Abstract
Despite the diversity of mammalian life histories, persistent patterns of covariation have been identified, such as the ‘fast–slow’ axis of life-history covariation. Smaller species generally exhibit ‘faster’ life histories, developing and reproducing rapidly, but dying young. Hormonal mechanisms with pleiotropic effects may mediate such broad patterns of life-history variation. Insulin-like growth factor 1 (IGF-1) is one such mechanism because heightened IGF-1 activity is related to traits associated with faster life histories, such as increased growth and reproduction, but decreased lifespan. Using comparative methods, we show that among 41 mammalian species, increased plasma IGF-1 concentrations are associated with fast life histories and altricial reproductive patterns. Interspecific path analyses show that the effects of IGF-1 on these broad patterns of life-history variation are through its direct effects on some individual life-history traits (adult body size, growth rate, basal metabolic rate) and through its indirect effects on the remaining life-history traits. Our results suggest that the role of IGF-1 as a mechanism mediating life-history variation is conserved over the evolutionary time period defining mammalian diversification, that hormone–trait linkages can evolve as a unit, and that suites of life-history traits could be adjusted in response to selection through changes in plasma IGF-1.
Keywords: insulin-like growth factor-1, mammals, life history, comparative analysis, fast–slow axis
1. Introduction
Life-history traits describe an organism's rate of development and reproduction, and are therefore intrinsically linked to fitness. Such traits vary greatly among and within species, yet persistent patterns of covariation among these traits have been widely documented [1,2]. These patterns of covariation are often referred to as ‘axes’ along which multiple life-history traits vary simultaneously. The most commonly described axis of life-history variation is referred to as the ‘fast–slow’ axis [1,2]. Species at the fast end of the axis generally are small, grow rapidly, mature early and reproduce rapidly, but die young [2,3]. A fast–slow axis persists after correcting for body mass [2,4], suggesting that the fast–slow axis is not simply a reflection of body mass, but of some variable that body mass and the mass-corrected fast–slow axis are both related to, possibly extrinsic mortality rates [1].
Mammals also exhibit other suites of covarying life-history traits that remain after accounting for the fast–slow life-history axis. For example, the iteroparity-semelparity gradient [2] is thought to be associated with the relative dispersion of lifetime reproductive effort across the lifespan [4,5]. At the iteroparous end, species produce a few precocial young distributed evenly over their lifetime, which pass rapidly through the juvenile period owing to intensive parental investment, whereas the opposite is true of species on the semelparous end. A similar, but not identical, ‘altricial–precocial’ axis has been suggested among mammals as well, which is generally defined by the degree of neonate independence, neonate mass, or gestation length relative to mass or to mass and the fast–slow axis [6]. Species at the altricial end of this continuum produce large litters of small offspring that have short gestation periods and grow more rapidly, whereas the opposite is true of species at the precocial end.
Given the close ties between life-history variation and fitness, it is important to understand the processes that create and maintain variation in these traits. Most research has focused on adaptive hypotheses that consider the energetic and ecological trade-offs that result in different life-history strategies [1–3,7,8]. Recently, the hypothesis that physiological mediators of life-history traits may constrain life-history variation has been considered [9,10]. Hormones have been proposed to play an important role by simultaneously regulating multiple life-history traits [9–12]. For example, testosterone influences both mating and parental effort, but in an opposing manner [10]. Although the effects of hormones on life-history variation have been well studied within species, we know less about the implications of hormonal pleiotropy for life-history variation across species. Because an adjustment in plasma hormones may affect all life-history traits in a uniform way, those traits sharing a common mechanism might not evolve independently and therefore constrain patterns of life-history variation [9–12]. However, trait expression is influenced not only by plasma hormones, but also by other factors [13], such as the sensitivity of target tissues to the hormone [14], which suggests an alternative route for the evolution of physiological systems that allows more independent changes in specific traits. Consequently, if selection on life-history traits affects endocrine traits (e.g. hormone receptor expression) other than by changing plasma hormones, multiple life-history traits affected by a single hormone may evolve independently and not constrain patterns of life-history variation [9,10].
