Abstract
In patients with diabetes, glycemic improvement by sodium-glucose cotransporter-2 inhibition depends on the kidney's ability to filter glucose. Dapagliflozin, a sodium-glucose cotransporter-2 inhibitor, reduces hyperglycemia in patients with diabetes and normal or mildly impaired renal function. In this randomized, double-blind, placebo-controlled study we assessed daily treatment with dapagliflozin in 252 patients with inadequately controlled type 2 diabetes and moderate renal impairment. The primary endpoint, the mean change in HbA1c, was not statistically different from placebo after 24 weeks (−0.41% and −0.44% for 5- and 10-mg doses, respectively, and −0.32% for placebo). The mean weight change from baseline was −1.54 and −1.89 kg for the 5- and 10-mg doses, respectively, and +0.21 kg for placebo. The mean systolic and diastolic blood pressure decreased in the dapagliflozin groups compared to placebo. Through 104 weeks, 13 patients receiving dapagliflozin and no patients receiving placebo experienced bone fracture. At 1 week, the mean serum creatinine increased with dapagliflozin 5 mg (+0.13 mg/dl) and 10 mg (+0.18 mg/dl) and did not change further after 104 weeks. Mean serum electrolytes did not change in any group, and there were fewer episodes of hyperkalemia with dapagliflozin than placebo. Thus, in patients with moderate renal impairment, dapagliflozin did not improve glycemic control, but reduced weight and blood pressure.
Keywords: diabetes mellitus, renal function
Current medications for treating type 2 diabetes mellitus (T2DM) target the pancreas, liver, intestines, muscle, or adipose tissue and act by increasing insulin secretion or action, or by improving insulin sensitivity.1 The sodium-glucose cotransporter-2 (SGLT2), located in the renal proximal tubule, reabsorbs the majority of filtered glucose.2, 3 Inhibition of renal glucose reabsorption via inhibition of SGLT2, an insulin-independent process, represents a novel approach to treating T2DM.
Several clinical trials with dapagliflozin, a potent and selective SGLT2 inhibitor, showed that it reduces hyperglycemia and improves glycemic control in patients with T2DM. These trials examined dapagliflozin as monotherapy4 or in combination with metformin,5 sulfonylureas,6, 7 thiazolidinediones,8 or insulin.9, 10 Treatment with dapagliflozin induces glucosuria, resulting in caloric elimination and weight loss.4, 5, 6, 7, 8, 9, 10, 11 A decrease in blood pressure has also been observed4, 5, 6, 7, 8, 9, 10, 11 and may occur as a consequence of osmotic diuresis and weight loss.
The mechanism of dapagliflozin depends on filtration of glucose at the glomerulus. Thus, with reduced renal function, dapagliflozin is expected to be less efficacious. Previous studies have shown efficacy of dapagliflozin in populations consisting primarily of patients with normal renal function or mild renal impairment. This study examines the efficacy and safety of dapagliflozin in patients with T2DM and moderate renal impairment.
RESULTS
Disposition and demographics
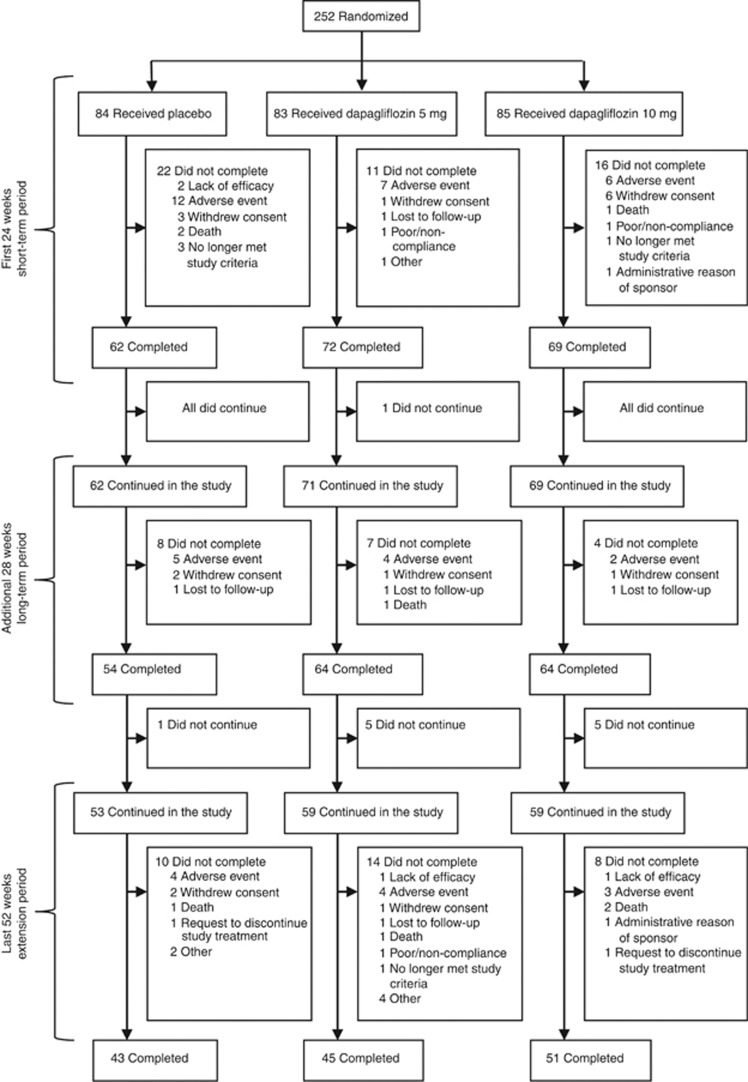
Of the 631 enrolled patients, 252 were randomized (Figure 1). A total of 203 patients completed the first 24 weeks (short-term period), 202 entered the additional 28-week period (long-term period) with 182 completing it, and 171 entered the second year (extension period), with 139 completing it (Figure 1). The short-term completion rate was lower for placebo (73.8%) than for dapagliflozin 5-mg (86.7%) and 10-mg (81.2%) groups, the main difference being higher rate of discontinuations for adverse events for placebo during the first 24 weeks than for dapagliflozin. Groups generally appeared to be balanced with respect to key demographic and baseline characteristics (Table 1).
Figure 1.
Trial profile through 104 weeks. A total of five patients left the trial because they ‘no longer met study criteria.' For the three patients in the placebo group, (1) the patient had insulin regimen altered, which is against the protocol guidance, (2) the patient met discontinuation criteria for sustained elevated serum creatinine, and (3) the patient was inappropriately randomized with a high screening potassium and removed a few days after starting treatment. The patient in the 5-mg group had myasthenia gravis, was administered prednisone for treatment and was asked to be discontinued by the medical monitor. The patient in the 10-mg group was discontinued owing to serum creatinine greater than two times the baseline at day 78.
