Abstract
Aims/Hypothesis
Levels and activity of atypical protein kinase C (aPKC) are elevated in hepatocytes of type 2 diabetic (T2DM) humans and cause excessive increases in expression of lipogenic and gluconeogenic enzymes; aPKC inhibitors largely correct these aberrations. Metformin improves hepatic gluconeogenesis by activating 5′-AMP-activated protein kinase (AMPK). However, metformin also activates aPKC in certain tissues; in liver, this activation could amplify T2DM aberrations and offset salutary effects of AMPK. Here, we examined whether metformin activates aPKC in human hepatocytes and metabolic its consequences.
Methods
We compared protein kinase activities and alterations of lipogenic and gluconeogenic enzyme expression during actions of AMPK activators, metformin and AICAR, relative to those of an aPKC-ι inhibitor, in hepatocytes of non-diabetic and T2DM humans.
Results
Metformin and AICAR activated aPKC at concentrations comparable to those required for AMPK activation. Moreover, both agents increased lipogenic enzyme expression by an aPKC-dependent mechanism. Thus, whereas insulin-dependent and T2DM-dependent increases in lipogenic enzyme expression were reversed by aPKC inhibition, such expression was increased in non-diabetic hepatocytes and remained elevated in T2DM hepatocytes following metformin and AICAR treatment. Also, whereas aPKC inhibition diminished gluconeogenic enzyme expression in the absence and presence of insulin in both non-diabetic and T2DM hepatocytes, metformin and AICAR increased gluconeogenic enzyme expression in non-diabetic hepatocytes, but nevertheless diminished gluconeogenic enzyme expression in insulin-treated T2DM hepatocytes.
Conclusions/Interpretations
Metformin and AICAR activate aPKC along with AMPK in human hepatocytes. aPKC activation increases lipogenic enzyme expression, alters gluconeogenic enzyme expression and therefore appears to offset salutary effects of AMPK.
Keywords: Metformin, AICAR, AMP-activated Protein Kinase, Protein Kinase C-iota, Type 2 Diabetes, Hepatocytes
Introduction
Metformin is widely used for treating type 2 diabetes mellitus (T2DM). Metformin improves hyperglycaemia primarily by diminishing expression of hepatic gluconeogenic enzymes, phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase), thereby reducing hepatic glucose output [1]. Metformin also increases glucose transport in muscle by improving insulin signalling [2] and by direct effects on glucose transport [3].
Metformin actions in liver and muscle are largely attributed to activation of 5′-AMP-activated protein kinase (AMPK) [3–5]. Although metformin apparently activates AMPK in mouse liver via LKB1 [6], in human hepatocytes, metformin activates AMPK by inhibiting mitochondrial respiratory chain activity and increasing 5′-AMP at the expense of ATP [7].
How AMPK diminishes gluconeogenic enzyme expression is uncertain. He and coworkers reported that, in mouse liver, metformin and AMPK activator, 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR), increase ser-436 phosphorylation of CREB binding protein (CBP) and disrupt formation of a complex between CBP, CREB and the target of rapamycin-C2 (TORC2) required for transcription of Pparγ-coactivator-1-α (PGC-1α) and PEPCK and G6Pase expression [8]. They proposed that AMPK increases CBP phosphorylation by activating atypical protein kinase C (aPKC), which directly phosphorylates ser-436-CBP [8]. Consonant with this idea, AICAR [3,9] and metformin [3] activate aPKC in rodent muscle independently of phosphatidylinositol 3-kinase (PI3K), but dependent on ERK and phospholipase D (PLD), which generates phosphatidic acid (PA), a directly activator of aPKCs-ι/λ/ζ [3,9].
As in previous reports [3,10–14], He et al [8] found that insulin activates hepatic aPKC by a PI3K-dependent mechanism, but further noted that this similarly leads to ser-436-CRB phosphorylation and disruption of the CREB/CBP/TORC2 complex. However, insulin also diminishes PEPCK and G6Pase expression by PI3K/Akt-dependent phosphorylation of ser-256-FoxO1, thereby causing nuclear exclusion and inactivation of FoxO1, which is co-required for CREB/CBP/TORC2/PGC-1α-induced increases in PEPCK/G6Pase expression [15,16]. The relative contributions of Akt-dependent Ser-256-FoxO1 vis-à-vis aPKC-dependent phosphorylation of Ser-436-CBP to diminish PEPCK/G6Pase expression during insulin action are presently uncertain.
Militating against the idea that aPKC activation diminishes PEPCK/G6Pase expression during metformin and insulin action is the finding that inhibition of hepatic aPKC by either adenovirally-mediated expression of kinase-inactive aPKC [13] or small-molecule inhibitors of aPKC [14,17] leads to decreased expression of PEPCK and G6Pase. Moreover, aPKC inhibition, like insulin, increases phosphorylation of ser-256-FoxO1 [14,17]. Although the mechanism underlying increases in FoxO1 phosphorylation during aPKC inhibition is uncertain, aPKC binds to and phosphorylates, and thus may inhibit, Akt [18]; in addition, aPKC (a) increases expression of TRB3, a pseudokinase that inhibits hepatic Akt [19], and (b) phosphorylates and inhibits IRS-1 [20], which is required for insulin activation of Akt, but not aPKC, in liver [21,22].
Another problem that may ensue from hepatic aPKC activation during metformin treatment arises from the fact that aPKC participates in mediating insulin-induced increases in expression of hepatic lipogenic genes [12–14,17]. Thus, metformin-induced increases in hepatic aPKC activity may increase expression of sterol receptor element binding protein-1c (SREBP-1c), which trans-activates expression of multiple lipogenic enzymes, including, fatty acid synthase (FAS).
