SUMMARY
Administration of exogenous L-Arginine (L-Arg) attenuates Angiotensin II (AngII)-mediated hypertension and kidney disease in rats. The present study assessed renal hemodynamics and pressure-diuresis-natriuresis in anesthetized rats infused with vehicle, AngII (20 ng/kg/min, iv) or AngII + L-Arg (300 µg/kg/min, iv). Increasing renal perfusion pressure (RPP) from approximately 100 to 140 mmHg resulted in a 9–10 fold increase in urine flow and sodium excretion rate in control animals. In comparison, AngII infusion significantly reduced renal blood flow (RBF) and glomerular filtration rate (GFR) by 40–42% and blunted the pressure-dependent increase in urine flow and sodium excretion rate by 54–58% at elevated RPP. Supplementation of L-Arg reversed the vasoconstrictor effects of AngII and restored pressure-dependent diuresis to levels not significantly different from control rats. Experiments in isolated aortic rings were performed to assess L-Arg effects on the vasculature. Dose-dependent contraction to AngII (10−10M to 10−7M) was observed with a maximal force equal to 27±3% of the response to 10−5M phenylephrine. Contraction to 10−7M AngII was blunted by 75±3% with 10−4M L-Arg. The influence of L-Arg to blunt AngII mediated contraction was eliminated by endothelial denudation or incubation with nitric oxide synthase inhibitors. Moreover, the addition of 10−3M cationic or neutral amino acids, which compete with L-Arg for cellular uptake, blocked the effect of L-Arg. Anionic amino acids did not influence the effects of L-Arg on AngII-mediated contraction. These studies indicate that L-Arg blunts AngII-mediated vascular contraction by an endothelial- and NOS-dependent mechanism involving cellular uptake of L-Arg.
Keywords: rats, blood pressure, nitric oxide, L-arginine
INTRODUCTION
Supplementation of extracellular L-arginine (L-Arg), the substrate for nitric oxide formation, enhances NO-dependent vasorelaxation or other indices of endothelial function in hypertension.1,2,3,4,5,6 Moreover, in Dahl salt sensitive rats, chronic oral, intravenous, or medullary interstitial administration of L-Arg prevented sodium dependent hypertension.1,3,4,5,7 Of clinical relevance, L-Arg has been shown to be beneficial in reducing arterial pressure and improving endothelial function in mild to moderate essential hypertension in patients.8,9 Together, these data indicate that exogenous L-Arg exerts beneficial effects in experimental and human hypertension.
AngII is a potent vasoconstrictor; infusion of AngII blunts the pressure-natriuretic-diuretic relationship10,11 and leads to hypertension when infused chronically into the systemic circulation.12,13,14 Previous studies from our laboratory demonstrated that supplementation of L-Arg attenuates AngII-mediated hypertension and associated renal damage in rats.13 We also demonstrated in the isolated perfused rat kidney and in cultured endothelial cells that cellular L-Arg uptake, mediated by y+ and y+L transport systems, can alter NO production and NO-dependent vasodilation.15 The link between these in vitro and in vivo observations has not been established. The present studies were performed to assess the mechanisms whereby L-Arg supplementation attenuates AngII-mediated effects in the renal vasculature.
The experiments in this study tested the hypothesis that exogenous L-Arg opposes the vasoconstrictor effects of AngII in the renal vasculature and shifts the pressure natriuretic response by opposing the vasoconstrictor effects of AngII. Initial studies examined the influence of exogenous L-Arg to modulate the effects of AngII on renal blood flow and glomerular filtration rate, the pressure-natriuretic response, and intrarenal blood flow distribution. Additional studies were then performed with isolated aortic ring segments to investigate the mechanisms whereby L-Arg opposes AngII’s vasoconstrictor responses. Together, the experiments in this study assessed the mechanisms of action of exogenous L-Arg to oppose the vasoconstrictor actions of AngII.
RESULTS
Figure 1 summarizes the changes in the urine flow rate, sodium excretion rate, and fractional sodium excretion rate as RPP was increased from low to high levels in rats administered saline (control) (n=10), Ang II (n=8) or Ang II plus L-Arg (n=12). In the saline infused group, urine flow rate and sodium excretion significantly increased by 9–10 times as RPP was increased from approximately 100 to 140 mmHg. Acute Ang II infusion blunted the pressure-natriuretic-diuretic relationship and suppressed urine flow rate and sodium excretion rate at the highest level of RPP. In rats in which L-Arg was co-infused with AngII, urine flow and sodium excretion rate were not different from the control group at any level of RPP. The fractional sodium excretion rate increased 8-fold in the control group as RPP was increased from low to high levels. Ang II infusion suppressed fractional sodium excretion rate at the medium and high levels of RPP. The fractional sodium excretion rate was not different from control in the L-Arg + AngII group.
Figure 1.
Effect of saline, Ang II (20 ng/kg/min, iv) or Ang II + L-Arg (300 µg/kg/min, iv) infusion on urine flow (top), urinary sodium excretion (middle), and fractional sodium excretion (lower) in response to increasing renal perfusion pressures (RPP) in volume expanded SD rats. †P<0.05 vs low and med RPP in the same grp, * P<0.05 vs saline and Ang II+ L-Arg at high RPP (n=8–12/group).
Glomerular filtration rate (GFR) and renal blood flow (RBF) data are presented in Figure 2. The GFR in the Ang II infused rats was significantly suppressed compared to the control group throughout the entire range of the RPP with a maximal reduction of approximately 42% at the highest RPP. GFR was not different from the control group at any level of RPP in rats co-infused with L-Arg and AngII. Similarly, acute intravenous infusion of Ang II (20 ng/kg/min) significantly reduced renal blood flow (RBF) from the control group, and RBF was not different from the control group in rats co-infused with L-Arg + AngII.