Comparative techniques can be used to test hypotheses about how hormones affect the evolution of life-history traits. If the relationship between hormones and life-history traits is similar within and among species, it suggests that the role of plasma hormones does not change over evolutionary timescales. As such, life-history traits regulated by the hormone in question will to some extent evolve in a concerted fashion. We refer to this as The ‘ECHoEs’ (evolutionary conservation of hormone effects) hypothesis, which proposes that interspecific relationships ‘echo’ those among individuals within species (figure 1). The ECHoEs hypothesis predicts that we should find a continuous relationship among species between plasma hormones and the phenotypes that it regulates, though only the strictest interpretation would suggest a linear relationship (figure 1). Support for the ECHoEs hypothesis would suggest that concentrations of a hormone evolve much more rapidly than the function of the hormone, as well as the relevant sensitivities in target tissues. Alternatively, if among-species relationships between life-history traits and hormones differ from those found in intraspecific studies, this suggests that hormones do not constrain the independent evolution of the multiple traits that they mediate (‘No ECHoEs’ hypothesis; figure 1). Our hypotheses are related to earlier ideas proposed by Hau [10] and by Ketterson et al. [9]. The ECHoEs hypothesis is similar to Hau's ‘evolutionary constraint’ or Ketterson et al.'s ‘phenotypic integration’ hypotheses, in which the pleiotropic functions of hormones promote phenotypic integration. Phenotypic integration between hormones and multiple traits could reduce the mutability of complex phenotypes, although like Ketterson et al. [9] we suggest this could also facilitate multivariate adaptation. Alternatively, the No ECHoEs hypothesis is similar to Ketterson's ‘phenotypic independence’ or Hau's ‘evolutionary potential’ in which the relationship between a hormone and a trait can change through other factors such as the evolution of differential tissue sensitivity to the hormone [13,14]. We are not proposing to replace these previous hypotheses [9,10], but rather we focus on whether interspecific relationships between hormones and life-history traits reflect those observed within species. Support for the ECHoEs hypothesis would suggest that fairly extensive multivariate adaptation in life-history parameters could occur simply through alteration of circulating concentrations of one hormone.
Figure 1.
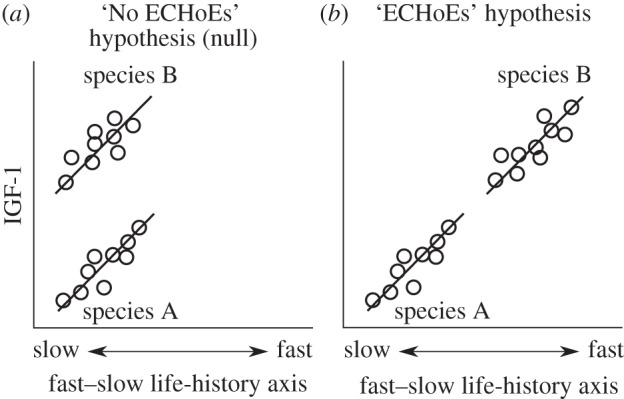
Two hypotheses for the evolution of phenotypic integration between circulating concentrations of hormones and the phenotypic traits that they mediate. As phenotypes evolve, (a) the patterns observed among species may be different from those observed within species or (b) the underlying physiological traits may evolve together with the traits they mediate such that interspecific correlations resemble intraspecific patterns. Each hypothesis makes different predictions about the nature of long-term evolution. (a) The ‘No ECHoEs’ hypothesis predicts that the relationship between a hormone and a trait is largely modified by other features than just a change in the circulating concentrations of hormones (e.g. hormone receptor expression in target tissues [13,14]). (b) The ‘ECHoEs’ hypothesis predicts that circulating concentrations of hormones evolve in a continuous ordinal manner with the traits they affect.
We tested the ECHoEs hypothesis by examining whether concentrations of the polypeptide hormone insulin-like growth factor-1 (IGF-1) explains life-history variation across Mammalia in a similar manner to that in which it does within species. We focused on the insulin/insulin-like growth factor pathway because it influences quantitative variation in life histories in diverse taxa through a variety of mechanisms. For example, in invertebrate organisms in which the associated mechanisms are best understood, increased insulin signalling inhibits forkhead box (FOXO) activity, which increases cellular growth and division, thereby enhancing somatic growth [15] but simultaneously decreasing longevity [16]. In laboratory studies in mammals, high plasma concentrations of IGF-1 or elevated IGF-1 receptor activity has mitogenic effects, and is associated with increased postnatal growth or adult body size and heightened reproductive output, but decreased lifespan [11]. IGF-1 may also influence mammalian life-history traits by mobilizing energy reserves or macronutrient stores [17], thereby mediating the energetic burden of lactation or other forms of parental care [18,19]. Although steroid hormones (glucocorticoids and androgens) may also affect life-history traits, IGF-1 could in some cases act as an intermediary mechanism [11].
We examined whether IGF-1 operates as a pleiotropic mediator of correlated suites of life-history traits across Mammalia using a comparative phylogenetic approach. Based on results from previous intraspecific studies [11,20], we predicted that species with high plasma IGF-1 concentrations would exhibit fast life histories. Although mammalian species at the altricial end of the altricial–precocial continuum may produce many small offspring with shorter prenatal and postnatal periods of parental investment, species with altricial young may actually invest more heavily in the growth of their offspring relative to the prenatal or postnatal periods of development than precocial species [9]. Because increases in plasma IGF-1 concentrations might increase reproductive effort [11], we therefore predicted that species exhibiting more altricial traits should exhibit increased IGF-1 concentrations. We then used interspecific path analyses to determine the direct and indirect effects that IGF-1 has on the individual life-history traits that comprise the fast–slow axis of life-history variation.
