Abstract
β-amyloid protein (Aβ)-induced neurotoxicity is the main component of Alzheimer’s disease (AD) neuropathogenesis. Inhalation anesthetics have long been considered to protect against neurotoxicity. However, recent research studies have suggested that the inhalation anesthetic isoflurane may promote neurotoxicity by inducing apoptosis and increasing Aβ levels. We therefore set out to determine whether isoflurane can induce dose- and time-dependent dual effects on Aβ-induced apoptosis: protection versus promotion. H4 human neuroglioma cells, primary neurons from naïve mice, and naïve mice were treated with Aβ and/or isoflurane, and levels of caspase-3 cleavage (activation), apoptosis, Bcl-2, Bax, and cytosolic calcium were determined. Here we show for the first time that the treatment with 2% isoflurane for six hours or 30 minutes potentiated, whereas the treatment with 0.5% isoflurane for six hours or 30 minutes attenuated, the Aβ-induced caspase-3 activation and apoptosis in vitro. Moreover, anesthesia with 1.4% isoflurane for two hours potentiated, whereas the anesthesia with 0.7% isoflurane for 30 minutes attenuated, the Aβ-induced caspase-3 activation in vivo. The high concentration isoflurane potentiated the Aβ-induced reduction in Bcl-2/Bax ratio and caused a robust elevation of cytosolic calcium levels. The low concentration isoflurane attenuated the Aβ-induced reduction in Bcl-2/Bax ratio and caused only a mild elevation of cytosolic calcium levels. These results suggest that isoflurane may have dual effects (protection or promotion) on Aβ-induced toxicity, which potentially act through the Bcl-2 family proteins and cytosolic calcium. These findings would lead to more systematic studies to determine the potential dual effects of anesthetics on AD-associated neurotoxicity.
Keywords: Anesthesia, Alzheimer’s disease, isoflurane, apoptosis, β-Amyloid protein, dual effects, cytosolic calcium
INTRODUCTION
Alzheimer’s disease (AD) is an insidious and progressive neurodegenerative disorder, which accounts for the vast majority of dementia and is one of the greatest public health problems in the United States. AD is characterized by global cognitive decline, and robust accumulation of amyloid deposits and neurofibrillary tangles in the brain [reviewed in 1].
Aβ, the key component of senile plaques in AD patients [2–4], was first isolated from meningovascular amyloid deposits in AD and Down syndrome [2, 5]. Genetic evidence, confirmed by neuropathological and biochemical findings, indicates that excessive production and/or accumulation of Aβ play a fundamental role in the pathology of AD [reviewed by 6, 7]. Moreover, increasing evidence suggests a role for caspase activation and apoptosis in AD neuropathogenesis [reviewed in 8, 9]. Finally, Aβ has been reported to induce caspase activation and apoptosis [10–16].
The commonly used inhalation anesthetic isoflurane has recently been reported to induce caspase activation and apoptosis, affect APP processing, increase Aβ levels, and enhance Aβ aggregation [17–22, reviewed in 23]. However, other reports have suggested that isoflurane may protect against apoptosis [24–33]. The reason for these different findings of isoflurane is not clear.
Several studies have suggested that isoflurane may induce apoptosis by facilitating calcium release from endoplasmic reticulum (ER) [18, 22, 34, reviewed in 35]. Recent research works have illustrated that ER-associated cytosolic calcium level may be involved in protection of apoptosis as well [36]. Other studies have shown that a short duration of isoflurane treatment can increase anti-apoptotic factor Bcl-2 levels in rats [25], whereas a long duration of isoflurane treatment can decrease Bcl-2 levels in cultured cells [18].
Given these observations, the aim of our current study was to assess whether different isoflurane treatments, e.g., high concentration and/or long duration versus low concentration and/or short duration, can have different effects on levels of cytosolic calcium, pro-apoptotic factor Bax, anti-apoptotic factor Bcl-2, caspase-3 activation, and apoptosis in naïve H4 human neuroglioma cells (H4 naïve cells), primary neurons, and brain tissues of mice. The dual effects of isoflurane (promotion versus protection) on the Aβ-induced caspase-3 activation and apoptosis were observed in vitro and in vivo. The other cell lines and other apoptotic insults e.g., ischemia, which may have different responses to isoflurane treatments, were not investigated in the current experiments.
MATERIAL AND METHODS
Cell Lines
We employed H4 human neuroglioma cells (H4 naïve cells) in the experiments. All cell lines were cultured in DMEM (high glucose) containing 9% heat-inactivated fetal calf serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine.
Primary Neurons
The protocol was approved by the Massachusetts General Hospital Standing Committee on Animals (Boston, Massachusetts) on the Use of Animals in Research and Teaching. Naïve mice with a gestation stage of day 15 were euthanized with carbon dioxide. We then performed a cesarean section to pull out the embryos and decapitate them in a 100-mm dish of phosphate-buffered saline. We placed the head on the top of a 100-mm dish and dissected out the cortices, removed the meninges, and placed the cortices into another 100-mm dish of phosphate-buffered saline. The neurons were dissociated by trypsinization and trituration. The dissociated neurons were resuspended in serum-free B27/neurobasal medium and were placed into six well plates with a confluent rate of 50%. Seven to ten days after the harvest, the neurons were exposed to isoflurane. The B27/neurobasal medium (Invitrogen, Carlsbad, CA) has shown excellent long-term viability of primary neurons. The glial cell growth at five days is reduced to less than 0.5% for a nearly pure neuronal population in the medium [37]. The 0.5 mM L-glutamine and 25 μM glutamate added to the B27/neurobasal medium can reduce glial cell growth.
Treatments for cells and primary neurons. The cells and primary neurons were treated with 2% isoflurane for six hours or 30 minutes (high concentration and long or short duration treatment) or 0.5% isoflurane for six hours or 30 minutes (low concentration and long or short duration treatment). Control conditions included 5% CO2 plus 21% O2 for six hours or 30 minutes, respectively, which did not affect caspase-3 activation and apoptosis (Data not shown). Treatment with 7.5 uM Aβ40 plus 7.5 uM Aβ42 for six hours was used to induce caspase-3 activation and apoptosis in the cells or neurons as previously described [20].
Lysis of Cells or Neurons and Protein Amount Quantification
The pellets of the cells or the neurons were detergent-extracted on ice using immunoprecipitation buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, 0.5% Non-idet P-40) plus protease inhibitors (1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin A). The lysates were collected, centrifuged at 12,000 × g for 10 min, and quantified for total proteins using the bicinchoninic acid protein assay kit (Pierce, Iselin, NJ).