Figure 2.
Effect of saline, Ang II (20 ng/kg/min, iv) or Ang II + L-Arg (L-Arg: 300 µg/kg/min, iv) infusion on glomerular filtration rate (GFR) (top) and renal blood flow (RBF) (bottom) in response to increasing renal perfusion pressures in volume expanded SD rats. †P<0.05 vs low and med RPP same group, * P<0.05 at respective RPP’s vs saline and Ang II + L-Arg group (n=8–12/group).
Superficial cortical blood flow (Figure 3-top) and outer medullary blood flow (Figure 3-middle) were significantly decreased by 13 and 18%, respectively, during AngII infusion. Renal cortical and outer medullary blood flow were not altered when L-Arg was co-infused with AngII. In contrast to the effects of AngII in the renal cortex and outer medulla, blood flow in the renal inner medulla increased significantly during AngII infusion. Co-infusion of L-Arg with AngII did not affect the blood flow response in the inner medulla. As an index of NO formation, total nitrite/nitrate excretion in the urine was increased by 35% in rats infused with L-Arg + AngII when compared to control animals treated with saline (Figure 4). Administration of AngII alone did not alter urinary nitrate/nitrite excretion.
Figure 3.
Effect of saline vehicle followed by Ang II (20 ng/kg/min, iv) or saline vehicle followed by Ang II+ L-Arg (L-Arg: 300 µg/kg/min, iv) infusion on renal cortical blood flow (top), renal outer medullary blood flow (middle), and renal inner medullary blood flow as measured by laser-Doppler flowmetry in volume-expanded SD rats. *P < 0.05 vs saline infusion (n=10/group).
Figure 4.
Urinary nitrate/nitrite excretion (nmol/min) during acute infusion of saline, Ang II (20 ng/kg/min, iv) and Ang II + L-Arg (300 µg/kg/min, iv) in the same group of rats. *P<0.05 vs saline and Ang II infusion (n=4)
Figure 5-top demonstrates the contractile force developed in response to incubation with 10−4M L-Arg or vehicle followed by incrementing doses (10−10M to 3×10−7M) of Ang II in aortic rings with an intact or physically-denuded endothelium. Approximately 27±4% of the maximal contractile response to 10−7M PE was attained with 10−7M AngII. The contraction to AngII was significantly blunted by pre-incubation with 10−4M L-Arg in endothelium-intact rings, reaching 7±1% of the PE response. Physical removal of the endothelium eliminated the effects of L-Arg on AngII-mediated contraction. Endothelial removal was documented by assessing the relaxation response to Ach. Aortic rings with an intact endothelium almost completely relaxed (by 80–90% from the PE-induced pre-contracted state) to 10−5M Ach; in contrast, the Ach-mediated relaxation was attenuated (29±4%) in the endothelial-denuded rings. Figure 5-middle demonstrates the stereospecificity of the response; pre-incubation with 10−5M D-Arg did not mimic the effects of L-Arg to blunt AngII-mediated contraction. The bottom panel of Figure 5 illustrates that the NOS inhibitor L-NAME (10−4M), which significantly attenuated the vasodilatory response to 10−5M Ach by approximately 80%, prevented L-Arg from blunting AngII-mediated contraction. The use of another NOS inhibitor, L-NMMA (10−4M), also prevented L-Arg from blunting AngII-mediated contraction; 10−7M AngII resulted in a contraction equivalent to 21 ± 2% of the maximal contraction in the rings treated with L-NMMA and 10−4M L-Arg.
Figure 5.
Comparison of contractile force generated in endothelium-intact or physically denuded (E-) aortic rings pre-incubated with vehicle or 10−4M L-Arg in response to incrementing doses of Ang II (10−10M to 3X10−7M) (top). Comparison of contractile force generated in aortic rings pre-incubated with vehicle, 10−4M L-Arg, or 10−4M D-Arg in response to incrementing doses of Ang II (10−10M to 3X10−7M) (middle). Comparison of contractile force generated in aortic rings pre-incubated with vehicle, 10−4M L-Arg, or 10−4M L-Arg + 10−4M L-NAME in response to incrementing doses of Ang II (10−10M to 3X10−7M) (bottom). Experiments were performed in parallel on separate aortic ring segments obtained from the same animal. * P<0.05 between groups (n=6–8/group).
