Abstract
Terminal maturation of invariant NKT (iNKT) cells from stage 2 (CD44+NK1.1–) to stage 3 (CD44+NK1.1+) is accompanied by a functional acquisition of a predominant IFN-γ–producing (iNKT-1) phenotype; however, some cells develop into IL-17–producing iNKT (iNKT-17) cells. iNKT-17 cells are rare and restricted to a CD44+NK1.1– lineage. It is unclear how iNKT terminal maturation is regulated and what factors mediate the predominance of iNKT-1 compared with iNKT-17. The tumor suppressor tuberous sclerosis 1 (TSC1) is an important negative regulator of mTOR signaling, which regulates T cell differentiation, function, and trafficking. Here, we determined that mice lacking TSC1 exhibit a developmental block of iNKT differentiation at stage 2 and skew from a predominantly iNKT-1 population toward a predominantly iNKT-17 population, leading to enhanced airway hypersensitivity. Evaluation of purified iNKT cells revealed that TSC1 promotes T-bet, which regulates iNKT maturation, but downregulates ICOS expression in iNKT cells by inhibiting mTOR complex 1 (mTORC1). Furthermore, mice lacking T-bet exhibited both a terminal maturation defect of iNKT cells and a predominance of iNKT-17 cells, and increased ICOS expression was required for the predominance of iNKT-17 cells in the population of TSC1-deficient iNKT cells. Our data indicate that TSC1-dependent control of mTORC1 is crucial for terminal iNKT maturation and effector lineage decisions, resulting in the predominance of iNKT-1 cells.
Introduction
The invariant NKT (iNKT) cells play important roles in both innate and adaptive immune responses (1–4). iNKT cells are generated in the thymus, and their development progresses from stage 0 (CD24+CD44–NK1.1–), to stage 1 (CD24–CD44–NK1.1–), to stage 2 (CD24–CD44+NK1.1–), and finally to stage 3 (CD24–CD44+NK1.1+) (5, 6). iNKT cells express the Vα14-Jα18 T cell receptor (iVα14TCR), which recognizes endogenous, microbial, and synthetic lipid ligands presented by CD1d. Signaling from the iV14TCR is crucial for early iNKT cell development (7–10). iNKT cell terminal maturation from stages 2 to 3 requires signal from the IL-15 and vitamin D receptors as well as the transcription factor T-bet and mediator subunit Med1 (11–14). How T-bet is regulated for iNKT terminal maturation is poorly understood.
One of the most striking features of iNKT cells is their ability to rapidly produce multiple cytokines, such as IL-4, IFN-γ, GM-CSF, IL-10, IL-13, and IL-17. These cytokines greatly affect innate immunity, shape adaptive immune responses, and contribute to the protective and detrimental roles of iNKT cells in various autoimmune, allergic, and inflammatory diseases, in defense against microbial infection, and in tumor surveillance (1–5). Remarkably, the CD44+NK1.1+ terminally matured iNKT cells, which account for about 80% to 90% of total iNKT cells, predominantly produce IFN-γ (referred to as iNKT-1) but not IL-17. IL-17–producing iNKT (iNKT-17) cells are rare and mostly confined to the minor CD4–NK1.1–neuropilin-1+ subset (15–18). The iNKT-17 fate is developmentally programmed, dependent on RORγt, and positively regulated by IL-17 receptor B (17, 19). In contrast, T-bet, which is critical for Th1 differentiation, is essential for iNKT-1 (20, 21). However, the relationship between these two iNKT effector lineages and the mechanisms dictating iNKT-1 predominance over iNKT-17 are poorly understood.
mTOR is a serine/threonine kinase with the ability to integrate environmental stimuli to regulate cell metabolism, survival, growth, and proliferation. mTOR forms two complexes, mTORC1 and mTORC2, with distinct signaling properties and sensitivities to rapamycin. mTORC1 phosphorylates S6K1 and 4EBP-1 to promote protein translation and is sensitive to rapamycin inhibition. mTORC2 phosphorylates AKT, PKC, and PKCθ and is less sensitive to acute rapamycin treatment (22, 23). In T cells, mTOR is activated via the PI3K/AKT and the RASGRP1/RAS/ERK1/2 pathways (24, 25). Deficiency and dysregulation of the RASGRP1/RAS/ERK1/2 pathways impairs iNKT cell development (26, 27). mTOR has been found to promote Th differentiation, control regulatory T cell generation and function, inhibit memory CD8+ T cell response, and regulate T cell trafficking in vivo (23, 25, 28–31).
The tuberous sclerosis 1 (TSC1) associates with TSC2 to form a complex, which inhibits mTORC1 activation by decreasing the active GTP-bound form of RHEB, a small GTPase critical for mTORC1 activation (32, 33). In addition, TSC1 promotes mTORC2 signaling in T cells through yet-to-be determined mechanisms. Deregulation of mTOR signaling due to TSC1 deficiency has been implicated in propensity to death, loss of quiescence, and resistance to anergy of T cells as well as abnormal function of mast cells and macrophages (34–41). While it is becoming clear that TSC1/mTOR signaling is involved in many aspects of T cell biology, the importance of TSC1/mTOR in iNKT cells is unclear. Although it was reported that TSC1-deficient mice contain decreased iNKT cells (35), how TSC1 deficiency affects iNKT cell development and effector function was not reported. We report here that TSC1 is critical for iNKT cell terminal differentiation and for iNKT-1 predominance over iNKT-17 via inhibiting mTORC1-mediated T-bet suppression and subsequent ICOS upregulation.
Results
TSC1 is critical for iNKT cell terminal maturation.
Using real-time quantitative PCR (qPCR), we detected 2- to 3-fold higher levels of Tsc1 and Tsc2 mRNA in iNKT cells than in conventional CD4+- or CD8+-TCRβ+CD1dTet– T (cαβT) cells (Figure 1A). To investigate the role of TSC1 for iNKT cells, we analyzed T cell–specific TSC1-deficient mice, specifically the Tsc1fl/fl-Cd4Cre (TSC1KO) mice (34). In TSC1KO mice, cαβT cell populations in the thymi are grossly normal but are decreased in peripheral lymphoid organs due to increased cell death (34–36). The percentages and total numbers of most thymic iNKT cells and all splenic and liver iNKT cells from TSC1KO mice were 2- to 5-fold lower than those of their Tsc1fl/fl-Cd4Cre– (WT) littermate controls (Figure 1, B and C). Moreover, a drastic decrease of CD44+NK1.1+ stage 3, but increase of CD44+NK1.1– stage 2, iNKT cells was observed in both percentages and absolute numbers in TSC1KO thymi, spleens, and livers (Figure 1, D and E). Although the percentages of CD24–CD44–NK1.1– stage 1 iNKT cells were increased in TSC1KO thymi and spleens, the absolute numbers of these cells were similar to those of the WT controls. iNKT cells contain CD4+CD8– single-positive (SP) and CD4–CD8– double-negative (DN) subsets, but CD4–CD8+ SP iNKT cells are extremely rare. No obvious increase of CD4–CD8+ iNKT cells or change in the ratio between CD4 SP and DN subsets was observed in TSC1KO mice (Figure 1F). Of note, TSC1KO iNKT cells expressed higher levels of iVα14TCR than WT controls (Figure 1B and Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI69780DS1), a phenotype that is also observed in T-bet–deficient iNKT cells (11). Together, these data indicate that TSC1 is critical for iNKT cell terminal maturation.
Figure 1. Critical role of TSC1 for iNKT cell terminal differentiation.
(A) Expression of Tsc1 and Tsc2 in iNKT cells and cαβT cells. mRNAs of indicated molecules from MACS- and FACS-sorted thymic CD1dTet+TCRβ+iNKT and CD1dTet–TCRβ+CD4 and CD8 T cells from WT mice were determined by real-time qPCR. Data shown (mean ± SEM) present values from triplicates and represent 2 experiments. (B) CD1dTet and TCRβ staining of WT and TSC1KO thymocytes (Thy), splenocytes (Spl), and liver MNCs (Liv). Representative dot plots of live-gated cells are shown. (C) Percentages and absolute numbers of CD1dTet+TCRβ+iNKT cells in WT and TSC1KO thymi, spleens, and livers (n = 5). (D) NK1.1 and CD44 staining on gated iNKT cells from B. (E) Percentages and absolute numbers of stage 1 to 3 iNKT cells from WT and KO mice (n = 5). (F) iNKT cell subsets based on CD4 and CD8 staining (n = 6). CD4SP, SP CD4 cells. (G) iNKT cell proliferation in vivo determined by BrdU staining. BrdU+ stage 1 to 3 iNKT cells from WT and TSC1KO thymi after 24-hour BrdU injection are shown (mean ± SEM) (n = 3). (H) Death rates of WT and TSC1KO stage 2 and 3 iNKT cells. *P < 0.05; **P < 0.01; ***P < 0.001, Student’s t test. (B–G) Data shown are representative or calculated from at least 3 experiments.