Table 1. Demographics and baseline characteristics.
| Placebo | Dapagliflozin 5 mg | Dapagliflozin 10 mg | |
|---|---|---|---|
| n | 84 | 83 | 85 |
| Age (years) | 67±8.6 | 66±8.9 | 68±7.7 |
| Men | 53 (63.1) | 55 (66.3) | 56 (65.9) |
| Women | 31 (36.9) | 28 (33.7) | 29 (34.1) |
| Geographic region | |||
| North America | 41 (48.8) | 51 (61.4) | 48 (56.5) |
| Latin America | 23 (27.4) | 15 (18.1) | 17 (20.0) |
| Europe | 11 (13.1) | 9 (10.8) | 9 (10.6) |
| Asia/Pacific | 9 (10.7) | 8 (9.6) | 11 (12.9) |
| Race | |||
| White | 69 (82.1) | 65 (78.3) | 77 (90.6) |
| African American | 1 (1.2) | 7 (8.4) | 4 (4.7) |
| Asian | 6 (7.1) | 4 (4.8) | 3 (3.5) |
| Other | 8 (9.5) | 7 (8.4) | 1 (1.2) |
| HbA1c (%) | 8.53±1.28 | 8.30±1.04 | 8.22±0.98 |
| FPG (mg/dl) | 149±48 | 161±56 | 164±66 |
| Weight (kg) | 89.6±20.0 | 95.2±20.9 | 93.2±17.3 |
| BMI, ⩾30 kg/m2 | 50 (59.5) | 59 (71.1) | 54 (63.5) |
| Duration of diabetes (years) | 15.7±9.5 | 16.9±9.0 | 18.2±10.1 |
| Pre-enrollment antihyperglycemic therapy | |||
| Insulin based | 55 (65.5) | 54 (65.1) | 55 (64.7) |
| Sulfonylurea based | 21 (25.0) | 21 (25.3) | 21 (24.7) |
| Thiazolidinedione based | 1 (1.2) | 1 (1.2) | 2 (2.4) |
| Other | 7 (8.3) | 7 (8.4) | 7 (8.2) |
| Chronic kidney disease stage: eGFR (ml/min per 1.73 m2) | |||
| Stage 4: <30 | 4 (4.8) | 4 (4.8) | 2 (2.4) |
| Stage 3B: ⩾30 and <45 | 34 (40.5) | 41 (49.4) | 47 (55.3) |
| Stage 3A: ⩾45 and <60 | 41 (48.8) | 35 (42.2) | 33 (38.8) |
| Stage 2: ⩾60 | 5 (6.0) | 3 (3.6) | 3 (3.5) |
| Urinary albumin:creatinine ratioa (mg/g) | 67 (20, 367) | 79 (13, 539) | 73 (9, 352) |
| Urinary protein:creatinine ratioa (mg/mg) | 0.75±1.77 | 0.72±1.22 | 0.63±1.12 |
| Patients with diabetic nephropathy | 60 (71.4) | 61 (73.5) | 58 (68.2) |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c.
Data are means±s.d. or n (%).
Measured by spot urine samples and urinary albumin:creatinine ratio is median (25, 75 percentiles).
Efficacy
In this moderate renal impairment population, the primary end point, mean change in hemoglobin A1c (HbA1c; % (s.e)) excluding data after glycemic rescue, was not statistically different from placebo at week 24 (−0.32 (0.17), −0.41 (0.17), and −0.44 (0.17) for placebo, 5 and 10-mg dapagliflozin, respectively, and P=0.561 and P=0.435 for dapagliflozin 5 and 10 mg vs. placebo, respectively; Table 2). Changes in HbA1c at 52 and at 104 weeks are shown in Table 3. Fasting plasma glucose (FPG) showed larger mean reductions for dapagliflozin 5 and 10 mg compared with placebo at 24 weeks (Table 2) and over 104 weeks (Table 3). Similar results for HbA1c and FPG were obtained in analyses that include data after glycemic rescue.
Table 2. Efficacy at 24 weeksa.
| Placebo (n=84) | Dapagliflozin 5 mg (n=83) | Dapagliflozin 10 mg (n=85) | |
|---|---|---|---|
| HbA1c (%) | |||
| nb | 82 | 83 | 82 |
| Baseline mean (s.d.) | 8.53 (1.29) | 8.30 (1.04) | 8.22 (0.97) |
| Mean change from baseline (s.e.) | −0.32 (0.17) | −0.41 (0.17) | −0.44 (0.17) |
| Difference from placebo (s.e.) | −0.08 (0.14) | −0.11 (0.15) | |
| P-value vs. placeboc | 0.561 | 0.435 | |
| FPG (mg/dl) | |||
| nb | 83 | 83 | 84 |
| Baseline mean (s.d.) | 150.2 (48.2) | 161.4 (55.9) | 164.8 (66.8) |
| Mean change from baseline (s.e.) | 8.4 (9.62) | −5.2 (9.55) | −0.6 (9.52) |
| Difference from placebo (s.e.) | −13.6 (8.14) | −9.0 (8.14) | |
Abbreviations: ANCOVA, analysis of covariance; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; LOCF, last observation carried forward.
Using ANCOVA with LOCF excluding data after rescue.
n is the number of randomized patients with non-missing baseline and week 24 (LOCF) values.
Primary end point (HbA1c) was tested at alpha=0.027 applying Dunnett's adjustment, and secondary end point (FPG) was tested following a sequential testing procedure at alpha=0.05.
