Abstract
We have previously reported that angiotensin receptor blockade reduces reperfusion hemorrhage in a suture occlusion model of stroke, despite increasing matrix metalloproteinase (MMP-9) activity. We hypothesized that candesartan will also decrease hemorrhage associated with delayed (6h) tissue plasminogen activator (tPA) administration after embolic stroke, widening the therapeutic time window of tPA. Adult male Wistar rats were subjected to embolic middle cerebral artery occlusion (eMCAO) and treated with either candesartan (1mg/kg) alone early at 3h, delayed tPA (10mg/kg) alone at 6h, the combination of candesartan and tPA, or vehicle control. Rats were sacrificed at 24 and 48h post-eMCAO and brains perfused for evaluation of neurological deficits, cerebral hemorrhage in terms of hemoglobin content (Hb), occurrence rate of hemorrhage, infarct size, tissue MMP activity and protein expression. The combination therapy of candesartan and tPA after eMCAO reduced the brain hemorrhage, and improved neurological outcome compared with rats treated with tPA alone. Further, candesartan in combination with tPA increased activity of MMP-9 but decreased MMP-3, nuclear factor kappa-B (NF-κB) and tumor necrosis factor-α (TNF-α) expression and enhanced activation of endothelial nitric oxide synthase (eNOS). An activation of MMP-9 alone is insufficient to cause increased hemorrhage in embolic stroke. Combination therapy with acute candesartan plus tPA may be beneficial in ameliorating tPA-induced hemorrhage after embolic stroke.
Keywords: candesartan, embolic stroke, matrix metalloproteinases, tissue plasminogen activator, hemorrhage
INTRODUCTION
Thrombolytic therapy with tissue plasminogen activator (tPA) remains the most effective treatment for ischemic stroke. Unfortunately, tPA is associated with a significant risk of hemorrhage and this, along with a narrow therapeutic time window, remains an important impediment to widespread adoption of the therapy [1].
It is hoped that combination therapy using tPA and a neuroprotective agent will achieve better outcomes than using either drug alone. Therefore, many neuroprotective agents have been tested in combination with tPA, and, while some of them have been able to protect the vascular unit and inhibit hemorrhage, others may increase hemorrhage [2]. Several recent studies have reinforced the observation that some drugs may increase hemorrhage when combined with tPA [2]. The most recent surprising finding was that of erythropoietin (EPO) in combination with tPA. Although a promising vascular protective agent alone [3], a recent clinical trial identified an increase in intracerebral hemorrhage and mortality when EPO was administered in combination with tPA [4]. Subsequently, it was reported that EPO promotes extracellular matrix degradation and edema formation in animals treated with tPA [5, 6]. The EPO development story points to the importance of preclinical combination investigations prior to clinical trials, in order to identify potential negative interactions of treatments.
Growing interest in the link between increase matrix metalloproteinases (MMPs) activation and tPA-thrombolytic therapy are at a higher risk for hemorrhage after ischemic stroke [7-9]. MMPs are a family of zinc-dependent proteases that degrade the extracellular matrix proteins [10, 11]. Although there are many MMPs, an important role for MMP-9 in particular has been suggested in hemorrhage after stroke. Stromelysin-1 (MMP-3) is a member of the MMP family that deserves particular attention for acute neurotoxicity after intracerebral hemorrhage (ICH) [12-14]. Moreover, increased MMP-3 in plasma is correlated with the majority of mortality after ICH in humans [15]. More recently, the intracranial bleeding after tPA treatment of stroke in mice was found to be attenuated in MMP-3 null, but not MMP-9 null, mice compared to wild-type controls, implicating MMP-3 in tPA-induced hemorrhage [16].
The angiotensin II type 1 receptor (AT1R) blocker, candesartan, has long been known to reduce injury due to ischemic stroke [17-19] and we have shown it to normalize blood pressure (BP), reduce neurovascular damage, and improve outcome in both permanent and transient stroke models [19-24]. However, the documented increase in the activity of the MMPs due to candesartan treatment, increased concern of a potential negative interaction with tPA. We hypothesized that the combination treatment of candesartan with delayed (6h) tPA will counteract the tPA-induced brain hemorrhage, despite increasing MMP-9 activity. This treatment will improve the safety and impact of tPA in a rat model of embolic stroke, one of the best-characterized and clinically relevant models of stroke [25, 26].
Materials and Methods
Animals and treatment regimen
Male Wistar rats (330–350g; Charles River Laboratories, Wilmington, MA) were used according to procedures approved by the Institutional Animal Care and Use Committee (IACUC) of the Charlie Norwood VA Medical Center (ACORP#11-03-032). The study was performed in two separate experiments. Experiment I was carried out to evaluate the effects of combination treatment on neurological outcome, hemorrhage, and infarct size at 48h post-embolic middle cerebral artery occlusion (eMCAO). The groups (N=8) were: eMCAO + early (3h) candesartan (1mg/kg) treatment only group I (cand only); eMCAO + vehicles treatment group II (vehicle only); embolic middle cerebral artery occlusion (eMCAO) + vehicle followed by tPA (10mg/kg, IV at 6h) treatment group III (tPA only); eMCAO + early (3h) candesartan (1mg/kg) followed by tPA (10mg/kg, IV at 6h) treatment group IV (cand 3h+tPA). Experiment II was carried out to evaluate the effects of combination treatments on the MMP activity and protein expression at 24h post-eMCAO. This experiment was divided into four groups (N=5), after eMCAO rats treated with either candesartan (1mg/kg) alone early at 3h, tPA (10mg/kg) alone at 6h, the combination of candesartan and tPA, or vehicle control.