Using BrdU incorporation to assess iNKT cell expansion in vivo, we observed increased proliferation of stage 1 but similar proliferation of stages 2 and 3 TSC1KO iNKT cells compared with those of WT controls (Figure 1G). These observations suggest that impaired terminal maturation of TSC1KO iNKT cells was not due to defective iNKT cell expansion. Although there was no statistically significant difference in death rates between WT and TSC1KO stage 2 iNKT cells, TSC1KO stage 3 iNKT cells had a 2.4-fold increase of death rate compared with that of WT controls (Figure 1H), which suggests that TSC1 may promote stage 3 iNKT cell survival. Although overexpression of a Bcl2 transgene in TSC1KO mice increased iNKT cell numbers (35), it failed to overcome the developmental blockade of TSC1KO iNKT cells at stage 2 (Supplemental Figure 2). Thus, the iNKT cell developmental blockade caused by TSC1 deficiency appears to be developmentally programed.
Although most TSC1KO mice had decreased iNKT cells in the thymus, a small portion of them had increased iNKT cell percentages and absolute numbers (Supplemental Figure 3, A and B). This increase of iNKT cells occurred only in the thymus and was at low frequency (11%) at 1 month of age but at higher frequencies (∼30%) after 2 months of age. Importantly, iNKT cells from these mice were hyperproliferative, indicated by elevated expression of Ki67, a marker for cell proliferation (Supplemental Figure 3C). These iNKT cells displayed phenotypic heterogeneity among different mice. Within individual mice, these cells were phenotypically predominant in one population, CD44–NK1.1–, CD44intNK1.1–, or CD44+NK1.1– but rarely CD44+NK1.1+. Functionally, they predominantly produced IFN-γ, IL-17, or neither (Wu and Zhong, unpublished observations). While these data suggest that these TSC1-deficient iNKT cells might represent transformed or lymphomatic cells, the cells failed to develop into tumors after adoptive transfer into syngeneic mice.
Reversal of iNKT-1/iNKT-17 dichotomy in TSC1-deficient iNKT cells.
The presence of iNKT cells in TSC1KO mice allowed us to examine how TSC1 deficiency may affect iNKT effector function. Following in vitro α-galactosylceramide (α-GalCer) stimulation for 72 hours, WT iNKT cells predominantly produced IFN-γ but minimally produced IL-17A, IL-17F, and IL-22. In striking contrast, TSC1KO iNKT cells predominantly produced these IL-17 family cytokines, with severely reduced IFN-γ production (Figure 2, A and B). A time course measurement of IFN-γ and IL-17A in culture supernatants further confirmed increased IL-17A but decreased IFN-γ production by TSC1KO iNKT cells (Figure 2C). In contrast to the cytokine abnormality, TSC1KO iNKT cells appeared to proliferate and expand similarly to WT iNKT cells (Supplemental Figure 4). Similar to thymocytes, iNKT cells from TSC1KO spleens also produced more IL-17A but less IFN-γ than did WT controls (Figure 2, D and E). A 5-hour stimulation of enriched WT and TSC1KO iNKT cells with PMA plus ionomycin (P + I) revealed similar increases of iNKT-17 cells and decreases of iNKT-1 cells in TSC1KO mice (Figure 2, F and G). Moreover, NK1.1– stage 2 TSC1KO iNKT cells, which contain the highest number of iNKT-17 cells (15–18), and stage 3 TSC1KO iNKT cells produced more IL-17 than did their respective WT counterparts (Figure 2H). Consistent with these in vitro observations, TSC1KO iNKT cells produced more IL-17A but less IFN-γ in vivo than WT iNKT cells following α-GalCer injection (Supplemental Figure 5). In contrast to iNKT-17 and iNKT-1, IL-4 production by iNKT cells appeared not affected by TSC1 deficiency (Figure 2I). In contrast to deletion of TSC1 during iNKT cell development, acute deletion of TSC1 in developed iNKT cells in Tsc1fl/fl-ERCre mice following tamoxifen treatment did not obviously affect iNKT-1/iNKT-17 balance (Supplemental Figure 6). Moreover, overexpression of BCL-2 was not able to revert the iNKT-17 predominance over iNKT-1 caused by TSC1 deficiency (Supplemental Figure 7). Together, these observations indicate that the reciprocal regulation of IL-17 and IFN-γ by TSC1 in iNKT cells was developmentally programmed, was not due to or at least not solely due to selective expansion of iNKT-17 cells following α-GalCer stimulation, and is not solely caused by an accumulation of stage 2 iNKT cells.
Figure 2. Reciprocal control of iNKT-1 and iNKT-17 by TSC1.
(A and B) Increased IL-17 but decreased IFN-γ production by TSC1KO iNKT cells. WT and TSC1KO thymocytes were stimulated with α-GalCer for 72 hours and with P + I and GolgiPlug in the last 5 hours. (A) Dot plots show indicated intracellular cytokines in gated iNKT cells. (B) Indicated cytokines from multiple experiments (mean ± SEM; IFN-γ and IL-17A, n = 5; IL-17F and IL-22, n = 3). (C) Time course analysis of IFN-γ and IL-17A secretion by WT and TSC1KO iNKT cells. WT and TSC1KO thymocytes were stimulated with α-GalCer for 24 or 48 hours. IFN-γ and IL-17A concentrations in cultural supernatants were measured by ELISA (n = 3). (D and E) Increased IL-17A but decreased IFN-γ production by TSC1KO splenic iNKT cells. WT and TSC1KO splenocytes were stimulated similarly and analyzed as in A and B. (F and G) Developmental programing of iNKT-1 predominance over iNKT-17 by TSC1. iNKT cells enriched from WT and TSC1KO thymocytes were stimulated with P + I and GolgiPlug for 5 hours. (F) Dot plots show indicated intracellular cytokines in gated CD1dTet+TCRβ+Lin–(GR1–B220–CD8–CD11c–CD11b–) iNKT cells. (G) Percentages of indicated cytokines in gated iNKT cells (mean ± SEM; n = 3). (H) Developmental stage analysis of iNKT-1/iNKT-17 in TSC1KO mice. (I) Effect of TSC1 deficiency on IL-4 production by iNKT cells. WT and TSC1KO thymocytes were similarly stimulated with α-GalCer as in A. *P < 0.05; **P < 0.01; ***P < 0.001, Student’s t test. Data shown are representative or calculated from at least 2 experiments.
Enhanced airway hyperresponsiveness in TSC1KO mice in response to α-GalCer challenge.
To determine whether the shift from iNKT-1 to iNKT-17 may affect iNKT cell function in vivo, we examined α-GalCer–induced changes in airway homeostasis with respect to airway hyperresponsiveness. In this model, IL-17 produced by iNKT cells induces contraction of bronchial smooth muscle and neutrophil infiltration, resulting in increased airway resistance (18, 42). Twenty-four hours after α-GalCer treatment, both TSC1KO and WT mice had significant elevations in their responsiveness to succeeding challenge doses of aerosolized methacholine above those of baseline controls; however, for the TSC1KO mice, the level of hyperresponsiveness was significantly increased above the level of airway hyperreactivity observed in the WT mice (Figure 3A). For treated mice, the total cell numbers present in the bronchoalveolar lavage (BAL) fluid from TSC1KO mice were significantly elevated as compared with those of the WT mice (Figure 3B). Neutrophils, but not macrophages, were particularly increased in TSC1KO BAL fluid (Figure 3, C and D). Moreover, following α-GalCer administration (Figure 3E), neutrophilic infiltration in interstitial lung tissue was more apparent for TSC1KO mice than for WT mice and occurred simultaneously with upregulation of IL-17 and downregulation of Ifng mRNA levels in TSC1KO mice (Figure 3F). Similarly, challenge of TSC1KO mice with Streptococcus pneumoniae, which may contain ligands to stimulate iNKT cells (43, 44), induced more severe neutrophil infiltration, correlated with increased IL-17 but decreased IFN-γ expression in iNKT cells, compared with that in WT mice (Figure 3, G and H). Taken together, these observations suggest that TSC1 deficiency results in enhanced IL-17 production in vivo following iNKT stimulation, leading to an enhancement of airway hyperresponsiveness and neutrophil infiltration.
Figure 3. Increased airway hyperreactivity, neutrophilic infiltration, and Il17a mRNA levels in the lungs of TSC1-deficient mice.