Table 3. Change from baseline in efficacy and renal parametersa.
| Placebo (n=84) | Dapagliflozin 5 mg (n=83) | Dapagliflozin 10 mg (n=85) | ||||
|---|---|---|---|---|---|---|
| |
n |
Value |
n |
Value |
n |
Value |
| HbA1c (%)b | ||||||
| Baseline | 84 | 8.53 (1.28) | 83 | 8.30 (1.04) | 83 | 8.23 (0.98) |
| Change at week 24 | 50 | −0.28 [0.13] | 63 | −0.38 [0.13] | 65 | −0.41 [0.13] |
| Change at week 52 | 13 | −0.06 [0.18] | 30 | −0.34 [0.16] | 35 | −0.34 [0.16] |
| Change at week 104 | 4 | −0.67 [0.41] | 8 | −1.21 [0.38] | 10 | −0.75 [0.22] |
| FPG (mg/dl)b | ||||||
| Baseline | 84 | 150 (48) | 83 | 161 (56) | 84 | 165 (67) |
| Change at week 24 | 50 | 2.53 [6.86] | 60 | −9.51 [6.35] | 65 | −9.29 [6.24] |
| Change at week 52 | 13 | −8.58 [9.00] | 29 | −14.98 [6.78] | 33 | −16.49 [6.54] |
| Change at week 104 | 4 | −18.25 [36.62] | 8 | −31.00 [13.55] | 10 | −22.10 [8.22] |
| 24-H urinary glucose:creatinine ratio (g/g)c | ||||||
| Baseline | 65 | 7.19 (23.15) | 72 | 3.16 (7.08) | 68 | 5.43 (12.75) |
| Change at week 6 | 63 | −2.20 (23.40) | 69 | 24.39 (18.75) | 63 | 24.78 (16.46) |
| Change at week 52 | 34 | −5.01 (25.07) | 40 | 26.84 (15.37) | 38 | 21.74 (17.47) |
| Change at week 104 | 36 | −4.81 (30.03) | 33 | 26.24 (14.60) | 38 | 21.97 (20.08) |
| Weight (kg)d | ||||||
| Baseline | 84 | 89.6 (20.1) | 83 | 95.2 (20.9) | 85 | 93.3 (17.3) |
| Change at week 24 | 63 | 0.68 [0.45] | 70 | −1.34 [0.43] | 69 | −1.72 [0.44] |
| Change at week 52 | 51 | 1.10 [0.60] | 64 | −1.17 [0.55] | 64 | −1.75 [0.56] |
| Change at week 104 | 42 | 2.63 [0.79] | 41 | −0.34 [0.75] | 51 | −1.10 [0.74] |
| eGFR (ml/min per 1.73 m2) | ||||||
| Baseline | 84 | 45.6 (10.0) | 83 | 44.2 (8.8) | 85 | 43.9 (10.6) |
| Change at week 24 | 62 | −0.25 [0.92] | 70 | −2.38 [0.84] | 69 | −4.80 [0.82] |
| Change at week 52 | 49 | −2.58 [1.16] | 64 | −2.08 [0.99] | 63 | −4.46 [0.97] |
| Change at week 104 | 42 | −2.38 [1.01] | 40 | −1.71 [1.23] | 50 | −3.50 [1.02] |
| CrCl (ml/min) | ||||||
| Baseline | 84 | 60.4 (17.3) | 83 | 62.3 (17.3) | 85 | 59.9 (17.8) |
| Change at week 24 | 62 | 0.03 [1.11] | 69 | −4.02 [1.10] | 69 | −7.38 [0.97] |
| Change at week 52 | 49 | −2.56 [1.31] | 64 | −4.15 [1.13] | 63 | −7.27 [1.23] |
| Change at week 104 | 42 | −2.35 [1.27] | 40 | −3.30 [1.69] | 50 | −6.32 [1.22] |
Abbreviations: CrCl, creatinine clearance; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c.
Data are mean (s.d.) or mean [s.e.].
Used longitudinal repeated-measure analysis excluding data after rescue.
Used descriptive statistics including data after rescue.
Used longitudinal repeated-measure analysis including data after rescue.
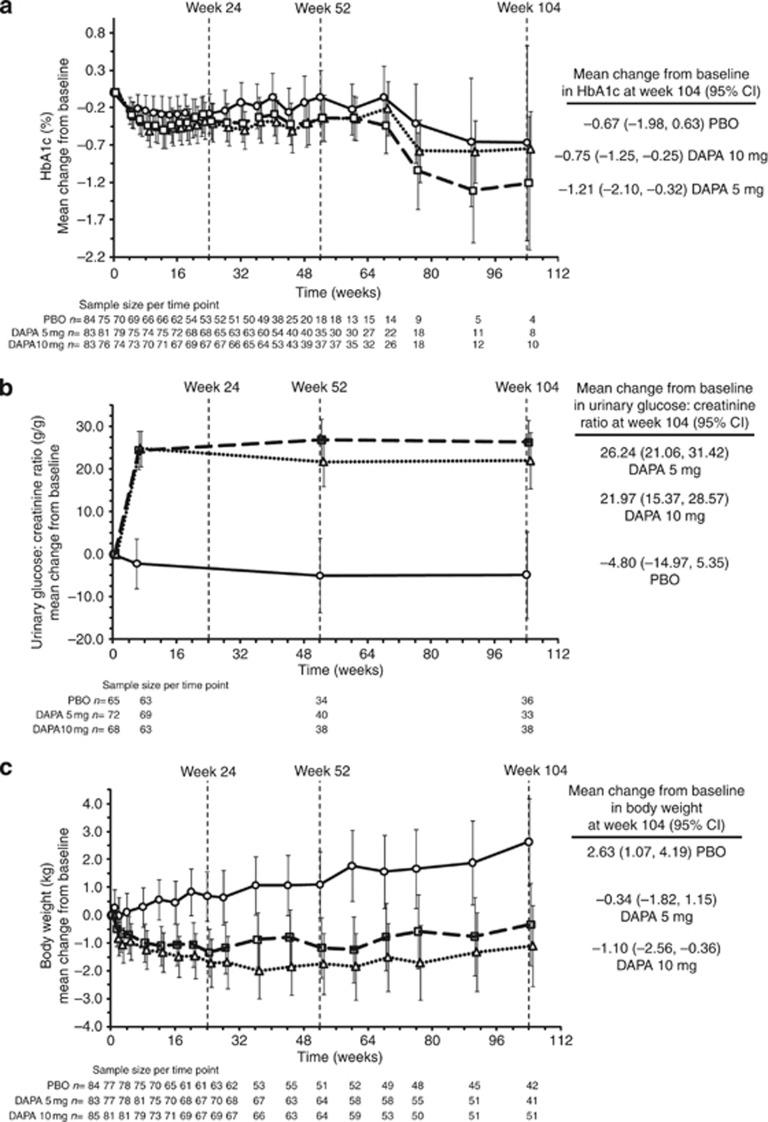
There was an increase in the 24-h urine glucose:creatinine ratio with dapagliflozin compared with placebo (Table 3; Figure 2). The dapagliflozin groups experienced mean weight loss, whereas there was an increase in the placebo group (Figure 2 and Table 3).
Figure 2.
