Abstract
Ventral pallidum (VP) is centrally positioned within mesocorticolimbic reward circuits, and its dense projection to ventral tegmental area (VTA) regulates neuronal activity there. However, VP is a heterogeneous structure, and how this complexity affects its role within wider reward circuits is unclear. Here we demonstrate that projections to VTA from rostral (RVP), but not caudal VP (CVP) are robustly Fos-activated during cue-induced reinstatement of cocaine seeking—a rat model of relapse in addiction. Moreover, designer receptor-mediated transient inactivation of RVP neurons, their terminals in VTA, or functional connectivity between RVP and VTA dopamine neurons blocks the ability of drug-associated cues (but not a cocaine prime) to reinstate cocaine seeking. In contrast, CVP neuronal inhibition instead blocked cocaine-primed, but not cue-induced reinstatement. This novel double dissociation in VP sub-regional roles in drug seeking is likely important for understanding mesocorticolimbic circuits underlying reward seeking and addiction.
Ventral pallidum (VP) is a crucial node in ventral striatopallidal circuits underlying reward-related behaviors, with major reciprocal connections to accumbens, extended amygdala, limbic thalamus, substantia nigra (SN) and ventral tegmental area (VTA)1–4. VP neurons respond to rewards and their cues in animals and humans, and are necessary for reward-seeking behaviors—leading to hypotheses that VP helps translate motivational states into appetitive behaviors5–7.
However, VP is a heterogeneous structure, exhibiting medial-lateral and rostral-caudal variation in histological markers and connectivity patterns1, 4, 8. Notably, ventromedial VP (most of which is located subcommissurally, rostral of bregma in rat: RVP) receives dense afferents from nucleus accumbens shell, and projects strongly to mediodorsal thalamus and VTA. Dorsolateral VP (most of which is located sublenticularlly, caudal of bregma: CVP) instead receives afferents from accumbens core, and projects to SN and subthalamic nucleus (STN), in addition to VTA1, 9. This topography implies involvement of VP subregions in functionally distinct mesolimbic circuits.
Functional heterogeneity between VP subregions has also been reported. In vitro, RVP and CVP neurons differ in several ways; many RVP neurons have spiny dendrites, glutamatergic afferents, hyperpolarized membrane potentials, and no spontaneous action potentials10. Intriguingly, these characteristics are more typical of accumbens medium spiny neurons than “classical” VP neurons. In contrast, CVP neurons have more “classical” pallidal characteristics, including aspiny dendrites, GABAergic inputs, depolarized membrane potentials, and spontaneous action potentials. Notably, neuronal firing during rat cocaine self-administration varies depending on mediolateral and rostrocaudal localization within VP11, and VP rostrocaudal variation exists in neuronal cytoarchitecture, in vivo firing rates, sensitivity to rewarding effects of electrical stimulation and microinjected drugs, opioid agonist-induced hedonic responses to tastes, and fMRI activation to emotionally arousing pictures12–19.
However, little is known about how such heterogeneity contributes to VP functions within wider mesolimbic circuits. In rat, CVP provides tonically-active GABA inputs to VTA and SN that gate firing of subpopulations of dopamine neurons and modulate reward seeking20. However, VP neurons are also acutely activated by reward-associated cues6, 21, 22, and VP projections to VTA are activated during cue-induced reinstatement of cocaine seeking23. How does functional-anatomical heterogeneity impact VP regulation of VTA, and the role of this circuit in drug seeking?
We sought to examine the roles of VP subregions and their VTA projections in relapse to cocaine seeking. We examined Fos activation of RVP and CVP projections to VTA, and inactivated these subregions or their projections to midbrain dopaminergic regions during reinstatement of cocaine seeking using Designer Receptors Exclusively Activated by Designer Drugs (DREADDs)24. DREADDs utilize endogenous G-protein signaling pathways, but do not affect neuronal activity in the absence of their otherwise inert, exogenously-administered ligand, clozapine-n-oxide (CNO). The Gi-coupled (hM4Di) DREADDs used here thereby allow transient inactivation of defined neuronal populations25. We also show that they can be used to manipulate axonal terminals of DREADD-expressing neurons to inactivate particular monosynaptic pathways (e.g. RVP-VTA), or to disrupt functional connectivity between specific neuronal populations (e.g. RVP and VTA dopamine neurons). We demonstrate a novel double-dissociation between rostral and caudal VP in cued vs. cocaine primed reinstatement respectively, and show that cued cocaine seeking is dependent upon RVP projections to VTA, and complex interactions with dopaminergic and non-dopaminergic neurons there.
Results
Validation of VP DREADDs
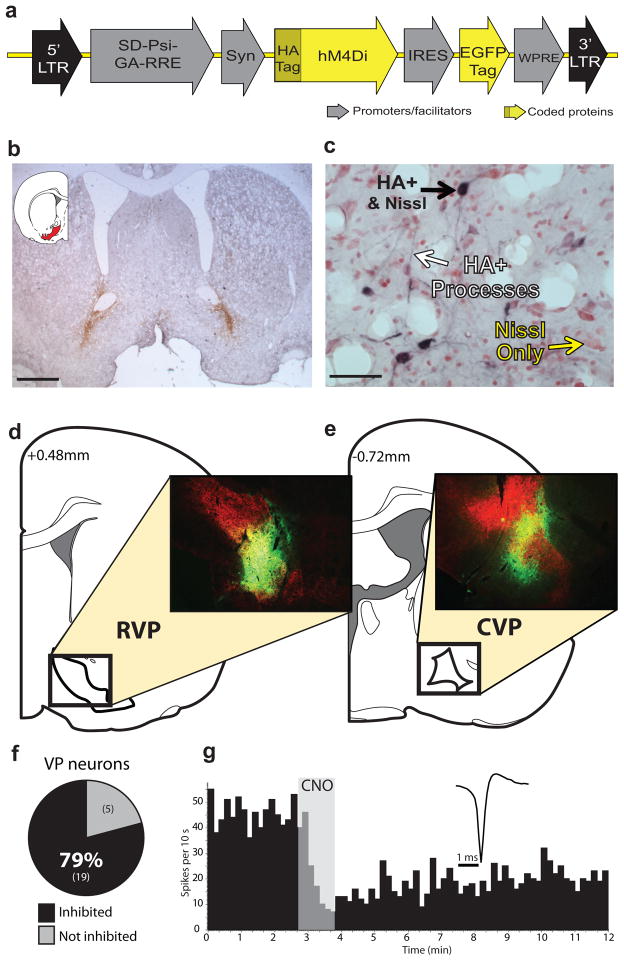
We used a DREADD-based strategy to remotely control VP neurons in vivo and manipulate the VP-VTA circuit. We injected a synapsin-driven lentiviral vector yielding expression of the Gi-coupled DREADD, hM4Di24, or a control virus lacking the DREADD gene (Syn-GFP) into RVP or CVP, and allowed animals to survive 6+ weeks before analyzing brain tissue for virus expression. The Syn-hM4Di-HA-GFP virus (Fig. 1a) caused robust hM4Di expression within RVP or CVP [visualized with immunoreactivity for the hemagglutinin (HA) tag, or green fluorescent protein (GFP) reporter]. These injections yielded limited expression outside the borders of VP (<30% labeling outside VP borders; Figs.1b–e, 2d, Supplemental Fig. 1). Syn-hM4Di-HA-GFP and Syn-GFP viruses yielded comparable zones of somatic GFP or hM4Di expression centered at the injection site [GFP: 0.82(0.21) mm3; hM4Di: 0.89(0.05) mm3].
Figure 1. hM4Di inhibitory DREADD expression and function.
a) Syn-hM4Di-HA-GFP lentivirus used to express hM4Di DREADDs under a neuronal-specific human synapsin promoter (Syn) in RVP or CVP. b) Typical staining for HA-tagged hM4Di after bilateral injections into RVP. Scale bar=1 mm. c) HA-tagged hM4Di receptors are expressed on cell bodies and processes (black staining) in VP (neutral red counterstain). Scale bar=50 μm. d) Typical HA expression (green) in RVP, largely contained within VP borders (defined with SP counterstain in red). e) Typical HA expression in CVP, largely contained within VP borders (as defined in d). Images in b–e are representative of DREADD expression seen in experimental groups, ns listed in Results. f) In animals with Syn-hM4Di-HA-GFP in RVP or CVP, local application of 100 μM CNO onto extracellularly recorded VP neurons in vivo (via double barrel glass pipette) inhibited firing rates in the vast majority of neurons tested (19/24). g) Typical change in discharge rate of a VP neuron (waveform inset) after local CNO application (shaded region).
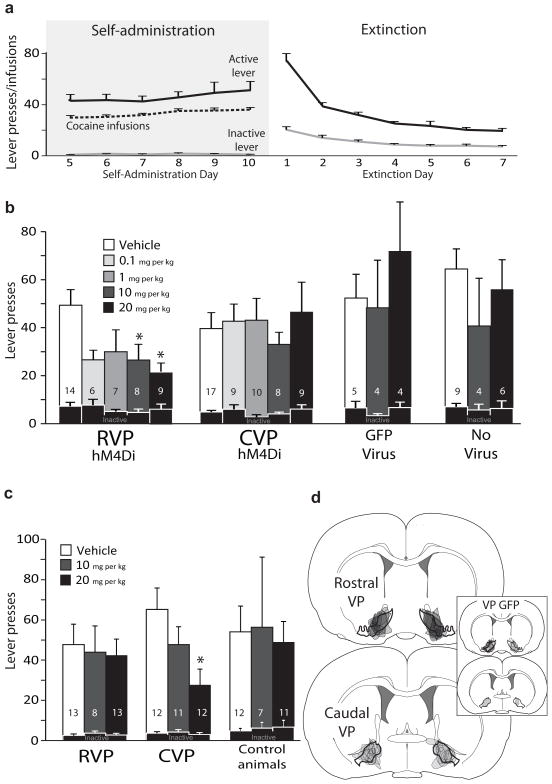
Figure 2. Effects of inactivating rostral or caudal VP on reinstatement of cocaine seeking.
a) Daily m±SEM cocaine infusions and active/inactive lever presses for the last 6 days of criterion self- administration (>10 infusions/day), and active/inactive lever presses for the first 7 days of extinction training. b) Active lever presses during cue-induced reinstatement following different doses of the DREADD agonist clozapine-n-oxide (CNO; dose=bar color; vehicle, 0.1, 1, 10, 20 mg/kg), in animals with bilateral RVP or CVP Syn-hM4Di-HA-GFP virus, bilateral VP control virus (Syn-GFP), or no virus expression. Inactive lever presses during reinstatement are shown with overlaid black bars. *Vehicle vs. 10 mg/kg CNO: p=0.001, vehicle vs. 20 mg/kg CNO: p=0.015. c) Active and inactive lever presses during cocaine primed reinstatement following different doses of CNO (Vehicle, 10, 20 mg/kg), in animals with RVP or CVP Syn-hM4Di-HA-GFP virus, or controls (Syn-GFP or no virus expression animals). *Vehicle vs. 20 mg/kg CNO: p=0.008. Bars and lines=m±SEM. d) The anatomical localization of virus expression sites (as visualized with HA or GFP immunoreactivity) is represented for animals injected with Syn-hM4Di-HA-GFP in RVP (black) or CVP (grey), or Syn-GFP (grey; inset).