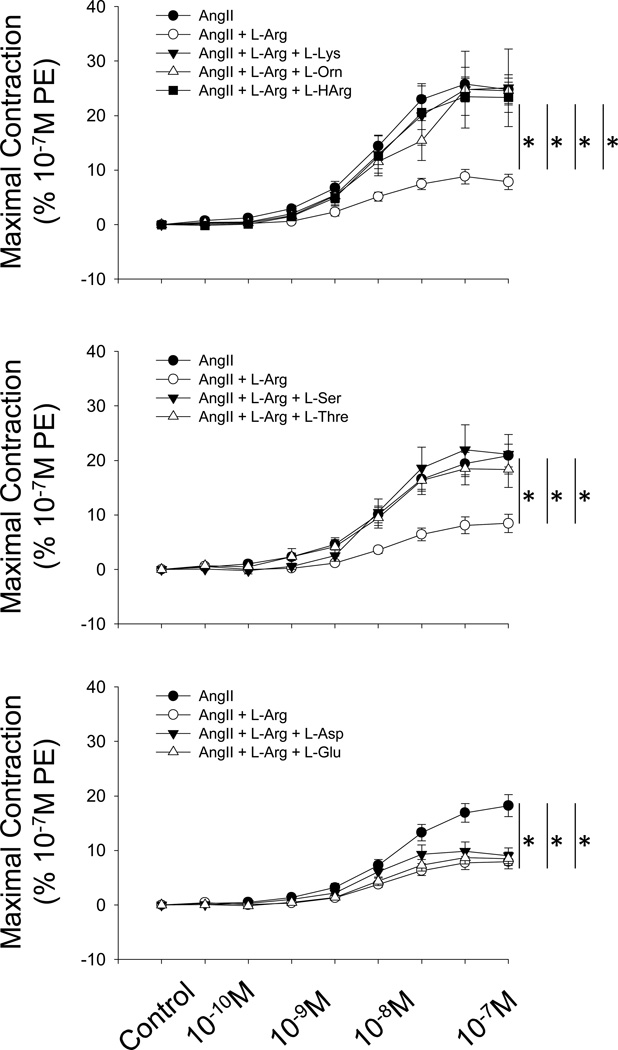
Figure 6 illustrates the effects of excess (10−3M) cationic amino acids (top), neutral amino acids (middle), and anionic amino acids (bottom) on Ang II mediated vasoconstriction in endothelium intact (E+) aortic rings. The presence cationic amino acids L-Orn, L-Lys, or L-Harg (which are known to be competitive inhibitors of L-Arg uptake) in excess of L-Arg in the vessel baths led to a sustained increase in contractile force in a dose dependent manner when treated with incrementing doses of Ang II. The peak contractile force averaged 24.3 ± 0.4% of the maximum contraction (HPSS+10−5 M PE) at an Ang II dose of 10−7M with any of the three cationic amino acids in the vessel baths. This contraction profile was similar to the group of rings (n=7) subjected to Ang II in the absence of any amino acid incubation and contrasted with the effect of L-Arg to significantly AngII-mediated contraction (n=7). Similar responses were obtained with addition of either 10−3M L-Thr or L-Ser, (neutral amino acids) in addition to 10−4M L-Arg in the vessel baths followed by Ang II stimulation (10−10M to 3X10−7M) in aortic rings. In contrast to the above observations, incubating endothelium-intact aortic rings with excess (10−3M) of the anionic amino acids L-Asp or L-Glu (n=10/grp) did not alter the effects of 10−4M L-Arg to blunt AngII-mediated contraction.
Figure 6.
Comparison of contractile force generated in aortic rings pre-incubated with vehicle, 10−4M L-Arg, or 10−4M L-Arg + excess cationic amino acids (10−3M L-Lys, L-Orn, or L-HArg) in response to incrementing doses of Ang II (10−10M to 3X10−7M) (top). Comparison of contractile force generated in aortic rings pre-incubated with vehicle, 10−4M L-Arg, or 10−4M L-Arg + excess neutral amino acids (10−3M L-Ser or L-Thr) in response to incrementing doses of Ang II (10−10M to 3X10−7M) (middle). Comparison of contractile force generated in aortic rings pre-incubated with vehicle, 10−4M L-Arg, or 10−4M L-Arg + excess anionic amino acids (10−3M L-Asp or L-Glu) in response to incrementing doses of Ang II (10−10M to 3X10−7M) (bottom). Experiments were performed in parallel on separate aortic ring segments obtained from the same animal. * P<0.05 between groups (n=6–8/group).
As a measure of NO production, the change in DAF fluorescence intensity was determined while aortic rings were incubated with 10−4M L-Arg prior to stimulation with a single dose of 10−6M Ang II (Figure 7). Incubating tissue sections with 10−4M L-Arg prior to addition of 10−6M Ang II, caused a significant increase in DAF fluorescence intensity (8.0 ± 1.2%, n=5), from baseline after 30 mins of addition of Ang II. The DAF fluorescence intensity reached in tissue sections incubated without L-Arg was significantly lower than the values obtained in the presence of L-Arg. Moreover, rings incubated with the NOS enzyme inhibitor L-NAME (10−4M) plus 10−4M L-Arg prior to Ang II stimulation had a significantly lower change in fluorescence (3.6 ± 2.1%) compared to the group incubated with L-Arg.
Figure 7.
Changes in DAF fluorescence intensity in rat aortic tissue sections incubated with 10−6M Ang II, 10−6M Ang II + 10−4M L-Arg, or 10−6M Ang II + 10−4M L-Arg + 10−4M L-NAME. *P< 0.05 vs 0 mins within the same group. †P< 0.05 vs Ang II + L-Arg or Ang II + L-Arg + L-NAME at the same time point (n=4/group).
METHODS
Experimental Animals
Protocols were conducted in accordance with the Medical College of Wisconsin Institutional Animal Care and Use Committee’s guidelines. Male Sprague Dawley rats weighing 275–300 grams were purchased from Harlan Sprague Dawley (Madison, WI) and housed in standard sized cages with 12 hour light: 12 hour dark cycle. The rats were given food (Purina 5001) and water ad libitum.
Surgical Preparation
For in vivo studies, the animals were anesthetized with Inactin (100 mg/kg ip) and placed on heated surgical tables to maintain body temperature at 37° C as previously described.16 The trachea was cannulated to facilitate respiration, and catheters were placed in the carotid and femoral artery to measure renal perfusion pressure (RPP). The jugular and femoral vein were cannulated for intravenous infusions. A midline abdominal incision was made, and a flow probe was placed around the renal artery for measurement of renal blood flow (RBF) using an ultrasonic Doppler flowmeter (model T206, Transonic Systems, Inc, Ithaca, NY). Adjustable clamps were placed around the aorta above and below the renal arteries, and a loose ligature was placed around the celiac and mesenteric arteries so RPP could be adjusted during the experiment. The left ureter was cannulated for urine collection in some rats. In other animals, the left kidney was placed in a holder and optical fibers for laser Doppler flowmetry were implanted to a depth of ~1.5 mm beneath the surface for measurements in the renal cortex, ~3.0 mm beneath the surface for measurements in the renal outer medulla, and ~4.0 mm for measurements in the renal inner medulla.