2. Material and methods
(a). Data collection and phylogeny acquisition
We collected life-history data and total plasma IGF-1 concentrations in 41 mammalian species from a variety of sources published up to January 2013 (see the electronic supplementary material). Life-history traits included in our analysis were body mass, age at female sexual maturity, gestation length, neonate mass, litter size, maximum lifespan and age at weaning. For phylogenetic analyses, we used a recent comprehensive mammalian phylogeny (electronic supplementary material, figure S1 [21]. Phylogeny manipulations were conducted in Mesquite [22] and R v. 2.13.0 [23] using the packages ‘ape’ [24], ‘picante’ [25] and ‘geiger’ [26]. All statistical analyses were conducted in R v. 2.13.0 [23].
(b). Phylogenetically corrected PCA
We log-transformed the life-history data before performing principal component analysis (PCA) because allometric relationships between life-history traits are generally log-linear [27]. We used phylogenetically corrected PCA [28] to describe multivariate axes of life-history variation for the life-history traits. We included body mass in our PCA rather than using mass-corrected life-history traits because the important growth-promoting activities of IGF-1 means that as it influences other life-history traits, it will invariably influence body size. Thus, the biological interpretation of how IGF-1 would change other life-history traits without changing body size is not clear. We calculated PC scores for the first three PC axes from this PCA and used these scores as variables in further analyses.
(c). Phylogenetically corrected regressions
We assessed the relationship between plasma IGF-1 and the first three PC axes by performing two multiple regressions with IGF-1 as the response and the first three PC axes as predictors [29,30] (see electronic supplementary material for details). For the first, we allowed λ to take its maximum-likelihood estimate (MLE). For the second, we fixed λ to 0, signifying no effect of phylogeny, equivalent to a standard regression. We then estimated Akaike's information criterion for each model, corrected for sample size (AICc). Allowing λ to take its MLE did not improve model fit (δAICc = 2.1; electronic supplementary material). Thus, we present results from the model with λ fixed to 0 (no phylogenetic signal), similar to previous comparative studies [31,32].
(d). Interspecific path analyses for IGF-1
Testing for a relationship between IGF-1 and multivariate life-history patterns can provide a test for the ECHoEs hypothesis, but it does not fully address the question of whether specific life-history traits themselves are directly affected by IGF-1 or relate to IGF-1 as a result of their relationship with the axes of life-history variation. We used interspecific path analysis to assess the direct and indirect effects IGF-1 has on specific life-history traits. We estimated 16 simple interspecific path models, each incorporating a univariate life-history variable, PC1 (fast–slow axis) and IGF-1 concentration. We used PC1 for these path analyses, but not PC2 or PC3, because PC1 explains the majority of variation among life-history traits (table 1). Each univariate life-history variable is included in two of the 16 path models (electronic supplementary material, table S1). One model associated with each variable depicts a direct link between IGF-1 and the variable, and the other suggests that IGF-1 and the life-history trait are related indirectly, owing to the life-history traits correlating to the fast–slow axis. We estimated goodness-of-fit measures for these path models using Shipley's d-sep method [33,34] with the appropriate PGLS models [35]. The goodness-of-fit standard for the d-sep test is similar to AICc, but following von Hardenberg & Gonzalez-Voyer [35] we refer to it as CICc. We included all life-history traits used in the calculation of PC1, as well as relative growth rate estimated from mechanistic Gompertz growth models and Basal metabolic rate (BMR), which necessitated a reduction in sample size to 26 species for the path models. We included these traits because there is strong evidence a priori that IGF-1 directly affects the quantitative values of these life-history traits within species [11].
Table 1.
Loadings for phylogenetically corrected PCA on life-history variables and the percentage of life-history variation explained by the three PC axes.
| life history traits | PC1 | PC2 | PC3 |
|---|---|---|---|
| % variance exp. | 66.5 | 11.6 | 8.6 |
| mass | −0.831 | −0.434 | −0.045 |
| maximum lifespan | −0.812 | −0.195 | 0.082 |
| neonate mass | −0.925 | −0.148 | 0.161 |
| age at female sex. mat. | −0.857 | −0.070 | −0.266 |
| litter size | 0.660 | −0.690 | −0.201 |
| gestation length | −0.862 | 0.060 | 0.382 |
| lactation length | −0.733 | 0.284 | −0.562 |
3. Results
The mean IGF-1 concentration was 331 (mean) ± 223 (1 standard deviation (s.d.)) ng ml−1, with muskoxen (Ovibos moschatus; 30 ng ml−1) having the lowest measured IGF-1 concentration included in our analysis and the brown rat (Rattus norvegicus; 975 ng ml−1) having the highest. The mean body mass was 255 ± 595 kg. The heaviest animal included in our sample was the Indian elephant (Elephas maximus; 3180 kg), while the lightest was the house mouse (Mus musculus; 0.023 kg). Mean values for other life-history traits included 31.8 ± 20.3 years (lifespan), 9.40 ± 19.4 kg (neonate mass), 1020 ± 945 days (age at female sexual maturity), 189 ± 127 days (gestation length), 222 ± 198 days (lactation length), 94.5 ± 108 (basal metabolic rate) and 0.0113 ± 0.0122 day−1 (relative postnatal growth rate).