The dose (1mg/kg, in saline) of candesartan used in this study was determined from our previous work demonstrating the neurovascular protection and improves functional outcome by decreases the acute stroke induced elevation of BP in suture model of stroke in Wistar rats [20, 23, 24].
tPA administration: For thrombolytic intervention, tPA (Activase-Genentech, Inc., San Francisco, CA, USA) was dissolved in sterile water and administered intravenously at a dose of 10mg/kg body weight with a 10% bolus and 90% continuous infusion over 30min started at 6h after embolism using a Harvard 11 Plus Syringe Pump (Harvard apparatus, USA). After tPA or vehicle administration, rats were returned to their cages. Body temperature was maintained at 37±0.5°C with heating pad duration of surgery. The mortality rate was determined at 48h. The relatively high dose of tPA was necessary to achieve a fibrinolytic effect in rats similar to that of thrombolytic therapy in humans. Because delayed reperfusion with tPA (10mg/kg) at 6h is reported to cause increased hemorrhage and worsen stroke outcome in this embolic model of stroke [5, 27], it is appropriate to determine the ability of candesartan therapy to add benefit and improve the safety of tPA, widening the therapeutic window.
Rat model of embolic stroke
Preparation of the clot
The blood donor rat was anesthetized with 1.5% isoflurane. Fresh arterial blood was withdrawn from the heart by 2 ml syringe. To increase the strength and uniformity of the fibrin rich core of the clot and stability of the occlusion, blood was supplemented with human thrombin (2 mg/mL), and retained in 25 cm of PE-50 tubing for overnight at room temperature and subsequently was stored at 4 °. Five centimeters of the PE-50 tubing containing clot was cut and attached to a system built of two syringes interconnected by 40 cm PE-50 tube filled with phosphate buffer saline (PBS). The clot was continuously shifted from one syringe to the other for 5 minutes. A single clot 35-40 mm was transferred to a PE-10 catheter filled with PBS.
Surgical procedure
Male Wistar rats were subjected to embolic middle cerebral artery occlusion (eMCAO) by placement of an embolus at the origin of the MCA, as described previously with slight modification [25, 26]. Briefly, prior to eMCAO, isoflurane anesthesia was induced by 5% and then maintained at 1.5–2% during surgery. Temperature was monitored and maintained (37 ±2°) during surgery by a homeothermic heating system (Fine Science Tools, Foster City, CA). A modified polyethylene (PE)-10 catheter containing a single fibrin rich clot (3.5 ±0.5cm length) was introduced into the external carotid artery (ECA) lumen through a small puncture and advanced into the internal carotid artery (ICA). The clot was gently injected with 100 μL of PBS. Catheter was removed immediately after embolization. After closure of the surgical site, the animals were allowed to awaken from anesthesia and subsequently transferred into the cage.
Cerebral perfusion imaging using laser doppler imaging system
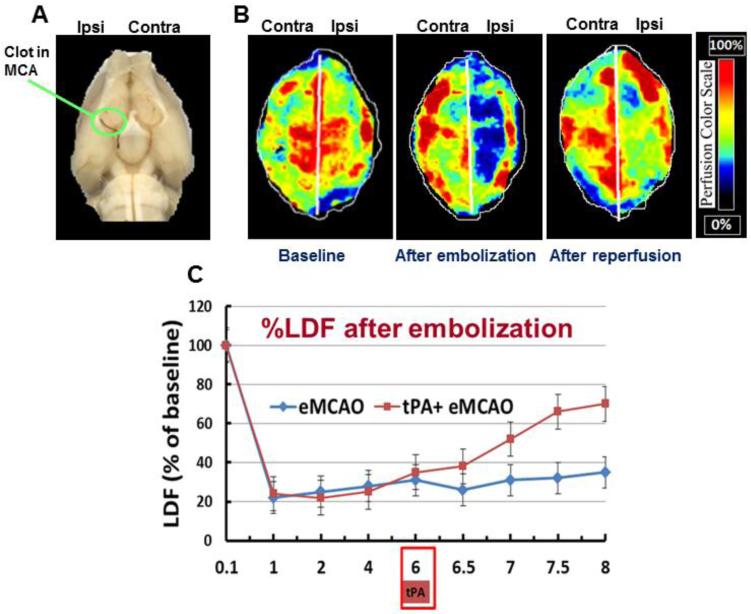
To ensure relative uniformity of the ischemic insult, in a separate sub-set of animals (N= 3), cerebral perfusion was measured using the Periscan PIM 3 System (Stockholm, Sweden). A skin incision was performed, and the skull was exposed and cleaned. Whole brain scan was performed using the PIM3 to measure cerebral perfusion in both hemispheres at baseline, after embolization and after reperfusion. A built-in photo detector assisted with LDPI win software (Perimed Inc) detected the reflected light from moving blood cells within 0.5 cm depth of the cortical surface. Color-coded images were acquired 3 times continuously, and the average CBF was calculated based on the concentration and mean velocity of the blood cells using the LDPI win software. On induction of ischemia, cerebral perfusion decreased to 20% to 30%, remaining stable throughout the 6h of MCA occlusion. Reperfusion was associated with a restoration of blood flow to 80-90% of baseline values in vehicle-treated control animals (Fig. 1).
Fig 1.