Here, we questioned whether metformin and AICAR activate aPKC in human hepatocytes, and whether increases in hepatic aPKC activity may offset the salutary effects that simple AMPK activation would otherwise have on hepatic gene expression. We compared the effects of two AMPK activators, metformin and AICAR, to those of an inhibitor of aPKC on expression of lipogenic and gluconeogenic factors in hepatocytes of non-diabetic and T2DM humans. In the latter regard, we recently reported, in hepatocytes of T2DM humans, that aPKC activity is elevated, protein and mRNA levels of aPKC-ι, are increased, and expression of gluconeogenic and lipogenic enzymes are increased [14]; moreover, PKC-ι inhibitors largely reverse the aberrant increases in expression of lipogenic and gluconeogenic factors in hepatocytes of T2DM humans [14] and livers of obese/T2DM mice [17].
Methods
Kinase Activators and Inhibitors
Metformin and AICAR were purchased from Sigma. PKC-ι inhibitor, [1H-imidazole-4-carboxamide, 5-amino-1-[2,3-dihydroxy-4-[(phosphono-oxy)methyl]cyclopentyl-[1R-(1a,2b,3b,4a)] (ICAP), was custom-synthesized by Southern Research, Birmingham, AL, USA or United Chemical Resources, Birmingham, AL, USA (>95% purity). We presently used ICAP instead of [1H-imidazole-4-carboxamide, 5-amino-1-[2,3-dihydroxy-4-[(phosphono-oxy)methyl]cyclopentyl-[1R-(1a,2b,3b,4a)] (ICAPP) [see 14,17], as ICAP synthesis is easier and much less costly, and, although ICAP is itself inactive, it can be converted to the active compound, ICAPP, by adenosine kinase (see below). In some cases, we also used a newly developed inhibitor of both PKC-ι and PKC-ζ, 2-acetyl-1,3-cyclopentanedione (ACPD) (Sigma); as will be reported separately, this inhibitor differs from ICAP in that it inhibits both recombinant PKC-ι/λ and PKC-ζ, but, like ICAPP, does not inhibit conventional or novel PKCs, Akt or AMPK.
Hepatocyte Incubations
Cryo-preserved hepatocytes (70–90% viability; purchased from Zen-Bio Corp, Research Triangle, North Carolina, USA) were harvested from perfused livers of non-diabetic subjects [2 females and 6 males; ages, 43–60 years, 51 ± 3 (mean ± SEM); BMI, 30 ± 2] and type 2 diabetic subjects [2 females and 4 males, ages, 46–68 years, 60 ± 4; BMI, 27 ± 2] maintained on life support as transplant donors (these hepatocytes were obtained from the same patient groups described previously [14]). Diabetic subjects were hyperglycaemic and undergoing insulin treatment, but other pertinent laboratory and clinical data are not available in transplant donors.
As described [14], unless otherwise indicsted, hepatocytes were incubated (106 cells/100mm plate) overnight (approx 16 hours) in Dulbecco’s minimal essential medium containing 5% fetal calf serum, 100units/ml sodium-penicillin,100μg/ml streptomycin-sulfate, 2μmol/l dexamethasone, then for 2 hours in William’s E medium (Sigma, St. Louis, Missouri, USA) containing Glutamax (Invitrogen, Carlsbad, California, USA),100 units/ml sodium-penicillin, 100μg/ml streptomycin-sulfate, 100nmol/l dexamethasone, then for 4 hours in similar medium supplemented with 25mg/ml transferrin, and 0.25μg/ml sodium selenite. Where indicated, 1μmol/l insulin and varying concentrations of ICAP, AICAR and metformin were also present in the media throughout all incubations. Note: (a) this concentration of insulin was needed to maintain a high level of insulin activation of aPKC during prolonged incubation; indeed, 100nmol/l insulin was considerably less effective than 1μmol/l insulin in maintaining increases in aPKC and Akt activity in non-diabetic hepatocytes; and (b) effects of metformin on AMPK activity develop slowly and reach maxima at 24 hours in rat and human hepatocytes [7].
In some studies, where indicated, we used a protocol described previously [14], viz., after overnight incubation in insulin-containing medium as described above, hepatocytes were incubated for 3 hours in similar but insulin-free Williams E medium, followed by 6 hours ± 100nmol/l insulin, ± 1 or 10mmol/l metformin, ± 100nmol/l ICAP.
After incubation, cells were sonicated in homogenizing buffer for protein studies or placed into Trizol reagent (Invitrogen) for mRNA studies.
All experimental procedures involving human materials were approved by the Institutional Review Board of the University of South Florida College of Medicine, and the James A. Haley Veterans Administration Medical Center Research and Development Committee, Tampa, Fl, and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice.