Mean change from baseline in HbA1c, urinary glucose:creatinine ratio, and body weight for placebo (circles, solid line), dapagliflozin 5-mg (squares, dashed line), and dapagliflozin 10-mg (triangles, dotted line) groups, all plus original pre-enrollment antidiabetic regimen up to 104 weeks. Data are means (95% confidence interval (CI)). Mean change from baseline in HbA1c after adjustment for baseline value (a), mean change from baseline in urinary glucose:creatinine ratio (g/g) after adjustment for baseline value (b), and mean change from baseline in body weight after adjustment for baseline value (c). HbA1c and weight obtained from longitudinal repeated-measure analysis; HbA1c excludes data after rescue; urinary glucose:creatinine ratio and weight include data after rescue. Treatment symbols shifted horizontally to prevent error bar overlapping. DAPA, dapagliflozin; HbA1c, hemoglobin A1c; PBO, placebo.
Post-hoc analysis by baseline chronic kidney disease (CKD) stage (Table 4) showed a larger reduction in adjusted mean change in HbA1c and FPG from baseline to week 24 for dapagliflozin vs. placebo for stage 3A CKD (estimated glomerular filtration rate (eGFR) ⩾45 and <60 ml/min per 1.73 m2) than for stage 3B CKD (eGFR ⩾30 and <45 ml/min per 1.73 m2). The placebo-corrected mean reduction from baseline in HbA1c at week 24 for dapagliflozin treatment groups in patients in stage 3A ranges between 0.33 and 0.37, whereas there is no change observed in patients in stage 3B. No difference was observed for change in body weight at week 24 between stages 3A and 3B (Table 4).
Table 4. Efficacy by baseline CKD stages 3A and 3B at 24 weeksa.
| HbA1c (%) | FPG (mg/dl) | Weight (kg) | ||||
|---|---|---|---|---|---|---|
| |
n |
Difference from placebo |
n |
Difference from placebo |
n |
Difference from placebo |
| This study | ||||||
| Stage 3B: eGFR ⩾30 and <45 ml/min per 1.73 m2 | ||||||
| Dapagliflozin 5 mg | 41 | 0.05 (0.21) | 41 | −2.66 (12.1) | 41 | −2.1 (0.69) |
| Dapagliflozin 10 mg | 45 | 0.07 (0.21) | 46 | −1.49 (11.8) | 47 | −2.2 (0.68) |
| Stage 3A: eGFR ⩾45 and <60 ml/min per 1.73 m2 | ||||||
| Dapagliflozin 5 mg | 35 | −0.37 (0.23) | 35 | −24.8 (12.4) | 35 | −1.9 (0.66) |
| Dapagliflozin 10 mg | 32 | −0.33 (0.24) | 33 | −24.4 (12.7) | 33 | −2.3 (0.68) |
Abbreviations: ANCOVA, analysis of covariance; CKD, chronic kidney disease; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; LOCF, last observation carried forward.
Data are difference in adjusted mean (s.e.) change from baseline of dapagliflozin vs. placebo (LOCF), excluding data after rescue based on an ANCOVA model with treatment group and stratum as main effects and baseline value as a covariate.
Safety
The proportions of patients experiencing an adverse event were similar for dapagliflozin and placebo (Table 5). More patients in the placebo group had adverse events leading to discontinuation (Table 5) than in dapagliflozin groups. Ten deaths were reported during the 104 weeks of treatment: 5 in placebo (1—traumatic brain injury, 2—acute myocardial infarction, 1—acute cardiac failure, myocardial infarction, and renal failure, and 1—general physical health deterioration), 2 in dapagliflozin 5 mg (both with myocardial infarction and cardiac arrest), and 3 in dapagliflozin 10 mg (1 each—myocardial infarction, cardiac failure, and sepsis). The proportions of patients with serious adverse events through the 104-week treatment period were similar for dapagliflozin and placebo. A higher incidence of genital infections was reported with dapagliflozin than placebo, and a similar incidence of patients experienced urinary tract infections with dapagliflozin and placebo (Table 5).
Table 5. Adverse and special interest events through week 104.
| Placebo | Dapagliflozin 5 mg | Dapagliflozin 10 mg | |
|---|---|---|---|
| n | 84 | 83 | 85 |
| Adverse event | 77 (91.7) | 80 (96.4) | 77 (90.6) |
| Related adverse event | 39 (46.4) | 39 (47.0) | 41 (48.2) |
| Adverse event leading to discontinuation | 22 (26.2) | 16 (19.3) | 11 (12.9) |
| At least one serious adverse event | 26 (31.0) | 21 (25.3) | 26 (30.6) |
| Deaths | 5 (6.0) | 2 (2.4) | 3 (3.5) |
| Adverse events of special interest | |||
| Suggestive of urinary tract infection | 12 (14.3) | 11 (13.3) | 12 (14.1) |
| Suggestive of genital tract infection | 3 (3.6) | 8 (9.6) | 7 (8.2) |
| Renal impairment or failure (total) | 6 (7.1) | 2 (2.4) | 8 (9.4) |
| Blood creatinine increased | 3 (3.6) | 0 | 5 (5.9) |
| Renal impairment | 0 | 1 (1.2) | 2 (2.4) |
| Renal failure chronic | 1 (1.2) | 0 | 1 (1.2) |
| Renal failure | 1 (1.2) | 1 (1.2) | 0 |
| Renal failure acute | 1 (1.2) | 0 | 0 |
| Hypotension/dehydration/hypovolemia | 5 (6.0) | 8 (9.6) | 11 (12.9) |
| Fracture | 0 | 5 (6.0) | 8 (9.4) |
| Hypertension | 6 (7.1) | 3 (3.6) | 5 (5.9) |
| Micturition urgency | 0 (0) | 5 (6.0) | 2 (2.4) |
| Hyperkalemia | 13 (15.5) | 10 (12.0) | 8 (9.4) |
| Events of hypoglycemia | |||
| Total patients with hypoglycemia | 43 (51.2) | 38 (45.8) | 33 (38.8) |
| Major episode of hypoglycemia a | 4 (4.8) | 0 | 2 (2.4) |
Data are means±s.d. or n (%) and include data after rescue.
Major episode defined as a symptomatic episode requiring third-party assistance owing to severe impairment in consciousness or behavior with a capillary or plasma glucose value <54 mg/dl and prompt recovery after glucose or glucagon administration.
Through 104 weeks, 13 (7.7%) patients experienced fracture in the dapagliflozin groups (5 at 5 mg and 8 at 10 mg) vs. 0 on placebo (Table 5). All fractures occurred after trauma and were mostly of low impact. Two of 13 fractures were assessed as serious adverse events (1 hip and 1 elbow, both with dapagliflozin 10 mg). None of these events led to discontinuation of study medication. Seven of 13 patients who sustained fracture had a history of diabetic neuropathy or exhibited orthostatic hypotension. Of the 13 patients reporting fracture, 5 had stage 3A CKD and 8 had stage 3B CKD.