We then used in vivo electrophysiology to functionally validate hM4Di receptor expression. In isofluorane-anesthetized rats, local CNO microinjection (60 nl,100 μM) inhibited VP neurons (n=24 cells; m=60.3 ± 6.6% decrease in firing; Z = −2.54; p=0.011; Fig. 1f–g). Local CNO similarly affected RVP (11/13 cells inhibited) and CVP (8/11 inhibited) neurons, with 58.2 ± 8.7% and 63.2 ± 10.6% inhibition, respectively. Pre-CNO firing rates in RVP (m=6.4 ± 2.0 Hz) were lower than in CVP (m=24.8 ± 5.3 Hz; p=0.007; t12=3.26), as previously reported10, 12.
Double Dissociation of VP Subregion Roles in Reinstatement
Next, we examined whether transient DREADD-mediated inhibition of RVP or CVP would attenuate either cued or cocaine primed (10 mg/kg) reinstatement of cocaine seeking following cocaine self-administration and extinction (Fig. 2a). CNO (0,0.1,1,10, or 20 mg/kg) attenuated cued reinstatement in rats with hM4Di expression in RVP, but not in animals with comparable DREADD expression in CVP [RVP (n=16 rats): F4,39=4.0, p=0.008; CVP (n=17 rats): F4,46=0.59, n.s.; Fig. 2b]. In contrast, CNO (0, 10 or 20 mg/kg) injections attenuated cocaine-primed reinstatement only in animals with hM4Di expression in CVP, but not in RVP (RVP: F2,29=0.45, n.s.; CVP: F2,31=3.48, p=0.04; Fig. 2c), revealing a novel double-dissociation between RVP and CVP for reinstatement elicited by cues vs. cocaine.
The zones in which hM4Di inhibition were most effective at reducing cued reinstatement were centered in RVP, while effective sites for cocaine-primed reinstatement were centered in CVP (cue—VP site x drug interaction: F1,16=7.35, p=0.015; prime—VP site x drug interaction: F1,25=4.85, p=0.037). VP inhibition was sufficient for these effects, as cases with minimal (<5%) hM4Di expression outside VP borders showed equivalent reinstatement effects as those with more (<30%) encroachment of expression into adjacent areas (Supplemental Fig. 1). CNO had no effects in animals with either GFP virus injections (Syn-GFP, lacking the hM4Di gene; n=5 rats; cue: F2,11=0.58, n.s.; prime: F1,8=0.05, n.s.), or no virus expression (n=9 rats; cue: F2,18=0.87, n.s.; prime: F2,24=0.28, n.s.; control groups combined: cue: F2,28=0.77, n.s.; prime: F2,31=0.22, n.s.; Fig. 2b–c)].
Fos Activation of RVP-VTA Projections During Reinstatement
We next asked whether the role for RVP in cued reinstatement behavior involves projections to VTA. First, we examined Fos expression in RVP or CVP VTA-projecting neurons during cued reinstatement, or control behaviors (Fig. 3a; Supplemental Fig. 2). The retrograde tracer cholera toxin beta subunit (CTb) was injected into rostral or caudal VTA. Animals (n=25) were trained to self-administer i.v. cocaine+cues, extinguished, then given a 2 h cue-induced reinstatement test or control behavioral test, as described in Online Methods. Animals were sacrificed immediately after this test session, and VP slices were co-stained for Fos and CTb.
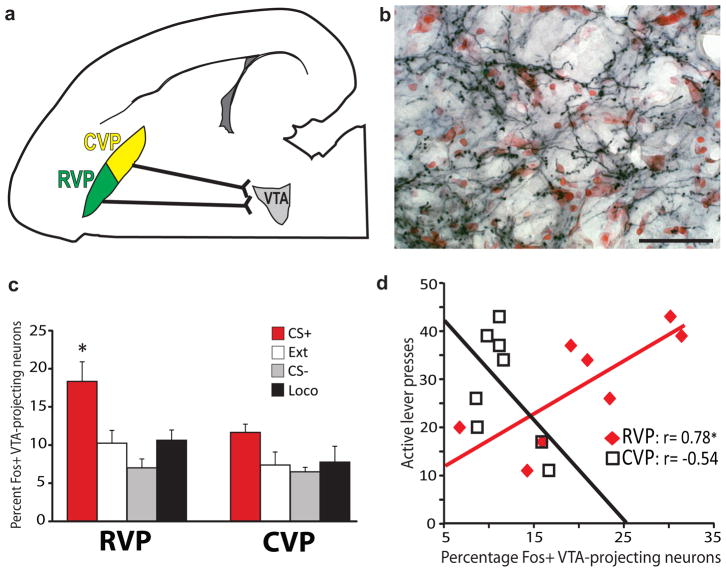
Figure 3. Rostral VP projections to VTA are Fos-activated during cued reinstatement.
a) Predominantly ipsilateral projections from RVP and CVP to VTA are diagrammed in the horizontal plane. b) Axon terminals in VTA express the anterogradely-transported, HA-tagged hM4Di receptor (black axonal processes) after Syn-hM4Di-HA-GFP injection in ipsilateral RVP (red Nissl counterstain in VTA). Coronal view, scale bar=50 μm. Image is representative of VP axonal DREADD expression in VTA of experimental animals, group ns listed in Results. c) Mean±SEM percentages of VTA-projecting (CTb+) RVP or CVP cells that express Fos after cue-induced reinstatement (CS+), or control behavioral conditions, where animals were exposed to: the extinguished self-administration environment without cues or cocaine (Ext), a tone/light stimulus not associated with cocaine (CS−), or a locomotion- enhancing novel environment (Loco). A greater proportion of VTA-projecting neurons in RVP were Fos-activated in CS+ than in control animals [*CS+ different from: Ext (p=0.01), CS− (p=0.005), and Loco (p=0.025)]. No significant behavior-specific activation of VTA-projecting neurons was detected in CVP. Bars=m±SEM. d) Cue-induced reinstatement behavior was positively correlated with Fos activation of VTA-projecting neurons in RVP (*Pearson correlation: p=0.02), but not CVP (not significant).
In RVP, VTA-projecting (CTb+) neurons were Fos-activated during cued cocaine seeking (CS+, n=8 rats), but not during exposure to the extinguished self-administration chamber (Ext., n=7), a novel environment (Loco, n=6), or a familiar, non-cocaine associated CS− stimulus (CS−, n=4; F3,21=6.6, p=0.002; Fig. 3c). Unlike RVP, CVP projections to VTA were not specifically activated during cued reinstatement (F3,21=2.3, n.s.; Fig. 3c; Supplemental Fig. 2). Moreover, in individual CS+ animals, the degree of Fos activation of VTA afferents from RVP, but not CVP was positively correlated with the degree of cue-induced cocaine seeking behavior (RVP: r=0.78, p=0.02; CVP: r=−0.54, n.s.; Fig. 3d; Supplemental Fig. 2d), indicating that more activity in RVP efferents to VTA is associated with more cue-triggered drug seeking.
Validating Inhibition of RVP-VTA Projection with DREADDs
The hM4Di DREADD is trafficked axonally, evidenced by numerous HA+ fibers and terminals reliably observed in ventral midbrain after hM4Di vector injection in RVP or CVP (Fig. 3b,4a, Supplemental Fig. 3d–e). We examined whether modulation of Gi-coupled signaling in VP axon terminals in VTA via local CNO application would alter activity of VTA neurons. First, we recorded spontaneous inhibitory postsynaptic currents (sIPSCs) from dopamine neurons (verified by tyrosine hydroxylase (TH) immunoreactivity) in VTA slices from rats that were injected 6+ weeks prior with the hM4Di vector in RVP (n=7 rats; Fig. 4b–c). Perfusion with 5 μM CNO had no effect on sIPSC amplitude (t6=0.94, n.s.; Fig. 5d), but decreased frequency (t6 = 2.50, p=0.046) and shifted the cumulative distribution of inter-event intervals (IEIs) to the right (main effect of IEI duration: F21,264=22.18, p=0.0001; and CNO treatment: F1,264=14.42, p=0.0002; Fig. 4e). In slices from Syn-GFP injected animals (n=5 cells), which also expressed GFP in RVP neurons and their VTA axons, CNO had no effect on sIPSC amplitude (t4 = 0.47, n.s.) or IEIs (t4 = 0.39, n.s.; Supplemental Fig. 4). These findings indicate that activation of hM4Di DREADDs on VP terminals in VTA decreased GABA release onto VTA dopamine neurons.
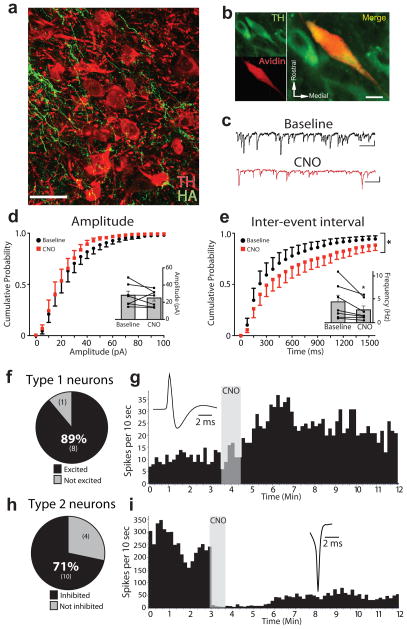
Figure 4. Inactivating rostral VP afferents modulates VTA cell firing.
a) hM4Di receptors (green) are expressed on RVP axon terminals in the vicinity of TH-labeled dopamine neurons (red) in VTA. Scale bar=30 μm. Image is representative of VP axonal DREADD expression in VTA of experimental animals, ns listed in Online Methods. b) In slice electrophysiology experiments, recorded neurons were filled with biotin, labeled with avidin (red), and identified as dopaminergic by co-labeling for TH (green). Scale bar=10 μm. c) Representative traces from VTA dopamine neurons are shown in baseline conditions (top, black), and after CNO (bottom, red; scale=20 pA, 200 ms). d) CNO had no effect on the cumulative distribution or mean (inset) sIPSC amplitude. Points=m±SEM, Lines in inset=individual raw values. e) CNO shifted the inter-event interval cumulative distribution to the right (*baseline vs. CNO, p=0.0002) and decreased mean sIPSC frequency (Inset, *baseline vs. CNO, p=0.046). Points=m±SEM, lines in inset=individual raw values. f) In vivo, 8/9 putatively dopaminergic “Type 1” neurons in VTA were excited by local application of CNO in the vicinity of hM4Di-expressing RVP terminals in VTA. g) Rate histogram demonstrating effect of local CNO application in vivo (60 nl; shaded region) on firing of a “Type 1” neuron (waveform inset) in VTA of RVP-hM4Di animal. h) 10/14 fast-firing, short-waveform “Type 2” neurons were inhibited by local application of CNO in vivo, in the vicinity of hM4Di-expressing RVP terminals in VTA. i) Example change in discharge rate of “Type 2” neuron (waveform inset) in VTA after local CNO application in vivo (60 nl; shaded region) in a RVP- hM4Di animal.