Tissue Preparation
As described [14], hepatocytes were homogenized in ice-cold buffer containing 0.25mol/l sucrose, 20mmol/l Tris/HCl (pH, 7.5), 2mmol/l EGTA, 2mmol/l EDTA, 1mmol/l phenlysulfonlyfluoride (PMSF), 20μg/ml leupeptin, 10μg/ml aprotinin, 2mmol/l Na4P2O7, 2mmol/l Na3VO4, 2mmol/l NaF, and 1μmol/l microcystin, and then supplemented with 1% TritonX-100, 0.6% Nonidet and 150mmol/l NaCl, and cleared by low-speed centrifugation.
aPKC, Akt, and AMPK Assays
As described [11–14,17], aPKCs were immunoprecipitated from lysates with rabbit polyclonal antiserum (Santa Cruz Biotechnologies, Santa Cruz, California, USA) which recognizes C-termini of PKC-ζ and PKC-λ/ι (PKC-ι is the human homolog of mouse PKC-λ with 98% homology; human and mouse muscle contain primarily PKC-ι/λ and little PKC-ζ; mouse and human liver contain substantial amounts of both PKC-ι/λ and PKC-ζ [23]). Immunoprecipitates were collected on Sepharose-AG beads (Santa Cruz Biotechnologies) and incubated for 8 min at 30°C in 100μl buffer containing 50mmol/l Tris/HCl (pH,7.5), 100μmol/l Na3VO4, 100μmol/l Na4 P2O4, 1mmol/l NaF, 100μmol/l PMSF, 4μg phosphatidylserine (Sigma, St. Louis, Missouri, USA), 50μmol/l [γ-32P]ATP (NEN Life Science Products, Beverly, Massachussetts, USA), 5mmol/l MgCl2, and, as substrate, 40μmol/l serine analogue of the PKC-ε pseudosubstrate (Millipore, Bedford, Massachussetts, USA). After incubation, 32P-labeled substrate was trapped on P-81 filter paper and counted. aPKC activation was also assessed by immunoblotting for phosphorylation of the auto(trans)phosphorylation site, thr-555/560 in PKC-ι/ζ, required for, and reflective of, activation [23].
As described [14], for assays of recombinant PKC-ι and PKC-ζ (5–10ng/assay; Biovision, Mountain, California, USA), 10fmol/l phosphatidylinositol-3,4,5-(PO4)3 (PIP3; Matreya, Pleasant Gap, Pennsylvania, USA) was added to activate and define aPKC activity.
Activation of AMPK was assessed by measurement of immunoprecipitable AMPK activity as described [3,14], and by immunoblotting for phosphorylation of both threonine-172-AMPK and the AMPK substrate, serine-79-acetyl-CoA carboxylase (ACC).
Western Analyses
Western analyses were conducted as described [11–14,17], using: anti-phospho-serine-473-Akt and glyceraldehyde-phosphate dehydrogenase (GAPDH) antisera (Santa Cruz Biotechnologies, Santa Cruz, CA, USA); anti-phospho-threonine-560/555-PKC-ζ/PKC-ι/λ antiserum (Invitrogen, Carlsbad, CA, USA); mouse monoclonal anti-PKC-ι/λ antibodies (Transduction Labs, Bedford, Massachussets, USA); and anti-phospho-threonine-172-AMPK; and anti-phospho-serine-79-ACC antisera (Millipore). Samples from experimental groups were compared on the same blots, and corrected for recovery as needed by measurement of GAPDH immunoreactivity.
mRNA Measurements
As described [11–14,17], tissues were added to Trizol reagent (Invitrogen) and RNA was extracted and purified with RNA-Easy Mini-Kit and RNAase-free DNAase set (Qiagen, Valencia, California, USA), quantified (A260/A280), checked for purity by electrophoresis on 1.2% agarose gels. mRNA was quantified by quantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR), using TaqMan reverse transcription reagent (Applied Biosystem, Carlsbad, California, USA) and SYBR Green (kit from Applied Biosystems,) with human nucleotide primers [see 13,14,17].
Statistical Evaluations
Data are expressed as mean ± SEM, and P values were determined by one-way ANOVA and least-significant multiple comparison methods.
Results
Activation of AMPK by Metformin and AICAR in Human Hepatocytes
Treatment of human hepatocytes over 24 hours with either metformin or AICAR increased AMPK activity, as evidenced by increases in: (a) phosphorylation of thr-172-AMPK and AMPK substrate, ser-79-ACC (Fig 1); and (b) immunprecipitable AMPK enzyme activity (Fig 2). Maximal activation of AMPK was seen at 1mmol/l metformin and 100nmol/l AICAR (Fig 2). Note that treatment with 10mmol/l metformin had variable effects on AMPK activity and, in some cases, diminished energy-dependent processes, e.g., aPKC activation (see below); this probably reflects varying degrees of limitation in ATP availability [7].
Figure 1.
Effects of ICAP, AICAR and Metformin on Phosphorylation of AMPK, ACC and aPKC in Human Hepatocytes. Hepatocytes of non-diabetic humans were treated for 24 hours with 100nmol/l ICAP, 100nmol/l AICAR or 1mmol/l metformin, and then examined for relative levels of phosho-threonine-172-AMPK, phospho-serine-79-ACC and phospho-threonine-555/560-PKC-ι/ζ. Representative immunoblots are shown above bargrams. Bargram relative values are Mean ± SEM of 5 determinations. Asterisks indicate: *, P<0.05; **, P<0.01; and ***, P<0.001.
Figure 2.
Dose-dependent effects of ICAP, AICAR and Metformin on AMPK Activity in Human Hepatocytes. Hepatocytes of non-diabetic humans were treated for 24 hours with indicated concentrations of ICAP, AICAR or metformin, and then examined for immunoprecipitable AMPK activity. Values are Mean ± SEM of 5 determinations.
Although we didn’t determine if metformin and AICAR activate aPKC through activation of ERK and PLD-mediated increases in phosphatidic acid (PA) in human hepatocytes, we found that, like PIP3, which mediates insulin effects on aPKC [23], PA activated recombinant aPKC, with potency comparable to that of PIP3 (Fig 3a).