Events of renal impairment or renal failure were uncommon during the 104-week period (Table 5). Reports of volume depletion events were more frequent in the dapagliflozin groups than in the placebo group (Table 5). Six patients (two on dapagliflozin 10 mg and four on placebo) experienced major episodes of hypoglycemia through the 104 weeks (Table 5).
Blood pressure
At week 1, there were mean reductions from baseline in seated systolic and diastolic blood pressure (−6.83 and −2.53 mm Hg, respectively) for dapagliflozin 10 mg. The magnitude of the mean reductions was generally stable for the first 52 weeks for this treatment group (−6.73 and −2.91 mm Hg, respectively). These reductions lessened somewhat after week 52 (Table 6). Although the proportion of patients with measured orthostatic hypotension at any assessment time during the 104 weeks was higher for the dapagliflozin groups (7.8% to 15.6%) than for the placebo group (2.9% to 10.5%), this difference appears to reflect a higher rate of orthostatic hypotension seen in dapagliflozin at baseline (14.5% and 14.1% for the dapagliflozin 5- and 10-mg groups, respectively, vs. 7.1% for placebo).
Table 6. Summary of laboratory parameters at 104 weeks.
| Placebo | Dapagliflozin 5 mg | Dapagliflozin 10 mg | |
|---|---|---|---|
| N | 84 | 83 | 85 |
| Serum creatinine (mg/dl) | |||
| Baseline | 1.46 (0.35) | 1.53 (0.33) | 1.52 (0.32) |
| Change at 104 weeks | 0.09 [0.039] | 0.06 [0.043] | 0.12 [0.044] |
| Urinary albumin:creatinine ratio (mg/g) | |||
| Baseline | 478 (1193) | 468 (851) | 401 (785) |
| Change at 104 weeks | 69.7 [80.1] | 78.0 [112.5] | −11.69 [148.6] |
| Urinary protein:creatinine ratio (mg/mg) | |||
| Baseline | 0.75 (1.77) | 0.72 (1.23) | 0.63 (1.12) |
| Change at 104 weeks | 0.09 [0.11] | 0.17 [0.19] | −0.03 [0.21] |
| Serum magnesium (mEq/l) | |||
| Baseline | 1.72 (0.23) | 1.67 (0.20) | 1.70 (0.18) |
| Change at 104 weeks | −0.06 [0.026] | 0.16 [0.034] | 0.12 [0.031] |
| Serum calcium (mg/dl) | |||
| Baseline | 9.55 (0.41) | 9.50 (0.49) | 9.52 (0.42) |
| Change at 104 weeks | −0.22 [0.080] | −0.15 [0.089] | −0.10 [0.067] |
| Serum phosphorus (mg/dl) | |||
| Baseline | 3.84 (0.61) | 3.65 (0.51) | 3.67 (0.52) |
| Change at 104 weeks | −0.13 [0.109] | 0.03 [0.076] | 0.28 [0.075] |
| Parathyroid hormone (pg/ml) | |||
| Baseline | 66.5 (53.0) | 70.2 (57.4) | 68.5 (48.9) |
| Change at 104 weeks | 3.67 [10.31] | 17.26 [8.84] | 15.13 [6.22] |
| Serum uric acid (mg/dl) | |||
| Baseline | 7.05 (1.94) | 7.30 (2.12) | 7.13 (1.71) |
| Change at 104 weeks | −0.16 [0.247] | −0.67 [0.254] | −0.39 [0.204] |
| Systolic blood pressure (mm Hg) | |||
| Baseline | 130.7 (14.1) | 131.8 (17.9) | 133.7 (17.0) |
| Change at 104 weeks | 4.14 (14.07) | −0.25 (18.30) | −2.51 (16.33) |
| Diastolic blood pressure (mm Hg) | |||
| Baseline | 73.6 (9.29) | 72.5 (9.45) | 73.8 (9.30) |
| Change at 104 weeks | −0.48 (9.60) | −0.55 (11.63) | −1.27 (9.49) |
Data are mean (s.d.) at baseline or mean change [s.e.], or (s.d.) for blood pressure, from baseline and include data after rescue.
Laboratory parameters with abnormalities
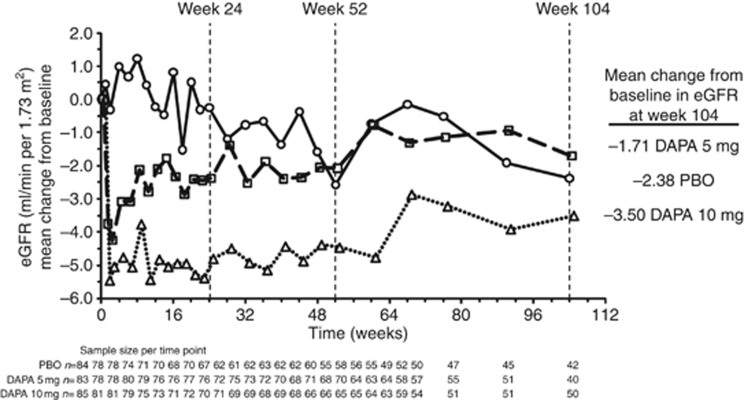
A mean increase from baseline in serum creatinine was observed at week 1 in dapagliflozin 5 mg (0.13 mg/dl) and 10 mg (0.18 mg/dl). The magnitude of this mean increase remained unchanged throughout the remainder of the study for dapagliflozin (Table 6). A similar pattern was seen for eGFR and creatinine clearance, with early mean decreases at week 1 followed by longer-term stability (Table 3 and Figure 3). Mean changes in eGFR through week 104 did not appear to differ for CKD stage 3A or stage 3B patients. The proportion of patients with a marked abnormality in serum creatinine (defined as a value ⩾2.5 or ⩾1.5 mg/dl increase from baseline) through 104 weeks of treatment was similar across the treatment groups. Over time, mean urinary albumin:creatinine and urinary protein:creatinine ratios fluctuated in all groups. Values of urinary albumin:creatinine ratio >1800 mg/g during the 104-week treatment period were reported in a higher proportion of patients in placebo (13.3%) than in dapagliflozin 5 or 10 mg (10.8% and 9.5%, respectively). In shift analyses, urinary albumin:creatinine ratio was characterized as belonging to one of three prespecified categories: 0 to <30 mg/g (normoalbuminuria), 30 to <300 mg/g (microalbuminuria), or ⩾300 mg/g (macroalbuminuria), and showed that for dapagliflozin-treated patients, relative to baseline, 38 patients shifted to a lower category at week 104 (18 patients from microalbuminuria to normoalbuminuria, 19 from macroalbuminuria to microalbuminuria, and 1 from macroalbuminuria to normoalbuminuria) compared with 18 patients who shifted to a higher category (12 patients from normoalbuminuria to microalbuminuria and 6 from microalbuminuria to macroalbuminuria). In the placebo group, the number of patients who shifted to a lower category (nine patients, five from microalbuminuria to normoalbuminuria and four from macroalbuminuria to microalbuminuria) was similar to the number of patients who shifted to a higher category (12 patients, 3 from normoalbuminuria to microalbuminuria and 9 from microalbuminuria to macroalbuminuria). For one patient each in the dapagliflozin groups and the placebo group, the shift was not reported, and the remaining patients did not exhibit a shift from baseline at week 104.
