Abstract
Decisions are said to be ‘risky’ when they are made in environments with uncertainty caused by nature. By contrast, a decision is said to be ‘trusting’ when its outcome depends on the uncertain decisions of another person. A rapidly expanding literature reveals economically important differences between risky and trusting decisions, and further suggests these differences are due to ‘betrayal aversion’. While its neural foundations have not been previously illuminated, the prevailing hypothesis is that betrayal aversion stems from a desire to avoid negative emotions that arise from learning one's trust was betrayed. Here, we provide evidence from an fMRI study that supports this hypothesis. In particular, our data indicate that the anterior insula modulates trusting decisions that involve the possibility of betrayal.
Keywords: betrayal aversion, trust, fMRI
1. Introduction
Social interactions are a critical component of human lives, having a strong impact on well-being and sense of achievement. Human interaction involves uncertainty about the consequences of one's own actions, and, in particular, the way one's actions impact other's decisions. An especially important example of this is trust [1]. It is well recognized that efficient economic interactions often rely on trust, a decision that can have substantially negative consequences for the trustor. However, many studies indicate that trust (involving strategic uncertainty) is behaviourally distinct from risk (involving state uncertainty) [2–7]. In addition to research exploring the neural foundations of trust [8–11] and risk [12–16], recent research explores how the brain distinguishes these two types of decisions [17–19]. Economically oriented trust research has also influenced research outside economics and basic neuroscience. For example, imaging work on trust environments has already had a major impact on fields including medicine [20] in helping to build a better understanding of behavioural psychological phenomenon such as borderline personality disorder [10], and other areas as well [21].
For instance, decisions made in environments that include strategic uncertainty, particularly trust, are processed differently by the brain than decisions made in risk environments that include only uncertainty about the outcome of a random device. Decisions in the latter seem to involve relatively greater posterior parietal cortex activation, while the decisions in the former seem to recruit the lateral prefrontal cortex [12,22,23]. Furthermore, a recent study by Lauharatanahirun et al. [24] traces differences in the source of uncertainty (social rather than non-social risk) to changes in amygdala activity [25]. While this is no doubt part of the story, behavioural evidence suggests the reason why the source of the uncertainty matters is probably the anticipated aversive emotions connected to possible betrayal of one's own trust by another human. Thus, it seems that not only the source of the uncertainty (social or non-social) but also how this uncertainty is resolved influences our willingness to trust in others.
We adapted the approach of Aimone & Houser [6] to examine the neural correlates of betrayal aversion. We used a design that allowed us to examine both within- and between-subject effects of betrayal aversion. While undergoing fMRI, 30 investors made 82 binary trust decisions of various stake sizes (figure 1). Investors chose either to trust or not to trust. Trusting decisions led the endowment to be tripled and to an ultimate pay-off determined by trustees’ decisions. Choosing not to trust split the endowment evenly between the investor and trustee. In particular, half (41) of the games were standard trust games where a decision to trust resulted in a payment based upon the investor's counterpart's decision to betray or reciprocate trust (binary decision of an uneven or even split of the tripled endowment). Betrayal aversion is known to influence trust decisions in these games. The other half were trust games where betrayal aversion could not influence decision-making (following [5–7]) through the introduction of a computer mediator.
Figure 1.
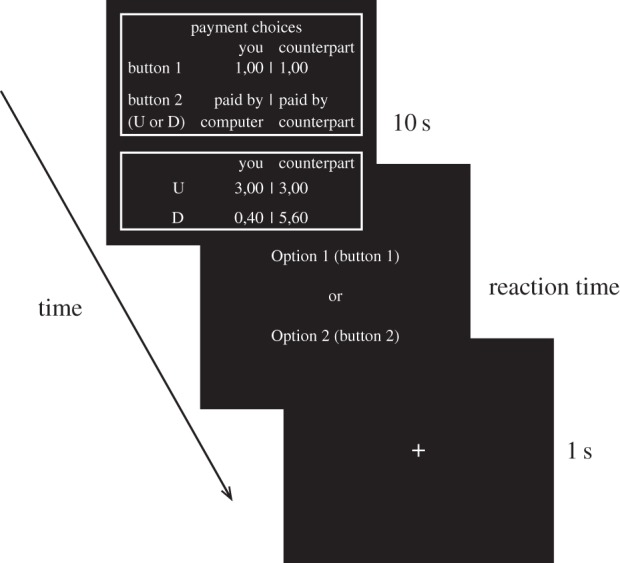
The experiment design.