Figure 3.
Effects of ICAPP, and ICAP ± adenosine (Ado) kinase on aPKC activity. Recombinant PKC-ι was incubated with 10fmol/l phosphatidylinositol-3,4,5-(PO4)3 (PIP3) (which maximally activated recombinant aPKC – see panel b) and indicated doses of ICAPP (panel a), ICAP (panel c), or ICAP + 100ng Ado-Kinase (panel d), along with other components of the aPKC assay system. Panel b shows similar dose-dependent effects of PIP3 and phosphatidic acid (PA) on recombinant aPKC activity.
Activation of aPKC by Metformin and AICAR in Human Hepatocytes
As in mouse liver [8], treatment of human hepatocytes with maximally effective concentrations of metformin or AICAR for 24 hours increased phosphorylation of thr-555/560-PKC-λ/ζ, the autophosphorylation site, reflective of, and required for, aPKC activation (Fig 1). Dose-dependent increases in immunoprecipitable aPKC enzyme activity were also seen following 24-hour treatments, with maximal increases seen at 1–3mmol/l metformin and 100nmol/l AICAR (Figs 4). In these comparisons, metformin- and AICAR-induced increases in aPKC activity were approximately 50–60% of those elicited by combined treatment with metformin or AICAR plus insulin; however, in individual comparisons, 1mmol/l metformin and 100nmol/l AICAR provoked increases in aPKC activity comparable in magnitude to those elicited by insulin (see Fig 6). Also note that treatment with 10mmol/l metformin in overnight incubations produced variable alterations, which, on the average, failed to increase basal aPKC activity, and, moreover, partially diminished insulin-stimulated aPKC activity (Fig 4). Indeed, even more marked inhibition of insulin-stimulated aPKC was seen in 6-hour incubations with 10mmol/l metformin, perhaps reflecting greater availability of metformin in shorter incubations (not shown).
Figure 4.
Effects of ICAP, AICAR and Metformin on aPKC Activity in Human Hepatocytes. Hepatocytes of 5 non-diabetic humans were treated for 24 hours with indicated concentrations of ICAP, AICAR or metformin, in the presence and absence of 1μmol/l insulin, and then examined for immunoprecipitable aPKC activity. Values are Mean ± SEM of 5 determinations. Asterisks indicate: *, P<0.05; **, P<0.01; and ***, P<0.001.
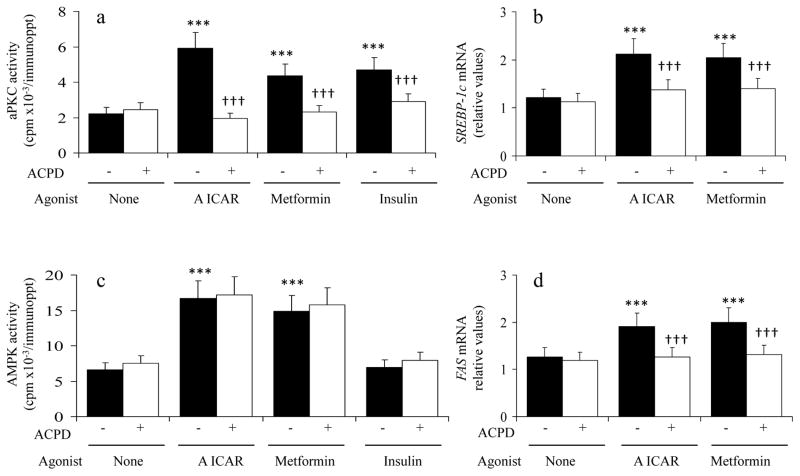
Figure 6.
AICAR and Metformin Increase Expression of Lipogenic Factors by an aPKC-dependent Mechanism in Human Hepatocytes. Hepatocytes of 4 non-diabetic humans were treated for 24 hours without or with 1μmol/l ACPD, 100nmol/l AICAR (A), 1 mmol/l metformin and 1μmol/l insulin, and then examined for mRNA levels of SREBP-1c and FAS, and activities of aPKC and AMPK. Values are Mean ± SEM of 4 determinations. Asterisks indicate: *, P<0.001, agonist-treated value versus the basal untreated value; and symbol € indicates, P<0.001, ACPD-inhibited value versus the adjacent uninhibited value.
Inhibition of aPKC Activity by ICAP in Human Hepatocytes
ICAP diminished insulin-stimulated aPKC activity by approx 50% in human hepatocytes (Figs 1 and 4), with maximal inhibition seen at 100nmol/l (Fig 4). However, ICAP itself did not directly inhibit recombinant PKC-ι (Fig 3c), indicating that ICAP must be converted intracellularly to the active inhibitory compound, ICAPP, which contains a phosphate group linked to the 4-methyl-hydroxy group, and which binds to the substrate binding site of PKC-ι/λ and specifically inhibits PKC-ι (Fig 3a) and 98% homologous PKC-λ (not shown), but no other PKCs, including aPKC-ζ (72% homology) and PKCs-α,β,δ,ε,θ [14]. Consonant with this idea: (a) AICAR is itself inactive but is phosphorylated intracellularly by adenosine kinase to the active compound, AICAR-PO4 (ZMP), which acts as an analogue of 5′-AMP; (b) ICAP is structurally identical to AICAR, except that ICAP has a cyclopentyl ring in place of the ribose ring in AICAR; (c) addition of adenosine kinase along with ICAP to the incubation of recombinant PKC-ι led to an inhibitory effect comparable to that of ICAPP (cf Figs 3d and 3a); and (d) incubation of ICAP with adenosine kinase and γ-32PO4-ATP yielded 32PO4–labeled ICAPP, as determined by purification with thin layer chromatography (Km, approx 1μmol/l). Also note in Fig 4 that: (a) insulin-stimulated aPKC activity resistant to ICAP probably reflects PKC-ζ, which is also present in human hepatocytes; and (b) the resistance of basal vis-à-vis insulin-stimulated aPKC activity to inhibition by ICAP may reflect that insulin-activated aPKC would be expected to have an open substrate-binding site that may be more sensitive to inhibitors than inactive closed aPKC, and/or a substantial amount of insulin-insensitive non-aPKC kinase(s) co-immunoprecipitates with aPKC.