ICV Injection of Aβ and Mice Anesthesia
Eight-month-old C57/BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were used in the experiments. All surgical procedures were performed with care to minimize pain and discomfort. The mice were anesthetized with an intraperitoneal (i.p.) injection of 1% avertin (10ml/kg body weight). Avertin has not been reported to interact with isoflurane on caspase activation and apoptosis. A Hamilton syringe was placed perpendicular to the skull at the following coordinate: anteroposterior, 0.5 mm; mediolateral, 1 mm; dorsoventral, 2.0 mm relative to bregma as described before [38] with modification. 250 ng human Aβ42 or DMSO (dissolved in artificial cerebrospinal fluid to make a working solution of 5 ul) was slowly injected at a rate of 0.5 ul/min; the needle was left undisturbed for 2 minutes before removal. The burr hole was covered by bone wax and the scalp was sutured. Then the mice received 1.4% isoflurane in 100% oxygen for 2 hours or 0.7% isoflurane in 100% oxygen for 30 minutes. The control group received 100% oxygen at an identical flow rate for either 2 hours or 30 minutes in an identical chamber. The mice breathed spontaneously, the concentration of isoflurane, O2 and CO2 were measured continuously (Datex, Tewksbury, MA). The temperature of the anesthetizing chamber was controlled to maintain rectal temperature of the animals at 37 ± 0.5°C. The isoflurane anesthesia did not significantly affect blood pressure or blood gas of mice [21]. Anesthesia was terminated by discontinuing isoflurane and placing animals in a chamber containing 100% oxygen until 20 minutes after the return of the righting reflex. They were then returned to individual home cage until sacrifice. Mice were euthanized by decapitation six hours after the anesthesia. The brain was removed rapidly and prefrontal cortex was dissected out and frozen in liquid nitrogen for subsequent processing for the determination of caspase-3 fragment levels.
Western Blots Analysis
The cells, neurons, or brain tissues of the mice were harvested at the end of the experiments and were subjected to Western blots analyses as described by Xie et al. [20]. A caspase-3 antibody (1:1,000 dilution; Cell Signaling Technology, Inc. Beverly, MA) was used to recognize the caspase-3 fragment [(17–20 kDa in vitro) and 12 kDa in vivo as previously demonstrated 39], which results from cleavage at the asparate position 175 and caspase-3 FL (35 – 40 kDa). Bax antibody (1:1,000 dilution; Cell Signaling Technology, Inc. Beverly, MA) was used to recognize Bax (20 kDa). Bcl-2 antibody (1:1,000 dilution; Cell Signaling Technology, Inc.) was used to recognize Bcl-2 (28 kDa). Antibody to non-targeted protein β-Actin (42 kDa, 1:5,000, Sigma, St. Louis, MO) was used to control for loading differences in total protein amounts. Each band in the Western blot represents an independent experiment. We have averaged the results from three to ten independent experiments. The intensity of signals in each Western blot was analyzed using the National Institute of Health image program (National Institute of Health Image 1.62, Bethesda, MD). We quantified the Western blots using two steps. First, we used levels of β-Actin to normalize (e.g., determine ratio of the amount of FL-caspase-3 to the amount of β-Actin) levels of Bax, Bcl-2, and caspase-3 to control for any loading differences in total protein amounts. Second, we presented changes in levels of Bax, Bcl-2, and caspase-3 in treated cells or mice as percentages of those in cells or mice from the control condition.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining-TMR red kit (Roche Diagnostics, Mannheim, Germany) was used for TUNEL staining. Specifically, cells or neurons were grown on coverslips overnight in an incubator. The cells or neurons were fixed in 4% paraformaldehyde for 30 minutes following the treatment with isoflurane plus Aβ or the control conditions. The coverslips were washed with phosphate buffered saline and were incubated with a permeabilization solution (0.2% TritonX - 100 in 0.1% sodium citrate) at 4 °C for 10 minutes, and then with a TUNEL reaction mixture for one hour at 37 °C in a humidified dark chamber. The negative control was prepared by incubating the coverslips in label solution only. The coverslips for the positive controls were incubated with DNase I recombinant. Finally, the coverslips were incubated with 10 μg/ml Hoechst 33342 in a humidified dark chamber for 10 minutes. Samples were then analyzed in a mounting medium under a fluorescence microscope. The TUNEL-positive cells were counted manually in five randomly selected areas under a 20X objective microscope lens by an investigator who was blinded to the experiments. The positive TUNEL cells were expressed as the fold of the number of nuclei. One fold of TUNEL positive cells in the figure refers to the control levels for the purposes of comparison to experimental conditions.
Cytosolic Calcium Measurement
Cytosolic calcium levels were determined as described by Wei et al. [34]. Specifically, naïve H4 cells were loaded with Fura-2 (Molecular probe, Eugene, OR), perfused with Tyrode buffer, and [Ca2+]i transients were recorded as changes in Fura-2 ratio (340/380 nm) using a spectrofluoroscope system (Ionoptix, Milton, MA). The cells were exposed to 0.57 mM isoflurane (2%) or 0.14 mM isoflurane (0.5%) solution for 100 seconds.
Statistics
Given the presence of background caspase-3 activation and apoptosis in cells cultured in serum free media, we did not use absolute values to describe changes in caspase-3 activation and apoptosis. Instead, changes in caspase-3 activation and apoptosis were presented as a percentage of those of the control group. 100% caspase-3 activation or cell viability refers to control levels for purposes of comparison to experimental conditions. Data were expressed as mean ± S.D. The number of samples varied from 3 to 10, and the samples were normally distributed. We used a two-tailed t-test to compare the difference between the experimental groups. P-values less than 0.05 (* or #) and 0.01 (** or ##) were considered statistically significant.
RESULTS
Low Concentration and Short Duration of Isoflurane Treatment Attenuates the Aβ-Induced Apoptosis In Vitro and In Vivo
We previously reported that the common inhalation anesthetic isoflurane can induce caspase activation and apoptosis in vitro [19, 20, 40] and in vivo [21]. However, many other studies have shown that isoflurane may protect against apoptosis [24–33]. One of the reasons for this discrepancy could be that different isoflurane treatments (e.g., different duration and concentration) may have different effects on caspase activation and apoptosis. Thus, we set out to determine the effects of low (0.5%) and high (2%) concentrations of isoflurane with long (six hours) and short (30 minutes) treatment times on the Aβ-induced apoptosis in vitro and in vivo.