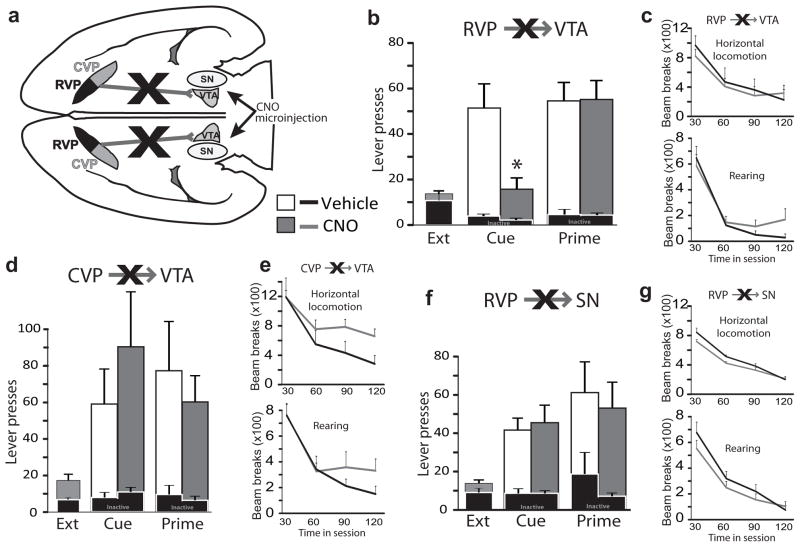
Figure 5. Inactivating rostral VP projection to VTA blocks cued reinstatement.
a) Projections from RVP to VTA or SN, or from CVP to VTA, were transiently inactivated by local midbrain microinjection of CNO amongst VP axon terminals expressing hM4Di receptors. b) Inactivation of VTA terminals from RVP blocked cue-induced (Cue), but not cocaine primed (Prime) reinstatement of cocaine seeking. Active lever pressing during the last day of extinction training (Ext., bars at left), and reinstatement after VTA vehicle (white bars) or VTA CNO (grey bars) are shown. Inactive lever presses are represented with black bars at bottom. * p<0.001. c) Inactivating RVP projections to VTA did not affect horizontal (top graph) or vertical (rearing; bottom graph) locomotion in a familiar environment. Locomotion (beam breaks) during 30 min bins in the 2 h session is shown after VTA microinjection of vehicle (black lines) or CNO (grey lines). d, e) Inactivation of CVP efferents to VTA did not affect cue-induced or cocaine-primed reinstatement, nor did it significantly affect horizontal locomotion or rearing. f, g) Inactivation of RVP projections to SN failed to affect reinstatement or locomotor behavior. Bars and lines=m±SEM.
We confirmed this finding in vivo, demonstrating that intra-VTA CNO microinjection in RVP-hM4Di rats significantly increased firing rate (m=82.5 ± 50.2% increase) in 8 of 9 “Type 1 neurons” (those meeting conventional electrophysiological criteria for VTA dopamine neurons26; p=0.03; t7 = 2.63; Fig. 4f–g). Conversely, 10 of 14 fast-spiking, short-waveform “Type 2” VTA neurons (not conventionally dopamine-like) were inhibited (m=−60.5 ± 12.0% change in discharge) after intra-VTA microinjection of CNO in RVP-hM4Di rats (p=0.003; t9 = 3.93; Fig. 4h–i).
Inhibiting RVP Terminals in VTA Blocks Cued Reinstatement
Next, we examined effects of inactivating VP terminals in VTA on cued or cocaine-primed reinstatement behavior. RVP hM4Di animals (n=28) were microinjected (in counterbalanced order on separate days) with CNO (1 mM/0.3 μl/side) or vehicle (artificial cerebrospinal fluid: aCSF) into VTA (n=17 rats) or SN (n=11), 5 min prior to cued or primed reinstatement (2 tests each/animal; Fig. 5a). CVP hM4Di animals (n=9) were also injected with CNO (0&1 mM/0.3 μl) into VTA, then tested on cued or primed reinstatement (Fig. 5a; cannulae sites in Supplemental Fig. 5). Inhibition of RVP terminals in VTA with local CNO selectively attenuated cued, but not primed reinstatement (cue: F1,16=27.87, p=0.000075; prime: F1,16=0.01, n.s.; Fig. 5b), indicating that activation of the RVP projection to VTA is required for drug cues to drive reinstatement of cocaine seeking. In contrast, inactivating RVP terminals in SN, or CVP terminals in VTA, had no effects on cued or primed reinstatement (RVP-SN inhibition, cue: F1,10=0.21, n.s., prime: F1,10=0.01, n.s.; CVP-VTA inhibition, cue: F1,8=0.84, n.s., prime: F1,8=0.37, n.s.; Fig. 5d,f). This indicates that although CVP is necessary for cocaine-primed reinstatement (Fig. 2c), its VTA targets are not essential for this action.
Inactivating RVP projections to VTA in the absence of cues or priming injections failed to affect drug seeking following extinction (n=4 rats; vehicle AL presses m±SEM=8.7±6.7, CNO m=4.3±1.7; F1,3=3.9, n.s.). In RVP (n=11) or CVP (n=6) hM4Di animals, neither intra-VTA (n=17) nor intra-SN (n=6) CNO had significant effects on general locomotor behavior in a familiar environment (no main effect of drug or drug X time interactions; Fs<1.7, n.s.; Fig. 5c,e,g), indicating that reinstatement effects of RVP-VTA inhibition are more likely motivational than motoric in nature.
RVP Interacts with VTA Dopamine Neurons in Reinstatement
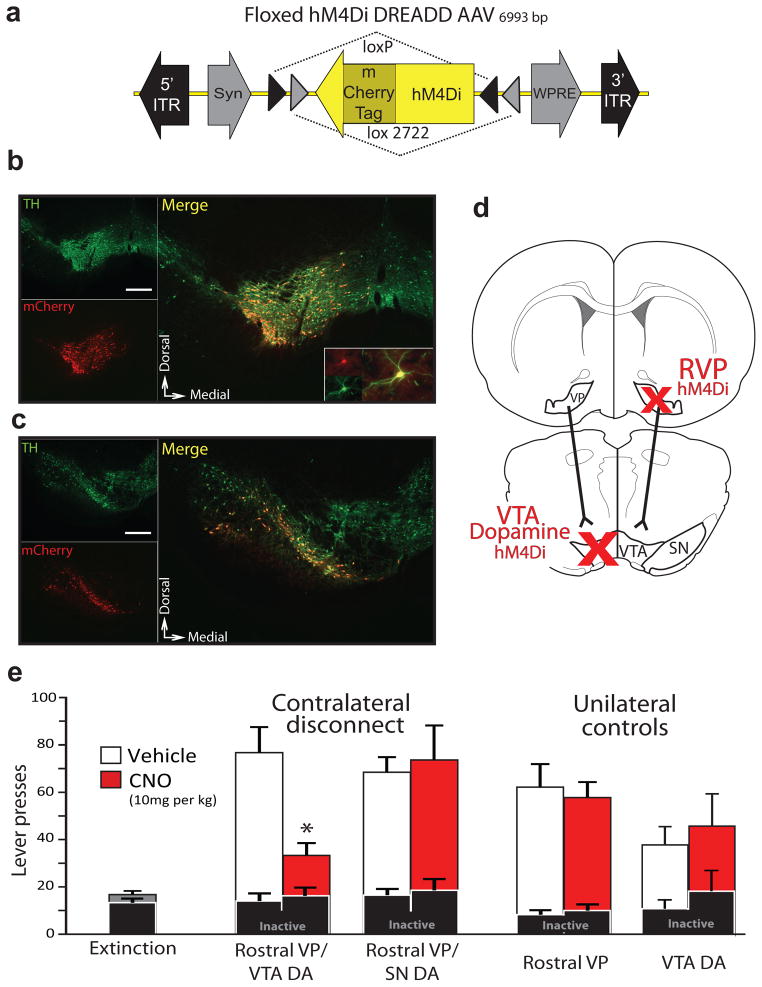
VTA is a complex structure, with dopamine, GABA and glutamate projection neurons, as well as interneurons and other local connectivity27–31. Because VP projections modulate dopamine and non-dopamine neuron firing in VTA32 (Fig. 4), we asked whether reinstatement behavior is affected by contrahemispheric disconnection of RVP from VTA dopamine neurons. In transgenic rats that express Cre recombinase in TH neurons (TH::Cre+ rats)33, we unilaterally transduced RVP neurons with the Syn-hM4Di-HA-GFP lentivirus described above. We also injected a double-floxed hM4Di AAV virus (DIO-Syn-hM4Di-mCherry; Fig. 6a) into the contralateral VTA (n=9 rats) or SN (n=8), yielding reliable and anatomically restricted hM4Di expression in unilateral dopamine neurons (Fig. 6b–c; Supplemental Fig. 6). A systemic CNO injection in these animals therefore functionally disconnected RVP inputs from midbrain dopamine cells bilaterally, while sparing RVP connectivity with non-dopaminergic VTA neurons (Fig. 6d). Control groups consisted of Cre+ animals with unilateral VTA DIO-Syn-hM4Di-mCherry injections but no virus in RVP (n=6 rats), or Cre− littermates (n=10) with unilateral RVP hM4Di and contralateral VTA injections of DIO-Syn-hM4Di-mCherry (but no VTA hM4Di expression).
Figure 6. Functional disconnection of rostral VP projection to VTA dopamine neurons blocks cue-induced reinstatement.
a) A DIO-Syn-hM4Di-mCherry AAV construct was used to selectively express mCherry-tagged hM4Di receptors in dopamine cells in TH::Cre+ transgenic rats. b) hM4Di expression is shown in a representative animal with DIO-Syn-hM4Di-mCherry injection in VTA. Immunolabeling for TH identifies dopamine neurons (green), and mCherry (red) identifies hM4Di-expressing neurons in VTA. Nearly all hM4Di-expressing neurons were dopaminergic. VTA-injected animals did not show substantial hM4Di expression laterally in SN, or in the contralateral VTA. Scale bar=500 μm. Inset shows hM4Di+TH+ cell at high magnification. c) hM4Di expression is shown in a representative animal with DIO-Syn-hM4Di-mCherry injections in SN. Minimal expression in VTA was observed. Scale bar=500 μm. Equivalent expression observed in behaviorally tested rats; VTA n=9, SN n=8. d) Unilateral Syn-hM4Di-HA-GFP injections were made in RVP, and DIO-Syn-hM4Di-mCherry injections were made in the contralateral VTA or SN of TH::Cre+ rats, or VTA of Cre- littermates. When systemic CNO is administered, serial connectivity between VP and midbrain dopamine populations is compromised bilaterally via unilateral hM4Di inhibition of RVP, and contralateral hM4Di inhibition of dopamine neurons. e) Active and inactive lever pressing during cue-induced reinstatement in RVP/VTA dopamine contralateral disconnect animals. Pressing during late extinction (Ext., grey bar at left), and cued reinstatement after vehicle (white bars) or CNO (10 mg/kg; red bars) are shown. All contralateral disconnect animals received unilateral RVP Syn-hM4Di-HA-GFP injections, and contralateral DIO-Syn-hM4Di-mCherry injections into VTA or SN of TH::Cre+ rats. Unilateral RVP inactivation rats (RVP hM4Di) were Cre negative, and received unilateral RVP hM4Di virus, plus contralateral VTA dopamine hM4Di virus (though the latter did not cause hM4Di expression in these Cre- rats). Unilateral VTA dopamine inactivation rats (VTA dopamine hM4Di) were Cre+, and received only unilateral VTA dopamine hM4Di virus. Only contralateral disconnection of RVP from VTA dopamine neurons reduced cued reinstatement below control levels. *p<0.001. Bars=m±SEM.