WT and TSC1KO mice were treated intranasally (i.n.) with 2 μg α-GalCer in 50 μl PBS. Twenty-four hours later, changes in airway resistance to succeeding doses of aerosolized methacholine were assessed. Mice were then euthanized for collection of BAL fluid, and lung tissues were harvested for histologic examination. (A) Airway response to methacholine. AvgRT, average total pulmonary resistance. (B) Total leukocyte numbers in BAL fluid. (C) Percentages of macrophages and neutrophils in the BAL fluid. (D) Representative H&E staining of WT and TSC1KO BAL fluid infiltrates. Arrows and arrowheads represent neutrophils and macrophages, respectively. (E) Enhanced interstitial infiltration in TSC1KO lungs. Representative H&E staining of lung thin sections is shown. (F) mRNA levels of Il17a (increased) and Ifng (decreased) in the lungs of TSC1KO mice 5 hours after α-GalCer treatment. (G) Neutrophil numbers in the lungs after S. pneumoniae infection. Ctrl, uninfected; Infect, infected. (H) mRNA levels of indicated cytokines in iNKT cells isolated from lungs after S. pneumoniae infection. *P < 0.05; **P < 0.01; ***P < 0.001, 2-way ANOVA (A); Student’s t test (B–H). Data are representative of 2 independent experiments with 4 mice (A–F) and 5 mice (G and H) per group in each experiment. Original magnification, ×400 (D); ×200 (E).
Contribution of cell-intrinsic mechanisms to the developmental defect and iNKT-17 predominance caused by TSC1 deficiency.
Because TSC1 was deficient in all αβ T cells in TSC1KO mice, the aforementioned iNKT cell terminal maturation defect and iNKT-1 to iNKT-17 change in these mice could be caused by extrinsic, intrinsic, or both mechanisms. To distinguish among these possibilities, we generated mixed BM chimeric mice by coinjecting WT CD45.1+and TSC1KO CD45.2+ BM cells at a 1:2.5 ratio into sublethally irradiated Tcra–/– mice (Supplemental Figure 8A). Eight weeks after reconstitution, DN, double-positive (DP), and SP thymocytes developed in the recipient mice (Figure 4A). The WT to KO ratios of DN, DP, and SP thymocytes were close to the original 1:2.5 ratio (Figure 4B), suggesting that TSC1 is not essential for cαβT cell maturation, even under a competitive environment. iNKT cells also developed in the mixed chimeric mice (Figure 4, C and D). Stage 1 and stage 2 iNKT cells predominantly originated from TSC1KO BM hematopoietic stem cells with the WT to KO ratios close to 1:4 to 1:6 (Figure 4E). The relative increases of TSC1KO stage 1 and stage 2 iNKT cells compared with DN, DP, and SP thymocytes were consistent with the increased proliferative potential of these TSC1KO iNKT cells, as shown in Figure 1G. However, the ratio was reversed in stage 3 iNKT cells, with WT-originated iNKT cells predominating over TSC1KO cells at a ratio of more than 2:1. Similar results were also observed in splenocytes and liver mononuclear cells (MNCs) from the chimeric mice (Supplemental Figure 8, B–G). These data confirm that TSC1 is critical for stage 2 to stage 3 maturation but dispensable for stage 1 to stage 2 maturation. The data further indicate that defective terminal maturation of TSC1KO iNKT cells was due to cell-intrinsic mechanisms. Similarly, TSC1KO iNKT cells from the chimeric mice produced less IFN-γ but more IL-17A than WT iNKT cells (Figure 4F), indicating that cell-intrinsic mechanisms led to iNKT-17 predominance over iNKT-1 of TSC1KO iNKT cells.
Figure 4. TSC1 inhibits mTORC1 to promote iNKT terminal maturation and iNKT-1 predominance over iNKT-17.
(A–G) TSC1KO iNKT cell developmental and functional abnormalities are due to cell-intrinsic mechanisms. Sublethally irradiated Tcra–/– mice were i.v. injected with WT (CD45.1) and TSC1KO (CD45.2) BM cells at a 1:2.5 ratio. Thymocytes from chimeric mice were analyzed with or without the indicated stimulations. (A) CD4 and CD8 staining. (B) CD45.1 staining of indicated subsets. (C) TCRβ and CD1dTet staining of live-gated Lin– (GR1–B220–CD8–CD11c–CD11b–) thymocytes. (D) CD44 and NK1.1 staining of gated iNKT cells. (E) CD45.1 staining of indicated iNKT subsets. (F) Intracellular IL-17 and IFN-γ staining in WT and TSC1KO iNKT cells following α-GalCer stimulation for 72 hours. (A–F) Data shown are representative of 3 chimeras from 2 independent experiments. (G) Increased mTORC1 signaling in TSC1KO iNKT cells. WT and TSC1KO thymocytes were stimulated with α-GalCer for 72 hours. Cell lysates from expanded and FACS-sorted live TCRβ+CD1dTet+iNKT cells were subjected to immunoblotting analysis with the indicated antibodies. (H) Rapamycin treatment partially restored iNKT terminal maturation and reverted iNKT-17 predominance to iNKT-1 predominance. WT and TSC1KO mice were i.p. injected with rapamycin (75 μg/kg, every other day 10 times) and euthanized for experiments 24 hours after the last injection. The top row shows TCRβ and CD1dTet staining of thymocytes. The middle row shows NK1.1 and CD44 expression on gated iNKT cells. The bottom row shows IL-17A and IFN-γ staining in iNKT cells following P + I stimulation for 5 hours. Data shown in G and H are representative of 2 independent experiments.
Contribution of enhanced mTORC1 signaling to abnormal iNKT cells development and function of TSC1-deficient iNKT cells.
To determine how TSC1 deficiency may affect mTOR signaling in iNKT cells, we sorted WT and TSC1KO iNKT cells following α-GalCer stimulation for immunoblotting analysis. mTORC1 phosphorylates S6K1, which in turn phosphorylates S6. S6 phosphorylation in TSC1KO iNKT cells was enhanced compared with that in WT controls. In contrast, AKT phosphorylation at S473, an mTORC2-dependent event, was similar between WT and TSC1KO iNKT cells (Figure 4G). Thus, TSC1 deficiency selectively increased mTORC1 signaling in iNKT cells. To determine whether enhanced mTORC1 activity contributed to the iNKT cell abnormalities manifest in TSC1KO mice, we administrated rapamycin to WT and TSC1KO mice every other day 10 times. Rapamycin treatment decreased the expression of the iVα14TCR in TSC1KO iNKT cells and increased stage 3 iNKT cells 5-folds in TSC1KO mice (Figure 4H). Moreover, the aberrant iNKT-17 predominance over iNKT-1 was substantially, although not completely, reversed in rapamycin-treated TSC1KO mice. Together, these observations suggest that TSC1 promotes iNKT cell terminal maturation and programs iNKT-1 predominance over iNKT-17, at least in part, by inhibiting mTORC1.
Altered expression of T-bet, RORγt, ICOS, and other molecules in TSC1KO iNKT cells.
T-bet is critical for iNKT cell terminal maturation and iNKT-1 generation (11, 20, 21). In contrast, RORγt is crucial for iNKT-17 differentiation (17). Consistent with the skewing of iNKT-1 to iNKT-17 lineage, TSC1KO iNKT cells expressed higher levels of RORγt but decreased T-bet protein (Figure 5A). Expression of CD122, a component of the IL-15 receptor that is important for iNKT terminal maturation and is positively controlled by T-bet (12), was also decreased in TSC1KO iNKT cells. Expression of ICOS, which promotes T cell activation, Th17 response, resistance to anergy of TSC1KO CD4 T cells, and iNKT cell survival (37, 45–47), was substantially increased.
Figure 5. Aberrant expression of key molecules involved in iNKT terminal maturation and effector function.
(A–C) Overlaid histograms show expression of T-bet, CD122, RORγt, and ICOS (A) in WT and TSC1KO thymic iNKT cells of different stages; (B) in thymic iNKT cells from WT and TSC1KO mixed BM chimeric mice, as described in Figure 4; (C) and in stage 2 and stage 3 thymic iNKT cells from rapamycin-treated and untreated WT and TSC1KO mice. (D) mRNA levels of indicated molecules in WT and TSC1KO iNKT cells. Expression of indicated mRNA from MACS- and FACS-sorted WT and TSC1KO iNKT cells from freshly isolated thymocytes was quantified by real-time qPCR. Mean ± SEM of triplicates are shown. *P < 0.05; **P < 0.01; ***P < 0.001, Student’s t test. Data shown represent 2 experiments.