Animals were volume expanded with an intravenous infusion of 1% bovine serum albumin in 0.9% NaCl at a rate of 1 ml/hr/100g bwt. The left renal artery was stripped of visible nerves and lightly coated with 10% phenol to eliminate neural influences on the kidney. The hormones aldosterone, vasopressin, corticosterone and norepinephrine were all fixed by intravenous infusion in 0.9% sodium chloride solution containing 1% bovine serum albumin (aldosterone: 6.67 ng/100g/min, vasopressin: 16.67 pg/100g/min, corticosterone: 3.33 µg/100g/min, norepinephrine: 33.3 ng/100g/min; all from Sigma Chemical, St. Louis MO). [3H] inulin (1µCi/mL; PerkinElmer) was added to the infusate to quantify glomerular filtration rate (GFR).
Preparation of the Aortic Rings
Aortic ring studies were performed as we previously described.17 Rats were deeply anaesthetized with sodium pentobarbital (50 mg/kg i.p). The thoracic aorta (a segment of length 3–5 cm) was removed, cleaned of adhering tissue, and cut into 3–4 mm wide rings while submerged in physiological salt solution (PSS). Each individual ring was mounted on a triangular wire holder and placed inside an individual vessel bath containing 5 ml of PSS (119mM NaCl, 4.7mM KCl, 1.2mM MgSO4, 1.6mM CaCl2, 24mM NaHCO3, 5.5mM dextrose, and 260.3mM HEPES). The chambers received continuous airflow (95% O2-5% CO2) and were maintained at 37°C. Tension (g) was recorded from each individual ring by a Grass FT-03 force transducer for data analysis with a Digi-Med Tissue Force Analyzer (Micro-Med Inc).
The mounted aortic rings were exposed to a passive tension of 1.5 gms and equilibrated 30 mins. The rings were first pre-constricted with 10−7M Phenylephrine (PE), followed by relaxation with 10−5M Acetylcholine (Ach). Only those rings that relaxed by 50% or more with Ach were considered to have an intact endothelium. After another 10 minute equilibration time in PSS the rings were subjected to a high potassium solution (HPSS containing 80 mM KCl) and 10−5M PE for a period of 10 minutes to test the maximum force of contraction. The rings were blotted and weighed at the conclusion of the experiments; contractile forces were normalized to the maximum force of contraction (while treated with 80 mM KCl +10−5 M PE) and tissue weight.
EXPERIMENTAL PROTOCOLS
Pressure Natriuretic Response
The influence of acute infusion of saline (n=12), Ang II (20 ng/kg/min; n=8), or Ang II (20 ng/kg/min) + L-Arg (300 µg/kg/min; n=12) on the pressure natriuresis-diuresis relationship was assessed. These AngII and L-Arg doses were utilized in a previous study in which we demonstrated that continuous administration of AngII results in sustained hypertension in conscious SD rats that is attenuated by exogenous L-Arg.13 Preliminary experiments demonstrated that L-Arg (300 µg/kg/min; n=5) alone had no influence on GFR, RBF, and the pressure diuretic response; moreover, we previously demonstrated that much higher intravenous infusion rates of L-Arg alone do not alter renal cortical or medullary blood flow.29 The animals were allowed to equilibrate for 45 minutes from the time of completion of the surgery with intravenous infusion of the experimental drug (i.e. either of saline, Ang II, or Ang II plus L-Arg prepared in 0.9% saline). The RPP was first lowered to 95–100 mmHg (about 20 mmHg below the control) by occluding the clamp above the renal artery. Following equilibration, urine was collected during two 20 minute clearance periods. The RPP was then allowed to return to control values (approximately 120 mmHg) by releasing the aortic clamp. After equilibration, urine was collected for two 15-minute clearance periods. Finally, the RPP was increased to approximately 140 mmHg by occluding the aorta below the kidney and ligating the celiac and superior mesenteric arteries. Following equilibration, urine was collected for two 10-minute periods. Plasma samples were also obtained at each of the three levels of RPP to quantify the plasma inulin concentration. The left kidney was collected and weighed at the conclusion of the experiment.
Urine flow rate (UFR) was determined gravimetrically. Urine sodium concentration was measured by flame photometry. The [3H] inulin content in the urine and plasma was measured by liquid scintillation counting (model 2450, Packard Instrument, Downers Grove, IL). The GFR was calculated as the product of the UFR and the plasma to urine inulin concentration ratio. The urine flow, sodium excretion, RBF, and GFR were all normalized to kidney weight (grams; gkwt).
Intrarenal Blood Flow Distribution
Following the pressure-natriuresis experiments, additional experiments were performed to examine regional renal blood flow in response to Ang II (20 ng/kg/min; n=10) or Ang II plus L-Arg (300 µg/kg/min; n=10) infusion. For this study the animals were surgically instrumented with catheters and infused with 1% BSA containing hormones as described above. Rats received 0.9% saline infusion in the control period. One hour was given as a stabilization period from the time of placement of the laser Doppler flow probes. After one hour of stabilization, baseline (control) values were recorded for two 5-minute time periods during infusion of isotonic saline. The animals were then infused with either of the experimental treatments for a period of 1 hour and the values were recorded again for two 5-minute periods. RPP was maintained at approximately 110 mmHg throughout the experiment. At the end of the experiment the left kidney was collected and weighed. The placement of the flow probes were verified by cross sectioning the kidney.