Since caspase-3 activation is one of the final steps of cellular apoptosis [41], we first assessed the effects of isoflurane plus Aβ on caspase-3 activation by quantitative Western blots analyses. The H4 naïve cells were treated with 7.5 uM Aβ40 plus 7.5 uM Aβ42 for 6 hours, or the Aβ treatment plus 0.5% isoflurane for the first 30 minutes then the Aβ treatment alone for the rest of the remaining 5.5 hours. The cells were harvested at the end of the experiment and were subjected to Western blot analysis. Caspase-3 immunoblotting revealed that the Aβ treatment induced caspase-3 activation Fig. (1A) as evidenced by increased ratios of cleaved (activated) caspase-3 fragment (17 kDa) to full-length (FL) (35 – 40 kDa) caspase-3. The treatment with 0.5% isoflurane for 30 minutes Fig. (1A) attenuated the Aβ-induced caspase-3 activation. Quantification of the Western blots, based on the ratio of caspase-3 fragment to FL caspase-3, revealed that the Aβ treatment led to caspase-3 activation as compared to control condition: 100% versus 167% (* P = 0.043), and the 0.5% isoflurane treatment attenuated the Aβ-induced caspase-3 activation: 167% versus 79% (# P = 0.028). These findings suggest that a low concentration and a short duration of isoflurane treatment may attenuate the Aβ-induced caspase-3 activation in the H4 naïve cells.
Fig. 1.
Low concentration and short duration of isoflurane treatment attenuates the Aβ-induced caspase-3 activation and apoptosis in H4 naïve cells and in brain tissues of naïve mice. A. Aβ treatment (lanes 3 and 4) induces caspase-3 cleavage (activation) by increasing caspase-3 fragment levels and decreasing FL-caspase-3 levels as compared to the control condition (lanes 1 and 2) in Western blots. 0.5% isoflurane (lanes 7 and 8) attenuates the Aβ-induced caspase-3 activation as compared to Aβ treatment (lanes 3 and 4) alone. B. Quantification of the Western blots shows that Aβ treatment (gray bar) induces caspase-3 activation, assessed by quantifying the ratio of caspase-3 fragment to FL-caspase-3, as compared to that of the control condition (white bar). 0.5% isoflurane (striped bar) attenuates the Aβ-induced caspase-3 activation as compared to Aβ treatment (gray bar). C. The Aβ treatment (column 3) increases the amount of TUNEL positive cells (apoptosis) as compared to the control conditions (column 1) in H4 naïve cells. Treatment with Aβ plus 0.5% isoflurane (column 4) decreases the amount of TUNEL positive cells as compared to the Aβ treatment alone (column 3). D. Quantification of the TUNEL image shows that the Aβ treatment (gray bar) increases the amount of TUNEL positive cells (apoptosis) as compared to control conditions (white bar) in H4 naïve cells. The treatment with Aβ plus 0.5% isoflurane induces fewer TUNEL positive cells (striped bar) as compared to Aβ treatment (gray bar) alone in H4 naïve cells. E. The Western Blots show that 0.7% isoflurane (lanes 4 to 6) attenuates the Aβ-induced caspase-3 activation by decreasing levels of caspase-3 fragment as compared to Aβ treatment alone (lanes 1 to 3) in vivo. F. Quantification of the Western blots shows that 0.7% isoflurane (black bar) attenuates the Aβ-induced caspase-3 activation (white bar).
Given that caspase-3 activation alone may not represent apoptotic cell damage [42], we also assessed the effects of the Aβ treatment and the Aβ treatment plus 0.5% isoflurane on cellular apoptosis using TUNEL studies. We found that the Aβ treatment increased TUNEL positive cells (apoptosis) as compared to the control condition Fig. (1C and ID): 1 fold versus 6.92 fold (** P = 0.001), and 0.5% isoflurane attenuated the Aβ-induced apoptosis: 6.92 fold versus 3.74 fold (## P = 0.003). These findings suggest that a low concentration and a short duration of isoflurane treatment may attenuate the Aβ-induced apoptosis.
Finally, we determined the in vivo relevance of these in vitro findings. 250 ng Aβ42 was administrated to the mouse brain through intraeerebroventricular (ICV) injection to create an in vivo model of Aβ-induced caspase-3 activation as evidenced by the fact that the Aβ treatment increased the levels of activated (cleaved) caspase-3 fragment Fig. (IE). Then, the Aβ-treated mice were exposed to 0.7% isoflurane or a control condition for 30 minutes. The brain tissues were harvested at the end of the experiment and were subjected to Western blot analysis. Caspase-3 immunoblotting showed that the treatment of 0.7% isoflurane for 30 minutes attenuated the Aβ-induced increases in the levels of caspase-3 fragment as compared to control condition Fig. (IE). The quantification of the Western blot showed that the anesthesia with 0.7% isoflurane for 30 minutes decreased the Aβ-induced caspase-3 activation in the brain tissues of naïve mice as compared to that of the control condition: 100% versus 22%, * P = 0.014 Fig. (IF). These in vivo findings have further illustrated that a low concentration and a short duration of isoflurane treatment can attenuate the Aβ-induced neurotoxicity.
High Concentration and Long Duration of Isoflurane Treatment Potentiates the Aβ-Induced Apoptosis In Vitro and In Vivo
Next, we asked whether a high concentration and a long duration of isoflurane treatment may have different effects on the Aβ-induced apoptosis. We thus assessed the effects of a treatment with 2% isoflurane for six hours on the Aβ-induced apoptosis in H4 naïve cells. We found that the treatment with 7.5 uM Aβ40 plus 7.5 uM Aβ42 for six hours induced caspase-3 activation Fig. (2A and 2B) and the treatment with 2% isoflurane for six hours potentiated the Aβ-induced caspase-3 activation: 272% versus 323%, # P = 0.04. We further found that Aβ led to apoptosis as compared to the control condition in the TUNEL studies: one fold versus 6.21 fold, ** P = 0.002. Finally, the treatment with 2% isoflurane for six hours potentiated the Aβ-induced apoptosis: 7.91 fold versus 6.21 fold, # P = 0.01. These results suggest that a high concentration and a long duration of isoflurane treatment may potentiate the Aβ-induced apoptosis.
Fig. 2.
High concentration and long duration of isoflurane treatment potentiates the Aβ-induced caspase-3 activation and apoptosis in H4 naïve cells and brain tissues of naïve mice. A. Aβ treatment (lanes 4 and 6) induces caspase-3 activation as compared to control condition (lanes 1 to 3) in Western blots analysis. 2% isoflurane (lanes 10 to 12) potentiates the Aβ-induced caspase-3 activation as compared to Aβ treatment alone (lanes 4 to 6). B. Quantification of the Western blots shows that Aβ treatment (gray bar) induces caspase-3 activation as compared to control condition (white bar). 2% isoflurane (striped bar) potentiates the Aβ-induced caspase-3 activation as compared to Aβ treatment (gray bar). C. The Aβ treatment (column 3) increases the amount of TUNEL positive cells (apoptosis) as compared to the control conditions (column 1). 2% isoflurane plus the Aβ treatment (column 4) induces a greater increase in the TUNEL positive cells as compared to the Aβ treatment alone (column 3). D. Quantification of the TUNEL image shows that the Aβ treatment (gray bar) increases TUNEL positive cells (apoptosis) as compared to the control conditions (white bar). The treatment with Aβ plus 2% isoflurane induces a greater amount of TUNEL positive cells (striped bar) as compared to Aβ treatment (gray bar) alone. E. 1.4% isoflurane (lanes 4 to 6) potentiates the Aβ-induced caspase-3 activation by increasing the levels of caspase-3 fragment as compared to Aβ treatment alone (lanes 1 to 3) in the Western blots. F. Quantification of the Western blots shows that 1.4% isoflurane (black bar) potentiates the Aβ-induced caspase-3 activation (white bar).