Such alterations were similarly observed in TSC1KO iNKT cells from the aforementioned WT and TSC1KO mixed BM chimeric mice (Figure 5B), indicating that cell-intrinsic mechanisms dictated the dysregulation of these molecules in TSC1KO iNKT cells. Moreover, in vivo rapamycin treatment was able to partially or almost completely reverse the aberrant expression of these molecules in TSC1KO iNKT cells (Figure 5C), suggesting that elevated mTORC1 activity contributed to these phenotypes. Similar to the abnormal expression of these proteins, mRNA levels for Rorgt, Rora, and Icos were increased, but Tbx21 mRNA was decreased in TSC1KO iNKT cells (Figure 5D), suggesting that TSC1 at least controls the expression of these molecules at the transcript level. Thpok, a transcription factor was that recently found to promote iNKT-1 but inhibit iNKT-17 (48, 49), was reduced about 50% in TSC1KO iNKT cells, which might also contribute to the iNKT-17 to iNKT-1 predominance in TSC1KO mice. However, the decrease of Thpok was not sufficient to shift the iNKT lineage to CD8+, as it did in THPOK-deficient mice. In addition, expression of Runx1, Il1r, and Il23r molecules promoting Th17 and iNKT-17 differentiation (50, 51) was also increased in TSC1KO iNKT cells, which may promote iNKT-17 differentiation during iNKT development. Socs1, which inhibits IFN-γ receptor signaling and Th1 differentiation (52, 53), was upregulated in TSC1KO iNKT cells. Socs3, which can suppress Th1 and Th17 differentiation (54, 55), was upregulated drastically in TSC1KO iNKT cells. However, Socs5, which suppresses Th2 responses (56), was not obviously altered in TSC1KO iNKT cells (Figure 5D).
Inhibition of iNKT-17 differentiation by T-bet.
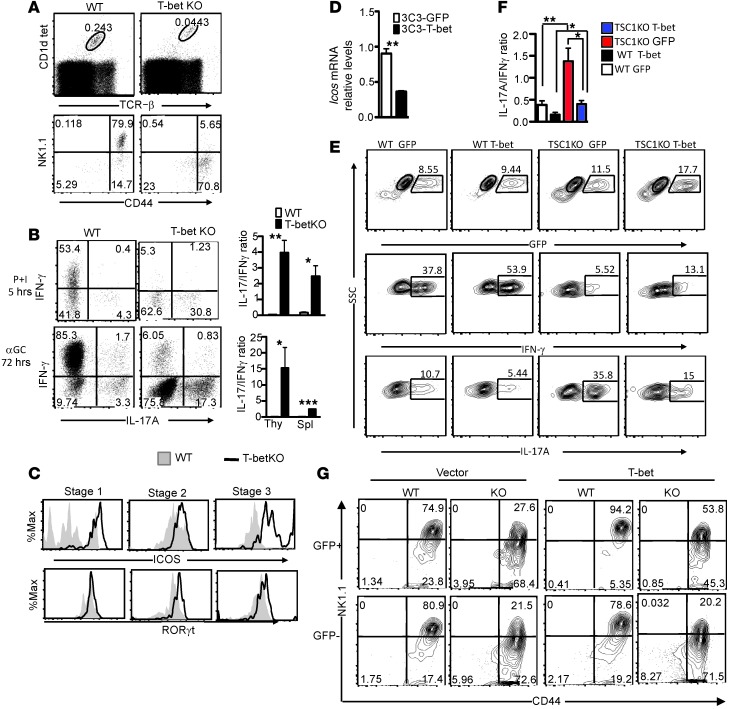
The decreased T-bet expression in TSC1KO iNKT cells prompted us to explore the role of T-bet in the control of iNKT-1/iNKT-17 dichotomy. Although it has been reported that T-bet is important for iNKT-1 differentiation (20, 21), its role in iNKT-17 is unknown. Tbx21–/– (T-betKO) iNKT cells also displayed stage 2 blockade and noted upregulation of iVα14TCR expression (11), similar to that of TSC1KO iNKT cells (Figure 6A). Moreover, T-betKO mice contained not only decreased iNKT-1 cells but also increased iNKT-17 cells (Figure 6B), which correlated with elevated RORγt levels (Figure 6C). Thus, T-bet not only is required for iNKT-1 differentiation but also inhibits iNKT-17 differentiation. Interestingly, ICOS expression was also increased in T-betKO iNKT cells (Figure 6C). Moreover, overexpression of T-bet in an iNKT cell hybridoma reduced Icos expression (Figure 6D), suggesting that T-bet negatively controls ICOS expression in iNKT cells.
Figure 6. TSC1 promotes T-bet expression to establish iNKT–IFN-γ predominance over iNKT-17 and for efficient iNKT terminal maturation.
(A) iNKT cell staining of WT and T-betKO iNKT cells in thymi. (B) IFN-γ and IL-17 staining in WT and T-betKO iNKT cells after P + I stimulation for 5 hours or α-GalCer for 72 hours (mean ± SEM from 4 pairs of mice). (C) ICOS and RORγt staining in WT and T-betKO iNKT cells. (D) Decreased Icos mRNA levels (mean ± SEM) in iNKT hybridoma (3C3) retrovirally transduced with T-bet. (E and F) Overexpression of T-bet promoted IFN-γ but inhibited IL-17 production by iNKT cells. WT and TSC1KO thymocytes were stimulated with α-GalCer in vitro and infected with GFP–expressing (vector) or GFP+T-bet–expressing (T-bet) retrovirus. Two days after infection and with the last 5-hour treatment with P + I, iNKT cells and cytokines were stained. (E) GFP expression in iNKT cells and IFN-γ and IL-17A expression in GFP+ iNKT cells. (F) IL-17A to IFN-γ ratios in GFP+-transduced iNKT cells (mean ± SEM). (G) Overexpression of T-bet partially restored TSC1KO iNKT terminal maturation. WT and TSC1KO BM cells, transduced with retrovirus expressing GFP or GFP+T-bet, were i.v. injected into sublethally irradiated Tcra–/– mice. Recipient mice were analyzed 6 to 8 weeks after reconstitution. Contour plots show CD44 and NK1.1 expression on GFP+ and GFP– live-gated CD1dTet+TCRβ+ iNKT cells. *P < 0.05; **P < 0.01; ***P < 0.001, 1-way ANOVA (F) and Student’s t test (B and D). Data are representative of 3 independent experiments.
TSC1 promotes iNKT cell terminal maturation and iNKT-1 predominance by maintaining T-bet expression.
The resemblance between T-betKO iNKT cells and TSC1KO iNKT cells led us to hypothesize that TSC1 ensures iNKT terminal maturation and dominant iNKT-1 over iNKT-17 fate by promoting T-bet expression. Expression of exogenous T-bet via retroviral transduction increased IFN-γ production but decreased IL-17 production in both WT and TSC1KO iNKT cells following α-GalCer stimulation (Figure 6, E and F), suggesting that decreased T-bet expression in TSC1KO iNKT cells contributed to the iNKT-17 predominance over iNKT-1.
To determine whether decreased T-bet expression may play a role in the defective terminal maturation of TSC1KO iNKT cells, we generated chimeric mice by reconstituting sublethally irradiated Tcra–/– mice with WT and TSC1KO BM cells transduced with T-bet plus GFP or GFP alone. Eight weeks after reconstitution, iNKT cells in the thymi of the recipient mice were analyzed for development based on GFP expression. As shown in Figure 6G, GFP–-untransduced or GFP+-vector control TSC1KO iNKT cells contained fewer stage 3 iNKT cells than WT controls, as expected. However, GFP+-T-bet–expressing TSC1KO iNKT cells contained more stage 3 and fewer stage 2 iNKT cells than GFP–-untransduced or GFP+-vector control TSC1KO iNKT cells, suggesting that decreased T-bet was partially responsible for the defective terminal maturation of TSC1KO iNKT cells. The inability of T-bet overexpression to fully restore TSC1KO terminal maturation could be due to the increased death of TSC1KO stage 3 iNKT cells or other T-bet–independent mechanisms affected by TSC1 deficiency.
ICOS and its regulation by TSC1 affect iNKT-17 and iNKT-1 balance.
The increase of ICOS expression in TSC1KO and T-betKO iNKT cells prompted us to investigate a potential role of ICOS in the regulation of iNKT-1/iNKT-17 balance. Enriched WT thymic iNKT cells were stimulated with P + I and then sorted into CD4+NK1.1–, CD4–NK1.1–, CD4+NK1.1+, and CD4–NK1.1+ subsets for assessing IFN-γ, IL-17A, and ICOS expression (Figure 7A). About a half of the NK1.1– iNKT cells were ICOS+, and IL-17A expression was strictly limited to ICOS+ iNKT cells regardless of CD4 expression. Within the NK1.1+ iNKT subset, fewer than 5% were ICOS+, and IL-17A–expressing cells were also limited to ICOS+ cells. In contrast to IL-17A, IFN-γ was expressed by both ICOS+ and ICOS– iNKT cells. Because more than 90% of iNKT cells were ICOS–, the ICOS– iNKT cell subset represented the major source of IFN-γ–producing cells.
Figure 7. TSC1 ensures the iNKT-1/iNKT-17 dichotomy in part via by inhibiting ICOS expression.