The first axis (PC1) recovered from the PCA was a fast–slow life-history axis in which body mass, lifespan, neonate mass, age at female sexual maturity, gestation and lactation length all load negatively, and litter size loads positively (PC1 in table 1). The second PC axis (PC2) represents a continuum that ranges from species with low adult body mass that produce small litters of small offspring whose prenatal (gestation length) and postnatal (lactation length) developmental periods are protracted despite a shorter lifespan to species with high adult body mass that produce large litters of large offspring with fairly short prenatal and postnatal developmental periods with respect to their longer lifespan (table 1). Generally, larger species have smaller litters, and this is captured in PC1, whereas PC2 captures the variation among species that do not fit this pattern. The third PCA axis (PC3) corresponds to an altricial–precocial axis. PC3 describes a transition from species that produce large litters of offspring that are small at birth and experience a short gestation period but experience an extended postnatal developmental period (specifically extended lactation length and later age at female sexual maturation) to species that produce small litters of precocial offspring that are large at birth and experience an extended gestation period but shortened period of postnatal development (short lactation length and earlier age at female sexual maturation; table 1).
Mammals (such as rodents) with higher plasma IGF-1 also exhibit faster life histories, whereas species such as the Indian elephant and most artiodactylid and perissodactylid species with lower plasma IGF-1 exhibit slower life histories (figure 2). Species with higher IGF-1 are smaller as adults, have an earlier age of female sexual maturity, produce larger litters of smaller offspring with shorter prenatal and postnatal periods of development, and have a shorter lifespan (figure 2). IGF-1 also increased with PC2 (figure 2), such that mammals with high IGF-1 (e.g. primates) produce fewer and smaller offspring that experience protracted prenatal and postnatal periods of development relative to their short lifespan (PC2 in figure 2). Finally, IGF-1 was associated with the altricial–precocial axis (PC3), though not significantly (p = 0.057). At a given position along the fast–slow axis, mammals such as rodents and primates with higher IGF-1 produced more but smaller offspring that experience short gestation periods but extended postnatal development (extended lactation length and delayed age of female sexual maturation; figure 2).
Figure 2.
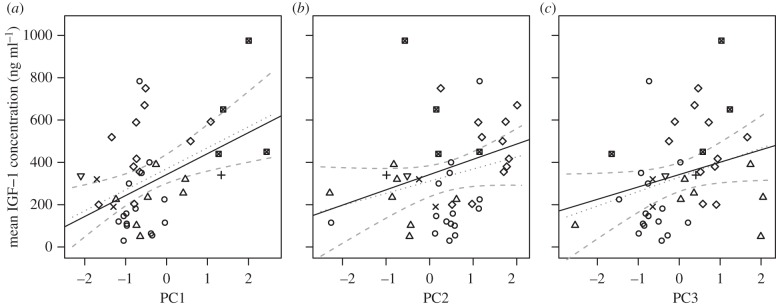
Regression plots of the relationship (±s.e.) of IGF-1 concentrations with (a) PC1 (β = 99.05 ± 29.85, t = 3.32, p = 0.002), (b) PC2 (β = 72.17 ± 29.6, t = 2.44, p = 0.020) and (c) PC3 (β = 58.48, ±29.71, t = 1.97, p = 0.057). PC axes are scaled by their standard deviations. Solid black lines represent best-fit lines from the phylogenetic multiple regression. Dotted grey lines represent best-fit lines from standard univariate regressions, and dashed lines 95% CIs from the same univariate regressions. R2 for the full model = 0.360. Symbols used: circles, artiodactyls; plus symbols, lagomorphs; inverted triangles, afrotheres; triangles, carnivorans; crosses, perissodactyls; diamonds, primates; crossed squares, rodents.
The ‘best’-fitting path models for each univariate life-history trait varied. IGF-1 appears to be more closely related to adult body mass, BMR and relative growth rate compared with the fast–slow life-history axis itself (figure 3; electronic supplementary material, table S1). This suggests that changes in plasma IGF-1 directly affect these single life-history traits and subsequently influence the placement of the species on the fast–slow life-history axis. For the six other life-history traits (lifespan, neonate mass, lactation length, age at female sexual maturity, litter size, gestation length), IGF-1 is more closely related to the fast–slow life-history axis itself (figure 3; electronic supplementary material, table S1), which suggests that IGF-1 has indirect effects on these individual life-history traits through its influence of the placement of the species on the fast–slow life-history axis. It is important to recognize that for some of these models, the dCICc is not greater than 2, which is generally considered to represent some evidence that the models are recognizably different. Thus, there is not a dichotomy here, but rather a range of confidences that a specific life-history trait is directly or indirectly tied to IGF-1.
Figure 3.