Rat embolic middle cerebral artery occlusion (eMCAO): (A) The clots were visualized in MCA origin with Evan’s Blue dye (arrowhead). (B) Representative images of cerebral perfusion detected with laser Doppler imaging system (Periscan PIM 3, laser scanner). The image illustrates the percentage of CBF change in ischemic (ipsilateral) versus contralateral hemisphere at 10 min before and after occlusion and 30min after reperfusion with tPA (C) The CBF over time as measured with Laser Doppler flowmeter (means ± SEM, n=3). CBF declines to 21±4% of baseline after embolization, and reperfusion with tPA overtime (8h).
Neurological assessment
Neurological deficits was evaluated in a blinded manner at 48 h (just before animals were sacrificed) using the Bederson score [28]. An animal with no apparent deficits obtained a 0; the presence of forelimb flexion, 1; decreased resistance to push, 2; and circling, 3.
Assessment of infarct size and hemorrhage
At 48h after the onset of eMCAO, anesthesia was performed with ketamine 44 mg/kg and xylazine 13 mg/kg administered intramuscularly; animals were then perfused with saline, killed, and their brains were removed. The brain tissue was sliced into seven 2mm-thick slices in the coronal plane and stained with a 2% solution of 2, 3, 5-triphenyltetrazolium chloride (Sigma, Chemical Co., St. Louis, Missouri, USA) for 15–20 min. Images of the stained sections were scanned using ImageJ analysis software (Image J, NIH) infarction zones were measured and the percentage infarct size was calculated and expressed as a percentage of the contralateral side ± SEM. Volume calculation with edema correction was performed blindly using the following formula: 100× (volume of ipsilateral hemisphere – volume of contralateral hemisphere)/volume of contralateral hemisphere.
Bleeding was quantified using two different methods. 1) A colorimetric hemoglobin detection assay (QuantiChrom Hemoglobin Assay Kit, BioAssay Systems; Haywood, CA) was performed on brain tissue. First, TTC-stained brain samples were separated in contralateral and ipsilateral hemispheres, and homogenized in a 10% glycerol-Tris 146 buffered saline solution containing Tween-20. Samples were prepared, read at 562 nm using a standard microplate reader, and hemoglobin concentration was calculated according to the manufacturer’s instructions. 2) Hemorrhagic occurrence rate (presence of macroscopic bleeding) by visual inspection at the time of sacrifice was also recorded and compared between the treatment groups.
Gelatin zymography
Substrate-specific zymography for determination of gelatinolytic activity of MMP-9 and MMP-2 was performed on brain homogenates taken 24 h after eMCAO as described previously [20]. The concentration of protein was adjusted equally in all the tissue samples. Samples were then mixed 1:1 with loading buffer (80 mmol/L Tris-HCl [pH 6.8], 4% SDS, 10% glycerol, and 0.01% bromphenol blue) and left standing for 10 min at room temperature. Proteins were separated by electrophoresis in a 10% SDS-PAGE gel containing 0.1% gelatin at 125 volts constant current. Gels were then washed to remove SDS with 2.5% Triton X-100 (Sigma) for 1 h and incubated at 37°C with developing buffer (50 mmol/L Tris-HCl [pH 7.5], 10 mmol/L CaCl2, 0.02% NaN3) for 36 h. Enzymatic bands were visualized after staining for 1h with Coomassie blue (BioRad) R-250 for 30 min, and de-stained with three changes of methanol: acetic acid: water (50:10:40). The gel was scanned and the bands of activity were quantified using Image J analysis software (Image J, NIH).
Western blotting
Brains were homogenized and processed for western blotting as previously described [21]. 50 ug of proteins were loaded in each lane and separated followed by transfer to nitrocellulose membranes. The membranes were blocked using 5% nonfat milk in TBST (1% tween 20 in tris buffered saline) and probed with the following antibodies active-MMP-3(1:500, abcam; Cambridge, MA), TNF-α (1:1000, 1:500, abcam; Cambridge, MA), p-NF-κBp65 (1:500, abcam; Cambridge, MA), p-eNOS/eNOS (1:1000, Cell Signaling; Danvers, MA). Expression was assessed by quantification of optical density of respective bands normalized to actin using NIH-image J software.
Statistical analysis
Calculations were obtained using GraphPad Prism software version 4.0. Data were presented as mean ± SEM. All measurements were assessed with one-way ANOVA, followed by Tukey’s test. P<0.05 was considered statistically significant.
RESULTS
Candesartan alone and in combination with tPA decreased Hb content, incidence of hemorrhage and neurological deficits
Delayed administration of tPA (6h) in the eMCAO model is known to be ineffective at reducing infarct size and results in significant hemorrhage [5]. We used embolic model to examine the effects of candesartan on tPA-induced severity (Hb content) and frequency of hemorrhage at 48h after eMCAO (Fig. 2A and 2B). The Hb content and occurrence rate hemorrhage were significantly (P<0.05) increased in tPA-alone eMCAO group compared to vehicle-eMCAO. Administration of candesartan alone and in combination with tPA significantly (P<0.05) reduced the Hb content and incidence of hemorrhage compared to tPA-alone eMCAO. Furthermore, there was no significant difference in the post-eMCAO mortality rates between combination therapy and tPA-alone groups (31.12% and 36.36% respectively).
Fig. 2.
Candesartan alone and in combination with tPA reduced Hb content, incidence of hemorrhage, neurological deficits and infarct size at 48h post-eMCAO. (A) The Hb content was significantly increased in tPA-alone group compared to vehicle. Candesartan alone and in combination with tPA reduced the Hb content compared to respective controls. (B) The incidence of hemorrhage was significantly increased in tPA-alone group compared to vehicle. Candesartan alone and in combination with tPA markedly decreased incidence of hemorrhage. (C) Candesartan alone and in combination with tPA attenuates neurological deficits. The graph shows the 3 point Bederson score. (D) Candesartan alone reduced infarct size while combination therapy failed to reduce infarct size. Values are mean ±SEM. a,b,c Pairs of means with different letters are statistically different, P<0.05, Tukey’s post- hoc test (n=8). NS indicates not statistically significant.