Effects of ICAP on AMPK Activity in Human Hepatocytes
Despite structural similarities to AICAR, ICAP, at concentrations that maximally inhibited aPKC (Fig 4), did not increase the phosphorylation of AMPK or ACC (Fig 1), or immunoprecipitable AMPK enzyme activity (Fig 2). Also, despite structural similarities to ICAP, AICAR, at concentrations that maximally activated AMPK (Fig 2), not only failed to inhibit, but, instead, increased aPKC phosphorylation at thr-555/560 (Fig 1) and aPKC enzyme activity (Fig 4). Further, although not shown, effects of 10μmol/l AICAR on both AMPK and aPKC activity were comparable to those elicited by 0.1–1μmol/l AICAR, indicating that increases in both activities had plateaued.
Effects of Metformin and AICAR versus ICAP on Lipogenic and Gluconeogenic Enzyme Expression in Hepatocytes of Non-Diabetic and T2DM Humans
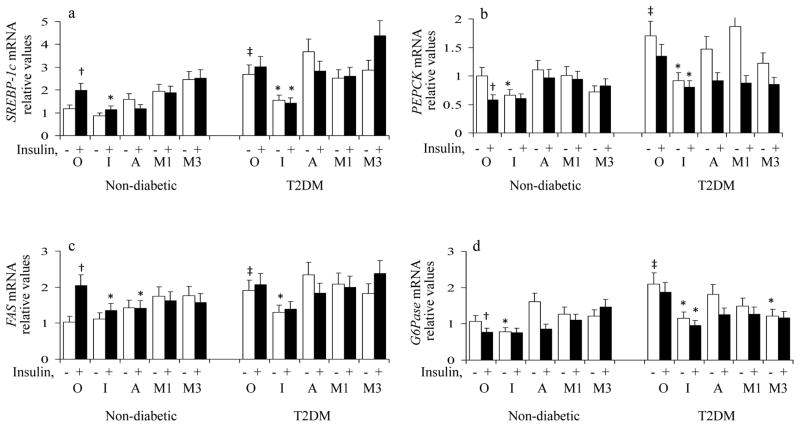
As in previous ICAPP studies [14]: (a) insulin provoked increases in expression of lipogenic factors, SREBP-1c and FAS, and decreases in expression of gluconeogenic enzymes, PEPCK and G6Pase, in non-diabetic hepatocytes; (b) the expression of these lipogenic and gluconeogenic factors was increased basally and insulin had no further effect on these factors in T2DM hepatocytes; and (c) 100nmol/l ICAP largely diminished both insulin-induced increases in expression of lipogenic factors, SREBP-1c and FAS, in non-diabetic hepatocytes, and diabetes-induced increases in both lipogenic and gluconeogenic factors in T2DM hepatocytes (Fig 5).
Figure 5.
Effects of ICAP versus AICAR versus Metformin on Expression of Lipogenic and Gluconeogenic Factors in Basal and Insulin-stimulated Hepatocytes of Non-diabetic and Type 2 Diabetic (T2DM) Humans. Hepatocytes of non-diabetic and T2DM humans were treated for 24 hours without (O) or with 100nmol/l ICAP (I), 100nmol/l AICAR (A) or 1 or 3 mmol/l metformin (1M and 3M), in the presence (solid bar) and absence (open bar) of 1μmol/l insulin, and then examined for mRNA levels of SREBP-1c, FAS, PEPCK and G6Pase. Relative values are Mean ± SEM of 5 determinations. Symbols indicate: *, P<0. 05, agent-treated basal or agent-treated insulin-stimulated value versus corresponding basal or insulin-stimulated value of the O group; †, P<0.05, insulin-treated value of the O group versus basal value of the O group; and ‡, P<0.05, basal diabetic value of the O group versus basal non-diabetic value of the O group.
In contrast to ICAP treatment, (a) basal expression of SREBP-1c and FAS increased following treatment of non-diabetic hepatocytes with 1mmol/l metformin, and 100nmol/l AICAR (Fig 6b and 6d), and concomitant insulin treatment did not provoke further increases in SREBP-1c/FAS expression (Fig 5), and (b) diabetes-dependent increases in expression of SREBP-1c and FAS were not improved by either 1–3mmol/l metformin or 100nmol/l AICAR treatment in T2DM hepatocytes (Fig 5).
As in ICAPP studies [14], treatment with 100nmol/l ICAP was attended by decreases in expression of PEPCK and G6Pase in hepatocytes of both non-diabetic and T2DM humans incubated in the absence of insulin; moreover, insulin did not elicit further decreases in PEPCK/G6Pase expression (Fig 5). In contrast to ICAP, basal expression of PEPCK and G6Pase trended higher following treatment of non-diabetic hepatocytes with 1–3mmol/l metformin and 100nmol/l AICAR, and concomitant insulin treatment failed to significantly improve PEPCK/G6Pase expression in non-diabetic hepatocytes (Fig 5). Also, 100nmol/l AICAR and 1mmol/l metformin did not diminish basal expression of PEPCK and G6Pase in T2DM hepatocytes (Fig 5). On the other hand, in T2DM hepatocytes, 1 and 3mmol/l metformin and 100nmol/l AICAR improved insulin effects on PEPCK/G6Pase expression (Fig 5).