The in vivo relevance studies showed that the anesthesia with 1.4% isoflurane for 2 hours potentiated the Aβ-induced increases in the levels of caspase-3 fragment in the brain tissues of mice as compared to that of the control condition Fig. (2E and 2F): 100% versus 168%, * P = 0.046. Taken together, these in vitro and in vivo results have revealed that the common inhalation anesthetic isoflurane may induce dose- and time-dependent dual effect, protection versus promotion, on the Aβ-induced neurotoxicity.
It should be emphasized that we only observed the dual effects of isoflurane in the naïve H4 cells and primary neurons in the current experiments. It is possible that isoflurane may show different effects in other cell lines. Moreover, we only demonstrated that isoflurane promoted or protected the Aβ-induced caspase activation and apoptosis with a dose- and time-dependent manner in the current experiments. It is also possible that isoflurane may not show such dual effects towards to other apoptotic insults, e.g., ischemia.
The effects of low concentration plus long duration or high concentration plus short duration isoflurane treatment on Aβ-induced caspase-3 activation.
Next, we asked whether the isoflurane treatment with either low concentration (0.5%) plus long duration (six hours) or high concentration (2%) plus short duration (30 minutes) can affect the Aβ-induced caspase-3 activation. As expected, the treatment with 7.5 uM Aβ40 plus 7.5 uM Aβ42 for six hours induced caspase-3 activation in the H4 naïve cells Fig. (3A and 3B): 225% versus 100%, P = 0.005. Whereas the treatment with 0.5% isoflurane for six hours alone did not induce caspase-3 activation (P = 0.284), this isoflurane treatment attenuated the Aβ-induced caspase-3 activation: 227% versus 80%, ## P = 0.002 Fig. (3A and 3B). Moreover, we were able to show that the treatment with 2% isoflurane for 30 minutes can promote the Aβ-induced caspase-3 activation: 174% versus 266%, ## P = 0.002 Fig. (3C and 3D), whereas the treatment with 2% isoflurane alone for 30 minutes did not induce the caspase-3 activation (P = 0.536). These results suggest that the dual effects of isoflurane (promotion versus protection) on the Aβ-induced caspase-3 activation may be dependent on concentration, but not duration.
Fig. 3.
The effects of isoflurane treatment with low concentration plus long duration or high concentration plus short duration on the Aβ-induced caspase-3 activation in H4 naïve cells. A. Aβ treatment (lanes 4 to 6) induces caspase-3 cleavage (activation) by increasing caspase-3 fragment levels and decreasing FL-caspase-3 levels as compared to the control condition (lanes 1 to 3) in Western blots. The treatment with 0.5% isoflurane for six hours (lanes 7 to 9) attenuates the Aβ-induced caspase-3 activation as compared to Aβ treatment (lanes 4 to 6) alone. B. Quantification of the Western blots shows that Aβ treatment (gray bar) induces caspase-3 activation as compared to control condition (white bar). The treatment with 0.5% isoflurane for six hours (striped bar) attenuates the Aβ-induced caspase-3 activation as compared to that of Aβ treatment alone (gray bar). C. Aβ treatment (lanes 4 to 6) induces caspase-3 activation as compared to the control condition (lanes 1 to 3) in Western blots. Treatment with 2% isoflurane for 30 minutes (lanes 7 to 9) promotes the Aβ-induced caspase-3 activation as compared to the Aβ treatment alone (lanes 4 to 6). D. Quantification of the Western blots shows that Aβ treatment (gray bar) induces caspase-3 activation as compared to the control condition (white bar). Treatment with 2% isoflurane for 30 minutes (striped bar) promotes the Aβ-induced caspase-3 activation as compared to the Aβ treatment alone (gray bar).
Low Concentration and Short Duration of Isoflurane Treatment Attenuates the Aβ-induced Decrease in the Bcl-2/Bax Ratio in Primary Neurons from Naïve Mice
Given that isoflurane may induce dual effects to either promote or protect the Aβ-induced neurotoxicity, we next investigated the potential underlying molecular mechanisms. Bcl-2 protein family members, including Bcl-2 and Bax, can regulate apoptosis by modulating outer mitochondrial membrane permeability [43, 44]. The pro-apoptotic protein Bax can promote a sustained and complete calcium release from the ER, and therefore cause an extreme/sustained elevation of cytosolic calcium levels, leading to apoptosis. In contrast, the anti-apoptotic protein Bcl-2 can induce an unregulated ER calcium leak, and therefore cause a mild/temporary elevation of cytosolic calcium levels, leading to the attenuation of apoptosis [45, 46]. Therefore, we set out to determine the effects of Aβ or Aβ plus isoflurane treatments on the levels of Bax, Bcl-2, and the ratio of Bcl-2 to Bax in the primary neurons from naïve mice.
We were able to show that the treatment with 7.5 uM Aβ40 plus 7.5 uM Aβ42 for six hours decreased Bcl-2 levels Fig. (4A) and increased Bax levels Fig. (4B) in the primary neurons, which can be attenuated by treatment with 0.5% isoflurane for 30 minutes Fig. (4A and 4B). The quantification of the Western blot illustrated that the Aβ treatment decreased the Bcl-2/Bax ratio: 100% versus 40% (** P = 0.0006), and the treatment with 0.5% isoflurane for 30 minutes protected the Aβ-induced reduction in the Bcl-2/Bax ratio: 40% versus 68% (## P = 0.004) Fig. (4C). These results suggest that Aβ may induce apoptosis by reducing the Bcl-2/Bax ratio, and a low concentration and a short duration of isoflurane treatment may attenuate the Aβ-induced apoptosis by mitigating the Aβ-induced reduction in the Bcl-2/Bax ratio.
Fig. 4.

Low concentration and short duration of isoflurane treatment attenuates the Aβ-induced decrease in the Bcl-2/Bax ratio in primary neurons from naïve mice. A. Aβ treatment (lanes 3 and 4) decreases Bcl-2 levels as compared to the control condition (lanes 1 and 2) in Western blots. Treatment of Aβ plus 0.5% isoflurane (lanes 5 and 6) increases the Bcl-2 levels as compared to the Aβ treatment alone (lanes 3 and 4). B. Aβ treatment (lanes 3 and 4) increases Bax levels as compared to the control condition (lanes 1 and 2) in Western blots. Treatment of Aβ plus 0.5% isoflurane (lanes 5 and 6) also increases the Bax levels as compared to the control condition (lanes 1 and 2). C. Quantification of the Western blots shows that Aβ treatment (black bar) decreases Bcl-2/Bax ratio as compared to control condition (white bar). The treatment with Aβ plus 0.5% isoflurane (gray bar) increases Bcl-2/Bax ratio as compared to the Aβ treatment alone (black bar).
