To the Editor:
Allergy and viral infection are the 2 main risk factors for the inception of persistent wheezing and asthma in early childhood and for acute exacerbations of established asthma.1 The underlying mechanisms of how these 2 distinct sources of inflammation contribute to asthma inception and exacerbation are incompletely understood.
Macrophages are the most numerous leukocyte (>90%) in the lower airways. Although rhinovirus (RV) does not replicate in macrophages, it directly interacts with macrophages in airway tissues.2 Once activated by the RV, macrophages can secrete various inflammatory chemokines, depending on the differentiation state and phenotype of the cells. For example, macrophages can secrete CXCL10 and CXCL11, which recruit adaptive immune cells that direct viral clearance,3 and/or CCL2 and CCL8, which can recruit myeloid cells such as monocytes and eosinophils.4 In mouse models of RV infection, macrophage responses contribute to airway inflammation and hyperresponsiveness. Furthermore, RV activation of monocytes and macrophages can potentiate antiviral responses of airway epithelial cells.5 These findings provide evidence that macrophages are important immunoregulatory cells during RV infections.
A recent study demonstrated reduced antiviral responses to RV stimulation in allergic asthmatic children, corresponding with increased susceptibility to RV-induced exacerbations.6 Therefore, we hypothesized that allergen exposure modifies RV-induced chemokine responses of airway macrophages to impair the antiviral response and promote inflammation. Together, these effects could increase the severity of viral respiratory infections and lead to lower respiratory tract symptoms in patients with asthma.
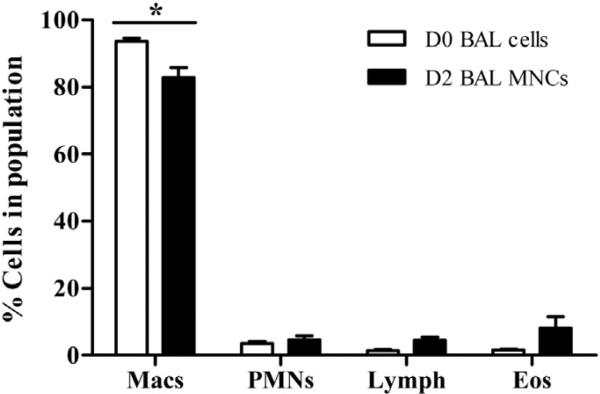
To test this hypothesis, in accordance with institutional review board–approved protocols from the Human Subjects Committee at the University of Wisconsin-Madison, bronchoalveolar lavage (BAL) was performed to obtain airway mononuclear cells (MNCs) from 10 donors with a history of mild atopic asthma (see Table E1 in this article's Online Repository at www.jacionline.org). BAL was performed before (D0) and 48 hours after (D2) segmental bronchoprovocation with allergen as previously described.7 Contaminating granulocytes were removed from D2 cells by Percoll density gradient centrifugation (see this article's Methods section in the Online Repository at www.jacionline.org), and cell populations were comparable between the 2 isolations (see Fig E1 in this article's Online Repository at www.jacionline.org). Macrophages were isolated from D0 cell and D2 MNC populations by adherence to plastic (2 hours).
TABLE E1.
Subject characteristics
| Subject | Sex | Age (y) | Methacholine PC20 (mg/mL)* | Baseline FEV1 (% pred)† | Allergen (Ag) | Ag PD20 (CBU)‡ | Medications§ |
|---|---|---|---|---|---|---|---|
| 1 | Male | 41 | 4.30 | 93 | Cat∥ | 5.59 | Albuterol, benadryl, sudafed, chlortrimetron, ibuprofen, naphcon |
| 2 | Female | 24 | 0.12 | 112 | HDM | 4.29 | Albuterol, singulair, ibuprofen, claritin, apri, omeprazole |
| 3 | Male | 21 | 2.66 | 75 | HDM | 8.60 | Albuterol, loratadine |
| 4 | Female | 25 | NR¶ | 88 | HDM | 16.19 | Albuterol, claritin, orthotricyclen, multivitamin |
| 5 | Male | 23 | 6.06 | 96 | Cat∥ | 10.95 | Albuterol, ibuprofen |
| 6 | Female | 21 | 1.99 | 93 | Cat∥ | 3.76 | Albuterol, ibuprofen, monessa, claritin, eczema cream |
| 7 | Male | 20 | NR¶ | 75 | HDM | 1.90 | Albuterol, sertaline, ambien, advil |
| 8 | Female | 21 | 0.35 | 89 | HDM | 8.74 | Albuterol, nasonex, depopovera, calcium, ibuprofen |
| 9 | Male | 32 | 0.23 | 92 | HDM | 34.46 | Albuterol, ibuprofen |
| 10 | Female | 24 | 1.20 | 75 | HDM | 14.74 | Albuterol, lutera, ibuprofen, multivitamin |
| 5 males; 5 females | 23.5 (21, 26.8)# | 1.6 (0.3, 3.9) | 90.5 (75, 93.8) | 3 Cat∥; 7 HDM | 8.7 (4.2, 15.1) |
CBU, Cumulative breath unit; HDM, house dust mite.