As shown in Fig. 6, cue-induced reinstatement was markedly attenuated by disrupting the RVP-VTA dopamine circuit in this manner (F1,8=37.97, p=0.0003; Fig. 6e). No effects were found after similarly disconnecting RVP from SN dopamine neurons (F1,7=0.29, n.s.), or after unilaterally inactivating either RVP (F1,9=0.06, n.s.) or VTA dopamine neurons (F1,5=0.57, n.s.; Fig. 6e). This indicates that intact connectivity between RVP (not CVP) and VTA (not SN) dopamine populations is required for conditioned cues to trigger cocaine seeking during reinstatement.
Further Examinations of the Nature of the RVP-VTA Circuit
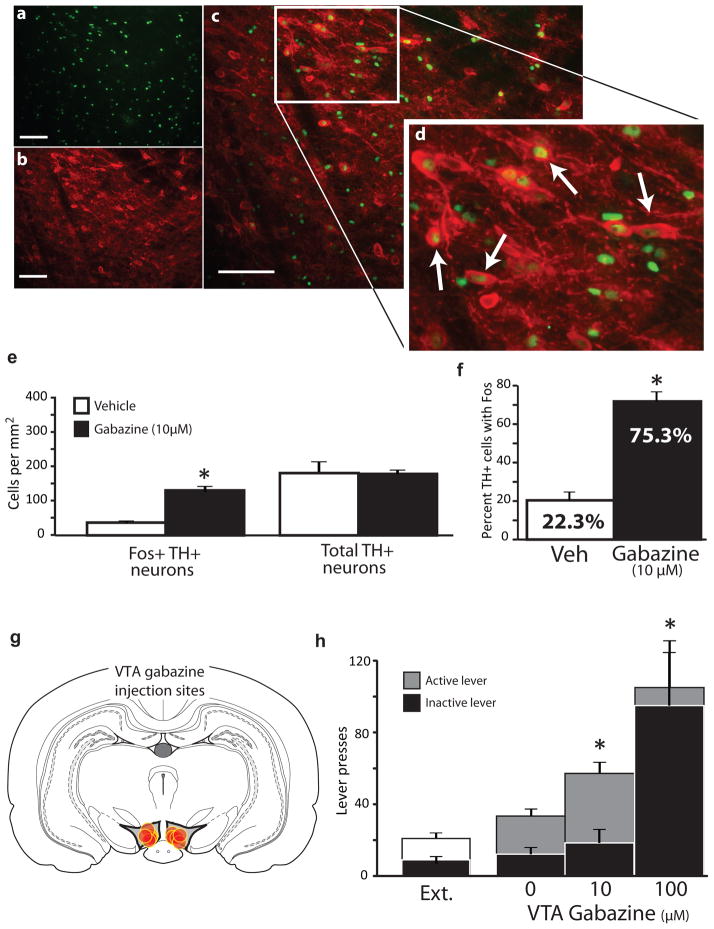
GABAergic VP projections to VTA can tonically inhibit VTA dopamine neurons5, and inactivating this projection with DREADDs can disinhibit dopamine neurons (Fig. 4). We therefore asked if disinhibition of VTA dopamine neurons could have contributed to the attenuation of reinstatement we observed after inactivating RVP inputs to VTA with DREADDs. If so, then direct disinhibition of VTA dopamine neurons with the GABAA antagonist gabazine should also attenuate reinstatement. Instead, we observed that intra-VTA gabazine (10 μM/0.3 μl) strongly increased cued reinstatement (n=6 rats; F2,17=9.43, p=0.002; veh vs. 10 μM gabazine active lever: t6=2.62, p=0.04; Fig. 7g–h), and robustly induced Fos in nearby dopamine neurons (veh n=3, gabazine n=5 rats; no effect on number of TH+ cells: t6=0.2, n.s.; total Fos+ TH+ cells: t4.4=5.55, p=0.001; percent Fos+ TH cells: t6=8.84, p=0.0001; Fig. 7a–f). This indicates that simple disinhibition of dopamine neurons is not likely the mechanism by which inhibiting RVP inputs to VTA reduces cued reinstatement.
Figure 7. GABAA-mediated disinhibition of VTA dopamine neurons enhances cue-induced reinstatement.
a) Example of Fos staining after nearby VTA gabazine microinjection (10 μM/0.3 μl). b) Example tyrosine hydroxylase (TH) staining of the same tissue. c) Overlay of Fos and TH staining. d) Magnified view of Fos+TH overlay. Arrows point to co-labelled neurons. n=5. e) Compared to vehicle microinjection, gabazine induced Fos in TH+ neurons, but did not affect the number of TH+ neurons. f) Gabazine increased the percentage of TH+ neurons that expressed Fos. g) Gabazine injection sites within VTA from cue-induced reinstatement animals. Gabazine was injected at equivalent sites as Fos/TH stained section in panel a. h) VTA gabazine (10 μM) increased active, but not inactive lever pressing during cued reinstatement. In a subset of animals, gabazine (100 μM) robustly increased pressing on both levers, which was very likely related to intense non-specific locomotor activation observed. *p<0.05. Bars=m±SEM. Scale bar=100 μm.
In addition to GABA projections, RVP sends numerous VGlut2+ efferents to VTA34; we asked whether this glutamatergic projection is required for cued reinstatement. We used unilateral DREADD-based RVP inhibition and contralateral VTA microinjection of a cocktail of the AMPA/NMDA antagonists CNQX/AP5 (0.7/1.6 mM) to bilaterally compromise glutamate projections from RVP to VTA. This did not affect cued reinstatement (n=5 rats; t4=1.28, n.s.; Supplemental Fig. 7). In contrast, reinstatement was attenuated when all VTA neurons (baclofen/muscimol; 0.3/0.03 mM) were inhibited contralaterally to a DREADD-inactivated RVP (RVP/VTA disconnect, n=10 rats: t9=2.81, p=0.02; no effect of unilateral VTA baclofen/muscimol alone, n=8: t7=1.03, n.s.; Supplemental Fig. 7). In slice, we also explored whether inhibiting RVP inputs to VTA would decrease spontaneous excitatory transmission (sEPSCs) onto dopamine neurons. CNO (5 μM) had no effects on sEPSC amplitude (t5=0.22, n.s.) or frequency (t5=0.43, n.s.) in TH+ cells (n=6 cells, Supplemental Fig. 8). These findings indicate that RVP glutamatergic projections to VTA do not directly modulate dopamine neurons, nor are they likely to be necessary for cued reinstatement.
Discussion
Here, we describe an RVP-VTA pathway activated by, and necessary for, conditioned cue-induced reinstatement of cocaine seeking in rats. VP’s involvement in reinstatement depends critically upon both the rostrocaudal position within VP, and the means by which drug seeking is reinstated (cues vs. cocaine prime). Cue-triggered drug seeking specifically requires both RVP projections to VTA, and functional interactions between RVP inputs and VTA dopamine and non-dopamine neurons. In addition to describing a novel double dissociation between VP subregions in drug seeking, we also validate the use of Gi-coupled DREADDs to inactivate specific neuronal pathways via modulation of DREADD-expressing terminals, and to functionally disconnect brain circuits in vivo.
VP Roles in Reward Seeking
Much prior research has focused on the portion of VP caudal of bregma in rat (CVP), which showed that VP is an important node in mesocorticolimbic reward circuits. Lesions or pharmacological inactivation of CVP reduce drug- or food-seeking13, 35–38. Many of these VP neurons are spontaneously active, and their GABAergic projection to VTA tonically inhibits populations of dopamine neurons there5.
VP efferents to VTA are also acutely activated by rewards and associated cues. For example, VP-VTA projections in rats are Fos-activated after acute amphetamine, cocaine self-administration, or cued reinstatement of cocaine seeking23, 39, 40, and VP neurons phasically fire when salient rewards and their cues are experienced6, 11. In primates, VP neurons also fire in relation to reward anticipation, and VP inactivation compromises modulation of reward seeking based upon reward expectation22. Similarly, VP is fMRI-activated by cues for drug or natural rewards in humans41. Our report therefore supports, and substantially expands upon the known roles for VP in reward-seeking.
Anatomical and Functional Heterogeneity Within VP
Anatomically, both rostrocaudal and mediolateral heterogeneity exists in VP cell morphology, transmitter content, sources of afferents, and efferent targets6, 10, 16, 42, 43. A large proportion of subcommissural RVP consists of a neurotensin-positive, ventromedial subregion, which receives afferents from medial accumbens shell, and sends efferents to mediodorsal thalamus and VTA. In contrast, a large proportion of sublenticular CVP consists of a calbindin-positive, dorsolateral subregion that receives accumbens core inputs, and projects to SN and subthalamic nucleus1. RVP (and adjacent caudal accumbens shell) has been described as a transition zone between accumbens and traditional (caudal) VP, with connectivity and morphological features distinct from rostral accumbens, pallidal, or extended amygdala regions10, 42. Supporting this view, RVP and caudal ventromedial accumbens shell project similarly to VTA, and Fos activation of both projections is correlated with cue-induced reinstatement behavior (Fig. 3)23.
Previous evidence supports a role for RVP in conditioned motivation, and CVP in the rewarding and priming effects of reinforcers themselves. For example, RVP (but not CVP) is Fos-activated during motivated behaviors triggered by Pavlovian cues for specific salient rewards, and is necessary for these cues to trigger seeking behaviors44, 45. RVP (not CVP) is fMRI-activated when people view highly salient, disgusting photographs—again indicating a role in conditioned motivational states18. In contrast, GABA agonists microinjected into CVP reduce cocaine-primed and stress-induced reinstatement of cocaine seeking35, 36, aligning with our observation that DREADD-based inhibition of CVP neurons blocked primed reinstatement (although ours is the first report for a role of VP, more specifically RVP, in cued reinstatement of cocaine seeking). Additionally, CVP (not RVP) has been linked to the hedonic evaluation of primary rewards in animals and humans18, 19.
Here, we examined the functions of RVP and CVP in cued vs. primed reinstatement of cocaine seeking. We showed that RVP (not CVP) is necessary for cues to trigger cocaine seeking, whereas CVP (not RVP) is necessary for a cocaine prime to elicit reinstatement. Therefore, VP in general is critically involved in promoting relapse-related drug seeking, but its rostral vs. caudal aspects differentially mediate the motivational properties of previously learned cues vs. cocaine itself.
RVP-VTA Projection Specifically Mediates Cued Drug Seeking
To examine the wider neural circuits with which RVP and CVP interact to drive reinstatement, we focused on VP projections to VTA. First, we showed that RVP but not CVP projections to VTA are Fos-activated in proportion to cued reinstatement. To test whether these RVP efferents to VTA are also necessary for reinstatement, we induced hM4Di DREADD expression in RVP or CVP neurons, then microinjected the DREADD agonist CNO directly into VTA. This allowed us to specifically inhibit VP-VTA projections, but not other VP efferents.