High concentration and long duration of isoflurane treatment potentiates the Aβ-induced decrease in the Bcl-2/Bax ratio in primary neurons from naïve mice.
Given that a high concentration and a long duration of isoflurane treatment can potentiate the Aβ-induced apoptosis, we next assessed whether such isoflurane treatment can also potentiate the Aβ-induced decrease in the Bcl-2/Bax ratio. Immunoblotting of Bcl-2 and Bax showed that the treatment with 7.5 uM Aβ40 plus 7.5 uM Aβ42 decreased levels of Bcl-2 Fig. (5A) and increased Bax levels Fig. (5B) in the primary neurons from naïve mice. The treatment with 2% isoflurane for six hours potentiated the Aβ-induced changes on the levels of Bcl-2 and Bax. The quantification of the Western blots showed that the Aβ treatment decreased the Bcl-2/Bax ratio: 100% versus 47% (** P = 0.003), and the treatment with the 2% isoflurane for six hours potentiated the Aβ-induced reduction in the Bcl-2/Bax ratio: 47% versus 20% (## P = 0.006) Fig. (5C). These results have suggested that Aβ may induce apoptosis by reducing the Bcl-2/Bax ratio. A high concentration and a long duration of isoflurane treatment may promote the Aβ-induced apoptosis by potentiating the Aβ-induced reduction in the Bcl-2/Bax ratio
Fig. 5.

High concentration and long duration of isoflurane treatment promotes the Aβ-induced decrease in the Bcl-2/Bax ratio in primary neurons from naïve mice. A. Aβ treatment (lanes 3 and 4) decreases Bcl-2 levels as compared to the control condition (lanes 1 and 2) in Western blots. Treatment with 2% isoflurane plus Aβ (lanes 5 and 6) decreases the Bcl-2 levels as compared to the control condition (lanes 1 and 2) and Aβ alone (lanes 3 and 4). B. Aβ treatment (lanes 3 and 4) increases Bax levels as compared to the control condition (lanes 1 and 2) in Western blots analysis. Treatment of Aβ plus 2% isoflurane (lanes 5 and 6) also increases the Bax levels as compared to the control condition (lanes 3 and 4). C. Quantification of the Western blots shows that Aβ treatment (black bar) decreases Bcl-2/Bax ratio as compared to the control condition (white bar). The treatment with Aβ plus 2% isoflurane (gray bar) further decreases Bcl-2/Bax ratio as compared to the Aβ treatment alone (black bar).
Isoflurane Induces a Dose-Dependent Alteration in Cytosolic Calcium Levels
Our previous studies have shown that the isoflurane may regulate cytosolic calcium levels, leading to caspase activation and apoptosis [22]. We, therefore, asked whether different concentrations of isoflurane treatment may cause different effects on the cytosolic calcium levels. We were able to show that 0.5 and 2% isoflurane treatment led to a mild and robust elevation of cytosolic calcium levels, respectively Fig. (6A), in the H4 naïve cells. The amplitude graph Fig. (6A) represents a single experiment and the bar graph Fig. (6B) was used for a better demonstration of the isoflurane-induced changes in cytosolic calcium levels. Quantification of the image showed that the 0.5% isoflurane treatment led to 139% increase in cytosolic calcium levels, whereas the 2% isoflurane treatment led to 229% increase in cytosolic calcium levels Fig. (6B). These results have suggested that low concentrations of isoflurane treatment may induce a mild elevation in cytosolic calcium levels, whereas high concentrations of isoflurane treatment may cause a robust elevation in cytosolic calcium levels.
Fig. 6.

Isoflurane induces a dose-dependent alteration in cytosolic calcium levels. A. 2% isoflurane treatment induces a robust increase in the fura-2 ratio (340nm/380nm) in H4 naïve cells. Treatment with 0.5% isoflurane induces a mild increase in the fura-2 ratio (340nm/380nm) in H4 naïve cells. The amplitude graph here represents a single experiment. B. Quantification of the amplitude suggests that the 2% isoflurane treatment leads to a 229% increase in cytosolic calcium levels, and the 0.5% isoflurane treatment leads to a 139% increase in cytosolic calcium levels. The bar graph represents a single experiment to better demonstrate the isoflurane-induced changes in cytosolic calcium levels.
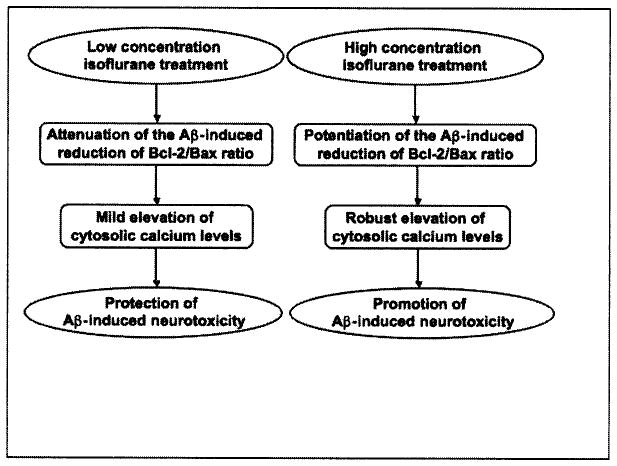
Collectively, these findings have suggested that a low concentration of isoflurane treatment (e.g., 0.5% isoflurane) may attenuate the Aβ-induced reduction in Bcl-2/Bax ratio and cause a mild elevation of cytosolic calcium levels, leading to the protection of Aβ-induced neurotoxicity. In contrast, treatment with a high concentration isoflurane (e.g., 2% isoflurane) may potentiate the Aβ-induced reduction in Bcl-2/Bax ratio and cause a robust elevation of cytosolic calcium levels, leading to promotion of Aβ-induced neurotoxicity Fig. (7).
Fig. 7.
The hypothesized pathway of the dual effects of isoflurane on Aβ-induced neurotoxicity. Low concentration isoflurane treatment and high concentration isoflurane treatment may induce different effects on the ratio of Bcl-2/Bax and cytosolic calcium levels, leading to either promotion or protection of the Aβ-induced neurotoxicity.
DISCUSSION
Our previous studies have shown that the common inhalation anesthetic isoflurane can induce caspase activation, apoptosis, and Aβ accumulation in vitro [19, 20, 40] and in vivo [21]. However, other reports have suggested different findings [24–33]. This difference could be due to different treatments of isoflurane, e.g., different concentration and duration of treatment. We therefore set out to compare the effects of high concentration isoflurane treatment versus low concentration treatment on the Aβ-induced caspase-3 activation and apoptosis.