Candesartan has been shown to improve neurological recovery in suture models of stroke [20, 23, 24]. To examine whether the combination of candesartan with tPA affects the neurological outcome, we used Bederson score at 48h after eMCAO (Fig. 2C). tPA treatment alone in the eMCAO + tPA group did not attenuate the neurological outcome significantly as compared to eMCAO + saline. Candesartan alone and in combination with tPA significantly (P<0.05) improved neurological outcome as compared to eMCAO + saline and eMCAO + tPA groups respectively.
We determined the effects of candesartan and tPA on infarct size at 48 h after eMCAO (Fig. 2D). There was no difference in infarct sizes between the control group and the tPA-treated groups. However, acute candesartan alone significantly (P<0.05) reduced infarction, which is consistent with our published study in suture model of stroke [20].
Candesartan in combination with tPA on MMP-9 and MMP-3
MMP-9 has been implicated in hemorrhage formation after tPA treatment for stroke [29, 30]. Gelatin zymography was performed to determine the impact of candesartan and tPA on MMP-9 activation. tPA alone did not influence the activity of MMP-9 compared to vehicle alone at 24h after eMCAO in our model. Further, candesartan alone and in combination with tPA didn’t significantly alter MMP-9 activation compared with respective controls, although a tendency for increased MMP-9 activity with candesartan was seen (Fig. 3). On the other hand, recent studies showed that MMP-3 is relatively more important than MMP-9 in the increase of tPA-induced hemorrhage [16] and proteolytic disruption of the blood-brain barrier (BBB) [31]. To examine whether combination therapy of candesartan and tPA affects MMP-3 expression was performed at 24h after eMCAO. tPA alone significantly (P<0.05) increased active MMP-3 compared to vehicle alone at 24h after eMCAO, suggesting the tPA specific activation of MMP-3. Candesartan in combination with tPA significantly (P<0.05) decreased the active-MMP-3 expression compared with tPA alone treated rats (Fig. 4), suggesting that tPA-induced MMP-3 activation may contribute to hemorrhage.
Fig. 3.
Candesartan alone and in combination with tPA did not alter MMP-9 activation compared with respective controls at 24h post-eMCAO. Values are mean ±SEM (n=5).
Fig. 4.
Candesartan alone and in combination with tPA reduced active MMP-3 at 24h post-eMCAO. Values are mean ±SEM. a,b,c Pairs of means with different letters are statistically different, P<0.05, Tukey’s post-hoc test (n=5). NS indicates not statistically significant.
Candesartan alone and in combination with tPA attenuated NF-κB p65, TNF-α and eNOS activation
NF-κBp65 plays a key role in endothelial cell activation and the inflammatory response after stroke. Further, activation of NF-κBp65 triggers upregulation of MMP-3 and acts as a primary transactivator for tPA-induced hemorrhage [32]. The combination treatment of candesartan and tPA significantly (P<0.05) inhibited the activation of NF-κB compared with tPA-alone (Fig. 5A). Activation of NF-κBp65 stimulates production of proinflammatory cytokines (e.g. TNF-α). TNF-α was significantly (P<0.05) expressed after embolic ischemia and reperfusion with tPA, and candesartan alone and in combination tPA significantly (P<0.05) decreased its expression (Fig. 5B). Activation of eNOS enhances thrombolysis, improves blood flow and reduces brain hemorrhage in the ischemic brain. Candesartan increases the eNOS protein levels in cerebral ischemia [33, 34]. The combination of candesartan with tPA significantly (P<0.05) increased p-eNOS expression at 24h after eMCAO (Fig. 5C).
Fig. 5.
Candesartan alone and in combination with tPA decreased (A) p-NFκB (B) TNF-α and increased (C) p-eNOS at 24h post-eMCAO. Values are mean ±SEM a,b Pairs of means with different letters are statistically different, P<0.05, Tukey’s post-hoc test (n=5). NS indicates not statistically significant.
DISCUSSION
The present study demonstrated that candesartan plus tPA combination therapy reduced brain hemorrhage and neurological deficits when treatment was initiated early (3h) with candesartan and delayed (6h) tPA in a rat model of eMCAO. Consistent with our previous studies in suture model [20, 23], candesartan alone was effective in reducing infarct size compared to vehicle-treated rats. Further, the beneficial effects of combination therapy of candesartan with tPA were associated with reducing the activation of MMP-3, NF-κB and TNF-α, and increasing eNOS activation.
Hemorrhage is an important complication of ischemic stroke and is responsible for most of the mortality associated with acute reperfusion therapy. Clinical and experimental studies have shown that MMPs are rapidly activated after cerebral ischemia [10, 29, 30]. Further, evidence suggests that tPA can upregulate and activate various members of the MMP family (especially MMP-2, MMP-3 and MMP-9) and thereby worsen stroke outcome [10, 16]. Although a broad spectrum of protease inhibitors have been used successfully in pathologic animal models [30, 35], low specificity and high toxicity, as well as a poor understanding of the role of individual MMPs, hampers their use in human stroke [36]. MMP-9 in particular has been cited as likely to be involved in hemorrhage after stroke because of its significant activation in human and animal stroke [29, 30]. Recent studies have shown that MMP-3, also known as stromelysin-1, plays a more critical role than MMP-9 in tPA-induced hemorrhage in a thrombolytic model of stroke [16]. MMP-3 has broader substrate specificity than MMP-9 [37]. Thus, although MMP-3 can contribute to the activation of proMMP-9 [38], the roles of these MMPs in hemorrhage induction appear to be different and tPA-specific.