Figure 3.
Mean change from baseline after adjustment for baseline value in eGFR for placebo (circles, solid line), dapagliflozin 5-mg (squares, dashed line), and dapagliflozin 10-mg (triangles, dotted line) groups, all plus original pre-enrollment antidiabetic regimen up to 104 weeks including data after rescue. DAPA, dapagliflozin; eGFR, estimated glomerular filtration rate; PBO, placebo.
No change from baseline was noted in mean serum sodium, potassium, bicarbonate, chloride, or calcium with dapagliflozin or placebo. No evidence of hyponatremia or hypokalemia was observed. Marked abnormalities of elevated potassium (⩾6 mEq/l) were more common in the placebo group (12.0%) than in dapagliflozin 5-mg (4.8%) or 10-mg groups (4.8%) during the study. More patients experienced marked abnormalities of hyperphosphatemia (⩾5.6 mg/dl for patients 17 to 65 years of age or ⩾5.1 mg/dl for patients ⩾66 years of age) on treatment with dapagliflozin 5 mg (15.7%) or 10 mg (14.3%) than placebo (8.4%). Patients receiving dapagliflozin had a larger mean decrease in serum uric acid than those receiving placebo (Table 6). The mean concentration of serum parathyroid hormone exceeded the upper limit of normal at baseline in all treatment groups, and there were larger mean increases in parathyroid hormone in dapagliflozin compared with placebo during the cumulative 104-week period (Table 6).
DISCUSSION
Dapagliflozin's ability to inhibit renal glucose reabsorption declines with decreasing GFR. Urinary glucose excretion is about 50% lower in patients with T2DM treated with dapagliflozin having CKD stage 3, as compared with patients with normal or mildly impaired renal function.11 Such GFR dependence is expected given the fall in filtered glucose load with worsening CKD stage. Consistent with the lower pharmacodynamic activity, dapagliflozin did not have a significant impact on glycemic control in patients with more advanced CKD, although there was a modest decrease in HbA1c and FPG in patients with a GFR of 45–59 ml/min.
Dapagliflozin showed improvements in weight despite lacking glycemic efficacy. Patients receiving dapagliflozin had modest weight loss in contrast to those receiving placebo, who had small weight gain. The placebo-subtracted weight changes observed here are similar to other studies in patients with normal to mildly impaired renal function,4, 5, 6, 7, 8, 9 and the weight loss in this study was similar between patients in CKD stages 3A and 3B. The apparent incongruity in glycemic and weight outcomes may be due to a variety of factors. Patients in this study may have already reached a threshold of glucosuria, beyond which further weight loss was not achieved because of compensatory mechanisms to defend caloric balance, or this patient population may be more susceptible to weight loss from glucosuria, requiring less glucosuria to drive weight change. Hormones regulating blood glucose (for example, glucagon, insulin, and cortisol) are different from those regulating weight (for example, ghrelin and leptin); therefore, one might expect to see different boundaries for these two outcomes. Further investigation is required to understand the dissociation of glycemic efficacy from weight efficacy seen in this trial. The FPG outcome points to the fact that there is still some glycemic efficacy in this group, although this study is not powered to empirically determine significance. The blood pressure response, which is the cumulative effect of weight loss, natriuresis, and osmotic diuresis, is not expected to be directly dependent on glycemic control, although it is partially dependent on weight change.
Adverse events were balanced among treatment and placebo groups through 104 weeks, except the following: (1) vulvovaginitis and balanitis, and (2) fractures. Genital infection was more frequent with dapagliflozin than with placebo, as has been seen in other studies.4, 5, 6, 7, 8, 9 Urinary glucose is thought to explain this increase in infections, serving as a growth substrate for genital fungal pathogens. The significance of the numeric increase in fractures observed with dapagliflozin in this study is uncertain. Many of the fractures were in places not suggestive of bone health issues (for example, toes and patella). The dapagliflozin groups had higher rates of neuropathy and orthostatic hypotension at baseline, conceivably predisposing them to falls. Given the small numbers, the increased fracture incidence could be a chance phenomenon. No increase in fractures was observed in pooled data from patients across the dapagliflozin clinical studies with stage 3A CKD, or in patients with normal to mildly impaired renal function.12, 13 In addition, dapagliflozin has been shown to have no effect on bone mineral density or on markers of bone turnover in patients with normal to mildly impaired renal function.14 Although there was no assessment of biomarkers for bone metabolism conducted for this study, the imbalance in fractures raises a safety question specific to patients with advanced (stage 3B or greater) CKD and calls for further study of the issue whether this population is going to be treated with the drug.
Mean eGFR and creatinine clearance fell after 1 week of dapagliflozin treatment, but stabilized thereafter through 104 weeks of therapy, whereas these parameters slowly declined in the placebo group. The initial reduction in eGFR associated with dapagliflozin may be related to a small antihypertensive and diuretic effect, as well as increased tubuloglomerular feedback.15 Importantly, the change in GFR from baseline at week 104 was similar in the dapagliflozin and placebo groups. Patients receiving dapagliflozin were more likely to regress to a lower albumin excretion category than patients receiving placebo. Thus, it will be of great interest to see whether dapagliflozin reduces renal functional loss and worsening of albuminuria.
Dapagliflozin was associated with fewer episodes of, and fewer discontinuations for, hyperkalemia compared with placebo. This protective effect is most likely due to osmotic diuresis induced by dapagliflozin in patients with restricted ability to excrete potassium.