The procedures governing our computer-mediated games have a number of unique features that distinguish them from many other ‘computer’ treatments. First, in the computer-mediated treatment investors continue to have a human counterpart, and in both environments trustees were paid based on their own decisions when an investor chose to trust. This feature ensures that altruism and other forms of payment comparison motivations (particularly inequity aversion) remain the same between treatments. Second, a decision to trust in these games paid investors based upon a computer mediator's random draw from the pool of that experiment's session's trustee decisions, instead of directly from one's own counterpart's decision. By drawing from this pool of trustee decisions (which includes one's own counterpart's decision), the investor's expectation-driven reference point remains identical between treatments. The advantage to this is that loss aversion cannot vary between treatments. Furthermore, both social and monetary risks are constant between environments. The reason is that subjective expectations over the pool of counterparts’ decisions must remain unchanged. Consequently, social and monetary risk preferences cannot underlie any between-treatment behavioural differences. Finally, as detailed by Aimone & Houser [6], computer mediation shields investors from knowing their own counterpart's betrayal decision, so that betrayal aversion cannot impact decisions.
Previous studies suggest an important role of the insula in the evaluation of trustworthiness and related behaviour [26]. Therefore, we hypothesized that insula activity might play an important role in mediating betrayal aversion as a strong motive in trust behaviour. Increased insular activity during the decision to trust may be a signal for stronger aversive emotions, which might lead to a tendency of people to avoid these situations.
2. Results
As described by figure 2, significantly more trust was observed when betrayal aversion does not influence decision-making (62.76% with the computer mediator) compared with when it does (49.43%; p < 0.01, two-tailed paired Wilcoxon). This result is observed to be driven largely by the responsiveness of males to the removal of betrayal aversion from the decision. Men (half the sample) are seen increasing trust from 51.38% when exposed to betrayal aversion to 71.22% when betrayal aversion is removed (p < 0.01). Conversely, women are not seen to have a significant reaction to the removal of betrayal aversion from the environment, with trust only increasing from 47.48% when exposed to their betrayal aversion to 54.31% when not exposed (p = 0.19).
Figure 2.
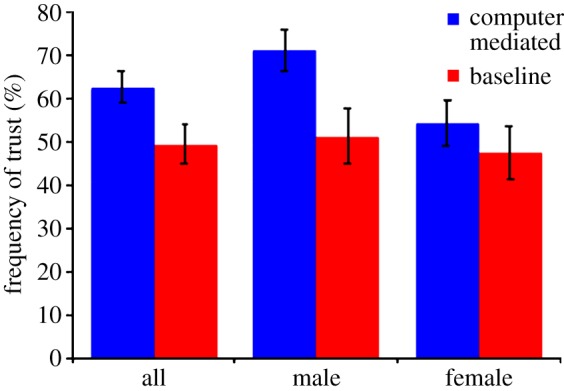
Frequency of trust by treatment and gender.
For initial evidence on the neural correlates of betrayal aversion, we compared the average blood-oxygen-level-dependent (BOLD) activity when playing with a human counterpart against playing with a computer mediator (figure 3). The results revealed activity in the right anterior insular cortex [40/22/3], as well as the medial frontal cortex [0/22/41] and right dorsolateral prefrontal cortex [35/44/33]. The latter two areas have, in previous studies, been implicated in emotion regulation [27], while the former has often been found to indicate heightened negative affective states [16,28]. This pattern is consistent with the hypothesized heightened negative emotions when in the human treatment, where investors are exposed to betrayal aversion. The opposite contrast did not show any significant activation.
Figure 3.
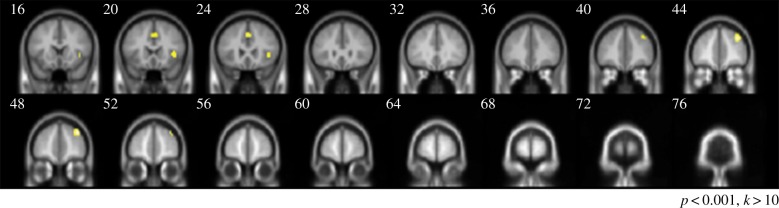
Brain areas displaying greater activation in the human than computer condition.
