maintenance of cortical astrocyte cultures. CSH Protoc. 2009;2009 doi: 10.1101/pdb.prot5273. pdb prot5273. [DOI] [PubMed] [Google Scholar]
- Albuquerque C, Joseph DJ, Choudhury P, MacDermott AB. Dissection, plating, and maintenance of dorsal horn neuron cultures. CSH Protoc. 2009;2009 doi: 10.1101/pdb.prot5274. pdb prot5274. [DOI] [PubMed] [Google Scholar]
- Albuquerque C, Joseph DJ, Choudhury P, MacDermott AB. Dissection, plating, and maintenance of dorsal root ganglion neurons for monoculture and for coculture with dorsal horn neurons. CSH Protoc. 2009;2009 doi: 10.1101/pdb.prot5275. pdb prot5275. [DOI] [PubMed] [Google Scholar]
- Albuquerque C, Joseph DJ, Choudhury P, MacDermott AB. Preparation of coverslips for neuronal cultures. CSH Protoc. 2009;2009 doi: 10.1101/pdb.prot5272. pdb prot5272. [DOI] [PubMed] [Google Scholar]
- Belcher S, Howe J. Characterization of RNA editing of the glutamate-receptor subunits GluR5 and GluR6 in granule cells during cerebellar development. Brain Res Mol Brain Res. 1997;52:130–138. doi: 10.1016/s0169-328x(97)00252-0. [DOI] [PubMed] [Google Scholar]
- Bernard A, Ferhat L, Dessi F, Charton G, Represa A, Ben-Ari Y, Khrestchatisky M. Q/R editing of the rat GluR5 and GluR6 kainate receptors in vivo and in vitro: evidence for independent developmental, pathological and cellular regulation. Eur J Neurosci. 1999;11:604–616. doi: 10.1046/j.1460-9568.1999.00479.x. [DOI] [PubMed] [Google Scholar]
- Catsicas M, Allcorn S, Mobbs P. Early activation of Ca(2+)-permeable AMPA receptors reduces neurite outgrowth in embryonic chick retinal neurons. J Neurobiol. 2001;49:200–211. doi: 10.1002/neu.1075. [DOI] [PubMed] [Google Scholar]
- Chang S, De Camilli P. Glutamate regulates actin-based motility in axonal filopodia. Nat Neurosci. 2001;4:787–793. doi: 10.1038/90489. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Egebjerg J, Heinemann SF. Ca2+ permeability of unedited and edited versions of the kainate selective glutamate receptor GluR6. Proc Natl Acad Sci U S A. 1993;90:755–759. doi: 10.1073/pnas.90.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M, Jennings E. The postnatal development of spinal sensory processing. Proc Natl Acad Sci U S A. 1999;96:7719–7722. doi: 10.1073/pnas.96.14.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TM, Spitzer NC. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature. 1999;397:350–355. doi: 10.1038/16927. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Zheng JQ. The molecular basis for calcium-dependent axon pathfinding. Nat Rev Neurosci. 2006;7:115–125. doi: 10.1038/nrn1844. [DOI] [PubMed] [Google Scholar]
- Henley J, Poo MM. Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol. 2004;14:320–330. doi: 10.1016/j.tcb.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettner JE. Glutamate receptor channels in rat DRG neurons: activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron. 1990;5:255–266. doi: 10.1016/0896-6273(90)90163-a. [DOI] [PubMed] [Google Scholar]
- Ibarretxe G, Perrais D, Jaskolski F, Vimeney A, Mulle C. Fast regulation of axonal growth cone motility by electrical activity. J Neurosci. 2007;27:7684–7695. doi: 10.1523/JNEUROSCI.1070-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskolski F, Coussen F, Nagarajan N, Normand E, Rosenmund C, Mulle C. Subunit composition and alternative splicing regulate membrane delivery of kainate receptors. J Neurosci. 2004;24:2506–2515. doi: 10.1523/JNEUROSCI.5116-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph DJ, Choudhury P, Macdermott AB. An in vitro assay system for studying synapse formation between nociceptive dorsal root ganglion and dorsal horn neurons. J Neurosci Methods. 2010;189:197–204. doi: 10.1016/j.jneumeth.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Wilding TJ, Huettner JE, Zhuo M. Kainate receptor subunits underlying presynaptic regulation of transmitter release in the dorsal horn. J Neurosci. 2002;22:8010–8017. doi: 10.1523/JNEUROSCI.22-18-08010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler M, Burnashev N, Sakmann B, Seeburg PH. Determinants of Ca2+ permeability in both TM1 and TM2 of high affinity kainate receptor channels: diversity by RNA editing. Neuron. 1993;10:491–500. doi: 10.1016/0896-6273(93)90336-p. [DOI] [PubMed] [Google Scholar]