In this study we found that candesartan treatment alone and in combination with tPA reduces hemorrhage without down-regulating MMP-9 activity compared to controls. We recently showed that treatments that reduced MMP-9 activity did not necessarily affect hemorrhage in the suture occlusion model suggesting that other factors contribute to hemorrhage [39]. Together, our findings suggest that inhibition of other proteolytic enzymes such as MMP-3, rather than MMP-9, may be contributing to the reduction of tPA-induced hemorrhage in embolic stroke. MMP-3 has been shown to be activated by neurons and microglia in the ischemic brain [40]. In a thrombolytic model of MCAO using MMP-3 and -9 knock-out mice, Suzuki et al.[16] demonstrated that tPA-induced hemorrhage was attenuated in MMP-3−/− but not MMP-9−/− mice, suggesting that MMP-3 is a more likely contributor than MMP-9 in tPA-induced hemorrhage. In our study, tPA did not alter MMP-9 activity when administered 6 hours after embolic stroke, indicating that MMP-9 contributes less than MMP-3 to an increase in tPA-induced hemorrhage. It is also conceivable that the induction of MMP-9 was not affected by tPA treatment because ischemia alone had already induced its maximal expression. Further, we found that tPA treatment increased active-MMP-3 expression after embolic stroke, suggesting that MMP-3 is a potential contributor to tPA-induced hemorrhage, and combination therapy of candesartan with tPA reduced the activation of MMP-3.
Regardless of the mechanisms implicated in the injury cascade following ischemic stroke, it is critical to determine whether the treatments are effective for better neurological recovery. Previous studies have shown that candesartan restored CBF and promoted neurological recovery in suture models of stroke [20, 23, 41]. Restoration of CBF is a promising target for the treatment of stroke [42]. Consistent with the earlier observations, we found that combination therapy with candesartan and tPA significantly improved neurological outcome.
The mechanisms by which candesartan provides vascular protection against tPA-induced hemorrhage after embolic stroke are not completely known. Thrombolysis with tPA upregulates MMP-3 in endothelial cells through the low-density lipoprotein receptor-related protein (LRP)/NF-κB pathway, and thereby exacerbates BBB disruption and hemorrhage [43]. Activation of NF-κB also stimulates production of proinflammatory cytokines such as TNF-α. Up-regulation of TNF-α after focal stroke plays a detrimental role in neuronal survival [44]. In contrast, inhibition of TNF-α in acute stroke has been shown to reduce the degree of ischemic injury [45]. Nitric oxide (NO) produced by eNOS is crucial for vascular function and homeostasis, and is considered to be neuroprotective after stroke [46]. Further, increased phosphorylation of eNOS has a broad range of effects, including the promotion of the proliferation of endothelial cells [47]. Candesartan increases eNOS and growth factors, inhibits TNF-α and exhibits vascular protection in experimental stroke [21, 22, 48]. Studies from our laboratory and others have demonstrated robust protective effects of candesartan in a number of ischemic stroke models [20, 23, 24]. Moreover, candesartan regulates the NF-κB signaling pathway that mediates candesartan-induced protection [49]. Thus, our data suggest that the combination therapy decreases NF-κBp65 activation and brain tissue inflammation, which may inhibit MMP-3 activation and upregulate eNOS activation, resulting in protection against vascular damage after ischemia and reperfusion with tPA (Fig. 6).
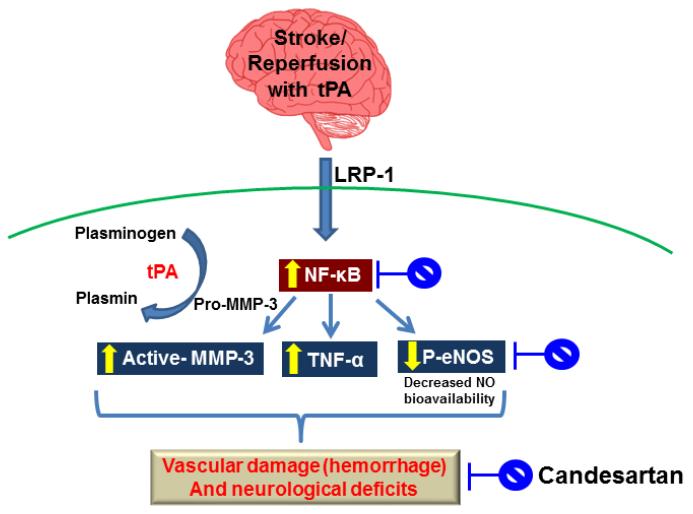
Fig. 6.
Schematic of experimental hypothesis for the possible mechanisms of candesartan-induced vascular protection. t-PA treatment induces matrix metalloproteinase-3 (MMP-3) through the binding to low-density lipoprotein receptor-related protein (LRP) and the activation of nuclear factor kappa-B (NF- κB) in endothelial cells (ECs) at the ischemic site, and t-PA activates plasmin, which in turn activates MMP-3, thereby contributing to greater vascular injury (i.e., hemorrhage) and neurological deficit. Abbreviations: tPA, tissue plasminogen activator; TNF-α, tumor necrosis factor- α p-eNOS, phospho- endothelial nitric oxide synthase; NO, nitric oxide.