When agents make the decision to trust, in contrast to the safe option to not trust, we observed increased activity in the right anterior insular cortex [41/8/3] and the mid-anterior cingulate [–5/16/40] (figure 4a). The interaction between the chosen option and the identity of the counterpart again reveals significantly higher activity in the right anterior and posterior insular cortex [41/17/1; 33/–10/19] when the subject decided to trust and the counterpart was a human player in contrast to a computer player, providing further evidence that the insula reflects the heightened negative state associated with betrayal aversion (figure 4b). When choosing the safe option in either treatment, there can be no betrayal. This is reflected in our neural results as no significant difference for the safe option was observed between the human and computer mediator.
Figure 4.
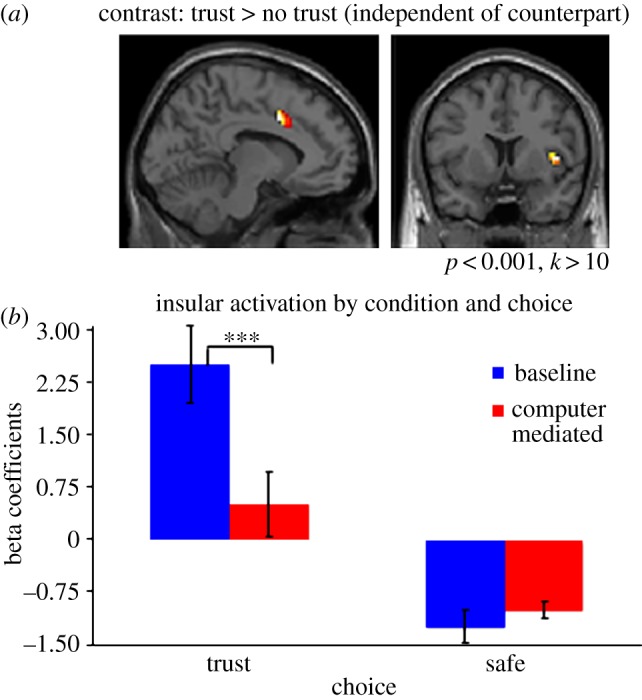
(a) Brain areas displaying greater activation when trusting than when not trusting (regardless of counterpart). (b) Level of insular activation by condition and choice.
In view of the significant behavioural differences, we split the subjects into those who are betrayal-averse (BA) and those who are non-betrayal-averse (NBA). The classification was based on whether the individual trust rate difference between the computer condition and the human condition was significant. Of the 30 subjects in the study, eight were significantly BA (7, p < 0.05; 1, p < 0.1). The BA subjects showed greater insular activity than NBA subjects when choosing the risky option while playing with a human counterpart [42/17/1], and we see no significant neural influence of gender.
These results are even more pronounced when using the difference in the number of safe options chosen between the human and computer mediated as a measure of the level of betrayal aversion, rather than using binary definition of betrayal aversion. As the level of betrayal aversion increases, subjects show greater insular activity when choosing the risky option while playing with a human counterpart than with a computer mediator (p < 0.05, FWE) [49/11/–2].
3. Discussion
These findings are the first evidence on the neural foundations of betrayal aversion. Insular activity is greater when making decisions that may result in betrayal, resulting in reduced willingness to participate in efficient social exchange. Furthermore, our results indicate that highly BA individuals display relatively greater insula activation when choosing to trust than do their less BA counterparts. These results support the understanding of betrayal aversion as a desire to avoid negative emotions associated with knowing one has been betrayed. Furthermore, our results suggest emotion regulation is the source of the behavioural gap between trusting and risky decisions.
The insular has been shown to be involved in trust evaluations. Winston et al. [26] showed that trustworthiness ratings of unfamiliar faces were correlated to BOLD signal changes in the anterior insula, which was interpreted as a marker for changing bodily states owing to negative emotions elicited by untrustworthy faces. A recent review underlines this view by highlighting the importance of the insula in social emotions [29]. In this and a previous paper [30], Singer and colleagues propose a model of the insula which is not only important for the evaluation of acute bodily states, but also for the prediction of future states (i.e. the anticipation of emotions [30] due, for example, to risky situations [15]). These studies fit nicely with our results. Indeed, our data could point to an aversive signal warning one to avoid future negative emotions that would arise should one experience either betrayal of trust or unfair treatment by another. Furthermore, recent results [31] showing betrayal aversion is distinct from related concepts like risk aversion and anxiety lend support to the view that the insular effects we are observing are a distinct effect of betrayal aversion.