- Lee CJ, Kong H, Manzini MC, Albuquerque C, Chao MV, MacDermott AB. Kainate receptors expressed by a subpopulation of developing nociceptors rapidly switch from high to low Ca2+ permeability. J Neurosci. 2001;21:4572–4581. doi: 10.1523/JNEUROSCI.21-13-04572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Paternain AV, Rodriguez-Moreno A, Lopez-Garcia JC. Molecular physiology of kainate receptors. Physiol Rev. 2001;81:971–998. doi: 10.1152/physrev.2001.81.3.971. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Dou P, Kater SB. Outgrowth-regulating actions of glutamate in isolated hippocampal pyramidal neurons. J Neurosci. 1988;8:2087–2100. doi: 10.1523/JNEUROSCI.08-06-02087.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger F, Wiese S, Sendtner M. Effect of glutamate on dendritic growth in embryonic rat motoneurons. J Neurosci. 1998;18:1735–1742. doi: 10.1523/JNEUROSCI.18-05-01735.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama M, von Schimmelmann MJ, Togashi K, Findley WM, Hong K. Membrane potential shifts caused by diffusible guidance signals direct growth-cone turning. Nat Neurosci. 2008;11:762–771. doi: 10.1038/nn.2130. [DOI] [PubMed] [Google Scholar]
- Olsen DP, Dunlap K, Jacob MH. Kainate receptors and RNA editing in cholinergic neurons. J Neurochem. 2007;101:327–341. doi: 10.1111/j.1471-4159.2006.04359.x. [DOI] [PubMed] [Google Scholar]
- Partin KM, Patneau DK, Winters CA, Mayer ML, Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron. 1993;11:1069–1082. doi: 10.1016/0896-6273(93)90220-l. [DOI] [PubMed] [Google Scholar]
- Paschen W, Dux E, Djuricic B. Developmental changes in the extent of RNA editing of glutamate receptor subunit GluR5 in rat brain. Neurosci Lett. 1994;174:109–112. doi: 10.1016/0304-3940(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Paschen W, Schmitt J, Dux E, Djuricic B. Temporal analysis of the upregulation of GluR5 mRNA editing with age: regional evaluation. Brain Res Dev Brain Res. 1995;86:359–363. doi: 10.1016/0165-3806(95)00042-c. [DOI] [PubMed] [Google Scholar]
- Pinheiro P, Mulle C. Kainate receptors. Cell Tissue Res. 2006;326:457–482. doi: 10.1007/s00441-006-0265-6. Epub 2006 Jul 2018. [DOI] [PubMed] [Google Scholar]
- Sato K, Kiyama H, Park HT, Tohyama M. AMPA, KA and NMDA receptors are expressed in the rat DRG neurones. Neuroreport. 1993;4:1263–1265. doi: 10.1097/00001756-199309000-00013. [DOI] [PubMed] [Google Scholar]
- Schmauss C, Howe JR. RNA editing of neurotransmitter receptors in the mammalian brain. Sci STKE. 2002;2002:pe26. doi: 10.1126/stke.2002.133.pe26. [DOI] [PubMed] [Google Scholar]
- Schmitz Y, Luccarelli J, Kim M, Wang M, Sulzer D. Glutamate controls growth rate and branching of dopaminergic axons. J Neurosci. 2009;29:11973–11981. doi: 10.1523/JNEUROSCI.2927-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg PH, Hartner J. Regulation of ion channel/neurotransmitter receptor function by RNA editing. Curr Opin Neurobiol. 2003;13:279–283. doi: 10.1016/s0959-4388(03)00062-x. [DOI] [PubMed] [Google Scholar]
- Sugiyama K, Muteki T. Local anesthetics depress the calcium current of rat sensory neurons in culture. Anesthesiology. 1994;80:1369–1378. doi: 10.1097/00000542-199406000-00025. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Dunaevsky A, Blazeski R, Mason CA, Yuste R. Bidirectional regulation of hippocampal mossy fiber filopodial motility by kainate receptors: a two-step model of synaptogenesis. Neuron. 2003;38:773–784. doi: 10.1016/s0896-6273(03)00299-x. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Tolle TR, Berthele A, Zieglgansberger W, Seeburg PH, Wisden W. The differential expression of 16 NMDA and non-NMDA receptor subunits in the rat spinal cord and in periaqueductal gray. J Neurosci. 1993;13:5009–5028. doi: 10.1523/JNEUROSCI.13-12-05009.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding TJ, Huettner JE. Activation and desensitization of hippocampal kainate receptors. J Neurosci. 1997;17:2713–2721. doi: 10.1523/JNEUROSCI.17-08-02713.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JQ, Wan JJ, Poo MM. Essential role of filopodia in chemotropic turning of nerve growth cone induced by a glutamate gradient. J Neurosci. 1996;16:1140–1149. doi: 10.1523/JNEUROSCI.16-03-01140.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