Determination of Urinary Nitrate/Nitrite Excretion
As an index of NO formation, urinary nitrate/nitrite excretion was assessed in SD rats (n=4) during the acute intravenous infusion of saline (vehicle) (1ml/100 g body wt/hr), Ang II (20 ng/kg/min) or Ang II plus L-Arg (300 µg/kg/min). The rats were surgically instrumented with catheters and volume expanded with intravenous infusion of 1% BSA in saline containing the hormones described above. The animals were intravenously infused with each treatment in a consecutive manner for one hour; urine samples were collected in a 10-minute clearance period at the end of every hour. The infusion order of the treatments was randomized in each animal. Urine samples were analyzed for nitrate/nitrite levels with a colorimetric assay kit (Cayman, Ann Arbor, Michigan).
Isolated Aortic Ring Studies
Isolated aortic rings were utilized to assess the vascular actions of L-Arg and AngII. The vascular reactivity of aortic rings incubated with 10−4M L-Arg prior to treatment with incremental doses (10−10M to 3×10−7M) of Ang II was initially evaluated. Rings were randomly chosen and incubated with PSS (vehicle) or PSS containing 10−4M L-Arg for 30 mins. After the incubation period, each of the individual rings was incubated with incrementing doses of Ang II (10−10M to 3×10−7M). An interval of 4 min was given between doses of Ang II and the force generated at each of the Ang II doses was recorded.
To verify the role of the endothelium in the response to L-Arg, some experiments were performed in aortic rings in which the endothelium was purposely damaged by physical denudation. These endothelium denuded (E-) rings were subjected to the Ach relaxation test to verify successful removal of the endothelium prior to L-Arg incubation and Ang II stimulation.
Additional experiments were performed to examine the stereospecificity of the response by substituting D-Arg (10−4M) for L-Arg. To assess the importance of NOS in the response, other experiments were performed in which the rings were pretreated with the NOS inhibitors L-NAME (10−4M) or L-NMMA (10−4M). The Ach relaxation test was used to assess NOS blockade in these experiments.
To assess the cellular uptake mechanisms, additional experiments were performed in aortic rings with an intact endothelium (assessed with the Ach relaxation test). Rings were incubated with an excess (10−3M) of individual cationic (L-Lys, L-Orn, L-Harg), anionic (L-Asp, L-Glu) or neutral (L-Leu, L-Gln, L-Ser) amino acids along with 10−4M L-Arg for 30 minutes prior to the addition of incrementing doses of Ang II (10−10M to 3×10−7M). The contractile force generated was recorded after addition of each successive dose of Ang II and normalized to the maximal force of contraction achieved with HPSS + 10−5M PE.
NO Production in Isolated Rat Aortic Rings
Experiments were performed in this protocol to document changes in NO production in the isolated aortic segments. Aortic segments were isolated, cleaned of adhering tissue, and flushed with PSS to remove the blood. The aorta was cut into 3–4 mm wide rings, sectioned longitudinally, and equilibrated at 37°C for an hour. The vessel were carefully handled to ensure that the endothelium remained intact during sectioning, and the tissue was pinned on a Sylgard-coated petri dish with the endothelial surface positioned upward and incubated for 10 minutes in HEPES buffered PSS. The vessel sections were then incubated with 10−5M DAF-2 diacetate, a cell permeable reagent that fluoresces in the presence of NO, for 30 minutes and rinsed with HEPES-buffered PSS. Background and stimulated fluorescence measurements were obtained with an epifluorescence microscope (Nikon E600) equipped with a 10× objective and 490 nm excitation and a 510–560 nm emission filter. Digital images were obtained with a PC-controlled camera and analyzed with Metamorph imaging and analysis software.
Once the tissues were loaded with DAF and the background image was obtained, the rings were incubated with different pharmacological agents and the change in fluorescence was recorded at 5 min interval for 30 mins. The tissues were incubated with 10−4M of L-Arg for 30 mins which was followed by addition of 10−6M Ang II. Some tissues were stimulated with 10−6M Ang II alone without incubation with L-Arg. Additional experiments were performed where the tissue was incubated for the same period of time (30 minutes) with 10−4M L-NAME serving as the negative control, in addition to 10−4M of L-Arg.
Statistical Analysis
Data are presented as mean ± SEM. A two-way repeated measures ANOVA with a Holm-Sidak post hoc test was used to test for differences in the pressure diuretic and natriuretic relationships and in the different groups of aortic ring segments. Paired t-tests were used to evaluate the alteration in the renal blood flows between the groups. A one-way ANOVA was used to evaluate the differences in urinary nitrate excretion. A probability value of P<0.05 was considered significant.
DISCUSSION
Previous studies from our laboratory indicated that supplementation of L-Arg blunted the hypertension and renal damage that occurred in SD rats continually infused with AngII.13 The present experiments were designed to elucidate the mechanism(s) of this effect. The in vitro studies in freshly isolated aortic rings demonstrated that exogenous L-Arg blunts AngII-mediated vascular constriction by increasing NO production. The in vivo experiments demonstrated that administration of L-Arg blunted AngII-mediated reductions in renal cortical blood flow, outer medullary blood flow, whole organ blood flow, and GFR. Correspondingly, the blunted pressure-natriuretic-diuretic response in rats administered AngII was reversed by administration of L-Arg. The present studies therefore provide evidence that the protective effects of L-Arg in AngII-mediated hypertension are mediated in part by increased uptake of L-Arg and increased NO production in endothelial cells, a reversal of AngII-mediated vasoconstriction, and a restoration of renal excretory function.