We have found that the treatment with 2% isoflurane for six hours can promote the Aβ-induced caspase-3 activation Fig. (2), whereas the treatment with 0.5% isoflurane for 30 minutes can protect the Aβ-induced caspase-3 activation Fig. (1) in naïve H4 cells. In the in vivo studies, we first identified that ICV administration of 250 ng of Aβ42 can induce more robust caspase-3 activation as compared to control condition six hours (versus two or 12 hours) after the administration (Supplemental Data). Then, we have shown that the anesthesia with 1.4% isoflurane for two hours promotes Fig. (2), whereas the anesthesia with 0.7% isoflurane for 30 minutes protects Fig. (1), this Aβ-induced caspase-3 activation in the brain tissues of mice. In addition, we have shown that the treatment with 0.5% isoflurane for six hours can protect the Aβ-induced caspase-3 activation; and the treatment with 2% isoflurane for 30 minutes can promote the Aβ-induced caspase-3 activation in the H4 naïve cells. Taken together, these results suggest that different isoflurane treatments may have different effects on caspase activation and apoptosis. Moreover, it seems that the dual effects (promotion versus protection) of isoflurane on Aβ-induced toxicity are dependent on concentration rather than duration because the treatment with 2% and 0.5% isoflurane for either six or 30 minutes promotes and protects the Aβ-induced caspase-3 activation, respectively, in our experiments Fig. (3).
Other studies support our findings. Wei et al. [47] showed that a treatment with isoflurane (0.6%, 1.2%, and 2.4%) for one hour inhibited the neurotoxicity induced by 2.4% isoflurane for 24 hours in vitro in a dose-dependent manner. These findings suggest that short duration of exposure to isoflurane may attenuate the isoflurane-induced neurotoxicity via induction of endogenous neuroprotective mechanisms (protection), while long duration of exposure to isoflurane induces neurotoxicity directly by its inherent neurotoxic effects [47]. In another study, Lee et al. [48] showed that isoflurane provided postconditioning effects on brain ischemia in vitro and in vivo. The studies further showed that 2% isoflurane provided postconditioning effects with 20- and 30-minute treatment times, but the postconditioning effects of 2% isoflurane disappeared when the isoflurane treatment time was increased to 60 minutes Fig. (3A) in [48]. Similarly, treatments with 1.5% and 2.0% isoflurane for 30 minutes provided the postconditioning effects, but these postconditioning effects disappeared when the isoflurane concentration was increased to 2.5% and 3.0% Fig. (3B) in [48]. These results support our findings that isoflurane may have concentration-dependent dual effects (promotion and protection) on the Aβ-induced caspase activation and apoptosis.
It has been reported that Bcl-2 protein family members, including Bcl-2 and Bax, can regulate apoptosis by modulating outer mitochondrial membrane permeability [43, 44]. The pro-apoptotic protein Bax can promote a sustained and complete calcium release from the ER, and therefore cause an extreme/sustained elevation of cytosolic calcium levels, leading to apoptosis. In contrast, the anti-apoptotic protein Bcl-2 can induce an unregulated ER calcium leak, and therefore cause a mild/temporary elevation of cytosolic calcium levels, leading to the attenuation of apoptosis [45, 46]. The anti-apoptotic protein Bcl-2 can also antagonize Bax by forming heterodimers that prevent the oligomerization of Bax, leading to inhibition of apoptosis [49]. In addition, both Bax and Bcl-2 can compete with each other for interaction with Inositol 1,4,5-Triphosphate Receptor (IP3R), resulting in either an extreme/sustained or mild/temporary elevations of cytosolic calcium levels [reviewed by [50].
In the current studies, we have first found that Aβ can decrease the ratio of Bcl-2 to Bax by decreasing the levels of Bcl-2 and increasing the levels of Bax Figs. (4 and 5). Then, we have found that the low concentration (0.5%) and short duration (30 minutes) of isoflurane treatment can mitigate this Aβ-induced reduction in the Bcl-2/Bax ratio Fig. (4), but the high concentration (2%) and long duration (6 hours) of isoflurane treatment can potentiate this Aβ-induced reduction in the Bcl-2/Bax ratio Fig. (5). Furthermore, we have found that high concentration (2%) isoflurane treatment can induce a robust increase, whereas low concentration (0.5%) isoflurane treatment can only cause a mild increase, in cytosolic calcium levels Fig. (6).
Taken together, these findings suggest that a mild/temporary elevation of cytosolic calcium levels following a lower concentration of isoflurane treatment could provide cytoprotection via up-regulating the host protection response [51–53]. However, an extreme/sustained elevation in cytosolic calcium levels following a high concentration of isoflurane can induce a caspase 12-, caspase 9- and caspase 3-associated form of apoptosis [18, 34, 54, 55, reviewed in 56] Fig. (7).
The mechanisms by which Aβ and isoflurane affect the levels of Bcl-2 and Bax are largely unknown. Recent studies by Zhang et al. [57] have shown that isoflurane can affect mRNA levels of Bcl-2 and Bax. Therefore, it is possible that Aβ and isoflurane may regulate the generation of Bcl-2 and Bax by affecting the gene expression of Bcl-2 and Bax, leading to the alterations in the ratio of Bcl-2/Bax. The future studies will include the assessment of the effects of Aβ and isoflurane on the gene expression of Bcl-2 and Bax, as well as other Bcl-2 protein family members.
Isoflurane may affect the cytosolic calcium levels by acting on NMDA receptors, IP3R and Sarco/Endoplasmic Reticulum Ca2+-ATPase (SERCA) [22, 34; reviewed in 35]. Therefore, the future studies will also include the investigation of the effects of different isoflurane treatments on the levels of NMDA receptors, IP3R, and SERCA, which may further elucidate the molecular mechanisms of the dual effects of isoflurane on Aβ-induced neurotoxicity.
One limitation to the current study is that we only investigated the potential dual effects of isoflurane on caspase-3 activation and apoptosis in naïve H4 cells and primary neurons of naïve mice treated with Aβ. It is possible that isoflurane may not show such dual effects in other cell lines and to other apoptotic insults, e.g., ischemia.
In conclusion, we have found that different isoflurane treatments could have different effects on the Aβ-induced neurotoxicity. Specifically, a low concentration isoflurane treatment may protect the Aβ-induced caspase-3 activation and apoptosis, whereas a high concentration isoflurane treatment may promote the Aβ-induced caspase-3 activation and apoptosis. Furthermore, different concentration and duration exposures of isoflurane may lead to different effects on the Aβ-induced reduction in Bcl-2/Bax ratio and cytosolic calcium levels, leading to the potential dual effects on the Aβ-induced neurotoxicity: protection and promotion. These findings should facilitate future studies to determine whether inhalation anesthetics may dose- and time-dependently induce neurotoxic and neuroprotective effects, which may eventually lead to safer anesthesia care for patients.