Methacholine concentration that resulted in a 20% reduction in FEV1.
Percentage of predicted value.
Provocative dose of allergen that resulted in a 20% decrease in lung function, measured in CBUs.
Subjects requested to hold medications (nonsteroidal anti-inflammatory drugs, corticosteroids, and antihistamines) for a week before BAL.
Cat dander.
Information not reported.
Median (25 and 75 percentiles).
FIG E1.
Cell differentials in BAL cell population on D0 and D2. Eos, Eosinophils; Lymph, lymphocytes; Macs, macrophages; PMNs, polymorphonuclear leukocytes. The values are means ± SEM from 9 individual subjects. *P < .05 by 2-way repeated-measures ANOVA (Bonferroni posttests).
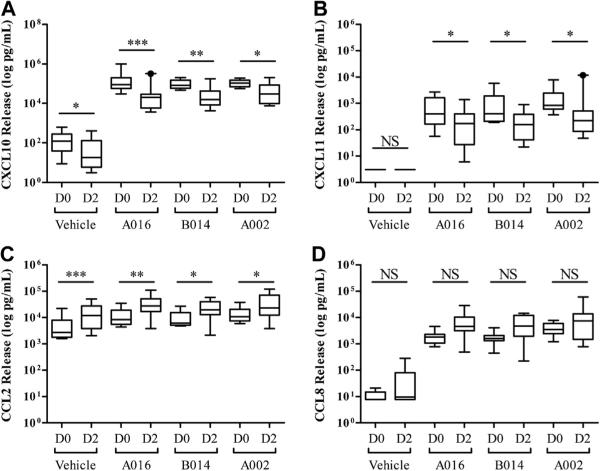
To determine the effect of allergen challenge on RV-induced macrophage responses, D0 and D2 BAL macrophages were incubated (24 hours) with RV A016, B014, and A002 (see this article's Methods section). The supernatants were then analyzed for CXCL10, CXCL11, CCL2, and CCL8 using ELISA (Fig 1). In the absence of virus, D0 macrophages secreted low levels of CXCL10 and CCL2 (geometric mean [GM], 95 and 3739 pg/mL, respectively), but not CXCL11 and CCL8, and incubation with RVA016, B014, and A002 significantly induced the secretion of all the 4 chemokines tested (P < .001).
FIG 1.
Allergen challenge alters RV-induced chemokine secretion in primary human BAL macrophages: CXCL10 (A), CXCL11 (B), CCL2 (C), and CCL8 (D). NS, Not significant; *P < .05, **P < .01, and ***P < .001 by 2-way repeated-measures ANOVA (Bonferroni posttests). The data are log transformed for normalcy and summarized as boxplots (whiskers, 10-90 percentile) from 9 individual subjects.