Translation of this combination treatment to human stroke patients is limited by recent clinical trials suggesting that the blood pressure lowering effects of AT1 receptor blockade in the acute stroke period could outweigh any benefit (Scandanavian Candesartan in Acute Stroke Trial “SCAST”) [50]. It is likely that most of the cerebrovascular protective effects of candesartan are mediated through stimulation of the AT2 receptor [51, 52], however, and direct AT2 receptor agonists are in development and are very promising [53].
This “proof of concept” study focused on the acute-stage treatment of ischemic injury, and did not include long-term follow-up. We have, however, validated our results in a clinically relevant model of embolic stroke designed to prevent hemorrhage and improve the safety and impact of tPA. Further in vitro studies are planned to explore in detail the underlying mechanisms of this therapy in attenuating tPA-induced neurovascular complications (hemorrhage) by looking at tPA-MMP interactions, vascular endothelial apoptosis and neuronal survival. In this study, we used the Bederson scale to evaluate the effect of combination therapy on neurological outcome. The effects of combination therapy on a variety of behavioral tasks (rotarod, grip strength and adhesive removal etc.) previously shown to be highly sensitive to ischemic injury warrant further studies.
In summary, this study showed that candesartan in combination with tPA significantly reduced tPA-associated hemorrhage after focal embolic stroke. The beneficial effects of candesartan–tPA combination therapy may be mediated, in part, by the suppression of MMP-3 activation, inflammation and NF-κB p65, and by increased eNOS activation. These findings will support the development of new therapeutic approaches to protect the brain from vascular damage secondary to tPA administration.
Acknowledgements
This study was supported in part by the Veterans Affairs Merit Review (SCF, BX000891 and AE, BX000347) and NIH – NINDS (SCF, NS063965 and AE, NS054688). Adviye Ergul is a research pharmacologist at the Charlie Norwood Veterans Affairs Medical Center in Augusta, Georgia.
Abbreviations
- eMCAO
embolic middle cerebral artery occlusion
- tPA
tissue plasminogen activator
- PE
polyethylene
- NF-κB
nuclear factor kappa-B
- TNF-α
tumor necrosis factor-α
- (p-eNOS)
phospho endothelial nitric oxide synthase
- MMP
matrix metalloproteinase
- AT1R
angiotensin II type 1 receptor
- Hb
hemoglobin
- TTC
2, 3, 5-triphenyltetrazolium chloride
- SEM
standard error of the mean
- CBF
cerebral blood flow
- HT
hemorrhagic transformation
- LRP
low-density lipoprotein receptor-related protein
- BBB
blood brain-barrier
- NO
Nitric oxide.
Footnotes
Disclosures/ Conflict of interest
SCF is a consultant for and has received funding from Pfizer. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- 1.Goldstein LB. Acute ischemic stroke treatment in 2007. Circulation. 2007;116:1504–1514. doi: 10.1161/CIRCULATIONAHA.106.670885. [DOI] [PubMed] [Google Scholar]
- 2.Ishrat T, Soliman S, Guan W, Saler M, Fagan SC. Vascular protection to increase the safety of tissue plasminogen activator for stroke. Curr Pharm Des. 2012;18:3677–3684. doi: 10.2174/138161212802002779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fagan SC, Hess DC, Machado LS, Hohnadel EJ, Pollock DM, Ergul A. Tactics for vascular protection after acute ischemic stroke. Pharmacotherapy. 2005;25:387–395. doi: 10.1592/phco.25.3.387.61592. [DOI] [PubMed] [Google Scholar]
- 4.Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, Schellinger PD, Bohn M, Becker H, Wegrzyn M, Jahnig P, Herrmann M, Knauth M, Bahr M, Heide W, Wagner A, Schwab S, Reichmann H, Schwendemann G, Dengler R, Kastrup A, Bartels C. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke; a journal of cerebral circulation. 2009;40:e647–656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- 5.Jia L, Chopp M, Zhang L, Lu M, Zhang Z. Erythropoietin in combination of tissue plasminogen activator exacerbates brain hemorrhage when treatment is initiated 6 hours after stroke. Stroke; a journal of cerebral circulation. 2010;41:2071–2076. doi: 10.1161/STROKEAHA.110.586198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zechariah A, ElAli A, Hermann DM. Combination of tissue-plasminogen activator with erythropoietin induces blood-brain barrier permeability, extracellular matrix disaggregation, and DNA fragmentation after focal cerebral ischemia in mice. Stroke; a journal of cerebral circulation. 2010;41:1008–1012. doi: 10.1161/STROKEAHA.109.574418. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Lee SR, Arai K, Lee SR, Tsuji K, Rebeck GW, Lo EH. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nature medicine. 2003;9:1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- 8.Pfefferkorn T, Rosenberg GA. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke; a journal of cerebral circulation. 2003;34:2025–2030. doi: 10.1161/01.STR.0000083051.93319.28. [DOI] [PubMed] [Google Scholar]
- 9.Montaner J, Alvarez-Sabin J, Molina C, Angles A, Abilleira S, Arenillas J, Gonzalez MA, Monasterio J. Matrix metalloproteinase expression after human cardioembolic stroke: temporal profile and relation to neurological impairment. Stroke; a journal of cerebral circulation. 2001;32:1759–1766. doi: 10.1161/01.str.32.8.1759. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50:329–339. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- 11.Romanic AM, Madri JA. Extracellular matrix-degrading proteinases in the nervous system. Brain pathology. 1994;4:145–156. doi: 10.1111/j.1750-3639.1994.tb00825.x. [DOI] [PubMed] [Google Scholar]