We identify increased activation in the anterior insula as a key neuro-correlate of betrayal aversion. Our design and results may be a step towards reconciling conflicting arguments regarding the role of oxytocin in betrayal. In particular, some have argued that oxytocin facilitates trust by reducing betrayal aversion [17–19], while others claim oxytocin modulates factors that improve how people cope with betrayal aversion [32]. The effect of oxytocin on insula activity seems to be context dependent, leading to different effects on behaviour. Riem et al. [33] have shown, for example that intranasal application of oxytocin increases insula activity elicited by infant crying. This might be related to increased responsiveness to the cries due to stronger aversive emotions. Also, Baumgartner et al. [18] described reduced insula activity after feedback in a trust game situation after oxytocin administration in comparison with placebo. The reduced insula activity in this context, together with decreased activity in the amygdala and other areas, could lead to a decrease in learning of betrayed trust and therefore less betrayal aversion. It might be very useful to combine endocrinological, pharmacological and imaging methods to further investigate the interaction of oxytocin, betrayal aversion and trust.
Another very interesting aspect of our results is the difference between genders. In our sample, women seem to be less affected by betrayal aversion than men. There might be many underlying reasons for this, which are also found in various other trust-related domains [34]. One very recent study showed that women with a history of substance abuse showed a decreased insula volume compared with men [35]. Strong evidence also exists for differential gender effects of oxytocin in animals as well as humans. This may at least partly explain differences in betrayal aversion between genders [36,37]. Future investigations of these gender differences may be fruitful.
The neural underpinnings of betrayal aversion we have uncovered imply that institutions that allow people to avoid knowing they have been betrayed should hold an advantage over those that do not. In particular, institutions that shield one from knowing they have been betrayed would be expected to reduce the level of unpleasant arousal associated with the decision-making process. This could be especially important in environments characterized by social dilemmas [38,39], and may also explain the widespread use of impersonal, institution-mediated exchange systems [7]. In particular, these institutions may be a response to the preference to trade in environments that mitigate negative emotions related to betrayal.
Impersonal and anonymous communication via the Internet, used for economic and social interaction with increasing regularity, might also mitigate adverse effects of betrayal aversion. At the same time, anonymous online interactions can carry significant risks [5]. Developing a better understanding of the economic costs and benefits of anonymous online interaction may be an especially profitable direction for future research [40,41].
4. Material and methods
(a). Subjects
Sixty participants were recruited, half of them at the Interdisciplinary Center for Economic Science at the George Mason University in Arlington, Virginia, USA. They made their decisions at a computer terminal. The other 30 participants (15 males and 15 females) were recruited at the University of Bonn (Germany) and made their decisions in an fMRI scanner. Their age ranged from 18 to 40 years with a mean of 25.2 and a standard deviation of 3.78 years. All participants were healthy and had no neurological or psychiatric diseases. All subjects were right-handed according to the Edinburgh Handedness Scale and provided written informed consent before the study. Prior to the task, participants were given written instructions about the experiment and two questionnaires—the Machiavellianism IV scale (Mach-IV) and the Consideration of Future Consequences (CFC). Two subjects had to be excluded from the neural analysis because they chose the same option on every trial.