When RVP projections to VTA (but not the adjacent SN) were inactivated in this manner, cue-induced reinstatement was abolished, but primed reinstatement was spared. In contrast, similar inhibition of CVP projections to VTA failed to affect either cued or primed reinstatement. Therefore, RVP projections to VTA are selectively recruited during cued reinstatement and are necessary for cues to trigger reinstatement, whereas CVP is necessary for a cocaine prime to promote reinstatement via VTA-independent circuitry.
RVP Indirectly Modulates Dopamine Neurons in Reinstatement
We identified a functional circuit containing RVP and VTA dopamine neurons (but not CVP, or SN dopamine neurons) that is necessary for cues to elicit reinstatement by functionally disconnecting RVP from VTA dopamine neurons via hM4Di inhibition of RVP in one hemisphere, and simultaneous hM4Di inhibition of VTA dopamine neurons in the contralateral hemisphere. This selective, bilateral circuit disruption reduced cue-induced reinstatement—indicating that serial connectivity between RVP and VTA dopamine neurons is required for cues to trigger cocaine seeking.
VP projections to VTA are predominantly GABAergic3, 5, 32, and consistent with previous reports32, we observed that DREADD-based inhibition of RVP inputs to VTA disinhibited dopamine neurons by decreasing sIPSC frequency. However, indiscriminate disinhibition of dopamine neurons is unlikely to account for attenuated reinstatement after we saw after RVP-VTA inactivation. When we directly disinhibited VTA dopamine neurons (as evidenced by robust Fos induction) with microinjections of the GABAA antagonist gabazine, cued reinstatement was instead increased (Fig. 7). It is therefore likely that reinstatement blockade by inhibition of RVP inputs to VTA involves differential modulation of heterogeneous VTA neuronal subpopulations, possibly including inhibition of non-dopaminergic VTA neurons. Interestingly, this modulation appears to be able to supersede reinstatement-promoting effects of dopamine neuron disinhibition, which can also occur when the RVP-VTA circuit is inactivated. Systematic mapping of RVP inputs to VTA neuronal subpopulations will be required to further characterize the complexities of this novel, addiction-related circuit.
In addition to GABA efferents, RVP sends substantial Vglut2-containing projections to VTA 34. However, DREADD-based inhibition of RVP-VTA projections did not reduce spontaneous excitatory transmission to TH+ cells, nor did a contralateral disconnect-based disruption of RVP glutamate projections to VTA affect cued reinstatement—arguing against a reinstatement-related glutamatergic RVP-VTA projection.
Conclusions
Here we showed that RVP projections to VTA are activated during, and are necessary for, cue-induced reinstatement, and that this requires modulation of VTA dopamine neurons. However, this modulation does not likely involve direct GABAergic or glutamatergic RVP projections onto dopamine neurons. Instead, inhibiting RVP inputs to VTA yielded both excitation of Type 1 VTA neurons, and inhibition of Type 2 neurons in vivo (Fig. 4). Type 1 neurons, which displayed conventionally dopaminergic electrophysiological characteristics, were excited by VP inhibition—consistent with our finding that inactivation of RVP inputs to VTA attenuates GABA inputs to TH+ neurons (Figure 4b–e). However, indiscriminate disinhibition of dopamine neurons with a GABAA antagonist did not impede reinstatement, but instead robustly facilitated it. Therefore, although our disconnection study revealed that dopamine neurons are needed for VP regulation of relapse, VP’s role in reinstatement does not depend upon general disinhibition of dopamine neurons in VTA.
One possibility is that VP neurons selectively regulate a select subpopulation of VTA dopamine neurons during cued drug seeking. Notably, VTA contains fast-firing, thin-waveform dopamine neurons46, 47, and it is possible that these could be the Type 2 neurons whose activity is suppressed after RVP-VTA inactivation (Fig. 4h–1). However, this seems unlikely because inhibitory inputs to dopamine neurons recorded in vitro here were nearly always reduced by DREADD-based inactivation of RVP-VTA projections (Fig. 4e). Therefore, we hypothesize that complex, subpopulation-specific VP modulation of VTA neurons is necessary for cues to elicit relapse to cocaine seeking. These findings call for additional research to characterize the relative connectivity of VP GABA, glutamate, acetylcholine, and peptide-containing neurons with VTA neuronal subpopulations, and to further define the roles for each in reward seeking.
In sum, we have described a novel pathway, the projection from RVP to VTA, which is necessary for conditioned drug seeking in a model of relapse in addiction. Furthermore, this circuit-behavior relationship requires RVP modulation of both non-dopamine and dopamine VTA neurons. We also showed that VP subregions play qualitatively different roles in drug seeking behavior elicited by either conditioned stimuli or drug itself. Together, these findings both expand the known circuitry of conditioned motivation48, and also highlight important next directions for untangling the neural substrates of reward seeking behavior—especially in appetitive disorders such as drug addiction.
Online Methods
Subjects
159 male Sprague Dawley rats (250–350g; Charles River), and 45 outbred male Long Evans TH::Cre rats (hemizygous Cre+ n=35; Cre− littermates n=10)33 were single or pair housed in a 12 h:12 h reverse light cycle vivarium (all tests conducted in dark period). No statistical methods were used to predetermine sample sizes, but they are similar to those reported in previous publications15, 19, 23, 49. All procedures were approved by MUSC’s Institutional Animal Care and Use Committee.
Surgical Procedures
Animals were anesthetized with ketamine/xylazine/meloxicam (56.5/8.7/1.0 mg/kg), and implanted with indwelling jugular catheters. They received 20–30 nl injections of 0.5% CTb unilaterally in VTA, or 1 μl uni- or bilateral virus injections into RVP, CVP or VTA. VTA CTb and VP lentivirus injections were made by pneumatic pressure via a glass pipette (for CTb: 15–20 μm tip; for lentivirus: 30–45 μm tip) over 5 min and left in place 15–20 min. In TH::Cre animals, midbrain injections of an AAV floxed DIO-Syn-hM4Di-mCherry virus were made over 10 min through a Hamilton microinjection syringe. For bilateral VTA CNO or gabazine, or unilateral VTA glutamate antagonist or GABA agonist microinjections in behaving animals, guide cannulae were implanted 2 mm dorsal to VTA or SN.
Viral Constructs
hM4Di Inhibition of Ventral Pallidum
To transduce VP neurons with hM4Di DREADDs, we used a synapsin promoter-driven lentiviral vector with a GFP reporter, custom packaged (VSV-G envelope) by University of Pennsylvania vector core. To assist with identification, the hM4Di has an N-terminal hemagglutinin (HA) tag, and an IRES GFP reporter (Syn-hM4Di-HA-GFP; Fig. 1a). A Syn-GFP lentivirus (lacking the DREADD gene) was used to control for non-specific virus effects (Fig. 2b–c; Supplemental Fig. 5).
hM4Di Inhibition of Midbrain Dopamine Neurons
In TH::Cre transgenic rats33, an AAV containing a double-floxed, inverted open reading frame hM4Di-mCherry sequence (DIO-Syn-hM4Di-mCherry; University of North Carolina Vector Core) was microinjected into ventral midbrain to express hM4Di receptors selectively in TH neurons of VTA or SN (Fig. 6a).
Localization of DREADD Expression
hM4Di receptor expression in cell bodies and processes was visualized with immunohistochemistry for HA, GFP, or mCherry tags (described below). Containment of transduction sites within RVP or CVP, or VTA or SN dopamine cells, was confirmed by co-staining for substance P (SP; defining borders of VP), or TH (defining VTA and SN), and a brain atlas50. A synapsin promoter virus was employed in VP, so some expression was observed outside the borders of RVP or CVP in most animals. Animals with more than 30% of DREADD expression observed outside RVP or CVP were excluded from analyses (n=7). For animals with RVP virus injections, no more than 20% of DREADD-expressing tissue encroached into CVP (i.e., caudal of bregma), or vice versa. Animals injected with the Syn-GFP control virus in VP had comparable GFP expression patterns to hM4Di animals. For TH::Cre rats, hM4Di expression was robust in either VTA or SN, and was restricted almost exclusively to TH+ cell bodies and processes [% mCherry+ neurons coexpressing TH: m±SEM=97.5±0.7%; Fig. 6b–c, Supplemental Fig. 6]. mCherry expression was not observed in the contralateral hemisphere, nor did expression extend substantially into the SN of VTA-injected animals, or into VTA of SN-injected animals (Fig. 6b–c).
Electrophysiological Validation of DREADD-Based Inhibition of VP Neurons and Efferents
In Vitro Electrophysiology in Brain Slices
Brain slices from VP Syn-hM4Di-HA-GFP (n=6 rats), and Syn-GFP animals (n=2) were prepared as previously described49. Slice physiology data collection and analysis was not performed blind to the experimental group. Horizontal sections containing VTA were transferred to a 32–34°C chamber containing a carbogen-bubbled aCSF solution.
Slices were transferred to a recording chamber and perfused with 32°C aCSF at 2 ml/min. Neurons were visually identified under infrared light using Dodt gradient contrast imaging. Recording pipettes with a resistance of 1–3 MΩ were filled with internal solution containing: (in mM): CsCl (120), HEPES (10), MgCl2 (2), EGTA (1), Na2ATP (2), NaGTP (0.3), 0.2% biocytin; 295 mOsm, pH = 7.3. To isolate sIPSCs, recording aCSF was supplemented with AMPA/NMDA antagonists (NBQX/AP5; 10 μM/100 μM; Abcam), and for sEPSC recordings, picrotoxin (100 μM; Tocris) was added to ACSF. Neurons medial to the nucleus of the accessory optic tract were targeted for patch clamp (in the vicinity of dense, GFP-expressing RVP afferents), and whole-cell mode was achieved while holding the cell at -−70mV.
Data were acquired using an Axon MultiClamp 700B amplifier and an ITC-18 digital interface (HEKA Instruments) controlled by AxographX software. Recordings were filtered at 4 kHZ and acquired at 10 kHz, and sIPSCs were detected and analyzed using the template-matching event-detection algorithm in AxographX. Detection parameters were set at amplitude >4 pA, and acquired events were visually inspected before averaging. We recorded at least 150 spontaneous currents during a baseline period, and following 10 min of 5 μM CNO perfusion. Series resistance (Rs) was monitored throughout the recording and an experiment was discontinued if Rs exceeded 25 MΩ or changed more than 25%.
In vivo electrophysiology
In vivo electrophysiology data collection and analysis were performed blind to experimental conditions. Glass recording pipettes (5–12 MΩ) were filled with 2% pontamine sky blue in 0.5 M sodium acetate. A glass injection pipette (20 μm tip) glued ~150 μm behind the recording tip delivered 60 nl of 100 μM CNO to VP or VTA neurons via brief pneumatic pulses. Multiple CNO applications occurred in each animal upon different cells, each separated by >30 min. Signals were amplified and filtered (0.3–5 kHz) using a Model 1600 Neuroprobe Amplifier (A-M Systems) and a BMA 200 Bioamplifier (CWE), before digital conversion and recording to Spike 2 (v5.21) via a Micro 1401 MkII interface (CED). Body temperature was maintained at 36.0–37.5°C throughout recording.