- 12.Grossetete M, Rosenberg GA. Matrix metalloproteinase inhibition facilitates cell death in intracerebral hemorrhage in mouse. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2008;28:752–763. doi: 10.1038/sj.jcbfm.9600572. [DOI] [PubMed] [Google Scholar]
- 13.Power C, Henry S, Del Bigio MR, Larsen PH, Corbett D, Imai Y, Yong VW, Peeling J. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Annals of neurology. 2003;53:731–742. doi: 10.1002/ana.10553. [DOI] [PubMed] [Google Scholar]
- 14.Wells JE, Biernaskie J, Szymanska A, Larsen PH, Yong VW, Corbett D. Matrix metalloproteinase (MMP)-12 expression has a negative impact on sensorimotor function following intracerebral haemorrhage in mice. The European journal of neuroscience. 2005;21:187–196. doi: 10.1111/j.1460-9568.2004.03829.x. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez-Sabin J, Delgado P, Abilleira S, Molina CA, Arenillas J, Ribo M, Santamarina E, Quintana M, Monasterio J, Montaner J. Temporal profile of matrix metalloproteinases and their inhibitors after spontaneous intracerebral hemorrhage: relationship to clinical and radiological outcome. Stroke; a journal of cerebral circulation. 2004;35:1316–1322. doi: 10.1161/01.STR.0000126827.69286.90. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki Y, Nagai N, Umemura K, Collen D, Lijnen HR. Stromelysin-1 (MMP-3) is critical for intracranial bleeding after t-PA treatment of stroke in mice. J Thromb Haemost. 2007;5:1732–1739. doi: 10.1111/j.1538-7836.2007.02628.x. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura Y, Ito T, Saavedra JM. Angiotensin II AT(1) blockade normalizes cerebrovascular autoregulation and reduces cerebral ischemia in spontaneously hypertensive rats. Stroke; a journal of cerebral circulation. 2000;31:2478–2486. doi: 10.1161/01.str.31.10.2478. [DOI] [PubMed] [Google Scholar]
- 18.Awad AS. Effect of combined treatment with curcumin and candesartan on ischemic brain damage in mice. J Stroke Cerebrovasc Dis. 2011;20:541–548. doi: 10.1016/j.jstrokecerebrovasdis.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Fu H, Hosomi N, Pelisch N, Nakano D, Liu G, Ueno M, Miki T, Masugata H, Sueda Y, Itano T, Matsumoto M, Nishiyama A, Kohno M. Therapeutic effects of postischemic treatment with hypotensive doses of an angiotensin II receptor blocker on transient focal cerebral ischemia. J Hypertens. 2011;29:2210–2219. doi: 10.1097/HJH.0b013e32834bbb30. [DOI] [PubMed] [Google Scholar]
- 20.Guan W, Kozak A, El-Remessy AB, Johnson MH, Pillai BA, Fagan SC. Acute Treatment with Candesartan Reduces Early Injury After Permanent Middle Cerebral Artery Occlusion. Transl Stroke Res. 2011;2:179–185. doi: 10.1007/s12975-010-0061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan W, Somanath PR, Kozak A, Goc A, El-Remessy AB, Ergul A, Johnson MH, Alhusban A, Soliman S, Fagan SC. Vascular protection by angiotensin receptor antagonism involves differential VEGF expression in both hemispheres after experimental stroke. PLoS One. 2011;6:e24551. doi: 10.1371/journal.pone.0024551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozak A, Ergul A, El-Remessy AB, Johnson MH, Machado LS, Elewa HF, Abdelsaid M, Wiley DC, Fagan SC. Candesartan augments ischemia-induced proangiogenic state and results in sustained improvement after stroke. Stroke; a journal of cerebral circulation. 2009;40:1870–1876. doi: 10.1161/STROKEAHA.108.537225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elewa HF, Kozak A, Johnson MH, Ergul A, Fagan SC. Blood pressure lowering after experimental cerebral ischemia provides neurovascular protection. J Hypertens. 2007;25:855–859. doi: 10.1097/HJH.0b013e3280149708. [DOI] [PubMed] [Google Scholar]
- 24.Fagan SC, Kozak A, Hill WD, Pollock DM, Xu L, Johnson MH, Ergul A, Hess DC. Hypertension after experimental cerebral ischemia: candesartan provides neurovascular protection. J Hypertens. 2006;24:535–539. doi: 10.1097/01.hjh.0000209990.41304.43. [DOI] [PubMed] [Google Scholar]
- 25.Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain Res. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- 26.Meng W, Wang X, Asahi M, Kano T, Asahi K, Ackerman RH, Lo EH. Effects of tissue type plasminogen activator in embolic versus mechanical models of focal cerebral ischemia in rats. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1999;19:1316–1321. doi: 10.1097/00004647-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke; a journal of cerebral circulation. 2008;39:3372–3377. doi: 10.1161/STROKEAHA.108.514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke; a journal of cerebral circulation. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 29.Rosell A, Ortega-Aznar A, Alvarez-Sabin J, Fernandez-Cadenas I, Ribo M, Molina CA, Lo EH, Montaner J. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke; a journal of cerebral circulation. 2006;37:1399–1406. doi: 10.1161/01.STR.0000223001.06264.af. [DOI] [PubMed] [Google Scholar]
- 30.Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke; a journal of cerebral circulation. 1998;29:1020–1030. doi: 10.1161/01.str.29.5.1020. [DOI] [PubMed] [Google Scholar]