As described above, intracellular transport of L-Arg occurs through specialized transport systems in the plasma membrane of epithelial and endothelial cells.15,18,19,20,21 In the endothelial cells of the renal vasculature, both the y+ and y+L transport systems are involved in L-Arg uptake.15 Aortic rings were utilized in the present study as an in vitro model to reflect the mechanisms of L-Arg uptake in the vasculature and its effects on Ang II mediated vasoconstriction. The effect of exogenous L-Arg to blunt Ang II-mediated vasoconstriction was dependent upon an intact endothelium, was prevented by NOS inhibition, and was stereospecific. Moreover, co-incubation of excess cationic or neutral amino acids, substrates for y+ and y+L transport systems, blunted the effects of L-Arg, indicating that L-Arg uptake was mediated by these cellular transport mechanisms. Finally, to document that the effect is mediated by NO, it was observed that L-Arg stimulated DAF fluorescence in this in vitro preparation. Together, these data indicate that cellular L-Arg uptake mechanisms in the endothelium increase intracellular availability of L-Arg as a substrate for NOS to increase NO production and blunt the vasoconstrictor effects of AngII.
In vivo experiments showed that acute infusion of AngII in volume-expanded SD rats caused a marked vasoconstriction with a reduced GFR and RBF when compared to the vehicle infused rats. The vasoconstrictor effects of Ang II were reversed by administration of L-Arg, demonstrating the vasodilator effects of L-Arg in the renal vasculature. Examination of intrarenal blood flow with laser Doppler flow probes showed a significant reduction in superficial cortical blood flow and outer medullary blood flow in response to acute infusion of Ang II. Co-infusion of L-Arg with Ang II restored these reduced blood flows to the control levels. To demonstrate that the reduced intravascular resistance with L-Arg administration was due to the generation of NO, urinary nitrate levels were examined as a measure of NO production. These experiments indicated significantly higher nitrate levels in the urine samples of rats administered L-Arg compared to rats infused with Ang II or saline. Our present data are consistent with previous data that demonstrate that chronic administration of L-Arg improved the suppressed PN in salt sensitive forms of hypertension3,5,22 with restoration of medullary blood flow.23
The present data document the effects of L-Arg to blunt AngII-mediated vasoconstriction in vitro and in vivo. Moreover, these studies, in the context of previous experiments, indicate that cellular uptake of L-Arg mediates the protective effect. These experiments do not address the mechanisms whereby increased intracellular L-Arg increases NO. The intracellular L-Arg concentration in endothelial cells greatly exceeds the Km of NOS for L-Arg.24,25 An explanation for this paradoxical observation is not apparent. Despite that, it has been demonstrated that alterations in cellular L-Arg uptake alter NO production in the isolated perfused kidney15,26 and in anesthetized and conscious SD rats.27,28 Further work remains to fully understand the intracellular mechanisms of this effect.
Consistent with our previous observations,29 L-Arg blunted AngII-mediated changes in renal cortical and outer medullary blood flow. In the present studies, with renal perfusion pressure fixed with aortic clamps, intravenous infusion of AngII significantly decreased blood flow in the renal cortex and outer medulla but slightly, though significantly, increased inner medullary blood flow. This is somewhat unexpected since we previously observed that this dose of AngII did not alter papillary blood flow in anesthetized Sprague-Dawley rats10 though we have reported that this dose of AngII significantly increased inner medullary blood flow in mice.30 One potential mechanism of interaction controlling blood flow in this region of the kidney is “cross-talk” between the tubules and vasculature. NO produced in the thick ascending limb of Henle can diffuse to adjacent vasa recta and alter vascular diameter,31 and this mechanism has been described to blunt AngII-mediated constriction of the vasa recta.32 Interactions between adjacent tubular and vascular structures are therefore another likely mechanism at work in the present study. Regardless of the mechanisms, the effects of AngII in the renal medullary circulation are somewhat controversial;33,34 the present data, however, are consistent with the general concept that the renal medullary circulation is relatively refractory to the constrictor effects of AngII.
Though the present experiments indicate that L-Arg supplementation blunts the renal vasoconstrictor effects of AngII, presumably by direct effects on the endothelium, it is critical to note that the renal tubular effects of AngII and L-Arg are also likely to play a role in the present observations. AngII has well-described effects to directly stimulate sodium reabsorption in the proximal tubule and has been reported to have similar effects in the thick ascending limb of Henle, the distal convoluted tubule, and in the collecting duct.35 We previously demonstrated that L-Arg supplementation increases L-Arg uptake and NO production in the renal medulla27,28 and in freshly isolated inner medullary collecting duct cells.21 Recent studies demonstrated that chronic AngII infusion independently increased ENaC-mediated sodium reabsorption in distal nephron segments, as evidenced by elevated plasma membrane αENaC in distal nephron segments in both the renal cortex and inner medulla.36 Experiments in the isolated perfused thick ascending limb demonstrated that chloride flux is inhibited following L-Arg administration, an effect which was blocked by pretreatment with L-NAME;37 while NO is released from medullary thick ascending loop of Henle in response to AngII.31 Together, these data indicate that NO production from epithelial cells in the thick ascending loop of Henle or the inner medullary collecting duct can be stimulated by L-Arg/NO or AngII, and both NO and AngII can alter tubular solute reabsorption. It is therefore likely that the effect of L-Arg to stimulate NO in renal tubules acts to oppose the direct tubular effects of AngII on epithelial sodium transport.
One weakness in the present experimental approach is the use of aortic rings as a model for the renal vasculature. A more accurate understanding of the modulatory effects of L-Arg uptake on AngII-mediated constrictor effects could have been obtained from isolated renal microvessels. The present data obtained in the aorta, however, are consistent with the in vivo renal hemodynamic data in the present study. Moreover, the modulation of NO-dependent effects are qualitatively in agreement with previous studies in which we examined the influence of manipulation of L-Arginine uptake on NO production and vascular resistance in the isolated perfused kidney,15 but it needs to be recognized that the observations in the aorta may be distinct from those in the renal vasculature.