(A) iNKT-17 cells are confined to the ICOS+ subset. CD1dTet-enriched WT thymic iNKT cells were stimulated with PMA and ionomycin for 5 hours in the presence of GolgiPlug followed by TCRβ, ICOS, NK1.1, CD4, and intracellular IFN-γ and IL-17A staining. Dot plots show IFN-γ or IL-17A and ICOS expression in live-gated CD1dTet+TCRβ+Lin– (CD8, CD11b, CD11c, B220) iNKT subsets. (B) ICOS+ and ICOS– iNKT cells in WT and TSC1KO thymi. (C) Expression of IFN-γ and IL-17A in ICOS+ and ICOS– iNKT subsets from WT and TSC1KO thymi. (D and E) Overexpression of ICOS increases IL-17 production by iNKT cells. Thymocytes from WT mice were similarly stimulated with α-GalCer in vitro, infected with retrovirus expressing either GFP or GFP plus ICOS, and analyzed for intracellular cytokine expression, as in Figure 6F. (D) Dot plots show IFN-γ and IL-17A expression in GFP+CD1dTet+TCRβ+ iNKT cells. (E) Percentages of IFN-γ+, IFN-γ+IL-17A+, and IL-17A+ cells in GFP+ iNKT cells (mean ± SEM, n = 4). (F and G) Absence of ICOS partially reverted the iNKT-17 predominance over iNKT-1 caused by TSC1 deficiency. iNKT cells enriched from thymi of indicated mice were similarly stimulated by P + I and analyzed as in A. (F) Contour plots show IFN-γ and IL-17A expression in live-gated CD1dTet+TCRβ+Lin– iNKT cells. (G) IL-17A+ to IFN-γ+ ratios in iNKT cells (mean ± SEM; n = 3). *P < 0.05; **P < 0.01; ***P < 0.001, Student’s t test (E) or 1-way ANOVA (G). Data shown represent 3 independent experiments.
Although the iNKT cell number was decreased in TSC1KO mice, the percentage of ICOS+ iNKT cells in TSC1KO mice was increased more than 20-fold compared with that of WT iNKT cells (Figure 7B). TSC1KO ICOS+ iNKT cells contained a slight decrease in iNKT-1 cells but similar percentages of iNKT-17 cells compared to the WT controls (Figure 7C). TSC1KO ICOS– iNKT cells also had decreased iNKT-1 cells but 10-fold more iNKT-17 cells than did the WT controls. However, TSC1KO ICOS– iNKT cells produced much less IL-17 than the ICOS+ subset. Thus, the increase of ICOS+ iNKT cells was mainly responsible for the iNKT-17 predominance in TSC1KO mice.
To determine whether ICOS plays an important role for iNKT effector differentiation/maintenance, we overexpressed ICOS in WT iNKT cells during in vitro α-GalCer–induced activation. Expression of exogenous ICOS in WT iNKT cells caused a slight decrease of IFN-γ but a 2-fold increase of IL-17A expression (Figure 7, D and E). Interestingly, iNKT cells expressing both IFN-γ and IL-17A, which were rare in WT iNKT cells, were also increased by ICOS overexpression, suggesting the possibility that ICOS may promote the transition of iNKT cells from IFN-γ– to IL-17–producing cells.
Using a complementary approach, we analyzed Icos–/– mice and mice doubly deficient for Icos/Tsc1 (Icos/Tsc1-DKO mice). Deficiency of Icos in TSC1KO mice did not obviously rescue the iNKT terminal maturation defect (Supplemental Figure 9). Icos–/– iNKT cells contained about 50% fewer iNKT-17 cells, without significant changes of iNKT-1 cell numbers compared with those of WT iNKT cells. Moreover, Icos/Tsc1-DKO mice had drastically decreased numbers of iNKT-17 cells but increased numbers of iNKT-1 cells compared with those of TSC1KO mice (Figure 7, F and G).
Together, these observations indicate that iNKT-17 cells are limited to the ICOS+ subset, ICOS positively contributes to iNKT-17 differentiation/maintenance, and elevated ICOS expression in TSC1KO iNKT cells plays a selective and critical role for iNKT-17 predominance over iNKT-1, without obvious contribution to their defective terminal maturation.
Discussion
In this report, we demonstrated that TSC1/mTOR signaling plays critical roles in iNKT cell development and effector lineage decision. We have shown that deficiency of TSC1 results in impaired iNKT terminal maturation, a reversal of the normal iNKT-1 predominance over iNKT-17, and enhanced airway hyperresponsiveness and neutrophil infiltration in vivo following iNKT cell activation.
iNKT cell terminal maturation is a critical step in iNKT cell ontogeny. Although T-bet is pivotal for iNKT cell terminal maturation (11), mechanisms controlling T-bet expression in iNKT cells have been poorly understood. We have demonstrated that TSC1 is crucial for iNKT terminal maturation via promoting T-bet expression. Because iNKT terminal maturation defect and decreased T-bet expression in iNKT cells caused by TSC1 deficiency can be reverted by rapamycin treatment, our data suggest that elevated mTORC1 causes defective terminal maturation and decreased T-bet expression.
Our findings that mTORC1 negatively controls T-bet expression and that TSC1 promotes T-bet expression via inhibiting mTORC1 in iNKT cells are surprising, as T-bet expression has been reported to be dependent on mTORC1 activity in both CD4 and CD8 T cells (28, 30). At present, how TSC1 promotes T-bet expression in iNKT cells is unclear. miR29s inhibit T-bet expression in CD4 T cells (57). We did not observe upregulated miR29 expression in TSC1KO iNKT cells (Wu and Zhong, unpublished observations). Moreover, IFN-γ and IL-12 signaling promotes T-bet expression during Th1 differentiation via STAT1 and STAT4, respectively (58, 59). In mice deficient of either IFN-γ or its receptor, we did not observe impaired iNKT terminal maturation or iNKT-1 to iNKT-17 switch (Wu and Zhong, unpublished observations). While we could not assess an effect of STAT4 deficiency in the BALB/c background on iNKT terminal maturation due to the lack of NK1.1 expression, we found that STAT4 deficiency did not result in decreased T-bet expression or iNKT-1 cells or increased iNKT-17 cells (Wu and Zhong, unpublished observations). Additionally, ablation of mTORC1 activity due to RHEB deficiency in CD4 T cells causes increased SOCS3 expression, leading to impaired Th1 and Th17 differentiation (28). However, TSC1KO iNKT cells with enhanced mTORC1 signaling also contain substantially increased SOCS3. Additional studies are needed to understand how TSC1/mTOR signaling controls T-bet expression in iNKT cells and whether SOCS3 plays a role in determining the iNKT-1/iNKT-17 dichotomy.
While iNKT-1 fate dominates over that of iNKT-17 in WT mice, such predominance is reverted in TSC1KO mice. The mutually exclusive relationship between iNKT-1 and iNKT-17 suggests that they are competing effector fates and that TSC1/mTOR signaling is a crucial regulator in iNKT-1/iNKT-17 lineage decision. The predominance of iNKT-1 over iNKT-17 may explain the suppressive effects of iNKT cells on Th17 responses, as IFN-γ produced by iNKT cells may promote Th1 while inhibiting Th17 (60). Given the multifold functions of mTOR, TSC1 likely promotes iNKT-1 predominance over iNKT-17 via multiple mechanisms. TSC1 may inhibit expression of RORγt and RORα via decreasing IL-1R and IL-23R expression in iNKT cells. It may also promote T-bet expression, not only to increase iNKT-1 generation/maintenance, but also to prevent iNKT-17 generation/maintenance, as T-bet can associate with RUNX1 to suppress RORGT transcription (61). Additionally, T-bet may compete directly with RORγt to prevent IL-17 production. In a NKT cell hybridoma, T-bet and RORγt compete with each other for directing IFN-γ and IL-17 expression, respectively (Supplemental Figure 10). THPOK has also been recently found to promote iNKT-1 but inhibit iNKT-17 and iNKT-2 differentiation (48, 49). The decreased THPOK in TSC1KO iNKT cells may also contribute to the iNKT-17 predominance over iNKT-1. However, in contrast to THPOK-deficient mice, CD8 SP iNKT cells were not increased and iNKT-2 cells were not decreased in TSC1KO mice. Finally, the developmental blockade at stage 2 of TSC1KO iNKT cells may extend the time window for iNKT-17 differentiation but limit optimal iNKT-1 differentiation. Of note, a very recent study reported that iNKT effector lineages diverge during early iNKT development and iNKT-1 and iNKT-17 cells display distinct phenotypes such as NK1.1+ for iNKT-1 and NK1.1– for iNKT-17 cells in C57BL/6J mice (62). It is possible that TSC1 deficiency may directly promote iNKT-17 but inhibit iNKT-1 lineage differentiation during early iNKT cell development. The impairment of iNKT-1 and the enhancement of iNKT-17 lineage differentiation may lead to severe reduction of NK1.1+ (stage 3) and increase of NK1.1– (stage 2) iNKT cells, respectively, in TSC1KO mice.