- 31.Gurney KJ, Estrada EY, Rosenberg GA. Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol Dis. 2006;23:87–96. doi: 10.1016/j.nbd.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki Y, Nagai N, Yamakawa K, Kawakami J, Lijnen HR, Umemura K. Tissue-type plasminogen activator (t-PA) induces stromelysin-1 (MMP-3) in endothelial cells through activation of lipoprotein receptor-related protein. Blood. 2009;114:3352–3358. doi: 10.1182/blood-2009-02-203919. [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Kitazato KT, Uno M, Yagi K, Kanematsu Y, Tamura T, Tada Y, Kinouchi T, Nagahiro S. Protective mechanisms of the angiotensin II type 1 receptor blocker candesartan against cerebral ischemia: in-vivo and in-vitro studies. J Hypertens. 2008;26:1435–1445. doi: 10.1097/HJH.0b013e3283013b6e. [DOI] [PubMed] [Google Scholar]
- 34.Engelhorn T, Doerfler A, Heusch G, Schulz R. Reduction of cerebral infarct size by the AT1- receptor blocker candesartan, the HMG-CoA reductase inhibitor rosuvastatin and their combination. An experimental study in rats. Neurosci Lett. 2006;406:92–96. doi: 10.1016/j.neulet.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 35.Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 37.Nagase H, Woessner JF., Jr. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg GA, Cunningham LA, Wallace J, Alexander S, Estrada EY, Grossetete M, Razhagi A, Miller K, Gearing A. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res. 2001;893:104–112. doi: 10.1016/s0006-8993(00)03294-7. [DOI] [PubMed] [Google Scholar]
- 39.Kelly-Cobbs AI, Prakash R, Li W, Pillai B, Hafez S, Coucha M, Johnson MH, Ogbi SN, Fagan SC, Ergul A. Targets of vascular protection in acute ischemic stroke differ in type 2 diabetes. Am J Physiol Heart Circ Physiol. 2013;304:H806–815. doi: 10.1152/ajpheart.00720.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jian Liu K, Rosenberg GA. Matrix metalloproteinases and free radicals in cerebral ischemia. Free Radic Biol Med. 2005;39 doi: 10.1016/j.freeradbiomed.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 41.Engelhorn T, Goerike S, Doerfler A, Okorn C, Forsting M, Heusch G, Schulz R. The angiotensin II type 1-receptor blocker candesartan increases cerebral blood flow, reduces infarct size, and improves neurologic outcome after transient cerebral ischemia in rats. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2004;24:467–474. doi: 10.1097/00004647-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Sutherland BA, Papadakis M, Chen RL, Buchan AM. Cerebral blood flow alteration in neuroprotection following cerebral ischaemia. J Physiol. 2011;589:4105–4114. doi: 10.1113/jphysiol.2011.209601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki Y. Role of tissue-type plasminogen activator in ischemic stroke. J Pharmacol Sci. 2010;113:203–207. doi: 10.1254/jphs.10r01cp. [DOI] [PubMed] [Google Scholar]
- 44.Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nature medicine. 2002;8:1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- 45.Shohami E, Ginis I, Hallenbeck JM. Dual role of tumor necrosis factor alpha in brain injury. Cytokine Growth Factor Rev. 1999;10:119–130. doi: 10.1016/s1359-6101(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 46.Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, Moskowitz MA. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1996;16:981–987. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- 47.Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest. 1998;101:731–736. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamakawa H, Jezova M, Ando H, Saavedra JM. Normalization of endothelial and inducible nitric oxide synthase expression in brain microvessels of spontaneously hypertensive rats by angiotensin II AT1 receptor inhibition. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2003;23:371–380. doi: 10.1097/01.WCB.0000047369.05600.03. [DOI] [PubMed] [Google Scholar]
- 49.Sanz-Rosa D, Oubina MP, Cediel E, de Las Heras N, Vegazo O, Jimenez J, Lahera V, Cachofeiro V. Effect of AT1 receptor antagonism on vascular and circulating inflammatory mediators in SHR: role of NF- kappaB/IkappaB system. Am J Physiol Heart Circ Physiol. 2005;288:H111–115. doi: 10.1152/ajpheart.01061.2003. [DOI] [PubMed] [Google Scholar]
- 50.Sandset EC, Bath PM, Boysen G, Jatuzis D, Korv J, Luders S, Murray GD, Richter PS, Roine RO, Terent A, Thijs V, Berge E. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet. 2011;377:741–750. doi: 10.1016/S0140-6736(11)60104-9. [DOI] [PubMed] [Google Scholar]
- 51.Alhusban A, Kozak A, Ergul A, Fagan SC. AT1 receptor antagonism is proangiogenic in the brain: BDNF a novel mediator. J Pharmacol Exp Ther. 2013;344:348–359. doi: 10.1124/jpet.112.197483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou J, Pavel J, Macova M, Yu ZX, Imboden H, Ge L, Nishioku T, Dou J, Delgiacco E, Saavedra JM. AT1 receptor blockade regulates the local angiotensin II system in cerebral microvessels from spontaneously hypertensive rats. Stroke; a journal of cerebral circulation. 2006;37:1271–1276. doi: 10.1161/01.STR.0000217404.64352.d7. [DOI] [PubMed] [Google Scholar]
- 53.Steckelings UM, Paulis L, Namsolleck P, Unger T. AT2 receptor agonists: hypertension and beyond. Curr Opin Nephrol Hypertens. 2012;21:142–146. doi: 10.1097/MNH.0b013e328350261b. [DOI] [PubMed] [Google Scholar]