Acknowledgments
FUNDING
This research was supported by K08NS048140, R21AG029856 and R01 GM088801 (National Institutes of Health), Jahnigen Career Development Award (American Geriatrics Society), Investigator Initiated Research Grant (Alzheimer’s Association) (to Z. X.), R01GM079360 (National Institutes of Health) (to FI), and China National Science Foundation Oversea young scholar collaboration research award NSF30928026 (to Y.Y. and Z. X.).
References
- 1.Tanzi RE, Bertram L. Alzheimer’s disease: The latest suspect. Nature. 2008;454:706–708. doi: 10.1038/454706a. [DOI] [PubMed] [Google Scholar]
- 2.Goate A, Chartier-Harlui MC, Mullan M, Brown J, Crowford F, Fidani L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 3.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beureuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selkoe DJ, Podlisny MB, Joachim CL, Vickers EA, Lee G, Fritz LC, et al. Beta-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci USA. 1988;85:7341–7345. doi: 10.1073/pnas.85.19.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glenner GG, Wong CW. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122:1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- 6.Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 8.Mattson MP. Contributions of mitochondrial alterations, resulting from bad genes and a hostile environment, to the pathogenesis of Alzheimer’s disease. Int Rev Neurobiol. 2002;53:387–409. doi: 10.1016/s0074-7742(02)53014-2. [DOI] [PubMed] [Google Scholar]
- 9.Raina AK, Hochman A, Ickes H, Zhu X, Ogawa O, Cash AD, et al. Apoptotic promoters and inhibitors in Alzheimer’s disease: Who wins out? Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:251–254. doi: 10.1016/S0278-5846(03)00020-4. [DOI] [PubMed] [Google Scholar]
- 10.Ivins KJ, Thornton PL, Rohn TT, Cotman CW. Neuronal apoptosis induced by beta-amyloid is mediated by caspase-8. Neurobiol Dis. 1999;6:440–449. doi: 10.1006/nbdi.1999.0268. [DOI] [PubMed] [Google Scholar]
- 11.Ivins KJ, Bui ET, Cotman CW. Beta-amyloid induces local neurite degeneration in cultured hippocampal neurons: evidence for neuritic apoptosis. Neurobiol Dis. 1998;5:365–378. doi: 10.1006/nbdi.1998.0228. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yanker BA, et al. Caspase-12 mediates endoplasimic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 13.Troy CM, Rabacchi SA, Friedman WJ, Frappier TF, Brown K, Shelanski ML. Caspase-2 mediates neuronal cell death induced by beta-amyloid. J Neurosci. 2000;20:1386–1392. doi: 10.1523/JNEUROSCI.20-04-01386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen JW, Eldadah BA, Huang X, Knoblach SM, Faden Al. Multiple caspases are involved in beta-amyloid-induced neuronal apoptosis. J Neurosci Res. 2001;65:45–53. doi: 10.1002/jnr.1126. [DOI] [PubMed] [Google Scholar]
- 15.Lu DC, Soriano S, Bredesen DE, Koo EH. Caspase cleavage of the amyloid precursor protein modulates amyloid beta-protein toxicity. J Neurochem. 2003;87:733–741. doi: 10.1046/j.1471-4159.2003.02059.x. [DOI] [PubMed] [Google Scholar]
- 16.Awasthi A, Matsunaga Y, Yamada T. Amyloid-beta causes apoptosis of neuronal cells via caspase cascade, which can be prevented by amyloid-beta-derived short peptides. Exp Neurol. 2005;196:282–289. doi: 10.1016/j.expneurol.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Eckenhoff RG, Johansson JS, Wei H, Camini A, Kang B, Wei W, et al. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–709. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Wei H, Kang B, Wei W, Liang G, Meng QC, Li Y, et al. Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Res. 2005;1037:139–147. doi: 10.1016/j.brainres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Xie Z, Dong Y, Maeda U, Alfille P, Culley DJ, Crosby G, et al. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology. 2006;104:988–994. doi: 10.1097/00000542-200605000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Xie Z, Dong Y, Maeda U, Moir RD, Xia W, Culley DJ, et al. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J Neurosci. 2007;27:1247–1254. doi: 10.1523/JNEUROSCI.5320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie Z, Culley DJ, Dong Y, Zhang G, Zhang B, Moir RD, et al. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann Neurol. 2008;64:618–627. doi: 10.1002/ana.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang G, Dong Y, Zhang B, Ichinose F, Wu X, Culley DJ, et al. Isoflurane-induced caspase-3 activation is dependent on cytosolic calcium and can be attenuated by memantine. J Neurosci. 2008;28:4551–4560. doi: 10.1523/JNEUROSCI.5694-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang J, Eckenhoff MF, Eckenhoff RG. Anesthesia and the old brain. Anesth Analg. 2010;110:421–426. doi: 10.1213/ANE.0b013e3181b80939. [DOI] [PubMed] [Google Scholar]
- 24.Xu X, Feng J, Zuo Z. Isoflurane preconditioning reduces the rat NR8383 macrophage injury induced by lipopolysaccharide and interferon gamma. Anesthesiology. 2008;108:643–650. doi: 10.1097/ALN.0b013e318167aeb4. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Peng L, Zuo Z. Isoflurane preconditioning increases B-cell lymphoma-2 expression and reduces cytochrome c release from the mitochondria in the ischemic penumbra of rat brain. Eur J Pharmacol. 2008 doi: 10.1016/j.ejphar.2008.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raphael J, Zuo Z, Abedat S, Beeri R, Gozal Y. Isoflurane preconditioning decreases myocardial infarction in rabbits via up-regulation of hypoxia inducible factor 1 that is mediated by mammalian target of rapamycin. Anesthesiology. 2008;108:415–425. doi: 10.1097/ALN.0b013e318164cab1. [DOI] [PubMed] [Google Scholar]
- 27.Zaugg M, Jamali NZ, Lucchinetti E, Shafiq SA, Siddique MA. Norepinephrine-induced apoptosis is inhibited in adult rat ventricular myocytes exposed to volatile anesthetics. Anesthesiology. 2000;93:209–218. doi: 10.1097/00000542-200007000-00032. [DOI] [PubMed] [Google Scholar]
- 28.Tyther R, Fanning N, Halligan M, Wang J, Redmond HP, Shorten G. The effect of the anaesthetic agent isoflurane on the rate of neutrophil apoptosis in vitro. Ir J Med Sci. 2001;170:41–44. doi: 10.1007/BF03167720. [DOI] [PubMed] [Google Scholar]