In conclusion, the present studies demonstrate that supplementation of exogenous L-Arg reverses AngII-mediated reductions in GFR and RBF and attenuates the effects of AngII to blunt the pressure-natriuretic-diuretic relationship in rats. The results indicate that the protective effects appear to be mediated by increasing cellular L-Arg uptake and NO production. Together, results of these studies provide evidence that the protective effects of L-Arg in AngII-mediated hypertension are mediated in part by increased uptake of L-Arg and increased NO production in endothelial cells, a reversal of AngII-mediated vasoconstriction, and a restoration of renal excretory function.
ACKNOWLEDGEMENTS
This work was partially supported by National Institutes of Health Grants HL-116264, DK-62803, and DK-96859.
Footnotes
CONFLICT OF INTEREST
None
REFERENCES
- 1.Chen PY, Sanders PW. Role of nitric oxide synthesis in salt sensitive hypertension in Dahl/Rapp rats. Hypertension. 1993;22:812–818. doi: 10.1161/01.hyp.22.6.812. [DOI] [PubMed] [Google Scholar]
- 2.Gokce N. L-arginine and hypertension. J. Nutr. 2004;134:2807S–2811S. doi: 10.1093/jn/134.10.2807S. [DOI] [PubMed] [Google Scholar]
- 3.Hu L, Manning RD., Jr Role of nitric oxide in regulation of long-term pressure-natriuresis relationship in Dahl rats. Am. J. Physiol. 1995;268:H2375–H2383. doi: 10.1152/ajpheart.1995.268.6.H2375. [DOI] [PubMed] [Google Scholar]
- 4.Miyata N, Zou AP, Mattson DL, Cowley AW., Jr Renal medullary interstitial infusion of L-arginine prevents hypertension in Dahl salt-sensitive rats. Am J Physiol. 1998;275:R1667–R1673. doi: 10.1152/ajpregu.1998.275.5.R1667. [DOI] [PubMed] [Google Scholar]
- 5.Patel AR, Granger JP, Kirchner KA. L-Arginine improves transmission of perfusion pressure to the renal interstitium in Dahl salt-sensitive rats. Am J Physiol. 1994;266:R1730–R1735. doi: 10.1152/ajpregu.1994.266.6.R1730. [DOI] [PubMed] [Google Scholar]
- 6.Siani A, Pagano E, Iacone R, Iacoviello L, Scopacasa F, Strazzullo P. Blood pressure and metabolic changes during dietary L-arginine supplementation in humans. Am. J. Hypertension. 2000;13:547–551. doi: 10.1016/s0895-7061(99)00233-2. [DOI] [PubMed] [Google Scholar]
- 7.Chen PY, Sanders PW. L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J. Clin. Invest. 1991;88:1559–1567. doi: 10.1172/JCI115467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higashi Y, Oshima T, Ozono R, Wantanabe M, Matsuura H, Kajiyama G. Effects of L-arginine infusion on renal hemodynamics in patients with mild essential hypertension. Hypertension. 1995;25:898–902. doi: 10.1161/01.hyp.25.4.898. [DOI] [PubMed] [Google Scholar]
- 9.Pagnotta P, Germano G, Grutter G, Leonardo F, Rosano G, Chierchia S. Oral L-arginine supplementation improves essential arterial hypertension. Circulation. 1997;96:538-I. [Google Scholar]
- 10.Mattson DL, Raff H, Roman RJ. Influence of Ang II on pressure natriuresis and renal hemodynamics on volume expanded rats. Am. J. Physiol. 1991;260:R1200–R1209. doi: 10.1152/ajpregu.1991.260.6.R1200. [DOI] [PubMed] [Google Scholar]
- 11.Van der Mark J, Kline RL. Altered pressure natriuresis in chronic Angiotensin II hypertension in rats. Am. J. Physiol. 1994;266:F739–F748. doi: 10.1152/ajpregu.1994.266.3.R739. [DOI] [PubMed] [Google Scholar]
- 12.Navar LG, Ichihara A, Chin SY, Imig JD. Nitric oxide-angiotensin II interactions in angiotensin II-dependent hypertension. Acta. Physiol. Scand. 2000;168:139–147. doi: 10.1046/j.1365-201x.2000.00630.x. [DOI] [PubMed] [Google Scholar]
- 13.Rajapakse N, Das S, De Miguel C, Lund H, Mattson DL. Exogenous L-arginine ameliorates angiotensin II-induced hypertension and renal damage in rats. Hypertension. 2008;52:1086–1090. doi: 10.1161/HYPERTENSIONAHA.108.114298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reckelhoff JF, Romero JC. Role of oxidative stress in angiotensin-induced hypertension. Am. J. Physiol. 2003;284:R893–R912. doi: 10.1152/ajpregu.00491.2002. [DOI] [PubMed] [Google Scholar]
- 15.Kakoki M, Kim H-S, Edgell C-JS, Maeda N, Smithies O, Mattson DL. Amino acids as modulators of endothelium-derived nitric oxide. Am. J. Physiol. 2006;291:F297–F304. doi: 10.1152/ajprenal.00417.2005. [DOI] [PubMed] [Google Scholar]
- 16.Pechman KR, De Miguel C, Lund H, Leonard EC, Basile DP, Mattson DL. Recovery from renal ischemia reperfusion injury is associated with altered renal hemodynamics, blunted pressure natriuresis, and sodium sensitive hypertension. Am. J. Physiol. 2009;297:R1358–R1363. doi: 10.1152/ajpregu.91022.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cholewa BC, Meister CJ, Mattson DL. Importance of the renin-angiotensin system in the regulation of arterial blood pressure in conscious mice and rats. Acta Physiol. Scand. 2005;183:309–320. doi: 10.1111/j.1365-201X.2004.01401.x. [DOI] [PubMed] [Google Scholar]