To determine whether stimulatory effects of metfromin and AICAR on SREBP-1c and FAS expression are dependent of aPKC, we used a newly developed inhibitor of PKC-ι and PKC-ζ, ACPD, instead of ICAP, as metfromin and AICAR activate both aPKCs [3], and to avoid competition ICAP and AICAR which are probably similarly transported and phosphorylated by adenosine kinase (see above). Indeed, in hepatocytes of non-diabetic humans, 1 μmol/l ACPD markedly inhibited the increases in aPKC activity elicited by metformin, AICAR and insulin (Fig 6a; note that metformin- and AICAR-induced increases in aPKC were equal to that of insulin). In contrast, ACPD did not diminish AMPK activation by AICAR and metformin (Fig 6c). Most importantly, ACPD largely inhibited AICAR- and metformin-induced increases in expression of both SREBP-1c (Fig 6b) and FAS (Fig 6d).
Effects of ICAP on Phosphorylation of FoxO1in Human Hrepatocytes
As in ICAPP studies in human hepatocytes [14] and mouse liver [17], ICAP provoked increases in phospho-threonine-256-FoxO1 in non-diabetic human hepatocytes that were comparable to or greater than those elicited by insulin (Fig 7). These effects of ICAP and insulin on FoxO1 phosphorylation were seen in 6-hour, but not 24-hour, incubations, perhaps reflecting an autoregulatory limitation consequent to prolonged suppression of gluconeogenic enzyme expression. As also portrayed in Fig 7, 100nmol/l insulin increased aPKC and Akt phosphorylation/activation, and 100nmol/l ICAP diminished aPKC, but not Akt, phosphorylayion in these 6-hour experiments.
Figure 7. Effects of ICAP on Phosphorylation of FoxO1 in Human Hepatocytes.

As described in Methods, hepatocytes of non-diabetic humans were incubated first for 16 hours with 1μmol/l insulin and then for 3 hours in insulin-free medium, and then treated for 6 hours ± 100nmol/l ICAP and ± 100nmol/l insulin, as indicated. Tissue extracts were examined by Western analysis for phospho-serine-256-FoxO1 and relative values are Mean ± SEM of 4 determinations. For comparison, effects of insulin and ICAP on activation of aPKC (phospho-threonine-555/560-PKC-ι/ζ) and Akt (phospho-serine-473-Akt1/2) in these conditions are shown.
Discussion
We found that metformin and AICAR activated aPKC in concentrations that activate AMPK in human hepatocytes. This is important, as hepatic aPKC inhibition by various means, including, adenoviral-mediated expression of kinase-inactive aPKC [12,13], or use of shRNA for knockdown of hepatic IRS-2, which controls hepatic aPKC [12], or small-molecule aPKC inhibitors [14,17], has salutary (inhibitory) effects on expression of hepatic lipogenic and gluconeogenic factors that contribute importantly to lipid and carbohydrate abnormalities in obesity, the metabolic syndrome and T2DM. Accordingly, except in states of maximal aPKC activation, hepatic aPKC activation by metformin and AICAR would be expected to diminish salutary effects that these agents might otherwise have on lipogenic and gluconeogenic factors by simple AMPK activation.
Activation of aPKC in human hepatocytes by metformin and AICAR most likely derives from AMPK activation, as activation profiles of aPKC and AMPK followed similar dose-response relationships. Consonant with this idea, in rodent muscle, aPKC activation by metformin and AICAR is dependent on AMPK, and AMPK activation by these agents is independent of aPKC [3,9]. Similarly, with a specific aPKC inhibitor, we presently found that AMPK activation is independent of aPKC in human hepatocytes (we were unable to use AMPK inhibitor, Compound C, as it unexpectedly inhibited aPKC).
In support of the idea that hepatic aPKC activation may diminish the therapeutically desirable effects of simple AMPK activation, both metformin and AICAR were less effective than aPKC inhibitor ICAP in diminishing insulin-dependent and diabetes-dependent increases in expression of lipogenic factors, SREBP-1c and FAS, in hepatocytes of non-diabetic and T2DM humans. Indeed, expression of these lipogenic factors increased following metformin and AICAR treatment in non-diabetic hepatocytes, and diabetes-dependent increases in expression of these lipogenic factors were not significantly improved by metformin and AICAR in hepatocytes of T2DM humans. In contrast, ICAP largely reversed both insulin-induced and T2DM-induced increases in these lipogenic factors. Of course, we cannot rule out the possibility that the failure of metformin and AICAR to improve SREBP-1c and FAS expression in diabetic hepatocytes resulted from an aPKC- independent mechanism.
The failure to find more significant salutary effects of metformin and AICAR on hepatic lipogenic factors in diabetic hepatocytes may explain why metformin has limited effects on weight loss and hyperlipidaemia in T2DM humans. This failure to improve lipogenic factor expression further suggests that salutary effects of metformin on lipid metabolism in vivo may reflect alterations in processes other than direct improvements of hepatic SREBP-1c and FAS expression, e.g., metformin-induced anorectic tendencies and decreases in hyperinsulinaemia (and thus decreases in hepatic aPKC activation) owing to improvements in hepatic and/or muscle glucose metabolism. In addition, AMPK directly phosphorylates/inhibits ACC, and this may increase fatty acid oxidation and diminish fatty acid synthesis.