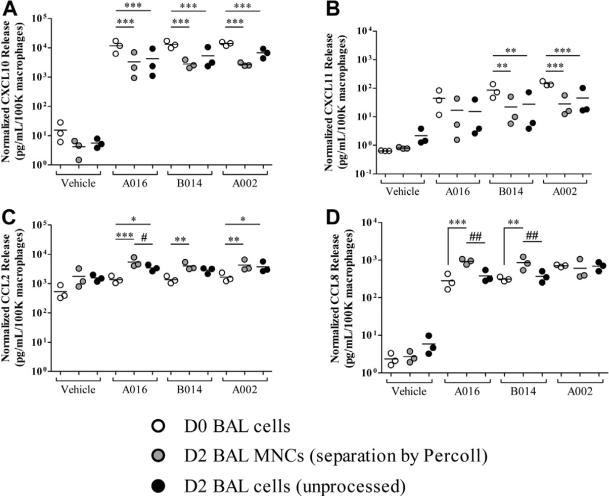
After allergen challenge, RV stimulation still significantly induced the secretion of CXCL10, CXCL11, CCL2, and CCL8 (P <.001 compared with D2 vehicle-treated cells), but there were several differences noted. Compared with D0 macrophages, D2 cells incubated in vehicle alone secreted less CXCL10 (GM, 26 vs 95 pg/mL; P < .05) and more CCL2 (GM, 11,392 vs 3,739 pg/mL; P < .001; Fig 1, A and C). After incubation with RV A016, D2 versus D0 macrophages secreted significantly less CXCL10 (GM, 17,271 vs 114,973 pg/mL; P < .001; Fig 1, A) and CXCL11 (GM; 117 vs 453 pg/mL; P < .05; Fig 1, B) and more CCL2 (GM, 27,264 vs 10,245 pg/mL; P < .01; Fig 1, C). In addition, these effects were similar for RV A016, B014, and A002 (P < .05; Fig 1). Allergen challenge had no significant effect on RV-induced CCL8 secretion (Fig 1, D). Preliminary experiments demonstrated that Percoll treatment did not alter the overall effect of allergen challenge on RV-induced macrophage chemokine response (see Fig E2 in this article's Online Repository at www.jacionline.org). These findings suggest that allergen exposure modifies RV-induced induction of specific chemokine responses from airway macrophages.
FIG E2.
Comparison of macrophage responsiveness to RV before and after Percoll separation: CXCL10 (A), CXCL11 (B), CCL2 (C), and CCL8 (D). White, D0 BAL cells; gray, D2 BAL MNCs (after separation by Percoll); black, D2 BAL cells (without Percoll processing). The data are normalized to 1 × 105 macrophages plated per treatment condition and displayed as 3 individual subjects and the mean. *P < .05, **P < .01, and ***P < .001 compared with D0 BAL cells and #P < .05 and ##P < .01 D2 BAL cells compared with D2 BAL MNCs; all comparisons tested by 2-way repeated-measures ANOVA with Bonferroni posttests.
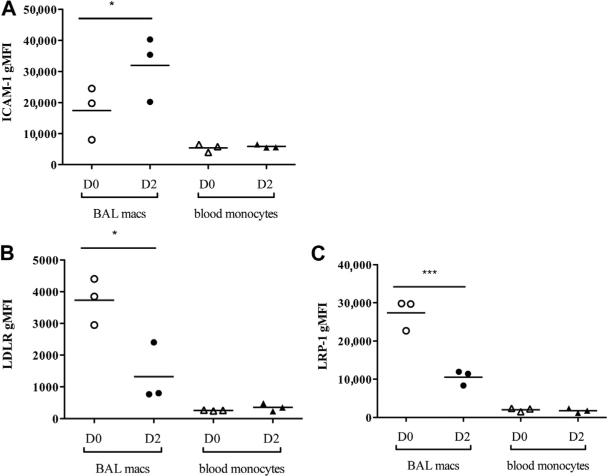
One mechanism by which allergens could alter antiviral responses is through altered expression of cellular receptors for RVs. Allergen challenge can induce expression of intercellular adhesion molecule-1 (ICAM-1), the major group RV receptor, but effects on minor group receptors (low-density lipoprotein receptor [LDLR] and LDLR-related protein-1 [LRP-1]) are unknown. Because allergen challenge had similar effects on RV-induced chemokine secretion induced by both major (A016 and B014) and minor (A002) group RVs, we hypothesized that differences in chemokine secretion were independent of effects on RV target receptors. To test whether allergen challenge affects RV receptor expression on airway macrophages, D0 BAL macrophages and D2 MNCs were stained for ICAM-1, LDLR, and LRP-1, and surface expression was measured using flow cytometry (Fig 2). As expected, allergen challenge significantly increased ICAM-1 expression on D2 than on D0 macrophages (Fig 2, A). In contrast, allergen challenge decreased both LDLR and LRP-1 surface expression on D2 BAL macrophages (Fig 2, B and C, respectively). Allergen challenge did not affect ICAM-1, LDLR, and LRP-1 surface expression on peripheral blood monocytes from the same patients, suggesting that D2 allergen-induced changes in receptor expression are localized to the airways. These findings indicate that allergen-induced effects on the RV-induced chemokines are independent of RV receptor utilization and raise the possibility that allergen challenge affects RV-induced intracellular signaling.
FIG 2.