- 18.Kilberg MS, Stevens BR, Novak DA. Recent advances in mammalian amino acid transport. Ann. Review Nutrition. 1993;13:137–165. doi: 10.1146/annurev.nu.13.070193.001033. [DOI] [PubMed] [Google Scholar]
- 19.Sala R, Rotoli BM, Colla E, Visigalli R, et al. Two-way arginine transport in human endothelial cells: TNF-alpha stimulation is restricted to system y(+) Am. J. Physiol. 2002;282:C134–C143. doi: 10.1152/ajpcell.2002.282.1.C134. [DOI] [PubMed] [Google Scholar]
- 20.Simon A, Plies L, Habermeier A, Martiné U, Reining M, Closs EI. Role of neutral amino acid transport and protein breakdown for substrate supply of nitric oxide synthase in human endothelial cells. Circ. Res. 2003;93:813–820. doi: 10.1161/01.RES.0000097761.19223.0D. [DOI] [PubMed] [Google Scholar]
- 21.Wu F, Cholewa BC, Mattson DL. Characterization of L-arginine transporters in rat renal inner medullary collecting ducts. Am. J. Physiol. 2000;278:R1506–R1512. doi: 10.1152/ajpregu.2000.278.6.R1506. [DOI] [PubMed] [Google Scholar]
- 22.Patel A, Layne S, Watts D, Kirchner KA. L Arg administration normalizes pressure natriuresis in hypertensive Dahl rats. Hypertension. 1993;22:863–869. doi: 10.1161/01.hyp.22.6.863. [DOI] [PubMed] [Google Scholar]
- 23.Miyata N, Cowley AW., Jr Renal intramedullary infusion of L-Arg prevents reduction in medullary blood flow and hypertension in Dahl salt-sensitive rats. Hypertension. 1999;33:446–450. doi: 10.1161/01.hyp.33.1.446. [DOI] [PubMed] [Google Scholar]
- 24.Baydoun AR, Emery PW, Pearson JD, Mann GE. Substrate-dependent regulation of intracellular amino acid concentrations in cultured bovine aortic endothelial cells. Biochem. Biophys. Res. Comm. 1990;173:940–948. doi: 10.1016/s0006-291x(05)80876-9. [DOI] [PubMed] [Google Scholar]
- 25.Pollock JS, Förstermann U, Mitchell JA, Warner TD, Schmidt HHHW, Nakane M, Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc. Nat. Acad. Sci. 1990;87:8612–8616. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radermacher J, Klanke B, Kastner S, et al. Effect of Arg depletion on glomerular and tubular kidney function: studies in isolated perfused rat kidneys. Am. J. Physiol. 1991;261:F779–F786. doi: 10.1152/ajprenal.1991.261.5.F779. [DOI] [PubMed] [Google Scholar]
- 27.Kakoki M, Kim H-S, Arendshorst W, Mattson DL. L-arginine uptake affects nitric oxide production and blood flow in the renal medulla. Am. J. Physiol. 2004;287:R1478–R1485. doi: 10.1152/ajpregu.00386.2004. [DOI] [PubMed] [Google Scholar]
- 28.Kakoki M, Wang W, Mattson DL. Cationic amino acid transport in the renal medulla and blood pressure regulation. Hypertension. 2002;39:287–292. doi: 10.1161/hy0202.102700. [DOI] [PubMed] [Google Scholar]
- 29.Rajapakse N, Mattson DL. Role of L-arginine uptake mechanisms in renal blood flow responses to angiotensin II in rats. Acta Physiol. 2011;203:391–400. doi: 10.1111/j.1748-1716.2011.02330.x. 2011. [DOI] [PubMed] [Google Scholar]
- 30.Mattson DL, Meister CJ. Renal cortical and medullary blood flow responses to L-NAME and angiotensin II in wild-type, nNOS null mutant, and eNOS null mutant mice. Am. J. Physiol. 2005;289:R991–R997. doi: 10.1152/ajpregu.00207.2005. [DOI] [PubMed] [Google Scholar]
- 31.Dickhout JG, Mori T, Cowley AW., Jr Buffering of Angiotensin II–induced medullary vasoconstriction. Circ Res. 2002;91:487–493. doi: 10.1161/01.res.0000035243.66189.92. [DOI] [PubMed] [Google Scholar]
- 32.O’Connor PM, Cowley AW., Jr Medullary thick ascending limb buffer vasoconstriction of renal outer-medullary vasa recta in salt-resistant but not salt-sensitive rats. Hypertension. 2012;60:965–972. doi: 10.1161/HYPERTENSIONAHA.112.195214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans RG, Head GA, Eppel GA, Burke SL, Rajapakse NW. Angiotensin II and neurohumoral control of the renal medullary circulation. Clin. Exp..Pharmacol. Physiol. 2010;37:e58–e69. doi: 10.1111/j.1440-1681.2009.05233.x. [DOI] [PubMed] [Google Scholar]
- 34.Sadowski J, Badzynska B. Specific features and roles of renal circulation: angiotensin II revisited. J. Physiol. Pharmacol. 2006;57:169–178. [PubMed] [Google Scholar]
- 35.Ballermann BJ, Onuigbo MAC. Angiotensins. Comprehensive Physiology. 2011:104–155. [Google Scholar]
- 36.Mamanko M, Zaika O, Prieto MC, Jensen VB, Doris PA, Navar LG, Pochynyuk O. Chronic angiotensin II infusion drives extensive aldosterone-independent epithelial Na+ channel activation. Hypertension. 2013;62:1111–1122. doi: 10.1161/HYPERTENSIONAHA.113.01797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plato CF, Stoos BA, Wang D, Garvin JL. Endogenous nitric oxide inhibits chloride transport in the thick ascending limb. Am J Physiol. 1999;276:F159–F163. doi: 10.1152/ajprenal.1999.276.1.F159. [DOI] [PubMed] [Google Scholar]