It was also important to find that, as with ICAPP [14,17], ICAP diminished expression of PEPCK and G6Pase basally, i.e., in the absence of insulin treatment, in hepatocytes of both non-diabetic and T2DM humans. In contrast, metformin and AICAR did not diminish basal expression of these gluconeogenic enzymes in non-diabetic hepatocytes, and seemed to provoke upward trends in these expressions that were not reversed by concomitant insulin treatment. On the other hand, metformin and AICAR did improve insulin-induced deceases in PEPCK and G6Pase expression in hepatocytes of T2DM humans, and this sensitizing mechanism may be important for metformin-induced improvements in hepatic gluconeogenesis in T2DM humans. That this salutary action required the presence of insulin correlates with the fact that metformin is most useful for treating earlier, but not later, phases of T2DM, when insulin secretion diminishes, or T1DM.
The mechanism whereby metformin and AICAR enhanced insulin effects on gluconeogenic enzymes in hepatocytes of T2DM humans is uncertain. One possibility is that metformin and AICAR increased phosphorylation and nuclear exclusion of TORC2 [6] independently of aPKC, and thereby restored the ability of insulin to disrupt the CREB/CBP/TORC2 complex needed for PEPCK/G6Pase expression. As another possibility, metformin and AICAR may have enhanced insulin effects on gluconeogenic enzymes by increasing aPKC-dependent phosphorylation and nuclear exclusion of CRB in accordance with the mechanism advanced by He et al [8]. This possibility, however, seems remote, as: (a) aPKC activity is substantially increased basally in hepatocytes of T2DM rodents [11–13,17] and humans [14 and present results]; and (b) as seen presently with ICAP and previously with other aPKC inhibitors [12–14,17], the inhibition of aPKC diminishes basal hepatic gluconeogenic enzyme expresssion. On the other hand, He et al [8] reported that, whereas insulin had little ability to phosphorylate CBP in high fat-fed mice, metformin was fully effective and, moreover, acutely lowered blood glucose levels. In this scenario, however, since overall hepatic aPKC activity is increased in hyperinsulinaemic high fat-fed mice (13), an important role for aPKC in mediating metformin effects in this model would require a remarkable degree of compartmentalization, i.e., an aPKC subset that is downregulated and unresponsive to hyperinsulinaemia, but responsive to metformin. Needless to say, other mechanisms may be operative in metformin-induced sensitization to insulin.
It was surprising to find that, despite structural similarity between ICAP and AICAR, ICAP did not increase AMPK activity, and AICAR did not diminish aPKC activity. This suggests that the one structural difference, viz., the oxygen atom in the ribose ring of AICAR-PO4, is not only important for AMPK activation, but also serves to prevent aPKC inhibition. On the other hand, the possibility that aPKC inhibition may occur when supra-optimal concentrations of metformin are used must be kept in mind, as aPKC inhibition rather than simple AMPK activation may underlie or contribute to salutary effects.
The ability of ICAP to maximally inhibit PKC-ι in intact human hepatocytes at a concentration only one order of magnitude greater than that of ICAPP [see 4,17] most likely reflects efficient cellular uptake of ICAP and subsequent conversion to the active phosphorylated compound, ICAPP, probably by the same adenosine transporter and kinase used by AICAR. In this regard, note that, in studies of intact mice, we found that ICAP, in doses slightly greater than those used in ICAPP studies: (a) specifically inhibited hepatic (but not muscle) PKC-λ, with no effects on hepatic Akt or AMPK; and (b) effectively inhibited aPKC-dependent expression of lipogenic and gluconeogenic factors in livers of T2DM mice (unpublished).
To summarize, in human hepatocytes, metformin and AICAR activated aPKC in concentrations comparable to those required for maximal AMPK activation. Since aPKC inhibition has salutary effects on, i.e., diminishes expression of, lipogenic and gluconeogenic factors in human hepatocytes, it was not surprising to find that the activation of aPKC during optimal metfomin and AICAR action on AMPK was attended by changes in expression of lipogenic and gluconeogenic factors that were less salutary than those elicited by ICAP, a specific inhibitor of PKC-ι. The activation of aPKC by metformin and AICAR appeared to explain why metformin and AICAR failed to reverse insulin- and T2DM-induced increases in lipogenic factors, SREBP-1c and FAS. Activation of aPKC by metformin and AICAR may also explain why these agents, blunted insulin effects on PEPCK and G6Pase expression in non-diabetic hepatocytes; accordingly, metformin usage in pre-diabetic states may be problematic. On the other hand, metformin and AICAR improved insulin effects on PEPCK and G6Pase in hepatocytes of T2DM humans, regardless of concomitant aPKC activation. Our findings may explain why metformin has only modest effects on lipid metabolism, and requires insulin for improvements in glucose metabolism.
Acknowledgments
Supported by funds from the Department of Veterans Affairs Merit Review Program and the National Institutes of Health Grants [DK 065969 to R.V.F.}
Footnotes
Contribution Statement
RV Farese conceived, designed and directed the studies, analyzed data, and wrote the paper. MP Sajan conducted studies and assays, and assembled and assisted in interpretation of data. RA Ivey conducted assays and assisted in interpretation of data.
Duality of Interest
There are no conflicts of interest amongst the authors.