The study has several limitations. The use of insulin-based regimens in almost two-thirds of patients, often with sliding-scale administration, made accurate capture of insulin dosing difficult. Unmeasured differential behavior with respect to sliding scale and other adjustments of insulin between placebo and dapagliflozin could blunt the apparent efficacy of dapagliflozin. A second limitation is the relatively small size of the study, which was powered for a 0.6% change in HbA1c and therefore limited in its ability to demonstrate smaller glycemic effects. Limited data regarding relevant effect size were available at the time this trial was designed. As we now know from the study of patients with relatively normal kidney function,5 and the diminished pharmacodynamic effect in patients with decreased renal function,16 this study may be considered underpowered and subject to type 2 error. Finally, lack of racial diversity in this study could potentially affect its generalizability.
Improvement of glycemic measures with dapagliflozin in this population was not statistically significant because of the inadequate renal function in these patients. The reduced glucose excretion in this population still appears sufficient to account for the weight loss and blood pressure reduction, which are important therapeutic considerations in patients with diabetes and CKD. Apart from the imbalance in fracture, the safety profile of dapagliflozin was similar to that observed in studies involving patients with T2DM and normal renal function to mild renal impairment.
MATERIALS AND METHODS
Trial design
This multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 2/3 study took place in 111 sites in United States, Argentina, Canada, India, Mexico, Peru, Italy, Australia, France, Spain, Denmark, Puerto Rico, and Singapore. Institutional review boards or independent ethics committees approved this protocol. This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Each patient provided written informed consent.
Male and female patients ⩾18 years old with T2DM and inadequate glycemic control defined as HbA1c ⩾7.0 and ⩽11.0% were enrolled from 19 June 2008 to 21 May 2009. Patients had eGFR values of 30 to 59 ml/min per 1.73 m2 and body mass index⩽45.0 kg/m2. Stable antidiabetic regimen was defined as diet and exercise therapy alone or in combination with a regimen of any approved antidiabetic medication(s), including insulin, in which either doses of oral antidiabetic medications, exenatide, or pramlintide had not changed during 6 weeks before enrollment, or doses of long-acting insulin or intermediate-acting insulin had not varied by >20% during 6 weeks before enrollment. Exclusion criteria included aspartate or alanine aminotransferases >3.0 times the upper limit of normal, serum total bilirubin >2.0 mg/dl, history of diabetes insipidus or diabetic ketoacidosis or hyperosmolar nonketotic coma, uncontrolled hypertension defined as systolic blood pressure ⩾180 mm Hg and/or diastolic blood pressure ⩾110 mm Hg, or specified cardiovascular/vascular diseases within 6 months of enrollment visit. Renal exclusion criteria included the need for hemodialysis or renal replacement therapy, history of rapidly progressing renal disease, lupus nephritis, renal or systemic vasculitis, renal artery stenosis, renal transplant, or hepatic disease. Diabetic nephropathy and hypertension were identified at the discretion of the treating physician, as no strict definitions were provided.
A 7-day lead-in period included diet and exercise counseling, which continued throughout the study. On day 1, patients were randomized in a double-blind manner to either placebo, dapagliflozin 5-mg, or dapagliflozin 10-mg daily, in addition to their original pre-enrollment antidiabetic regimen. Randomization was stratified based on pre-enrollment antihyperglycemic therapy (strata were: insulin-based regimen, sulfonylurea-based regimen, thiazolidinedione-based regimen, or other regimen). During the first 24 weeks (short-term period), patients received rescue medication (any approved antidiabetic agent except metformin) if FPG >270 mg/dl (weeks 4–6), >240 mg/dl (weeks 6–12), or >200 mg/dl (weeks 12–24). Patients completing the first 24 weeks were eligible to continue into an additional 28-week (long-term) period and were eligible to receive rescue medication if HbA1c >8.0%. Patients completing the first 52 weeks (the short-term plus long-term periods) were eligible to continue into the extension period (an additional 52 weeks) and received rescue medication if HbA1c >7.5% (weeks 52–76) and >7.0% (weeks 76–104).
Outcomes of interest
Efficacy analyses included or excluded data after glycemic rescue as indicated. The primary efficacy objective compared the change from baseline in HbA1c with each dose of dapagliflozin vs. placebo at 24 weeks. The secondary objectives compared the following: (1) change from baseline in FPG and weight for each dose of dapagliflozin vs. placebo at 24 weeks; and (2) change from baseline in eGFR (Modification of Diet in Renal Disease Study equation (four-variable)) and creatinine clearance (Cockcroft and Gault method) for each dapagliflozin dose vs. placebo at 52 weeks.
All safety analyses included data after glycemic rescue. Safety outcomes included serious and nonserious adverse events, discontinuations owing to adverse events, hypoglycemia, laboratory abnormalities, electrocardiograms, and vital signs. Seated blood pressure and heart rate determinations were made in triplicate at each study visit, and supine and standing blood pressure measurements were taken at baseline, and at weeks 1, 12, 24, 52, and 104. Urinary albumin:creatinine ratio and urinary protein:creatinine ratio were based on untimed spot urine sample collections. Patients were actively monitored throughout the trial for clinical signs and symptoms suggestive of urinary tract infection and genital infection, with questioning by the investigator at every visit.
A central laboratory evaluated the assays for the efficacy and safety outcomes, using high-performance liquid chromatography for HbA1c and colorimetric indicator for plasma creatinine and urinary albumin:creatinine ratio.
Statistical analysis
The primary efficacy analysis was based on an analysis of covariance model with treatment group and pre-enrollment antihyperglycemic therapy stratum as fixed effects and the baseline value as a covariate. For patients with no week 24 measurement or rescue before week 24, the last available post-baseline measurement (last observation carried forward) before rescue and before week 24 was used.17 Treatment-by-baseline interaction and treatment-by-stratification factor interaction were tested at the 0.10 level of significance, and no significant interaction was found. Several sensitivity analyses (completers, longitudinal repeated-measure analysis with or without data after rescue) were performed. Over 104 weeks, longitudinal repeated-measure analysis was used to estimate the change in HbA1c, FPG, and total body weight from baseline; the model included categorical fixed effects of treatment, strata based on pre-enrollment antidiabetic therapy category, week, and treatment-by-week interaction, as well as continuous fixed covariates of baseline measurement and baseline measurement-by-week interaction.
With 80 patients per treatment group, there was at least 88% power to detect a difference of 0.6% in mean change from baseline in HbA1c between each dapagliflozin treatment group and placebo at a significance level of 0.027, using Dunnett's adjustment and assuming an s.d. of 1.1%. Assuming that 5% of the patients would not have a post-baseline assessment, a total of 252 patients (84 patients per treatment group) needed to be randomized.