Recorded VP neurons were located nearby prior virus injections, and identified by their firing rates and waveforms, as previously reported12. For confirmation of recording locations, markings 1 mm above and immediately below recording tracks in each VP and VTA site were made by iontophoretic dye deposits immediately prior to perfusion. VTA “Type 1” neurons were identified by electrophysiological criteria traditionally describing dopamine neurons26, including (1) biphasic or triphasic waveform >2.5 ms in duration, (2) >1.1 ms from spike onset to negative trough, and (3) spontaneous firing rate <10 Hz. All cells included in analyses were from confirmed recordings in VP or VTA.
Drugs and Tract Tracers
Cocaine HCl (NIDA) was dissolved in 0.9% sterile saline. CTb (Sigma) was dissolved at 0.5% in 0.1 M PBS. CNO was obtained from the NIH RAID program (Jürgen Wess; NIDDK). For systemic administration, CNO (0, 0.1, 1, 10 & 20 mg/kg) was dissolved in 5% DMSO, then diluted to a 1 ml/kg volume with 0.9% saline. For intracranial administration, CNO was dissolved in aCSF to 100 μM (in vivo electrophysiology) or 1 mM (behavioral microinjection), or 5 μM in vitro. Gabazine (10&100 μM), baclofen/muscimol (0.3/0.03 mM), and CNQX/AP5 (0.7/1.6 mM; all from Sigma) were dissolved in aCSF for 0.3 μl VTA microinjections.
Behavioral Training and Testing Procedures
Behavioral training and testing took place in standard Med Associates operant chambers described elsewhere23. Rats underwent 10 daily 2 h self-administration sessions (>10 cocaine infusions/day, 0.2 mg/50 μl infusion). Pressing one lever yielded a 3.6 s cocaine infusion, tone, and light above the active lever (FR1 schedule), followed by a 20 s timeout, when pressing was recorded but did not yield cocaine or cues (Figure 2a). Inactive lever presses were recorded, but had no consequences. Animals then received 7+ d of extinction training (until criterion: <25 presses for 2 consecutive days), where lever presses yielded neither cocaine nor cues.
During 2 h cue-induced reinstatement tests, active lever presses yielded cocaine cues, but no cocaine. For cocaine priming tests, 10 mg/kg i.p. cocaine was administered immediately prior to the 2 h test, where lever presses yielded neither cocaine nor cues. For the CTb/Fos experiment, animals were perfused immediately after one of the following 2 h test sessions: CS+ reinstatement, an additional extinction session, exposure to a novel environment, or exposure to a discrete CS− (details in23). Behavior during the first 30 min of tests (which most influences Fos protein measured 90 min later) was compared to neuronal activation (Fig. 3).
Animals that received systemic CNO had 3 cued, and 3 cocaine primed reinstatement tests each. 30 min prior to each test, RVP (n=16) or CVP (n=17) Syn-hM4Di-HA-GFP animals, VP Syn-GFP animals (n=5), and animals with no virus expression (n=9) received counterbalanced i.p. injections of vehicle, and 2 doses of CNO [0.1 mg/kg: n=25; 1.0 mg/kg: n=25; 10 mg/kg: n=26; 20 mg/kg: n=30). Other animals received counterbalanced intra-VTA (after RVP or CVP Syn-hM4Di-HA-GFP) or intra-SN (after RVP Syn-hM4Di-HA-GFP) microinjections of vehicle (aCSF) and CNO (1 mM/0.3 μl) 5 min prior to each of 2 cued, and 2 primed reinstatement tests. Some of these animals were later habituated to an open field for 2 d, then locomotor activity after vehicle and CNO was tested over 2 d, 48 h+ apart (intra-VTA CNO: 0&1 mM/0.3 μl, n=23). TH::Cre animals (RVP Syn-hM4Di-HA-GFP +VTA DIO-Syn-hM4Di-mCherry: Cre+ n=9, Cre- n=10; RVP Syn-hM4Di-HA-GFP+SN DIO-Syn-hM4Di-mCherry: Cre+ n=8; VTA DIO-Syn-hM4Di-mCherry only n=6) received i.p. vehicle or CNO (10 mg/kg) 30 min before each of two cue-induced reinstatement tests. Animals with unilateral RVP Syn-hM4Di-HA-GFP +contralateral VTA cannulae underwent 3 cued reinstatement tests after unilateral VTA vehicle+i.p. CNO (10 mg/kg), and two of the following: intra-VTA baclofen/muscimol+ i.p. CNO (n=12 rats), intra-VTA CNQX/AP5+ i.p. CNO (n=5), intra-VTA baclofen/muscimol+ i.p. vehicle (n=8). For bilateral VTA gabazine, animals also underwent 2–3 reinstatement tests, after vehicle and 10 μM (n=7 rats), and 100 μM (n=4) gabazine (high dose testing was discontinued due to intense nonspecific locomotor activation). Vehicle/CNO injection order was counterbalanced in all cases.
Tissue Preparation and Staining
Animals were deeply anesthetized and perfused with 0.9% saline, followed by 4% paraformaldehyde. Brains were postfixed in 4% paraformaldehyde, cryoprotected, and sliced at 30–40 μm on a cryostat. For CTb/Fos staining, IHC procedures were described previously23. To visualize hM4Di expression, we performed immunohistochemistry for HA, mCherry, and/or GFP tags. VP borders were defined via immunoreactivity for SP4, and dopamine cells were identified as TH immunoreactive. All tissue was first incubated in 3% normal donkey serum PBS-Triton (PBST; 2 h), then in primary antibodies in PBST overnight at RT. The following primary antibodies were used in these experiments (1 or 2 antibodies/tissue sample): mouse anti HA (1:500; Covance catalogue number: MMS-101P), rabbit anti DsRed (mCherry tag; 1:500; Clontech catalogue number: 632496), chicken anti GFP (1:2000; Abcam catalogue number: ab13970), rabbit anti SP (1:5000; Immunostar catalogue number: 20064), and mouse anti TH (1:1000, Immunostar catalogue number: 22941). For fluorescent stains, sections were incubated 4 h at RT in fluorescent conjugated secondary antibodies [donkey anti mouse 488 (HA and TH: 1:500; Invitrogen catalogue number: A21202), donkey anti rabbit 594 (Ds Red, TH, and SP: 1:500; Invitrogen catalogue number: A21207), donkey anti chicken 488 (GFP, Fos: 1:500, Jackson catalogue number: 703545155)]. Slices were mounted and coverslipped with Citifluor mounting medium. For DAB visualization of HA, slices were incubated 2 h in a biotinylated donkey anti mouse secondary antibody (1:500; Jackson catalogue number: 715066151), and amplified with an avidin-biotin complex (1:500; Vector). Tissue was dehydrated and coverslipped with Permount. After slice physiology experiments, tissue was fixed 48h in 4% paraformaldehyde, sectioned, incubated in 5% normal goat serum PBST (7min), rabbit anti TH (1:1000; Millipore catalogue number: AB152) and Avidin TexasRed (1:500; Life Technologies catalogue number: A820) overnight at 4°C, and Alexafluor 488 goat anti-rabbit IgG (Life Technologies catalogue number: A11008) for 2h. All antibodies were previously validated for specificity, as described on the manufacturer’s websites and the Journal of Comparative Neurology antibody database.
Quantification of CTb/Fos staining
Fos expression in CTb+ cells was analyzed as a function of rostral/caudal position within VP, using a quantification strategy described in a previous report, where other analyses of this tissue were described23. Coronal sections at ~250 μm intervals throughout the rostral/caudal extent of VP were photographed at 10X magnification, and montaged with the Stereo Investigator Virtual Slice Module (MBF Bioscience) to preserve anatomical landmarks. All CTb+ neurons (with or without Fos) within VP ipsilateral to VTA CTb injection were counted on each slice by an observer blind to experimental group, and primary analyses examined the percentage of total CTb neurons that were Fos+ (Fig. 3, Supplemental Fig. 2), or average number of CTb cells per rostro-caudal level (Supplemental Fig. 3). To quantify VTA gabazine (vehicle or 10 μM)-induced Fos, z-stack images were taken at 20X magnification of TH (red) and Fos (green) immunoreactivity, 90–110 μm lateral of VTA microinjection sites (Fig. 7). TH+ and Fos+ neurons were quantified in Photoshop using ImageJ-deconvolved images. 2–3 images/microinjection were quantified and averaged for each animal.
Analysis and Statistics
To examine reinstatement-related Fos expression in VTA-projecting neurons within RVP and CVP, total CTb+, and CTb+/Fos+, cells in VP (ipsilateral of VTA CTb injections) were quantified at ~250 μm intervals in each animal. One-way ANOVA (with Tukey posthoc comparisons) were used to analyze percentages of Fos+ CTb neurons between behavioral groups. Average percentages of Fos+ CTb neurons in RVP (rostral of bregma) or CVP (caudal of bregma) for each animal were also correlated with active lever pressing in the first 30 min of cued reinstatement, 90 min before sacrifice (Pearson’s test). For gabazine-induced Fos in VTA, average TH+, and TH+/Fos+ neurons were computed for each animal after bilateral vehicle (n=3 rats) or gabazine (n=5), and compared with between-subjects t-tests.
Effects of systemic CNO on cued or primed reinstatement were analyzed with mixed ANOVAs for CNO dose (between subjects variable) X lever (active or inactive; within subjects) for each behavioral group (RVP Syn-hM4Di-HA-GFP, CVP Syn-hM4Di-HA-GFP; Syn-GFP, or no virus). Repeated measures drug X lever ANOVAs tested effects of CNO in VTA or SN on cued or primed reinstatement in RVP or CVP Syn-hM4Di-HA-GFP animals, and systemic CNO on cued reinstatement in TH::Cre rats. One way ANOVAs were used to test effects of bilateral VTA gabazine, and contralaterally disconnecting RVP from VTA, or VTA glutamate, on cued reinstatement. Tukey or Bonferroni-corrected t-test posthoc analyses were used as appropriate to determine the nature of significant main effects and interactions. All tests were two-tailed, and normality of data distribution was tested with Mauchly’s test of Sphericity (Greenhouse-Geisser corrected if necessary).
For in vitro electrophysiology, the change in firing rate of VP or VTA neurons was determined from 100 s epochs immediately before vs during CNO application with paired samples t-tests comparing average pre- vs. post- CNO sIPSC or sEPSC amplitude or frequency, and 2-way ANOVAs with Bonferroni posthocs. Results were normalized to pre-CNO firing rate (100%) for each cell. In vivo, two-tailed t-tests or Wilcoxon signed-rank paired sample tests were used to compare pre- and post-CNO firing rates in VP and VTA (depending on normality computed by D’Agostino-Pearson omnibus test). Silenced neurons were included in analyses if the original waveform returned within 200 s post-CNO application. Baseline firing rates of VP neurons were compared using a two-tailed unpaired t-test with Welch’s correction due to a significant F-test for unequal variance.