References
- 1.Hundal RS, Krssak M, Dufour S, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luna V, Casauban L, Sajan MP, et al. Metformin improves atypical protein kinase C activation by insulin and phosphatidylinositol-3,4,5- (PO4) in diabetic muscle. Diabetologia. 2006;49:375–382. doi: 10.1007/s00125-005-0112-4. [DOI] [PubMed] [Google Scholar]
- 3.Sajan MP, Bandyopadhyay G, Miura A, et al. AICAR and metformin, but not exercise, increase muscle glucose transport through AMPK-, ERK- and PDK1-dependent activation of atypical PKC. Am J Physiol Endocrinol Metab. 2010;298:E179–E192. doi: 10.1152/ajpendo.00392.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musi N, Hirshman MF, Nygren J, et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 6.Shaw RJ, Lamia KA, Vasquez D, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephenne X, Foretz M, Talcux N, et al. Metformin activates AMP-activated kinase in primary human hepatocytes by decreasing cellular energy status. Diabetologia. 2011;54:3101–3110. doi: 10.1007/s00125-011-2311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He L, Sabet A, Djedjos S, et al. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell. 2009;15:635–646. doi: 10.1016/j.cell.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen HC, Bandyopadhyay G, Sajan MP, et al. Activation of the ERK pathway and atypical protein kinase C isoforms in exercise- and AICAR-stimulated glucose transport. J Biol Chem. 2002;277:23554–23562. doi: 10.1074/jbc.M201152200. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto M, Ogawa W, Akimoto K, et al. PKCλ in liver mediates insulin-induced SREBP-1c expression and determines both hepatic lipid content and overall insulin sensitivity. J Clin Invest. 2003;112:935–944. doi: 10.1172/JCI18816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Standaert ML, Sajan MP, Mirua A, et al. Insulin-induced activation of atypical protein kinase C, but not protein kinase B, is maintained in diabetic (ob/ob and Goto-Kakazaki) liver. Contrasting insulin signaling patterns in liver versus muscle define phenotypes of type 2 diabetic and high-fat-induced insulin-resistant states. J Biol Chem. 2004;279:24929–24934. doi: 10.1074/jbc.M402440200. [DOI] [PubMed] [Google Scholar]
- 12.Sajan MP, Standaert ML, Rivas J, et al. Role of atypical protein kinase C in activation of sterol regulatory element binding protein-1c and nuclear factor kappa B (NFkappaB) in liver of rodents used as model of diabetes, and relationships to hyperlipidaemia and insulin resistance. Diabetologia. 2009;52:1197–1207. doi: 10.1007/s00125-009-1336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sajan MP, Standaert ML, Nimal S, et al. Critical role of atypical protein kinase C in activating hepatic SREBP-1c and NFκB in obesity. J Lipid Res. 2009;50:1133–1145. doi: 10.1194/jlr.M800520-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sajan MP, Farese RV. Insulin Signalling in Hepatocytes of Type 2 Diabetic Humans. Excessive Expression and Activity of PKC-ι and Dependent Processes and Reversal by PKC-ι Inhibitors. Diabetologia. 2012;55:1446–1457. doi: 10.1007/s00125-012-2477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitamura Y, Accilli D. New insights into the integrated physiology of insulin action. Rev Endocr Metab Disord. 2004;5:143–149. doi: 10.1023/B:REMD.0000021436.91347.93. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto M, Pocal A, Rossetti L, et al. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor foxo 1 in liver. Cell Metab. 2007;6:208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Sajan MP, Nimal S, Mastorides S, et al. Correction of Metabolic Abnormalities in a Rodent Model of Obesity, Metabolic Syndrome and Type 2 Diabetes by Inhibitors of Hepatic Protein Kinase C-iota. Metabolism. 2012;61:459–469. doi: 10.1016/j.metabol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doombos RP, Theelan M, van der Hoeven PC, et al. Protein kinase Czeta is a negative regulator of protein kinase B activity. J Biol Chem. 1999;274:8589–8596. doi: 10.1074/jbc.274.13.8589. [DOI] [PubMed] [Google Scholar]
- 19.Ding J, Kato S, Du K. PI3K activates negative and positive signals to regulate TRB3 expression in hepatic cells. Exp Cell Res. 2008;314:1566–1574. doi: 10.1016/j.yexcr.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 20.Liu YF, Paz K, Herschkovitz A, Alt A, et al. Insulin stimulates PKCzeta-mediated phosphorylation of insulin receptor substrate-1 (IRS-1). A self-attenuated mechanism to negatively regulate IRS proteins. J Biol Chem. 2001;276:14459–14465. doi: 10.1074/jbc.M007281200. [DOI] [PubMed] [Google Scholar]
- 21.Sajan MP, Standaert ML, Miura A, et al. Tissue-specific differences in activation of atypical protein kinase C and protein kinase B in muscle, liver, and adipocytes of insulin receptor substrate-1 knockout mice. Mol Endocrinol. 2004;18:2513–2521. doi: 10.1210/me.2004-0045. [DOI] [PubMed] [Google Scholar]
- 22.Guo S, Copps KD, Park S, et al. The Irs1 branch of the insulin signaling cascade plays a dominant role in hepatic nutrient homeostasis. Mol Cell Biol. 2009;29:5070–5083. doi: 10.1128/MCB.00138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farese RV, Sajan MP. Metabolic Functions of Atypical Protein Kinase C: “Good and Bad” as Defined by Nutritional Status. (Invited Review) Am J Physiol Endocrinol Metab. 2010;298:E385–394. doi: 10.1152/ajpendo.00608.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]