Allergen challenge differentially affects major and minor RV target receptor expression. Live (propidium iodide negative), CD14 positive BAL macrophages (macs), and blood monocytes. Open circles, D0 BAL macs; solid circles, D2 BAL macs; open triangles, D0 blood monocytes; solid triangles, D2 blood monocytes. ICAM-1 (A), LDLR (B), and LRP-1 (C) surface expression measured using flow cytometry and represented as geometric mean fluorescent intensity (gMFI) from 3 individual subjects. *P < .05 and ***P < .001 by 1-way ANOVA (Bonferroni posttests).
In summary, allergen exposure modifies the quality and quantity of RV-induced chemokine secretion independent of allergen effects on RV target receptors. These findings complement and extend earlier observations, which showed that allergen challenge during RV infection leads to enhanced airway eosinophil recruitment and CXCL8.8,9 Our data demonstrate that in vivo allergen challenge decreases ex vivo macrophage responses to RV by inhibiting CXCL10 and CXCL11 and enhancing CCL2 secretion. By decreasing RV-induced CXCL10 and CXCL11 secretion, allergen challenge may dampen the antiviral response in the airways by decreasing the influx of active T cells, which direct the adaptive immune response to viral infections and viral clearance. On the other hand, by increasing CCL2 and maintaining CCL8 secretion, allergen exposure may further promote the recruitment of proinflammatory cells, such as eosinophils, neutrophils, and macrophages, to the airways. If similar processes occur in vivo, these allergen-induced changes may lead to greater airway inflammation and a less effective antiviral response, and thereby promote more severe respiratory illness and exacerbations of asthma.
METHODS
Segmental bronchoprovocation with antigen and BAL
BAL and segmental bronchoprovocation were performed as previously described.E1 In addition, the MNC population was enhanced from the BAL fluid on the second day (D2) by Percoll density centrifugation (1.085 g/mL Percoll; 700g, 20 minutes, 25°C).
RV production and purification
RV A016, B014, and A002 were grown in HeLa cells and purified by centrifugation through a 30% sucrose cushion as previously described.E2 Titers of virus were determined by measuring the infectivity of the virions in HeLa cell monolayers (plaque-forming units). All experiments were done using the same viral stock preparation that was aliquoted and stored at –80°C.
Chemokine detection
BAL macrophages were plated (5 × 105 cells/well, 12-well CoStar plates) in RPMI 1640 supplemented with 10% FBS, 100 U/mL penicillin/streptomycin, 2 mM sodium pyruvate, 2 mM l-glutamine, and 0.25 μg/mL amphotericin B (Mediatech, Herndon, Va). To enhance purity, macrophages were allowed to adhere (2 hours, 37°C) and then washed twice with HBSS. Following overnight incubation, macrophages were treated (24 hours, 34.5°C) with vehicle (0.00025% human serum albumin/HBSS) or RV (multiplicity of infection = 10). After incubation, supernatants were collected, centrifuged (15800g, 1 minute, 4°C), and analyzed for the presence of CXCL10, CXCL11, CCL2, and CCL8 via ELISA, as previously described.E3 Capture and biotinylated antibodies and recombinant protein for CXCL10/IP-10 and CCL8/MCP-2 were purchased from R&D Systems (Minneapolis, Minn), CXCL11/I-TAC from PeproTech, Inc (Rocky Hill, NJ), and CCL2/MCP-1 from BD Biosciences (San Jose, Calif). The assay sensitivity level was 3.1 pg/mL for CXCL10, CXCL11, and CCL2 and 7.8 pg/mL for CCL8. All determinations were performed in technical duplicates.
Flow cytometry
D0 BAL cells and D2 BAL MNCs were suspended in 1% FBS/RPMI and immunostained (30 minutes, 4°C) with V450-conjugated anti-CD14 (Clone MΦPg, BD Biosciences), phycoerythrin-conjugated anti-CD54 (ICAM-1, Clone HA58, BD Biosciences), antigen-presenting cell–conjugated anti-LDLR (Clone 472413, R&D Systems), or fluorescein isothiocyanate–conjugated anti-CD91 (LRP-1, Clone A2MR-a2, BD Biosciences). Cells were washed with 1% FBS/RPMI and suspended in PBS. Propidium iodide (3 μg/mL) staining was used to exclude dead cells, and 10,000 events were assessed on an LSRII flow cytometer (Becton–Dickinson, Bedford, Mass). After gating for CD14+ cells, geometric mean fluorescence intensity was determined for each receptor by using FlowJo software (TreeStar, Ashland, Ore).