Supplementary Material
a) RVP, CVP, and surrounding structures are represented in the horizontal plane. Boxed area is enlarged in panels b&c. ac: anterior commissure; IPAC: interstitial nucleus of the posterior limb of the anterior commissure; Lat. Shell: lateral nucleus accumbens shell; LH: lateral hypothalamus; POA: preoptic hypothalamic area; SLEA: sublenticular extended amygdala. b) Top panel shows m±SEM active lever pressing during cue-induced reinstatement in animals with containment of hM4Di expression >95% within RVP or CVP, after vehicle or CNO (20 mg/kg, i.p.). *p=0.015. Bottom panel represents effects in individual animals of CNO microinjections into various parts of VP on cued reinstatement. Location of each oval represents site of virus injections in behaviorally tested animals (bilateral virus placement represented on a unilateral map; two ovals/animal). Size and shape of ovals represents the average zone of hM4Di expression around virus injection sites [m±SEM spread: dorso-ventral: m=1.18(0.02) mm; medio-lateral: m=0.72(0.02); rostro-caudal: m=0.95(0.02)]. Color of ovals represents change in cue-induced reinstatement after CNO injection (expressed as percentage of vehicle day lever pressing in the same animal). Blues indicate decreases from vehicle day due to hM4Di inhibition, yellows and reds represent increases in reinstatement responding over vehicle day. c) Using the same symbol logic as panel b, effects of CNO (20 mg/kg, i.p.) on cocaine-primed reinstatement are shown for RVP and CVP hM4Di animals. Bars=m±SEM. *p<0.05
a) Coronal 40 μm thick section from RVP (~0.6 mm rostral of bregma) showing typical staining for Fos (blue-black nuclear stain) and CTb (brown somatic stain, indicating VTA-projecting neurons). Scale bar=1 mm. Expression typical of behaviorally tested CS+ group, n=8. b) Coronal 40 μm thick section from CVP (~0.4 mm caudal of bregma) showing typical staining for Fos and CTb. Scale bar=1 mm. Expression typical of behaviorally tested CS+ group, n=8. c) Higher magnification view of Fos and CTb staining in RVP. Arrows indicate co-labeled cells. Scale bar=50 μm. d) Fos activation of VTA-projecting cells (%CTb cells that are Fos+; y-axis) in brain slices taken from various rostro-caudal levels of VP (mm rostral or caudal of bregma; x-axis). Symbols indicate the behavioral test prior to sacrifice (red diamonds: CS+ reinstatement, blue squares: control CS- cue, pink triangles: extinction test, black Xs: novel environment locomotor test). Note that the samples in which the greatest proportion of VTA-projecting neurons expressed Fos were in CS+ animals, and were located rostral of bregma (in RVP).
a) RVP and CVP projections to rostral and caudal VTA are illustrated in the horizontal plane. b) Mean±SEM CTb+ cells/sample located in RVP (left) or CVP (right), projecting to either rostral or caudal VTA. CTb injections were localized to VTA either entirely rostral of the interpeduncular nucleus (blue bars), or entirely caudal of it (red bars). These animals also supplied data for a previous publication23, where additional details on CTb injection sites can be found. c) Mean±SEM total CTb+ cells/slice at various levels throughout the rostro-caudal axis of VP after CTb injection in VTA. Lines=m±SEM. d) Example of axonal labeling for the HA-tagged hM4Di receptor in midbrain after injection of Syn-hM4Di-HA-GFP into RVP. Strong axonal hM4Di expression (black) was seen in lateral VTA, and ventromedial SN (pars compacta). Scale bar=500 μm. Inset shows 60X magnification of axon terminals expressing HA (black) in the vicinity of VTA cells (red Nissl stained), scale bar=50 μm. The sample sizes of experimental groups with equivalent axonal DREADD expression in ventral midbrain are listed in Results. (e) Example of axonal labeling for the HA-tagged hM4Di receptor in midbrain after injection of Syn-hM4Di-HA-GFP into CVP. The strongest axonal hM4Di expression was seen in SN (especially pars reticulata), though fibers were also observed in VTA (inset). Scale bar=500 μm. Inset shows 60X magnification of axon terminals expressing HA (black) in the vicinity of VTA cells (red Nissl stained), scale bar=50 μm. The sample sizes of experimental groups with equivalent axonal DREADD expression in ventral midbrain are listed in Results.
a) Syn-GFP control lentivirus used to express GFP under a neuronal-specific human synapsin promoter (Syn) in RVP. b) Representative traces from VTA dopamine neurons are shown in baseline conditions (left, black), and after CNO (right, red; scale=20 pA, 200 ms). c) CNO had no effect on mean sIPSC amplitude. d) CNO had no effect on mean sIPSC frequency. Lines=individual raw values.
Midbrain cannulae placements for microinjections of CNO into VTA or SN in animals tested for reinstatement of cocaine seeking. Animals with placements within VTA borders are shown with filled black circles, animals with placements outside VTA (including in SN) are shown with unfilled (white) circles.
Confocal photomicrographs of VTA in TH::Cre rats after injection of DIO-Syn-hM4Di-mCherry. Equivalent expression seen in behavioral tested animals, n=9. Dopamine cells are labeled for TH (green, top left), and nearly all co-express the mCherry-tagged hM4Di receptor (red, bottom left). Yellow cells indicate cells and their processes co-expressing dopamine and DREADDs. Scale bar=50 μm.
a) Animals received unilateral RVP Syn-hM4Di-HA-GFP, and a chronic cannula into contralateral VTA, and were tested for cue-induced reinstatement on separate days after i.p. 10 mg/kg CNO and intra-VTA vehicle (Veh-CNO; unilateral RVP inactivation control group), i.p. CNO plus unilateral intra-VTA cocktail of the AMPA/NMDA antagonists CNQX/AP5 (A/C-CNO: RVP/VTA glutamate disconnect group), or i.p. CNO plus unilateral intra-VTA cocktail of GABAA/B agonists baclofen and muscimol (B/M-CNO: RVP/VTA disconnect group). Some animals also received unilateral intra-VTA B/M + i.p. veh. (B/M-Veh: Unilateral VTA inactivation control group). b) Disconnection of RVP from VTA reduced reinstatement relative to unilateral RVP inhibition control, but neither unilateral VTA inactivation, nor disconnecting RVP from glutamate inputs did so. *p<0.05 Bars=m±SEM
a) Representative traces from VTA dopamine neurons are shown in baseline conditions (black), and b) after CNO (red; scale=20 pA, 200 ms). c) CNO had no effect on mean sIPSC amplitude. d) CNO had no effect on mean sIPSC frequency. Lines=individual raw values.
Acknowledgments
We thank Phong Do, Meghin J. Gilstrap, and Edwin C. Lin for assistance with behavioral testing and immunohistochemistry, and Bryan L. Roth for DREADD constructs and consultation on DREADDs. Clozapine-n-oxide was obtained from the NIH as part of the Rapid Access to Investigative Drug Program funded by the NINDS. Research was supported by NIH grants: F32 DA026692, K99 DA035251 (SVM), F31 DA030891 (JTB), R21 DA025837 (GA-J & SPW), R01 DA013951 (JJW), R37 DA006214, P50 DA015369 (GA-J). This project was supported by the National Center for Research Resources and the Office of the Director of the National Institutes of Health through Grant Number C06 RR015455.
Footnotes
Author Contributions: SVM attained funding, designed and conducted experiments, performed surgeries, analyzed data, and wrote the manuscript. EMV designed and conducted anesthetized electrophysiology experiments, analyzed these data, and wrote the manuscript. JTB designed and conducted slice electrophysiology experiments, analyzed these data, and wrote the manuscript. CRK conducted behavioral experiments and wrote the manuscript. EMM performed surgeries for, and conducted behavioral and immunohistochemical experiments. Jennifer Kaufling conducted immunohistochemical and confocal microscopy experiments. SPW attained funding, and contributed the Syn-GFP viral construct. KD contributed the TH::Cre transgenic rat line. JJW attained funding, designed and conducted slice electrophysiology experiments and wrote the manuscript. GA-J attained funding, designed experiments, and wrote the manuscript.
References
- 1.Zahm DS, Heimer L. Two transpallidal pathways originating in the rat nucleus accumbens. J Comp Neurol. 1990;302:437–446. doi: 10.1002/cne.903020302. [DOI] [PubMed] [Google Scholar]
- 2.Zahm DS, Williams E, Wohltmann C. Ventral striatopallidothalamic projection: IV. Relative involvements of neurochemically distinct subterritories in the ventral pallidum and adjacent parts of the rostroventral forebrain. J Comp Neurol. 1996;364:340–362. doi: 10.1002/(SICI)1096-9861(19960108)364:2<340::AID-CNE11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 3.Kalivas PW, Churchill L, Klitenick MA. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience. 1993;57:1047–1060. doi: 10.1016/0306-4522(93)90048-k. [DOI] [PubMed] [Google Scholar]
- 4.Haber SN, Nauta WJ. Ramifications of the globus pallidus in the rat as indicated by patterns of immunohistochemistry. Neuroscience. 1983;9:245–260. doi: 10.1016/0306-4522(83)90291-9. [DOI] [PubMed] [Google Scholar]
- 5.Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 6.Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behavioural brain research. 2009;196:155–167. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mogenson GJ, Brudzynski SM, Wu M, Yang CY, Yim CY. From motivation to action: A review of dopaminergic regulation of limbic to nucleus accumbens to ventral pallidum to pedunculopontine nucleus circuitries involved in limbic-motor integration. In: Kalivas PW, Barnes CD, editors. Limbic Motor Circuits in Neuropsychiatry. CRC Press; Boca Raton, FL: 1993. pp. 193–236. [Google Scholar]
- 8.Churchill L, Kalivas PW. A topographically organized gamma-aminobutyric acid projection from the ventral pallidum to the nucleus accumbens in the rat. J Comp Neurol. 1994;345:579–595. doi: 10.1002/cne.903450408. [DOI] [PubMed] [Google Scholar]
- 9.Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- 10.Kupchik YM, Kalivas PW. The rostral subcommissural ventral pallidum is a mix of ventral pallidal neurons and neurons from adjacent areas: an electrophysiological study. Brain Struct Funct. 2012 doi: 10.1007/s00429-012-0471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Root DH, et al. Differential roles of ventral pallidum subregions during cocaine self-administration behaviors. J Comp Neurol. 2013;521:558–588. doi: 10.1002/cne.23191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang CR, Mogenson GJ. Ventral pallidal neuronal responses to dopamine receptor stimulation in the nucleus accumbens. Brain Res. 1989;489:237–246. doi: 10.1016/0006-8993(89)90856-1. [DOI] [PubMed] [Google Scholar]
- 13.McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- 14.Panagis G, Miliaressis E, Anagnostakis Y, Spyraki C. Ventral pallidum self-stimulation: a moveable electrode mapping study. Behav Brain Res. 1995;68:165–172. doi: 10.1016/0166-4328(94)00169-g. [DOI] [PubMed] [Google Scholar]
- 15.Bengtson CP, Osborne PB. Electrophysiological properties of cholinergic and noncholinergic neurons in the ventral pallidal region of the nucleus basalis in rat brain slices. J Neurophysiol. 2000;83:2649–2660. doi: 10.1152/jn.2000.83.5.2649. [DOI] [PubMed] [Google Scholar]
- 16.Zahm DS, Heimer L. Ventral striatopallidal parts of the basal ganglia in the rat: I. Neurochemical compartmentation as reflected by the distributions of neurotensin and substance P immunoreactivity. J Comp Neurol. 1988;272:516–535. doi: 10.1002/cne.902720406. [DOI] [PubMed] [Google Scholar]