Acknowledgments
The study team acknowledges the patients for their participation and commitment during the study. We also thank the investigators and contributors from each study site. We express sincere gratitude to Dr Bruce Leslie for his dedication, involvement, and insights in this study. This Bristol-Myers Squibb and AstraZeneca-supported study is also known as Study MB102029 and is registered with ClinicalTrials.gov, number NCT00663260, available at http://clinicaltrials.gov. Professional medical writing and editorial assistance was provided by Carolyn Carroll, PhD, and Ann L Davis, MPH, employees of Bristol-Myers Squibb. Data from this study were presented at the American Society of Nephrology, Philadelphia, PA, USA, 8–13 November, 2011.
APPENDIX
DEK—consultant for Bristol-Myers Squibb, PF—consultant for BristolMyers Squibb and participant in boards for Boehringer Ingelheim, JFL and WT—employees and shareholders of Bristol-Myers Squibb.
Principal investigator list: Argentina: Fabio Massari, Alejandra Moisello, Alejandra Oviedo, Silvia Saavedra, Georgina Sposetti, Maria Rosa Ulla, Marisa Vico; Australia: Anne Corbould, Timothy Davis, Greg Fulcher, William Jeffries, Robert Moses, Dennis Yue; Canada: Suzan Abdel-Salam, Ronnie Aronson, Andre Belanger, Patrick Duffy, Jean Garon, Chantal Godin, Ronald Goldenberg, Stuart A Ross, Sheldon Tobe, Vincent Woo; Denmark: Thure Krarup, Lise Tarnow, Sten Madsbad; France: Beatrice Duly-Bouhanick, Jean-Francois Gautier, Samy Hadjadj, Veronique Kerlan, Michel Marre, Alfred Penfornis, Pierre-Jean Saulnier; India: V Balaji, Ganapathi Bantwal, Vaishali Deshmukh, Mala Dharmalingam, Jugal Bihari Gupta, Sunil Jain, Shailaja Kale; Italy: Nicola Carulli, Agostino Consoli, Eleuterio Ferrannini, Paola Fioretto, Francesco Dotta, Carlo Bruno Giorda, Gabriele Perriello, Giuseppe Pugliese; Mexico: Manuel Aguilera, Carlos Cano Ramirez, Jose Ricardo Correa Rotter, Jose Gerardo Gonzalez Gonzalez, Marisol Herrera Marmolejo, Guadalupe Morales, Victor Hugo Olmedo Canchola, Juan Rosas Guzman; Peru: Jose Burga, Jose Caballero, Wilson Gallardo, Luis Zapata; Puerto Rico: Jose L Cangiano, Eugenia Galindo, Luis J Quesada; Singapore: Kok Onn Lee; Spain: Fernando De Alvaro, Sonia Gaztambide, Esteban Poch, Ramon Romero; USA: Intekhab Ahmed, Robert J Anderson, Stephen Aronoff, Akhtar Ashfaq, Richard I Bernstein, Samuel S Blumenthal, D Eric Bolster, Gholamreza Bonabi, James J Brown, Anne M Carrol, Richard S Cherlin, James Cimo, William R Cook, Michael E Campolo, Rajesh Kantilai Desai, Donald Eagerton, Steven Fishbane, Jeremy Flood, John Gerich, Harold H Gillum, Daniel Goodman, Simin Goral, Ronald J Graf, Elizabeth L Helfer, Jennifer Hone, Randall T Huling Jr, Eli Ipp, Rajeev Jain, Eric J Klein, Andrew E Lazar, Joseph JC Lee, Dennis HJ Linden, Howard A Lippes, Norman Martin Lunde, Timothy J Lyons, Zuhayr T Madhun, Samuel O Mayeda, Nicholas J Messina III, Jeffrey Lynn Miller, Jesus Ovidio Navarro, Alan E Nolasco, Paul Norwood, John Chip H Reed, III, Daniel B Scheerer, John A Spence, George Tomy, Aaron I Vinik, Peter N Weissman, Thomas Wiegmann.
All the authors declared no competing interests.
References
- Tahrani AA, Bailey CJ, Del Prato S, et al. Management of type 2 diabetes: new and future developments in treatment. Lancet. 2011;378:182–197. doi: 10.1016/S0140-6736(11)60207-9. [DOI] [PubMed] [Google Scholar]
- Wood IS, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr. 2003;89:3–9. doi: 10.1079/BJN2002763. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Lee WS, You G, et al. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest. 1994;93:397–404. doi: 10.1172/JCI116972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E, Ramos SJ, Salsali A, et al. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33:2217–2224. doi: 10.2337/dc10-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CJ, Gross JL, Pieters A, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:2223–2233. doi: 10.1016/S0140-6736(10)60407-2. [DOI] [PubMed] [Google Scholar]
- Strojek K, Yoon KH, Hruba V, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011;13:928–938. doi: 10.1111/j.1463-1326.2011.01434.x. [DOI] [PubMed] [Google Scholar]
- Nauck MA, Del Prato S, Meier JJ, et al. Dapagliflozin vs. glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34:2015–2022. doi: 10.2337/dc11-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstock J, Vico M, Wei L, et al. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35:1473–1478. doi: 10.2337/dc11-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding JP, Norwood P, T'Joen C, et al. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment. Diabetes Care. 2009;32:1656–1662. doi: 10.2337/dc09-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding JP, Woo V, Soler NG, et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156:405–415. doi: 10.7326/0003-4819-156-6-201203200-00003. [DOI] [PubMed] [Google Scholar]
- List JF, Woo V, Morales E, et al. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32:650–657. doi: 10.2337/dc08-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Administration FaD. FDA Brief Document NDA 202293 Dapagliflozin tablets, 5 and 10 mg2011. Available from http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM262994.pdf .
- AstraZeneca B-MSa. Endocrinologic and Metabolic Drugs Advisory Committee Meeting2012. Available from http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM264314.pdf .
- Ljunggren O, Bolinder J, Johansson L, et al. Dapagliflozin has no effect on markers of bone formation and resorption or bone mineral density in patients with inadequately controlled type 2 diabetes mellitus on metformin. Diabetes Obes Metab. 2012;14:990–999. doi: 10.1111/j.1463-1326.2012.01630.x. [DOI] [PubMed] [Google Scholar]
- Thomson SC, Rieg T, Miracle C, et al. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012;302:R75–R83. doi: 10.1152/ajpregu.00357.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasichayanula S, Liu X, Benito MP, et al. The influence of kidney function on dapagliflozin exposure, metabolism, and efficacy in healthy subjects and in patients with type 2 diabetes mellitus. Br J Clin Pharmacol. 2012;76:432–444. doi: 10.1111/bcp.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Administration FaD. Guidance for industry diabetes mellitus: developing drugs and therapeutic biologics for treatment and prevention 2008: Available from www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071624.pdf .