Statistical analysis
Measurements of chemokine secretion were log transformed to approximate normal distribution. Two-way and 1-way repeated-measures ANOVA (Bonferroni posttests) were used to evaluate treatment effects. Analysis was performed with Prism 5, version 5.01 (GraphPad Software, Inc, La Jolla, Calif), and a 2-sided P value of .05 was considered statistically significant.
Acknowledgments
We thank Yury Bochkov and Wai-Ming Lee for preparation of virus stocks and Sameer Mathur, Elizabeth Schwantes, and Lei Shi for support and technical assistance.
This study was supported by National Institutes of Health grants U19 AI070503, P01 HL088594, T32 GM008688, T32 DK007665, and M01 RR003186.
L. C. Denlinger has received research support from the National Institutes of Health, has received consultancy fees from Novartis, and has a patent with WARF. J. E. Gern has received consultancy fees from GlaxoSmithKline, Biota, Centocor, Boehringer Ingelheim, MedImmune, Theraclone, Merck, and Gilead and has received research support from Merck, AstraZeneca, and GlaxoSmithKline.
Footnotes
Disclosure of potential conflict of interest: The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Gavala ML, Bashir H, Gern JE. Virus/allergen interactions in asthma. Curr Allergy Asthma Rep. 2013;13:298–307. doi: 10.1007/s11882-013-0344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bentley JK, Sajjan US, Dzaman MB, Jarjour NN, Lee WM, Gern JE, et al. Rhinovirus colocalizes with CD68- and CD11b-positive macrophages following experimental infection in humans. J Allergy Clin Immunol. 2013;132:758–61. e3. doi: 10.1016/j.jaci.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melchjorsen J, Sorensen LN, Paludan SR. Expression and function of chemokines during viral infections: from molecular mechanisms to in vivo function. J Leukoc Biol. 2003;74:331–43. doi: 10.1189/jlb.1102577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smit JJ, Lukacs NW. A closer look at chemokines and their role in asthmatic responses. Eur J Pharmacol. 2006;533:277–88. doi: 10.1016/j.ejphar.2005.12.064. [DOI] [PubMed] [Google Scholar]
- 5.Korpi-Steiner NL, Valkenaar SM, Bates ME, Evans MD, Gern JE, Bertics PJ. Human monocytic cells direct the robust release of CXCL10 by bronchial epithelial cells during rhinovirus infection. Clin Exp Allergy. 2010;40:1203–13. doi: 10.1111/j.1365-2222.2010.03546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durrani SR, Montville DJ, Pratt AS, Sahu S, DeVries MK, Rajamanickam V, et al. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol. 2012;130:489–95. doi: 10.1016/j.jaci.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gavala ML, Kelly EA, Esnault S, Kukreja S, Evans MD, Bertics PJ, et al. Segmental allergen challenge enhances chitinase activity and levels of CCL18 in mild atopic asthma. Clin Exp Allergy. 2013;43:187–97. doi: 10.1111/cea.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calhoun WJ, Dick EC, Schwartz LB, Busse WW. A common cold virus, rhinovirus 16, potentiates airway inflammation after segmental antigen bronchoprovocation in allergic subjects. J Clin Invest. 1994;94:2200–8. doi: 10.1172/JCI117581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greiff L, Andersson M, Svensson C, Linden M, Myint S, Persson CG. Allergen challenge-induced acute exudation of IL-8, ECP and alpha2-macroglobulin in human rhinovirus-induced common colds. Eur Respir J. 1999;13:41–7. doi: 10.1034/j.1399-3003.1999.13a09.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.Gavala ML, Kelly EA, Esnault S, Kukreja S, Evans MD, Bertics PJ, et al. Segmental allergen challenge enhances chitinase activity and levels of CCL18 in mild atopic asthma. Clin Exp Allergy. 2013;43:187–97. doi: 10.1111/cea.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Lee WM, Monroe SS, Rueckert RR. Role of maturation cleavage in infectivity of picornaviruses: activation of an infectosome. J Virol. 1993;67:2110–22. doi: 10.1128/jvi.67.4.2110-2122.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Kelly EA, Rodriguez RR, Busse WW, Jarjour NN. The effect of segmental bronchoprovocation with allergen on airway lymphocyte function. Am J Respir Crit Care Med. 1997;156:1421–8. doi: 10.1164/ajrccm.156.5.9703054. [DOI] [PubMed] [Google Scholar]