- 17.Johnson PI, Stellar JR, Paul AD. Regional reward differences within the ventral pallidum are revealed by microinjections of a mu opiate receptor agonist. Neuropharmacology. 1993;32:1305–1314. doi: 10.1016/0028-3908(93)90025-x. [DOI] [PubMed] [Google Scholar]
- 18.Calder AJ, et al. Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods. Eur J Neurosci. 2007;25:3422–3428. doi: 10.1111/j.1460-9568.2007.05604.x. [DOI] [PubMed] [Google Scholar]
- 19.Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Tindell AJ, Berridge KC, Aldridge JW. Ventral pallidal representation of pavlovian cues and reward: population and rate codes. J Neurosci. 2004;24:1058–1069. doi: 10.1523/JNEUROSCI.1437-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tachibana Y, Hikosaka O. The primate ventral pallidum encodes expected reward value and regulates motor action. Neuron. 2012;76:826–837. doi: 10.1016/j.neuron.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahler SV, Aston-Jones GS. Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 2012;32:13309–13326. doi: 10.1523/JNEUROSCI.2277-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson SM, Neumaier JF. Grateful DREADDs: engineered receptors reveal how neural circuits regulate behavior. Neuropsychopharmacology. 2012;37:296–297. doi: 10.1038/npp.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ungless MA, Grace AA. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012 doi: 10.1016/j.tins.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morales M, Pickel VM. Insights to drug addiction derived from ultrastructural views of the mesocorticolimbic system. Ann N Y Acad Sci. 2012;1248:71–88. doi: 10.1111/j.1749-6632.2011.06299.x. [DOI] [PubMed] [Google Scholar]
- 29.Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- 30.van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stamatakis AM, et al. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron. 2013;80:1039–1053. doi: 10.1016/j.neuron.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hjelmstad GO, Xia Y, Margolis EB, Fields HL. Opioid modulation of ventral pallidal afferents to ventral tegmental area neurons. J Neurosci. 2013;33:6454–6459. doi: 10.1523/JNEUROSCI.0178-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witten IB, et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robledo P, Koob GF. Two discrete nucleus accumbens projection areas differentially mediate cocaine self-administration in the rat. Behav Brain Res. 1993;55:159–166. doi: 10.1016/0166-4328(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 38.Farrar AM, et al. Forebrain circuitry involved in effort-related choice: Injections of the GABAA agonist muscimol into ventral pallidum alter response allocation in food-seeking behavior. Neuroscience. 2008;152:321–330. doi: 10.1016/j.neuroscience.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colussi-Mas J, Geisler S, Zimmer L, Zahm DS, Berod A. Activation of afferents to the ventral tegmental area in response to acute amphetamine: a double-labelling study. Eur J Neurosci. 2007;26:1011–1025. doi: 10.1111/j.1460-9568.2007.05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geisler S, et al. Prominent activation of brainstem and pallidal afferents of the ventral tegmental area by cocaine. Neuropsychopharmacology. 2008;33:2688–2700. doi: 10.1038/sj.npp.1301650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Childress AR, et al. Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS ONE. 2008;3:e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zahm DS. Is the caudomedial shell of the nucleus accumbens part of the extended amygdala? A consideration of connections. Critical reviews in neurobiology. 1998;12:245–265. doi: 10.1615/critrevneurobiol.v12.i3.50. [DOI] [PubMed] [Google Scholar]
- 43.Tripathi A, Prensa L, Mengual E. Axonal branching patterns of ventral pallidal neurons in the rat. Brain Struct Funct. 2012 doi: 10.1007/s00429-012-0451-0. [DOI] [PubMed] [Google Scholar]
- 44.Leung BK, Balleine BW. The ventral striato-pallidal pathway mediates the effect of predictive learning on choice between goal-directed actions. J Neurosci. 2013;33:13848–13860. doi: 10.1523/JNEUROSCI.1697-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson MJ, Berridge KC. Instant transformation of learned repulsion into motivational “wanting”. Current biology : CB. 2013;23:282–289. doi: 10.1016/j.cub.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lammel S, et al. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 47.Luo AH, Georges FE, Aston-Jones GS. Novel neurons in ventral tegmental area fire selectively during the active phase of the diurnal cycle. Eur J Neurosci. 2008;27:408–422. doi: 10.1111/j.1460-9568.2007.05985.x. [DOI] [PubMed] [Google Scholar]
- 48.Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 49.Beckley JT, Evins CE, Fedarovich H, Gilstrap MJ, Woodward JJ. Medial prefrontal cortex inversely regulates toluene-induced changes in markers of synaptic plasticity of mesolimbic dopamine neurons. J Neurosci. 2013;33:804–813. doi: 10.1523/JNEUROSCI.3729-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press/Elsevier; Amsterdam ; Boston: 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
a) RVP, CVP, and surrounding structures are represented in the horizontal plane. Boxed area is enlarged in panels b&c. ac: anterior commissure; IPAC: interstitial nucleus of the posterior limb of the anterior commissure; Lat. Shell: lateral nucleus accumbens shell; LH: lateral hypothalamus; POA: preoptic hypothalamic area; SLEA: sublenticular extended amygdala. b) Top panel shows m±SEM active lever pressing during cue-induced reinstatement in animals with containment of hM4Di expression >95% within RVP or CVP, after vehicle or CNO (20 mg/kg, i.p.). *p=0.015. Bottom panel represents effects in individual animals of CNO microinjections into various parts of VP on cued reinstatement. Location of each oval represents site of virus injections in behaviorally tested animals (bilateral virus placement represented on a unilateral map; two ovals/animal). Size and shape of ovals represents the average zone of hM4Di expression around virus injection sites [m±SEM spread: dorso-ventral: m=1.18(0.02) mm; medio-lateral: m=0.72(0.02); rostro-caudal: m=0.95(0.02)]. Color of ovals represents change in cue-induced reinstatement after CNO injection (expressed as percentage of vehicle day lever pressing in the same animal). Blues indicate decreases from vehicle day due to hM4Di inhibition, yellows and reds represent increases in reinstatement responding over vehicle day. c) Using the same symbol logic as panel b, effects of CNO (20 mg/kg, i.p.) on cocaine-primed reinstatement are shown for RVP and CVP hM4Di animals. Bars=m±SEM. *p<0.05
a) Coronal 40 μm thick section from RVP (~0.6 mm rostral of bregma) showing typical staining for Fos (blue-black nuclear stain) and CTb (brown somatic stain, indicating VTA-projecting neurons). Scale bar=1 mm. Expression typical of behaviorally tested CS+ group, n=8. b) Coronal 40 μm thick section from CVP (~0.4 mm caudal of bregma) showing typical staining for Fos and CTb. Scale bar=1 mm. Expression typical of behaviorally tested CS+ group, n=8. c) Higher magnification view of Fos and CTb staining in RVP. Arrows indicate co-labeled cells. Scale bar=50 μm. d) Fos activation of VTA-projecting cells (%CTb cells that are Fos+; y-axis) in brain slices taken from various rostro-caudal levels of VP (mm rostral or caudal of bregma; x-axis). Symbols indicate the behavioral test prior to sacrifice (red diamonds: CS+ reinstatement, blue squares: control CS- cue, pink triangles: extinction test, black Xs: novel environment locomotor test). Note that the samples in which the greatest proportion of VTA-projecting neurons expressed Fos were in CS+ animals, and were located rostral of bregma (in RVP).
a) RVP and CVP projections to rostral and caudal VTA are illustrated in the horizontal plane. b) Mean±SEM CTb+ cells/sample located in RVP (left) or CVP (right), projecting to either rostral or caudal VTA. CTb injections were localized to VTA either entirely rostral of the interpeduncular nucleus (blue bars), or entirely caudal of it (red bars). These animals also supplied data for a previous publication23, where additional details on CTb injection sites can be found. c) Mean±SEM total CTb+ cells/slice at various levels throughout the rostro-caudal axis of VP after CTb injection in VTA. Lines=m±SEM. d) Example of axonal labeling for the HA-tagged hM4Di receptor in midbrain after injection of Syn-hM4Di-HA-GFP into RVP. Strong axonal hM4Di expression (black) was seen in lateral VTA, and ventromedial SN (pars compacta). Scale bar=500 μm. Inset shows 60X magnification of axon terminals expressing HA (black) in the vicinity of VTA cells (red Nissl stained), scale bar=50 μm. The sample sizes of experimental groups with equivalent axonal DREADD expression in ventral midbrain are listed in Results. (e) Example of axonal labeling for the HA-tagged hM4Di receptor in midbrain after injection of Syn-hM4Di-HA-GFP into CVP. The strongest axonal hM4Di expression was seen in SN (especially pars reticulata), though fibers were also observed in VTA (inset). Scale bar=500 μm. Inset shows 60X magnification of axon terminals expressing HA (black) in the vicinity of VTA cells (red Nissl stained), scale bar=50 μm. The sample sizes of experimental groups with equivalent axonal DREADD expression in ventral midbrain are listed in Results.
a) Syn-GFP control lentivirus used to express GFP under a neuronal-specific human synapsin promoter (Syn) in RVP. b) Representative traces from VTA dopamine neurons are shown in baseline conditions (left, black), and after CNO (right, red; scale=20 pA, 200 ms). c) CNO had no effect on mean sIPSC amplitude. d) CNO had no effect on mean sIPSC frequency. Lines=individual raw values.
Midbrain cannulae placements for microinjections of CNO into VTA or SN in animals tested for reinstatement of cocaine seeking. Animals with placements within VTA borders are shown with filled black circles, animals with placements outside VTA (including in SN) are shown with unfilled (white) circles.
Confocal photomicrographs of VTA in TH::Cre rats after injection of DIO-Syn-hM4Di-mCherry. Equivalent expression seen in behavioral tested animals, n=9. Dopamine cells are labeled for TH (green, top left), and nearly all co-express the mCherry-tagged hM4Di receptor (red, bottom left). Yellow cells indicate cells and their processes co-expressing dopamine and DREADDs. Scale bar=50 μm.
a) Animals received unilateral RVP Syn-hM4Di-HA-GFP, and a chronic cannula into contralateral VTA, and were tested for cue-induced reinstatement on separate days after i.p. 10 mg/kg CNO and intra-VTA vehicle (Veh-CNO; unilateral RVP inactivation control group), i.p. CNO plus unilateral intra-VTA cocktail of the AMPA/NMDA antagonists CNQX/AP5 (A/C-CNO: RVP/VTA glutamate disconnect group), or i.p. CNO plus unilateral intra-VTA cocktail of GABAA/B agonists baclofen and muscimol (B/M-CNO: RVP/VTA disconnect group). Some animals also received unilateral intra-VTA B/M + i.p. veh. (B/M-Veh: Unilateral VTA inactivation control group). b) Disconnection of RVP from VTA reduced reinstatement relative to unilateral RVP inhibition control, but neither unilateral VTA inactivation, nor disconnecting RVP from glutamate inputs did so. *p<0.05 Bars=m±SEM
a) Representative traces from VTA dopamine neurons are shown in baseline conditions (black), and b) after CNO (red; scale=20 pA, 200 ms). c) CNO had no effect on mean sIPSC amplitude. d) CNO had no effect on mean sIPSC frequency. Lines=individual raw values.