(b). Task
Subjects performed 82 trials of a one-shot binary trust game described in figure 1, previously shown by Aimone & Houser [5–7] to capture betrayal aversion. The software Presentation (Neurobehavioural Systems, San Francisco, CA) was used for stimulus presentation. For each trial, three different screens were shown, the first one with information about the counterpart and the different amounts of money both the investor and trustee could earn (option-screen), the second with the decision-screen showing which button subjects had to push for which option. A fixation-screen followed. Participants in Bonn played 82 rounds in the investor role; participants in Arlington played 41 rounds in the trustee role. Participants in Bonn (in Room A) were first told that they were playing with a human counterpart in another room (Room B) who had previously made their decisions and were awaiting the Room A player's decisions. The trustees’ decisions had been elicited before in Arlington. The trustees’ decisions were matched during the experiment with answers of the investors in Bonn. The results contained decisions for 41 rounds which were used for the 41 human trials in Bonn. For the other 41 trials, investor subjects were informed that the trustee decisions would be randomly drawn (without replacement) by a computer from the actual human choices made by the Room A counterparts. There were 41 different endowment pay-off amounts shown on the screen, appearing in both environments, which ranged from 3 EUR to 7 EUR in steps of 0.10 EUR. Before the experiment, participants received written instructions describing the different options the investors could choose. They also received the instructions of their counterparts in Arlington. In addition, investors had to draw lots for the number of the trustee whom they played with. In the scanner, they completed two sample trials to ensure comprehension of the instructions. To choose between the two options, participants got response grips (Nordic Neurolab, Bergen, Norway) with two buttons for every hand. At the end of the experiment, participants (in both Arlington and Bonn) were paid according to their decision in one randomly chosen trial from the 82 Room A decisions. Ten EUR (seven US Dollars) were additionally paid in Bonn (Arlington) for participating in the experiment as show-up fee.
(c). fMRI data acquisition
Scanning was performed on a 3.0 T Trio Scanner (Siemens) using a standard 8-channel head coil. Slices were in axial orientation in line with the AC–PC direction and covered all of the brain, including the midbrain. T2*-weighted echo planar images with a time to repeat (TR) of 2500 ms and a time to echo (TE) of 45 ms were acquired. Images were taken of slices of the brain with a thickness of 3 mm, and a 64 × 64 matrix was used. The resulting scanning time was approximately 25 min with a number of approximately 400 scans.
(d). fMRI data preprocessing
Analysis of the fMRI data were performed by using statistical parametric mapping (SPM v. 8; www.fil.ion.ucl.ac.uk). For preprocessing, the functional images were realigned to the first image of the first session of each time series and again realigned to the mean image after first realignment. Images were then slice-time corrected, normalized to the canonical EPI template used in SPM8 and smoothed with an 8 mm Gaussian kernel. After normalization images were resampled to a voxel size of 3 × 3 × 3 mm.
(e). fMRI model
For first-level analysis, one general linear model was designed for every subject with the factors counterpart (with two levels: human and computer) and options (also with two levels: Option 1 and Option 2). For the second-level analysis, a full factorial model was created. In this way, differences in brain activity in the two environments and for the two options could be identified. Regions were anatomically identified using the WFU Pickatlas (Functional MRI Laboratory at the Wake Forest University School of Medicine, NC). For further analysis, subjects were divided into different groups: (i) male/female, and (ii) BA/NBA to investigate differences in the brain activity between gender and participants that showed BA behaviour or not.
Acknowledgements
For valuable comments, the authors thank Ming Hsu, Soo Hong Chew, Richard Ebstein, Lusha Zhu, Nina Laharatanahirun and Brooks King-Casas, as well as seminar participants at National University of Singapore, Society for Neuroeconomics 2011.
Funding statement
This research was supported by grants from the International Foundation for Research in Experimental Economics (to D.H.), The Mercatus Center, National Science Foundation Research Grant SES-0851250 (to J.A.A), The National Institutes of Health LRP (NIMH) (to J.A.A.), and the Deutsche Forschungsgemeinschaft (DFG) with a Heisenberg grant (WE 4427/3-1) (to B.W.)