ICOS provides costimulation for T cell activation, Th17 cell differentiation, and iNKT cell survival (47). We have revealed elevated ICOS expression in TSC1-deficient iNKT cells. Similar to that in iNKT cells, TSC1 also inhibits ICOS upregulation in conventional T cells via downregulation of mTORC1 activity (37, 40). Both transcriptional and posttranscriptional mechanisms regulate ICOS expression (63). Because Icos mRNA is increased in TSC1KO iNKT and T cells, TSC1 may modulate ICOS expression at least through transcriptional regulation. Further studies are needed to illustrate the exact mechanisms by which TSC1 negatively controls ICOS expression. Apparently, the elevated ICOS expression is not sufficient to rescue TSC1KO iNKT cells from death. In TSC1-deficient conventional T cells, the intrinsic death pathway is activated (32, 34, 35), which may also occur in TSC1KO iNKT cells and limit ICOS from preventing death in these cells.
Evidence from our studies reveals an important role of ICOS for the abundance of iNKT-17 cells. First, iNKT-17 cells reside strictly in the ICOS+ subset of iNKT cells. Second, ICOS deficiency leads to a 50% decrease of iNKT-17 cells. Third, overexpression of ICOS during in vitro iNKT stimulation increases IL-17 production. Fourth, the percentage of ICOS+ iNKT-17 cells is increased greatly within TSC1KO iNKT cells. Last, compound ICOS and TSC1 deficiency reverts the iNKT-17 predominance over iNKT-1 caused by TSC1 deficiency. One potential mechanism for the predominance of iNKT-17 over iNKT-1 in TSC1KO mice is increased death of stage 3 iNKT cells, which predominantly contain iNKT-1 cells. Since ICOS deficiency fails to rescue terminal maturation defect but is able to revert iNKT-17 predominance to iNKT-1 predominance caused by TSC1 deficiency, the data suggest that increased death of stage 3 iNKT cells is not the cause of, or at least is not the sole causal factor, for iNKT-17 predominance in TSC1KO mice. At present, whether ICOS participates in iNKT effector lineage fate decision or in the maintenance/expansion of the iNKT-17 lineage remains unclear. The ability of ICOS overexpression to increase iNKT-17 during in vitro iNKT activation suggests that ICOS at least can increase IL-17 production or expansion of already committed iNKT-17 cells. Since ICOS deficiency selectively reverses iNKT-17 predominance of TSC1KO iNKT cells to iNKT-1 predominance, without rescuing their terminal maturation defect, this suggests that the iNKT-17 predominance over iNKT-1 of TSC1KO iNKT cells is unlikely caused by increased death of TSC1KO iNKT-1 cells. In CD4 T cells, ICOS promotes Th17 via inducing c-MAF expression (45, 46). Elevated Maf in TSC1KO iNKT cells (Figure 5D) may strengthen iNKT-17 responses. Interestingly, c-MAF also promotes ICOS transcription (46), suggesting that ICOS and c-MAF may form a positive feedback loop to enhance iNKT-17 in TSC1KO iNKT cells. Of note, ICOS expression is also increased in T-bet–deficient iNKT cells, suggesting that T-bet may directly suppress ICOS transcription. Alternatively, the developmental blockade at stage 2 of T-betKO and TSC1KO iNKT cells may also lead to increased ICOS expression, because ICOS is expressed at the highest level at this stage during iNKT ontogeny.
In summary, iNKT terminal maturation and proper effector function is dependent on TSC1 and its tight control of mTORC1 activity. TSC1 inhibits RORγt and iNKT-17 via negative control of mTORC1 signaling. In contrast to cαβT cells, mTORC1 inhibits T-bet expression in iNKT cells. By inhibiting mTORC1, TSC1 ensures that T-bet expression not only promotes iNKT terminal maturation but also establishes iNKT-1 predominance over iNKT-17. Additionally, downregulation of ICOS by TSC1, possibly via T-bet, contributes greatly to the iNKT-1/iNKT-17 dichotomy (Figure 8). Given the importance of iNKT cells in the pathogenesis of a variety of diseases, including asthma, autoimmune diseases, cancer, and obesity, manipulating TSC1/mTOR and ICOS signaling in iNKT cells may provide new strategies for treatment of these diseases.
Figure 8. Schematic model of TSC1/mTOR signaling in iNKT cell development and iNKT-1/iNKT-17 lineage differentiation (see Discussion for details).

Methods
Mice and cells.
Tsc1fl/fl-Cd4Cre+ and WT mice as well as Tsc1fl/fl-ERCre+ and Tsc1fl/fl-ERCre– mice backcrossed to C57BL/6J background for 8 to 9 generations were previous reported (34, 38, 64). Tcra–/–, Tbx21–/–, Ifng–/–, Ifngr–/–, Stat4–/–, and Icos–/– mice were purchased from The Jackson Laboratory. C57BL/6J and CD45.1+ congenic mice were generated by in-house breeding. Bcl2 transgenic mice and Tsc1fl/fl-Cd4Cre-Bcl2 mice were previously reported (35). Mice used for functional studies were 6 to 8 weeks old and had lower occurrence of “iNKT cell tumor” than older mice. Mice with “tumor-like” increases of iNKT cells (marked by strong Ki67 staining) were excluded from functional studies. All mice were housed in a pathogen-free facility and used according to protocols approved by the IACUC of Duke University and St. Jude Children’s Research Hospital. Tsc1fl/fl-ERCre+ and Tsc1fl/fl-ERCre– mice were intraperitoneally injected with tamoxifen (100 mg/kg body weight) on days 1, 2, and 5 and then euthanized for experiments on day 8 to delete TSC1 in mature iNKT cells. Splenocytes, thymocytes, LN cells, and liver MNCs were made according to previously published protocols (26, 27).
Reagents, plasmids, and antibodies.
Iscove’s modified Dulbecco’s medium (IMDM) was supplemented with 10% (vol/vol) FBS, penicillin/streptomycin, and 50 μM 2-mercaptoethanol (IMDM-10). PE- or APC-conjugated mouse CD1d tetramers loaded with PBS-57 were provided by the NIH Tetramer Facility. Cell death was determined by adding the Live/Dead Fixable Violet Dead Cell Stain (Invitrogen) during iNKT cell staining according to the manufacturer’s protocol and was analyzed by flow cytometry. Dead cells stained positive for Live/Dead. Fluorescence-conjugated anti-mouse CD24 (M1/69), CD44 (IM7), NK1.1 (PK136), CD4 (GK1.5), CD8 (53-6.7), TCRβ (H57-597), CD45.1 (A20), Thy1.1 (OX-7), Thy1.2 (30-H12), CD122 (TM-β1), ICOS (C398.4A), Gr-1 (RB6-8C5), CD11c (N418), CD11b (M1/70), T-bet (4B10), RORγt (ATKJS-9), IFN-γ (XMG1.2), IL-17A (TC11-18 H10.1), IL-17F (9D3.1C8), IL-22 (Poly5164), and IL-4 (11B11) antibodies were purchased from BioLegend. Recombinant murine IL-6 (catalog no. 200-06), SCF (catalog no. 250-03) and IL-3 (catalog no. 213-13) were purchased from PeproTech.
Cell surface staining was performed with 2% FBS-PBS. Intracellular staining for T-bet, RORγt, and Ki67 (BD Biosciences) was performed using the eBioscience Foxp3 Staining Buffer Set. Ki67 was detected with Alexa Fluor 488–conjugated goat anti-mouse IgG (H+L) (Invitrogen). Intracellular staining for IFN-γ, IL-17A, IL-17F, IL-22, and IL-4 was performed using the BD Biosciences Cytofix/Cytoperm and Perm/Wash solutions. All flow cytometry data were collected using a FACS Canto-II (BD Biosciences) and analyzed with FlowJo software. A solution of 0.5% Tween-20-PBS was used to dissolve α-GalCer (Enzo Life Science). Rapamycin was intraperitoneally injected into mice at 75 μg/kg body weight every other day starting on day 1, and mice were euthanized for experiments on day 22. Murine Icos cDNA, amplified from murine T cell RNA using Icos-specific primers (forward, 5′-CTGCTCCTGGCAGACATGAA-3′; reverse, 5′-GTTCCAGCTTATGAGGTCACA-3′) was cloned into the MIGR1 retroviral vector. MIGR1, pRV-T-bet, Migr1-Thy1.1, and Migr1-Thy1.1-RORγt were provided by Warren Pear (University of Pennsylvania, Philadelphia, Pennsylvania, USA), Steve Reiner (University of Pennsylvania), Alejandro Villarino (UCSF, San Francisco, California, USA), and Abul Abbas (UCSF), respectively.