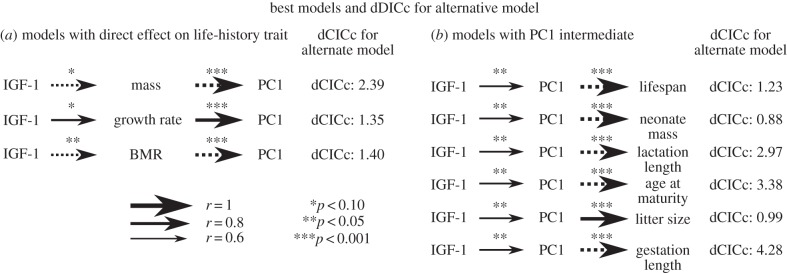
(a,b) Best path models for each life-history variable (dCICc = 0). Each variable only has two possible models: one with IGF-1 predicting PC1, and through PC1 the life-history variable, and the other with IGF-1 predicting the life-history variable, and PC1 through the life-history variable. dCICc values given to the right of each row belong to the alternative model (shown in electronic supplementary material, table S1 and figure S2). Number of asterisks represents statistical significance. Solid arrows connecting traits denote a positive relationship, and dashed lines denote a negative relationship. Arrow width is proportional to the absolute value of the correlation coefficient (r) for the model.
4. Discussion
Within mammalian species, increased plasma IGF-1 concentrations are associated with increased growth, rapid development and increased reproduction, but reduced lifespan (reviewed in [11]). We show that similar patterns hold across diverse mammalian species. Mammalian species with higher IGF-1 concentrations exhibit faster life histories and more altricial patterns of reproduction characterized by rapid development, small body size, the production of many but smaller offspring, reduced prenatal and postnatal periods of investment in offspring, and a short lifespan (table 1 and figure 2).
Our results support the ‘ECHoEs’ hypothesis (figure 1), suggesting that hormone–trait linkages may evolve as a unit and the values of suites of life-history traits can therefore be adjusted in response to selection through increases or decreases in plasma IGF-1 concentrations. The nature of the relationship between hormones and the traits they influence as they evolve has important consequences for whether the vertebrate neuroendocrine system constrains or facilitates evolutionary change [9–11,36,37]. Owing to their pleiotropic effects, hormones can promote phenotypic integration [9,38], but could also constrain the rate of evolutionary change if selection is orthogonal to the greatest axes of covariance among life-history traits produced by that hormone. Our results suggest that IGF-1 concentrations can be used to predict life-history patterns across mammalian taxa, and that the functional significance of increasing IGF-1 increases linearly with log-transformed life-history traits (‘ECHoEs’ hypothesis in figure 1). The systematic and linear/log-linear nature of these relationships across Mammalia suggests that the responsiveness of life-history traits to important hormones must evolve to some extent concomitantly with circulating hormones. In contrast to the prediction of Ketterson et al. [9], our results suggest that the integration of multiple life-history traits promoted by hormones, at least for IGF-1, persists through the formation of new species. Although future studies must assess the role of other components of the IGF-1 axis (receptors, binding proteins, etc.) and the validity of this hypothesis for other hormones, our results provide initial support for the ‘ECHoEs’ hypothesis (figure 1) that consistent intraspecific patterns of hormone–trait correlations translate into interspecific correlations across larger taxonomic groups.
Model comparisons among the interspecific path models we estimated are consistent with the hypothesis that IGF-1 in mammals directly affect some individual life-history traits, such as adult body size, growth rate and basal metabolic rate, and these variables then relate to the fast–slow life-history axis (figure 3a). Nevertheless, other individual life-history traits appear to be indirectly affected by IGF-1 owing to their correlation with other life-history traits in the form of the fast–slow axis, rather than by a direct link (figure 3b). The results from our multiple regression and interspecific path analyses may appear to contradict one another at first, but because the relationship between a phenotypic trait and plasma hormones is complex [13,14], it is not surprising that model selection techniques would indicate variation in the goodness of fit between IGF-1 and different life-history traits. The purpose of the path analyses was to establish the relative goodness of fit of the relationships between IGF-1 and the various life-history traits we studied, and identify possible IGF-1/life-history linkages that seem to evolve in an integrated manner. Life-history traits that are indirectly linked to IGF-1, but still correlated, may reach their observed values by evolving to new optima that are related to traits directly mediated by IGF-1, such as body size, which is often thought to be central to the fast–slow axis. Other life-history traits probably attain their observed values as a direct result of ‘primary’ life-history variation (e.g. age at maturity may be heavily dependent upon postnatal growth rate), or their quantitative values may be primarily influenced by other endocrine mechanisms.
Our results are supported by previous intraspecific studies showing strong mechanistic links between IGF-1 and specific life-history traits. For example, experimentally increasing IGF-1 activity increases mitogenesis in structural or reproductive tissues [11]. Our results for the interspecific path analyses support the hypothesis that IGF-1 directly influences growth rate and body size, perhaps by influencing mitogenesis. By contrast, although IGF-1 appears to play a role in lactation [39], it is not clear that upregulating IGF-1 systemically or for a longer period of time will have a simple additive effect on lactation length within species. Our results from the interspecific path analyses suggest that the relationship between IGF-1 and weaning age or gestation length among species appears indirect. Future studies experimentally manipulating IGF-1 with the implicit goal of understanding its direct and indirect effects of life-history traits would be a valuable addition to our results (reviewed in [11]).