- 29.Wise-Faberowski L, Raizada MK, Sumners C. Oxygen and glucose deprivation-induced neuronal apoptosis is attenuated by halothane and isoflurane. Anesth Analg. 2001;93:1281–1287. doi: 10.1097/00000539-200111000-00051. [DOI] [PubMed] [Google Scholar]
- 30.Wise-Faberowski L, Aono M, Pearlstein RD, Warner DS. Apoptosis is not enhanced in primary mixed neuronal/glial cultures protected by isoflurane against N-methyl-D-aspartate excitotoxicity. Anesth Analg. 2004;99:1708–1714. doi: 10.1213/01.ANE.0000136474.35627.FF. table of contents. [DOI] [PubMed] [Google Scholar]
- 31.de Klaver MJ, Manning L, Palmer LA, Rich GF. Isoflurane pretreatment inhibits cytokine-induced cell death in cultured rat smooth muscle cells and human endothelial cells. Anesthesiology. 2002;97:24–32. doi: 10.1097/00000542-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Kawaguchi M, Drummond JC, Cole DJ, et al. Effect of isoflurane on neuronal apoptosis in rats subjected to focal cerebral ischemia. Anesth Analg. 2004;98:798–805. doi: 10.1213/01.ane.0000105872.76747.f6. table of contents. [DOI] [PubMed] [Google Scholar]
- 33.Gray JJ, Bickler PE, Fahlman CS, Zhang X, Schuyler JA. Isoflurane neuroprotection in hypoxic hippocampal slice cultures involves increases in intracellular Ca2+ and mitogen-activated protein kinases. Anesthesiology. 2005;102:606–615. doi: 10.1097/00000542-200503000-00020. [DOI] [PubMed] [Google Scholar]
- 34.Wei H, Liang G, Yang H, Wang Q, Hawkins B, Madesh M, et al. The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology. 2008;108:251–260. doi: 10.1097/01.anes.0000299435.59242.0e. [DOI] [PubMed] [Google Scholar]
- 35.Wei H, Xie Z. Anesthesia, calcium homeostasis and Alzheimer’s disease. Curr Alzheimer Res. 2009;6:30–35. doi: 10.2174/156720509787313934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bickler PE, Fahlman CS, Gray J, McKleroy W. Inositol 1,4,5-triphosphate receptors and NAD(P)H mediate Ca2+ signaling required for hypoxic preconditioning of hippocampal neurons. Neuroscience. 2009;160:51–60. doi: 10.1016/j.neuroscience.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brewer GJ. Isolation and culture of adult rat hippocampal neurons. J Neurosci Methods. 1997;71:143–155. doi: 10.1016/s0165-0270(96)00136-7. [DOI] [PubMed] [Google Scholar]
- 38.Thakker DR, Weatherspoon MR, Harrison J, Keene TE, Lane Ds, Kaemmerer WF, et al. Intracerebroventricular amyloid-beta antibodies reduce cerebral amyloid angiopathy and associated micro-hemorrhages in aged Tg2576 mice. Proc Natl Acad Sci USA. 2009;106:4501–4506. doi: 10.1073/pnas.0813404106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du J, Wang X, Miereles C, Bailey JL, Debigore R, Zheng B, et al. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest. 2004;113:115–123. doi: 10.1172/JCI200418330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie Z, Dong Y, Maeda U, Moir R, Inouye Sk, Culley DJ, et al. Isoflurane-induced apoptosis: a potential pathogenic link between delirium and dementia. J Gerontol A Biol Sci Med Sci. 2006;61:1300–1306. doi: 10.1093/gerona/61.12.1300. [DOI] [PubMed] [Google Scholar]
- 41.Thornberry NA. Caspases: key mediators of apoptosis. Chem Biol. 1998;5:R97–103. doi: 10.1016/s1074-5521(98)90615-9. [DOI] [PubMed] [Google Scholar]
- 42.McLaughlin B, Hartnett KA, Erhardt JA, Legos JJ, White RF, Barone FC, et al. Caspase 3 activation is essential for neuroprotection in preconditioning. Proc Natl Acad Sci USA. 2003;100:715–720. doi: 10.1073/pnas.0232966100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jurgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredsen D, Reed JC, et al. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutskopoulu V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zong WX, Li C, Hatzivassiliou G, Lindsten T, Yu QC, Yuan J, et al. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol. 2003;162:59–69. doi: 10.1083/jcb.200302084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oakes SA, Scorrano L, Opferman JT, Bassik NC, Nishino M, Pozzan T, et al. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci USA. 2005;102:105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei H, Liang G, Yang H. Isoflurane preconditioning inhibited isoflurane-induced neurotoxicity. Neurosci Lett. 2007;425:59–62. doi: 10.1016/j.neulet.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JJ, Li L, Jung HH, Zuo Z. Postconditioning with isoflurane reduced ischemia-induced brain injury in rats. Anesthesiology. 2008;108:1055–1062. doi: 10.1097/ALN.0b013e3181730257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, et al. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 50.Lao Y, Chang DC. Study of the functional role of Bcl-2 family proteins in regulating Ca(2+) signals in apoptotic cells. Biochem Soc Trans. 2007;35:1038–1039. doi: 10.1042/BST0351038. [DOI] [PubMed] [Google Scholar]
- 51.Bickler PE, Zhan X, Fahlman CS. Isoflurane preconditions hippocampal neurons against oxygen-glucose deprivation: role of intracellular Ca2+ and mitogen-activated protein kinase signaling. Anesthesiology. 2005;103:532–539. doi: 10.1097/00000542-200509000-00016. [DOI] [PubMed] [Google Scholar]
- 52.Bickler PE, Fahlman CS. The inhaled anesthetic, isoflurane, enhances Ca2+-dependent survival signaling in cortical neurons and modulates MAP kinases, apoptosis proteins and transcription factors during hypoxia. Anesth Analg. 2006;103:419–429. doi: 10.1213/01.ane.0000223671.49376.b2. table of contents. [DOI] [PubMed] [Google Scholar]
- 53.Zhan X, Fahlman CS, Bickler PE. Isoflurane neuroprotection in rat hippocampal slices decreases with aging: changes in intracellular Ca2+ regulation and N-methyl-D-aspartate receptor-mediated Ca2+ influx. Anesthesiology. 2006;104:995–1003. doi: 10.1097/00000542-200605000-00016. [DOI] [PubMed] [Google Scholar]
- 54.Paschen W, Mengesdorf T. Endoplasmic reticulum stress response and neurodegeneration. Cell Calcium. 2005;38:409–415. doi: 10.1016/j.ceca.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 55.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 56.Mattson MP. Calcium and neurodegeneration. Aging Cell. 2007;6:337–350. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, Dong Y, Wu X, Lu Y, Xu Z, Knapp A, et al. The mitochondrial pathway of anesthetic isoflurane-induced apoptosis. J Biol Chem. 2010;285:4025–4037. doi: 10.1074/jbc.M109.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]