Purification of iNKT cells and real-time qPCR.
iNKT cells were enriched with CD1dTet and MACS-beads according to a previously published protocol (26). Enriched iNKT cells were stained with anti-TCR, anti-ICOS, anti-NK1.1, and 7-AAD. TCRβ+CD1dTet+7 total or NK1.1+ICOS– and NK1.1–ICOS+ iNKT cells were sorted using MoFlo with greater than 98% purity and were immediately lysed in TRIzol for RNA preparation. cDNA was made using the iScript Select cDNA Synthesis Kit (Bio-Rad). Real-time qPCR was performed and analyzed as previously described (39). Expressed levels of target mRNAs were normalized with β-actin and calculated using the 2–ΔΔCT method. Primers are listed in Supplemental Table 1.
Stimulation of iNKT cells.
For α-GalCer stimulation, thymocytes (10 million), splenocytes (5 million), or liver MNCs (2 million) were seeded in a 48-well plate in 1 ml IMDM-10, left unstimulated, or stimulated with α-GalCer (125 ng/ml) for 72 hours, with the addition of PMA (50 ng/ml) and ionomycin (500 ng/ml) as well as GolgiPlug (1 ng/ml) in the last 5 hours. For short-term P + I stimulation, MACS-bead–enriched iNKT cells from thymocytes and splenocytes or density-enriched liver MNCs were seeded in a 96-well V-bottom plate (0.5–1 million per well in 200 μl IMDM-10). Cells were stimulated with P + I for 5 hours in the presence of GolgiPlug. After stimulation, cells were stained with CD1dTet, TCRβ, CD44, NK1.1, IFN-γ, IL-17A, IL-17F, and IL-22. iNKT cells were gated on live (L/D–) and B220–, Gr1–, CD11b–, CD11c–, and CD8– cells.
Western blot analysis.
Following α-GalCer stimulation for 72 hours, iNKT cells were sorted by MoFlo and lysed in lysis buffer (1% nondiet P-40, 150 mM NaCl, 50 mM Tris, pH 7.4) with freshly added protease and phosphatase inhibitors. Cell lysates were subjected to immunoblotting analysis using anti–phospho-S6 S235/236 (2F9), anti-S6, anti–phospho-AKT S473, and anti–β-actin (Cell Signaling Technology) according to a previously published protocol (24).
Airway hyperresponsiveness and inflammation.
Airway hyperresponsiveness to methacholine challenge and pressure-volume curves were measured 24 hours after intranasal injection of WT and TSC1KO mice with 2 μg α-GalCer in 50 μl PBS according to published protocols (65). Immediately after pulmonary function measurements, mice were euthanized, BAL fluids were collected, and differentials were determined. Lungs were fixed in formalin, and H&E staining of lung thin sections was performed following standard protocols. Total RNA, extracted from the lungs 5 hours after α-GalCer injection, was prepared using TRIzol reagents and was used to quantify Il17 and Ifng mRNA levels.
S. pneumoniae infection.
WT and TSC1KO mice were intranasally injected with 5 × 105 CFU S. pneumoniae WU2 strain (provided by Karen Haas, Wake Forest University, Winston-Salem, North Carolina, USA) in 50 μl PBS. Lung from each infected mouse was harvested 5 hours after infection, cut into small pieces, and digested in 1.5 ml IMDM-10 medium with collagenase type IV (3.3 mg/ml, Worthington Biochemical Corporation) and DNase (0.33 mg/ml, Sigma-Aldrich) for 60 minutes. Single cell suspension was stained with CD45, CD11b, and Ly-6G followed by FACS analysis to identify CD45+CD11bhiLy6Ghi neutrophils. In addition, single cell suspension was stained with CD1dTet, 7AAD, and anti-CD45, CD24, and TCRβ antibodies. Live CD45+CD24–TCRβ+CD1dTet+ iNKT cells were sorted using an Astrios sorter (Beckman Coulter) following Percoll gradient separation. Total RNA from sorted iNKT cells was prepared using the TRIzol reagent for real-time qPCR analysis.
BrdU incorporation.
Mice were intraperitoneally injected with 1 mg BrdU (BD Biosciences) in 200 μl PBS. Twenty-four hours later, thymocytes were cell surface stained with CD1dTet, TCRβ, CD44, and NK1.1, followed by intracellular staining for BrdU using a BrdU Flow Kit (BD Biosciences) according to the manufacturer’s protocol.
Retroviral transduction of iNKT and BM cells.
Retroviruses were made using the Phoenix-Eco packaging cell line. Thymocytes (10 million) were seeded in 24-well plates in 1 ml IMDM-10 and stimulated with 125 ng/ml α-GalCer for 24 hours. After replacing 500 μl cultural medium with retroviral supernatants containing polybrene (5 μg/ml final concentration), cells were spin infected at 25°C, 1,250 g, for 1.5 hours. After incubation at 37°C for 6 hours, culture supernatants were replaced with fresh IMDM-10 containing α-GalCer. After overnight culture, cells were spin infected again and cultured for an additional 48 hours before being used for staining.
For transduction of BM cells, 6- to 12-week-old WT and Tsc1fl/fl-Cd4Cre+ mice were sacrificed 4 days after 5-FU treatment. BM cells were seeded in 24-well plate (2–5 million in 1 ml IMDM-10) and stimulated with 10 ng/ml IL-3, 10 ng/ml IL-6, 50 ng/ml SCF. Following stimulation for 24 and 48 hours, BM cells were similarly infected with T-bet–expressing retroviruses, as described above, and were cultured for at least 6 hours after the last infection before injected into sublethally irradiated Tcra–/– mice.
To infect iNKT hybridoma, 5 × 105 NKT hybridoma 3C3 (provided by Kim Nichols, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania, USA, and Mitchell Kronenberg, La Jolla Institute For Allergy and Immunology, La Jolla, California, USA) were seeded in 24-well plates in 1 ml IMDM-10. Cells were spin infected twice in the same manner as the primary iNKT cells. Forty-eight hours after infection, cells were stimulated with P + I for 5 hours to detect cytokine production. Infected cells were also sorted to make RNA for assessing Icos mRNA levels.
BM chimeric mice.
Tcra–/– mice were sublethally irradiated (6 Gy) and intravenously injected with a mixture of WT (CD45.1+) and TSC1KO (CD45.2+) BM cells at indicated ratios or with retrovirally transduced BM cells. The recipient mice were analyzed 8 weeks later.
Statistics.
Data are presented as mean ± SEM, and statistical significance was determined by 2-tailed Student’s t test or by 1-way or 2-way ANOVA. A P value of less than 0.05 was considered statistically significant.
Study approval.
Animal studies were performed according to protocols approved by the IACUC of Duke University and by the IACUC at St. Jude Children’s Research Hospital.
Supplementary Material
Acknowledgments
We thank Kim Nichols and Mitchell Kronenberg for iNKT hybridomas; Steve Reiner, Alejandro Villarino, and Abul Abbas for providing T-bet, RORγt, and Thy1.1 retroviral vectors; Karen Haas for providing S. pneumoniae; the NIH Tetramer Core Facility for CD1d tetramers; and the flow cytometry core facility at Duke University for cell sorting. The study is supported by the NIH (AI076357, AI079088, and AI101206) and the American Cancer Society (RSG-08-186-01-LIB) to X.-P. Zhong.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article:J Clin Invest. 2014;124(4):1685–1698. doi:10.1172/JCI69780.