The interspecific relationship between IGF-1 and the fast–slow axis of mammalian life histories that we uncovered largely matches the pattern predicted by intraspecific studies. The relationships between IGF-1 and age at reproductive maturity, growth rate and lifespan generally conform among and within species [11,40]. The inter- and intraspecific associations between litter size, gestation length and IGF-1 appear to be the same, though the evidence for the intraspecific association is sparse [11]. Evidence for a relationship between IGF-1 and gestation length is complicated because fetal size and relative gestational age appear be affected by IGF-1, which can subsequently influence gestation length indirectly [41]. Our results also indicate that species with higher plasma IGF-1 produce smaller offspring, which is supported by some intraspecific studies (reviewed in [11]) but not others [42–44]. The main exception between intra- and interspecific studies is the relationship between adult body mass and IGF-1. Our results confirm those of Stuart & Page [45], who also documented a negative relationship between body mass and IGF-1 among species using a similar set of species but different statistical methods. By contrast, within-species increased IGF-1 is generally associated with increased body mass [11]. Overall, our results indicate that the general relationship between IGF-1 and life-history traits other than body mass within species can be extrapolated to among-species comparisons.
The relationship between IGF-1 and the other multivariate axes of life-history variation (PC2 and PC3) suggest that greater investment in individual young, greater total postnatal investment or greater ‘daily’ or instantaneous parental investment increases with IGF-1. Daily parental investment simply describes the degree of investment in offspring for a given time period, regardless of the total duration of parental care. We found a significant positive relationship between IGF-1 and PC2, which suggests that at a given point along the fast–slow axis, IGF-1 concentrations are higher in species that are small, but for their size produce litters of few small offspring that exhibit fairly protracted prenatal and postnatal development and shorter lifespan. Species exhibiting this set of life-history traits in our sample include many primates, which may invest relatively more per offspring in fewer offspring. Conversely, species on the other end of PC2 with low IGF-1 concentrations include carnivores, rabbits and pigs, which are species that may invest relatively little in individual offspring given their adult body mass. For PC3, species at the altricial end of the altricial–precocial continuum may invest more heavily in their offspring on a per-day basis relative to their shorter prenatal or postnatal periods of development [6], which could be mediated by increases in plasma IGF-1 that increase reproductive effort. We propose that the role of IGF-1 in relation to these axes of life-history variation may be in its ability to mobilize resources or otherwise mediate intensity of parental investment pre- and postnatally.
To date, few studies have measured plasma IGF-1 concentrations. Our analysis is therefore not entirely representative of extant mammalian diversity. For example, ungulates are over-represented in our dataset (41% of the species our analysis) and rodents (7% of species) are underrepresented compared with their abundance in the order Mammalia (ungulates: approx. 9% of extant mammalian species; rodents: approx. 45% of extant species [46]). Although our results should be re-examined when plasma IGF-1 has been measured in more species, our results are comprehensive for mammals for which both IGF-1 and life-history measures are currently available.
Acknowledgements
We thank Kay Holekamp for encouraging us to pursue this research, Austin Dreyer, Dieter Lukas and Emilie Snell-Rood for helpful comments on a previous version of the manuscript, as well as three anonymous reviewers.
Data accessibility
All code and data are accessible in non-proprietary format in the electronic supplementary material.
Funding statement
E.M.S. was supported by a Michigan State University Distinguished fellowship and a National Science Foundation Postdoctoral Research Fellowship in Biology.