References
- 1.Fehr E. 2009. On the economics and biology of trust. J. Eur. Econ. Assoc. 7, 235–266 (doi:10.1162/JEEA.2009.7.2-3.235) [Google Scholar]
- 2.Bohnet I, Zeckhauser R. 2004. Trust, risk and betrayal. J. Econ. Behav. Organ. 55, 467–484 (doi:10.1016/j.jebo.2003.11.004) [Google Scholar]
- 3.Bohnet I, Grieg F, Herrmann B, Zeckhauser R. 2008. Betrayal aversion: evidence from Brazil, China, Oman, Switzerland, Turkey, and the United States. Am. Econ. Rev. 98, 294–310 (doi:10.1257/aer.98.1.294) [Google Scholar]
- 4.Houser D, Schunk D, Winter J. 2010. Distinguishing trust from risk: an anatomy of the investment game. J. Econ. Behav. Organ. 74, 72–81 (doi:10.1016/j.jebo.2010.01.002) [Google Scholar]
- 5.Aimone JA, Houser D. 2011. Beneficial betrayal aversion. PLoS ONE 6, e17725 (doi:10.1371/journal.pone.0017725) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aimone JA, Houser D. 2012. What you don't know won't hurt you: a laboratory analysis of betrayal aversion. Exp. Econ. 15, 571–588 (doi:10.1007/s10683-012-9314-z) [Google Scholar]
- 7.Aimone JA, Houser D. 2013. Harnessing the benefits of betrayal aversion. J. Econ. Behav. Organ. 89, 1–8 (doi:10.1016/j.jebo.2013.02.001) [Google Scholar]
- 8.McCabe K, Houser D, Ryan L, Smith V, Trouard T. 2001. A functional imagin study of cooperation in two-person reciprocal exchange. Proc. Natl Acad. Sci. USA 98, 11 832–11 835 (doi:10.1073/pnas.211415698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King-Casas B, Tomlin D, Anen C, Camerer CF, Quartz SR, Montague PR. 2005. Getting to know you: reputation and trust in a two-person economic exchange. Science 308, 78–83 (doi:10.1126/science.1108062) [DOI] [PubMed] [Google Scholar]
- 10.King-Casas B, Sharp C, Lomax-Bream L, Lohrenz T, Fonagy P, Montague PR. 2008. The rupture and repair of cooperation in borderline personality disorder. Science 321, 806–810 (doi:10.1126/science.1156902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riedl R, Javor A. 2012. The biology of trust: integrating evidence from genetics, endocrinology, and functional brain imaging. J. Neurosci. Psychol. Econ. 5, 63–91 (doi:10.1037/a0026318) [Google Scholar]
- 12.Huettel SA, Stowe CJ, Gordon EM, Warner BT, Platt ML. 2006. Neural signatures of economic preferences for risk and ambiguity. Neuron 49, 765–775 (doi:10.1016/j.neuron.2006.01.024) [DOI] [PubMed] [Google Scholar]
- 13.Huettel SA, Platt ML. 2008. Risky business: the neuroscience of decision making under uncertainty. Nat. Neurosci. 11, 398–403 (doi:10.1038/nn2062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christopoulos GI, Tobler PN, Bossaerts P, Dolan RJ, Schultz W. 2009. Neural correlates of value, risk, and risk aversion contributing to decision making under risk. J. Neurosci. 29, 12 574–12 583 (doi:10.1523/JNEUROSCI.2614-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudorf S, Preuschoff K, Weber B. 2012. Neural correlates of anticipation risk reflect risk preferences. J. Neurosci. 32, 16 683–16 692 (doi:10.1523/JNEUROSCI.4235-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu CC, Sacchet MD, Knutson B. 2012. Toward an affective neuroscience account of financial risk taking. Front. Neurosci. 6, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. 2005. Oxytocin increases trust in humans. Nature 435, 673–676 (doi:10.1038/nature03701) [DOI] [PubMed] [Google Scholar]
- 18.Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. 2008. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron 58, 639–650 (doi:10.1016/j.neuron.2008.04.009) [DOI] [PubMed] [Google Scholar]
- 19.DeDreu CKW. 2012. Oxytocin modulates the link between adult attachment and cooperation through reduced betrayal aversion. Psychoneuroendocrinology 37, 871–880 (doi:10.1016/j.psyneuen.2011.10.003) [DOI] [PubMed] [Google Scholar]
- 20.Javor A, Koller M, Lee N, Chamberlain L, Ransmayr G. 2013. Neuromarketing and consumer neuroscience: contributions to neurology. BMC Neurol. 13, 13 (doi:10.1186/1471-2377-13-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glimcher P, Fehr E. (eds). 2014. Neuroeconomics: decision making and the brain, 2nd edn, pp. 19–34 New York, NY: Academic Press [Google Scholar]