References
- 1.Brutkiewicz RR, Sriram V. Natural killer T (NKT) cells and their role in antitumor immunity. Crit Rev Oncol Hematol. 2002;41(3):287–298. doi: 10.1016/S1040-8428(01)00198-6. [DOI] [PubMed] [Google Scholar]
- 2.Van Kaer L. NKT cells: T lymphocytes with innate effector functions. Curr Opin Immunol. 2007;19(3):354–364. doi: 10.1016/j.coi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Cerundolo V, Kronenberg M. The role of invariant NKT cells at the interface of innate and adaptive immunity. Semin Immunol. 2010;22(2):59–60. doi: 10.1016/j.smim.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Alonzo ES, Sant’Angelo DB. Development of PLZF-expressing innate T cells. Curr Opin Immunol. 2011;23(2):220–227. doi: 10.1016/j.coi.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 6.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11(3):197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 7.Kawano T, et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 8.Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol. 2001;2(10):971–978. doi: 10.1038/ni710. [DOI] [PubMed] [Google Scholar]
- 9.Mendiratta SK, Martin WD, Hong S, Boesteanu A, Joyce S, Van Kaer L. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6(4):469–477. doi: 10.1016/S1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 10.Das R, Sant’Angelo DB, Nichols KE. Transcriptional control of invariant NKT cell development. Immunol Rev. 2010;238(1):195–215. doi: 10.1111/j.1600-065X.2010.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Townsend MJ, et al. T-bet regulates the terminal maturation and homeostasis of NK and Vα14i NKT cells. Immunity. 2004;20(4):477–494. doi: 10.1016/S1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 12.Gordy LE, et al. IL-15 regulates homeostasis and terminal maturation of NKT cells. J Immunol. 2011;187(12):6335–6345. doi: 10.4049/jimmunol.1003965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yue X, Izcue A, Borggrefe T. Essential role of Mediator subunit Med1 in invariant natural killer T-cell development. Proc Natl Acad Sci U S A. 2011;108(41):17105–17110. doi: 10.1073/pnas.1109095108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu S, Cantorna MT. The vitamin D receptor is required for iNKT cell development. Proc Natl Acad Sci U S A. 2008;105(13):5207–5212. doi: 10.1073/pnas.0711558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coquet JM, et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc Natl Acad Sci U S A. 2008;105(32):11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milpied P, et al. IL-17-producing invariant NKT cells in lymphoid organs are recent thymic emigrants identified by neuropilin-1 expression. Blood. 2011;118(11):2993–3002. doi: 10.1182/blood-2011-01-329268. [DOI] [PubMed] [Google Scholar]
- 17.Michel ML, et al. Critical role of ROR-γt in a new thymic pathway leading to IL-17-producing invariant NKT cell differentiation. Proc Natl Acad Sci U S A. 2008;105(50):19845–19850. doi: 10.1073/pnas.0806472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michel ML, et al. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204(5):995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watarai H, et al. Development and function of invariant natural killer T cells producing Th2- and Th17-cytokines. PLoS Biol. 2012;10(2):e1001255. doi: 10.1371/journal.pbio.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuda JL, Zhang Q, Ndonye R, Richardson SK, Howell AR, Gapin L. T-bet concomitantly controls migration, survival, and effector functions during the development of Vα14i NKT cells. Blood. 2006;107(7):2797–2805. doi: 10.1182/blood-2005-08-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HY, et al. The development of airway hyperreactivity in T-bet-deficient mice requires CD1d-restricted NKT cells. J Immunol. 2009;182(5):3252–3261. doi: 10.4049/jimmunol.0803339. [DOI] [PubMed] [Google Scholar]
- 22.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee K, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32(6):743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorentla BK, Wan CK, Zhong XP. Negative regulation of mTOR activation by diacylglycerol kinases. Blood. 2011;117(15):4022–4031. doi: 10.1182/blood-2010-08-300731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinclair LV, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9(5):513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen S, Chen Y, Gorentla BK, Lu J, Stone JC, Zhong XP. Critical roles of RasGRP1 for invariant NKT cell development. J Immunol. 2011;187(9):4467–4473. doi: 10.4049/jimmunol.1003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen S, et al. Tight regulation of diacylglycerol-mediated signaling is critical for proper invariant NKT cell development. J Immunol. 2011;187(5):2122–2129. doi: 10.4049/jimmunol.1100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delgoffe GM, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12(4):295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460(7251):108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32(1):67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 2013;499(7459):485–490. doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Brien TF, Zhong XP. The role and regulation of mTOR in T-lymphocyte function. Arch Immunol Ther Exp (Warsz). 2012;60(3):173–181. doi: 10.1007/s00005-012-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37(Pt 1):217–222. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Brien TF, et al. Regulation of T-cell survival and mitochondrial homeostasis by TSC1. Eur J Immunol. 2011;41(11):3361–3370. doi: 10.1002/eji.201141411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang K, Neale G, Green DR, He W, Chi H. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat Immunol. 2011;12(9):888–897. doi: 10.1038/ni.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Q, et al. The tuberous sclerosis complex-mammalian target of rapamycin pathway maintains the quiescence and survival of naive T cells. J Immunol. 2011;187(3):1106–1112. doi: 10.4049/jimmunol.1003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie DL, Wu J, Lou YL, Zhong XP. Tumor suppressor TSC1 is critical for T-cell anergy. Proc Natl Acad Sci U S A. 2012;109(35):14152–14157. doi: 10.1073/pnas.1119744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan H, O’Brien TF, Zhang P, Zhong XP. The role of tuberous sclerosis complex 1 in regulating innate immunity. J Immunol. 2012;188(8):3658–3666. doi: 10.4049/jimmunol.1102187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin J, Pan H, Zhong XP. Regulation of mast cell survival and function by tuberous sclerosis complex 1. Blood. 2012;119(14):3306–3314. doi: 10.1182/blood-2011-05-353342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong XP. An expanded role of the tumor suppressor TSC1 in T cell tolerance. Cell Cycle. 2012;11(21):3909–3910. doi: 10.4161/cc.22235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12(5):325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kudo M, et al. IL-17A produced by alphabeta T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med. 2012;18(4):547–554. doi: 10.1038/nm.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kinjo Y, et al. Invariant natural killer T cells recognize glycolipids from pathogenic Gram-positive bacteria. Nat Immunol. 2011;12(10):966–974. doi: 10.1038/ni.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brigl M, et al. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med. 2011;208(6):1163–1177. doi: 10.1084/jem.20102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bauquet AT, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10(2):167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akbari O, et al. ICOS/ICOSL interaction is required for CD4+ invariant NKT cell function and homeostatic survival. J Immunol. 2008;180(8):5448–5456. doi: 10.4049/jimmunol.180.8.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Enders A, et al. ZBTB7B (Th-POK) regulates the development of IL-17-producing CD1d-restricted mouse NKT cells. J Immunol. 2012;189(11):5240–5249. doi: 10.4049/jimmunol.1201486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Engel I, Zhao M, Kappes D, Taniuchi I, Kronenberg M. The transcription factor Th-POK negatively regulates Th17 differentiation in Vα14i NKT cells. Blood. 2012;120(23):4524–4532. doi: 10.1182/blood-2012-01-406280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doisne JM, et al. Cutting edge: crucial role of IL-1 and IL-23 in the innate IL-17 response of peripheral lymph node NK1.1- invariant NKT cells to bacteria. J Immunol. 2011;186(2):662–666. doi: 10.4049/jimmunol.1002725. [DOI] [PubMed] [Google Scholar]
- 51.Rachitskaya AV, et al. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180(8):5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diehl S, et al. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity. 2000;13(6):805–815. doi: 10.1016/S1074-7613(00)00078-9. [DOI] [PubMed] [Google Scholar]
- 53.Alexander WS, et al. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell. 1999;98(5):597–608. doi: 10.1016/S0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- 54.Egwuagu CE, Yu CR, Zhang M, Mahdi RM, Kim SJ, Gery I. Suppressors of cytokine signaling proteins are differentially expressed in Th1 and Th2 cells: implications for Th cell lineage commitment and maintenance. J Immunol. 2002;168(7):3181–3187. doi: 10.4049/jimmunol.168.7.3181. [DOI] [PubMed] [Google Scholar]
- 55.Chen Z, et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103(21):8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seki Y, et al. Expression of the suppressor of cytokine signaling-5 (SOCS5) negatively regulates IL-4-dependent STAT6 activation and Th2 differentiation. Proc Natl Acad Sci U S A. 2002;99(20):13003–13008. doi: 10.1073/pnas.202477099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steiner DF, et al. MicroRNA-29 regulates T-box transcription factors and interferon-gamma production in helper T cells. Immunity. 2011;35(2):169–181. doi: 10.1016/j.immuni.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Afkarian M, et al. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3(6):549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 59.Yang Y, Ochando JC, Bromberg JS, Ding Y. Identification of a distant T-bet enhancer responsive to IL-12/Stat4 and IFNgamma/Stat1 signals. Blood. 2007;110(7):2494–2500. doi: 10.1182/blood-2006-11-058271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mars LT, et al. Invariant NKT cells inhibit development of the Th17 lineage. Proc Natl Acad Sci U S A. 2009;106(15):6238–6243. doi: 10.1073/pnas.0809317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lazarevic V, et al. T-bet represses Th17 differentiation by preventing Runx1-mediated activation of the gene encoding RORγt. Nat Immunol. 2011;12(1):96–104. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14(11):1146–1154. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Glasmacher E, et al. Roquin binds inducible costimulator mRNA and effectors of mRNA decay to induce microRNA-independent post-transcriptional repression. Nat Immunol. 2010;11(8):725–733. doi: 10.1038/ni.1902. [DOI] [PubMed] [Google Scholar]
- 64.Pan H, et al. Critical Role of the Tumor Suppressor Tuberous Sclerosis Complex 1 in Dendritic Cell Activation of CD4 T Cells by Promoting MHC Class II Expression via IRF4 and CIITA. J Immunol. 2013;191(2):699–707. doi: 10.4049/jimmunol.1201443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Foster WM, Adler KB, Crews AL, Potts EN, Fischer BM, Voynow JA. MARCKS-related peptide modulates in vivo the secretion of airway Muc5ac. Am J Physiol Lung Cell Mol Physiol. 2010;299(3):L345–L352. doi: 10.1152/ajplung.00067.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.