References
- 1.Promislow DEL, Harvey PH. 1990. Living fast and dying young: a comparative analysis of life-history variation among mammals. J. Zool. 220, 417–437 (doi:10.1111/j.1469-7998.1990.tb04316.x) [Google Scholar]
- 2.Gaillard J-M, Pontier D, Allaine D, Lebreton J-D, Trouvilliez J, Clobert J. 1989. An analysis of demographic tactics in birds and mammals. Oikos 56, 59–76 (doi:10.2307/3566088) [Google Scholar]
- 3.Harvey PH, Read AF, Promislow DEL. 1989. Life-history variation in placental mammals: unifying data with theory. Oxf. Surv. Evol. Biol. 6, 13–31 [Google Scholar]
- 4.Stearns SC. 1983. The influence of size and phylogeny on patterns of covariation among life-history traits in the mammals. Oikos 41, 173–187 (doi:10.2307/3544261) [Google Scholar]
- 5.Derrickson EM. 1992. Comparative reproductive strategies of altricial and precocial eutherian mammals. Funct. Ecol. 6, 57–65 (doi:10.2307/2389771) [Google Scholar]
- 6.Martin RD, Maclarnon AM. 1985. Gestation period, neonatal size and maternal investment in placental mammals. Nature 314, 220–223 (doi:10.1038/313220a0) [Google Scholar]
- 7.Read AF, Harvey PH. 1989. Life-history differences among the eutherian radiations. J. Zool. 219, 329–353 (doi:10.1111/j.1469-7998.1989.tb02584.x) [Google Scholar]
- 8.Stearns SC. 1991. The evolution of life histories. Oxford, UK: Oxford University Press [Google Scholar]
- 9.Ketterson ED, Atwell JW, McGlothlin JW. 2009. Phenotypic integration and independence: hormones, performance, and response to environmental change. Integr. Comp. Biol. 49, 365–379 (doi:10.1093/icb/icp057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hau M. 2007. Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. BioEssays 29, 133–144 (doi:10.1002/bies.20524) [DOI] [PubMed] [Google Scholar]
- 11.Dantzer B, Swanson EM. 2012. Mediation of vertebrate life histories via insulin-like growth factor-1. Biol. Rev. 87, 414–429 (doi:10.1111/j.1469-185X.2011.00204.x) [DOI] [PubMed] [Google Scholar]
- 12.Ricklefs RE, Wikelski M. 2002. The physiology/life-history nexus. Trends Ecol. Evol. 17, 462–468 (doi:10.1016/S0169-5347(02)02578-8) [Google Scholar]
- 13.Ball GF, Balthazart J. 2008. Individual variation and the endocrine regulation of behaviour and physiology in birds: a cellular/molecular perspective. Phil. Trans. R. Soc. B 363, 1699–1710 (doi:10.1098/rstb.2007.0010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosvall KA, Burns CMB, Barske J, Goodson JL, Schlinger BA, Sengelaub DR, Ketterson ED. 2012. Neural sensitivity to sex steroids predicts individual differences in aggression: implications for behavioural evolution. Proc. R. Soc. B 279, 3547–3555 (doi:10.1098/rspb.2012.0442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang HY, Smith-Caldas MSB, Driscoll MV, Salhadar S, Shingleton AW. 2011. FOXO regulates organ-specific phenotypic plasticity in Drosophila. PLoS Genet. 7, e1002373 (doi:10.1371/journal.pgen.1002373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenyon CJ. 2010. The genetics of ageing. Nature 464, 504–512 (doi:10.1038/nature08980) [DOI] [PubMed] [Google Scholar]
- 17.Poggi C, Lemarchandbrustel Y, Zapf J, Froesch ER, Freychet P. 1979. Effects and binding of insulin-like growth factor-1 in the isolated soleus muscle of lean and obese mice: comparison with insulin. Endocrinology 105, 723–730 (doi:10.1210/endo-105-3-723) [DOI] [PubMed] [Google Scholar]
- 18.Travers MT, Madon RJ, Vallance AJ, Barber MC. 1990. Circulating concentrations and hepatic expression of IGF-1 during pregnancy and lactation in the mouse. Biochem. Soc. Trans. 18, 1268. [DOI] [PubMed] [Google Scholar]
- 19.Thompson IM, et al. 2010. Effects of lactation and pregnancy status on concentrations of insulin and IGF-1, and correlations with metabolic indicators in Holstein dairy cattle. J. Dairy Sci. 93, 4006–4018 (doi:10.3168/jds.2009-2941) [DOI] [PubMed] [Google Scholar]
- 20.Sparkman AM, Vleck CM, Bronikowski AM. 2009. Evolutionary ecology of endocrine-mediated life-history variation in the garter snake Thamnophis elegans. Ecology 90, 720–728 (doi:10.1890/08-0850.1) [DOI] [PubMed] [Google Scholar]
- 21.Bininda-Emonds ORP, et al. 2007. The delayed rise of present-day mammals. Nature 446, 507–512 (doi:10.1038/nature05634) [DOI] [PubMed] [Google Scholar]
- 22.Maddison WP, Maddison DR.2004. Mesquite: a modular system for evolutionary analysis, v. 1.05. See http://mesquiteproject.org.