- 22.Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer C. 2005. Neural systems responding to degrees of uncertainty in human decision-making. Science 310, 1680–1683 (doi:10.1126/science.1115327) [DOI] [PubMed] [Google Scholar]
- 23.Bach DR, Seymour B, Dolan RJ. 2009. Neural activity associated with the passive prediction of ambiguity and risk for aversive events. J. Neurosci. 29, 1648–1656 (doi:10.1523/JNEUROSCI.4578-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauharatanahirun N, Christopoulos GI, King-Casas B. 2012. Neural computations underlying social risk sensitivity . Front. Hum. Neurosci. 6, 213 (doi:10.3389/fnhum.2012.00213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Honk J, Eiseneggerc C, Terburgab D, Steinb DJ, Morgane B. 2013. Generous economic investments after basolateral amygdala damage. Proc. Natl Acad. Sci. USA 110, 2506–2510 (doi:10.1073/pnas.1217316110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winston JS, Strange BA, O'Doherty J, Dolan RJ. 2002. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat. Neurosci. 5, 277–283 (doi:10.1038/nn816) [DOI] [PubMed] [Google Scholar]
- 27.Delgado M, Nearing K, Ledoux J, Phelps E. 2008. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron, 59, 829–838 (doi:10.1016/j.neuron.2008.06.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhnen CM, Knutson B. 2005. The neural basis of financial risk taking. Neuron 47, 763–770 (doi:10.1016/j.neuron.2005.08.008) [DOI] [PubMed] [Google Scholar]
- 29.Lamm C, Singer T. 2010. The role of anterior insular cortex in social emotions. Brain Struct. Funct. 214, 579–591 (doi:10.1007/s00429-010-0251-3) [DOI] [PubMed] [Google Scholar]
- 30.Singer T, Lamm C. 2009. The social neuroscience of empathy. Ann. NY Acad. Sci. 1156, 81–96 (doi:10.1111/j.1749-6632.2009.04418.x) [DOI] [PubMed] [Google Scholar]
- 31.Aimone JA, Ball S, King-Casas B. 2014. Anxiety, risk preferences, betrayal aversion, and the growth of interpersonal trust. See http://ssrn.com/abstract=2402413 [Google Scholar]
- 32.Bartz J. 2012. Oxytocin, attachment, betrayal and self-interest: a commentary on ‘Oxytocin modulates the link between adult attachment and cooperation through reduced betrayal aversion’. Psychoneuroendocrinology 37, 1106–1110 (doi:10.1016/j.psyneuen.2012.03.003) [DOI] [PubMed] [Google Scholar]
- 33.Riem MM, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MA, Vermeiren RR, Rombouts SA. 2011. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: a randomized controlled trial. Biol. Psychiatry 70, 291–297 (doi:10.1016/j.biopsych.2011.02.006) [DOI] [PubMed] [Google Scholar]
- 34.Riedl R, Hubert M, Kenning P. 2010. Are there neural gender differences in online trust? An fMRI study on the perceived trustworthiness of eBay offers. MIS Q. 34, 397–428 [Google Scholar]
- 35.Tanabe J, York P, Krmpotich T, Miller D, Dalwani M, Sakai JT, Rojas DC. 2013. Insula and orbitofrontal cortical morphology in substance dependence is modulated by sex. Am. J. Neuroradiol. 34, 1150–1156 (doi:10.3174/ajnr.A3347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Francis DD, Young LJ, Meaney MJ, Insel TR. 2002. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. J. Neuroendocrinol. 14, 349–353 (doi:10.1046/j.0007-1331.2002.00776.x) [DOI] [PubMed] [Google Scholar]
- 37.Barraza JA, Zak PJ. 2009. Empathy toward strangers triggers oxytocin release and subsequent generosity. Ann. NY Acad. Sci. 1167, 182–189 (doi:10.1111/j.1749-6632.2009.04504.x) [DOI] [PubMed] [Google Scholar]
- 38.Isaac M, McCue K, Plott C. 1985. Public goods provision in an experimental environment. J. Public Econ. 26, 51–74 (doi:10.1016/0047-2727(85)90038-6) [Google Scholar]
- 39.Houser D, Kurzban R. 2002. Revisiting kindness and confusion in public goods experiments. Am. Econ. Rev. 92, 1062–1069 (doi:10.1257/00028280260344605) [Google Scholar]
- 40.Dimoka A. 2010. What does the brain tell us about trust and distrust? Evidence from a functional neuroimaging study. MIS Q. 34, 373–396 [Google Scholar]
- 41.Riedl R, Mohr P, Kenning P, Davis F, Heekeren H. 2011. Trusting humans and avatars: behavioral and neural evidence. ICIS 2011 Proc., Paper 7. See http://aisel.aisnet.org/icis2011/proceedings/hci/7 [Google Scholar]