- 23.R Development Core Team 2011. R: a language and environment for statistical computing, v. 2.13.0. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org [Google Scholar]
- 24.Paradis E. 2006. Analysis of phylogenetics and evolution with R. New York, NY: Springer [Google Scholar]
- 25.Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO. 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464 (doi:10.1093/bioinformatics/btq166) [DOI] [PubMed] [Google Scholar]
- 26.Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W. 2008. GEIGER: investigating evolutionary radiations. Bioinformatics 24, 129–131 (doi:10.1093/bioinformatics/btm538) [DOI] [PubMed] [Google Scholar]
- 27.Calder WA. 1984. Size, function, and life history. Cambridge, MA: Harvard University Press [Google Scholar]
- 28.Revell LJ. 2009. Size-correction and principal components for interspecific comparative studies. Evolution 63, 3258–3268 (doi:10.1111/j.1558-5646.2009.00804.x) [DOI] [PubMed] [Google Scholar]
- 29.Grafen A. 1989. The phylogenetic regression. Phil. Trans. R. Soc. Lond. B 326, 119–157 (doi:10.1098/rstb.1989.0106) [DOI] [PubMed] [Google Scholar]
- 30.Martins EP, Hansen TF. 1997. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 149, 646–667 (doi:10.1086/286013) [Google Scholar]
- 31.Swanson EM, Holekamp KE, Lundrigan BL, Arsznov BM, Sakai ST. 2012. Multiple determinants of whole and regional brain volume among terrestrial carnivorans. PLoS ONE 7, e38447 (doi:10.1371/journal.pone.0038447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White CR, Blackburn TM, Seymour RS. 2009. Phylogenetically informed analysis of the allometry of mammalian basal metabolic rate supports neither geometric nor quarter-power scaling. Evolution 63, 2658–2667 (doi:10.1111/j.1558-5646.2009.00747.x) [DOI] [PubMed] [Google Scholar]
- 33.Shipley B. 2009. Confirmatory path analysis in a generalized multilevel context. Ecology 90, 363–368 (doi:10.1890/08-1034.1) [DOI] [PubMed] [Google Scholar]
- 34.Shipley B. 2000. A new inferential test for path models based on directed acyclic graphs. Struct. Equ. Modeling Multidiscip. J. 7, 206–218 (doi:10.1207/S15328007SEM0702_4) [Google Scholar]
- 35.von Hardenberg A, Gonzalez-Voyer A. 2013. Disentangling evolutionary cause–effect relationships with phylogenetic confirmatory path analysis. Evolution 67, 378–387 (doi:10.1111/j.1558-5646.2012.01790.x) [DOI] [PubMed] [Google Scholar]
- 36.Adkins-Regan E. 2008. Do hormonal control systems produce evolutionary inertia? Phil. Trans. R. Soc. B 363, 1599–1609 (doi:10.1098/rstb.2007.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGlothlin J, Ketterson E. 2008. Hormone-mediated suites as adaptations and evolutionary constraints. Phil. Trans. R. Soc. B 363, 1611–1620 (doi:10.1098/rstb.2007.0002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin LB, Liebl AL, Trotter JH, Richards CL, McCoy K, McCoy MW. 2011. Integrator networks: illuminating the black box linking genotype and phenotype. Integr. Comp. Biol. 51, 514–527 (doi:10.1093/icb/icr049) [DOI] [PubMed] [Google Scholar]
- 39.Neville MC, McFadden TB, Forsyth I. 2002. Hormonal regulation of mammary differentiation and milk secretion. J. Mammary Gland Biol. Neoplasia 7, 49–66 (doi:10.1023/A:1015770423167) [DOI] [PubMed] [Google Scholar]
- 40.Gay E, Seurin D, Babajko S, Doublier S, Cazillis M, Binoux M. 1997. Liver-specific expression of human insulin-like growth factor binding protein-1 in transgenic mice: repercussions on reproduction, ante- and perinatal mortality and postnatal growth. Endocrinology 138, 2937–2947 [DOI] [PubMed] [Google Scholar]
- 41.Ashton IK, Zapf J, Einschenk I, Mackenzie IZ. 1985. Insulin-like growth factors (IGF)-1 and (IGF)-2 in human fetal plasma and relationship to gestational age and fetal size during midpregnancy. Acta Endocrinol. 110, 558–563 [DOI] [PubMed] [Google Scholar]
- 42.Gluckman PD, Morel PC, Ambler GR, Breier BH, Blair HT, McCutcheon SN. 1992. Elevating maternal insulin-like growth factor-I in mice and rats alters the pattern of fetal growth by removing maternal constraint. J. Endocrinol. 134, R1–R3 (doi:10.1677/joe.0.134R001) [DOI] [PubMed] [Google Scholar]
- 43.Clapp JF, Schmidt S, Paranjape A, Lopez B. 2004. Maternal insulin-like growth factor-I levels (IGF-1) reflect placental mass and neonatal fat mass. Am. J. Obstet. Gynecol. 190, 730–736 (doi:10.1016/j.ajog.2003.09.061) [DOI] [PubMed] [Google Scholar]
- 44.Kenyon PR, Blair HT, Breier BH, Gluckman PD. 2007. The influence of maternal IGF-1 genotype on birthweight and growth rate of lambs. New Zealand Journal of Agricultural Research 50, 291–297 (doi:10.1080/00288230709510297) [Google Scholar]
- 45.Stuart JA, Page MM. 2010. Plasma IGF-1 is negatively correlated with body mass in a comparison of 36 mammalian species. Mech. Aging Dev. 131, 591–598 (doi:10.1016/j.mad.2010.08.005) [DOI] [PubMed] [Google Scholar]
- 46.Wilson DE, Reeder DM. 2005. Mammal species of the world, a taxonomic and geographic reference, 3rd edn Baltimore, MD: Johns Hopkins University Press [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All code and data are accessible in non-proprietary format in the electronic supplementary material.


